Introduction
The incidence of asthma remains high worldwide. As a
chronic airway inflammatory disease, asthma not only harms
patient's physical and mental health, but is also considered a
burden to society (1,2). There are several clinical
interventions used to treat asthma, including traditional treatment
with glucocorticoids; however, the effects of treatment are not
always satisfactory. Glucocorticoid therapy is an effective
treatment for asthma; however, its side effects greatly constrain
clinical applications (3).
Therefore, it is necessary to explore novel therapeutic strategies
for the treatment of asthma.
Various cells, including eosinophils, basophils and
cluster of differentiation (CD)4+ T cells, and several
proinflammatory mediators, such as tumor necrosis factor-α and
interleukin (IL)-17, are involved in the chronic respiratory
inflammatory responses of asthma (4,5).
However, the mechanism underlying asthma remains largely unknown.
CD4+ T cells are critical cellular mediators of asthma
and inflammation. The CD4+ T cell subset can be divided
into T helper (Th)1 and Th2 cells, according to the type of
cytokines they release and their function (6). The impaired balance of Th1/Th2, and
the abnormal polarization of Th2 are considered the most important
mechanisms of asthma (7). During
the development of asthma, Th2 cells orchestrate the inflammatory
microenvironment via the production of Th2 cytokines (IL-4, IL-5
and IL-13), thus contributing to the pathological process of asthma
(8). The balance between Th1 and
Th2 cells is finely tuned and their differentiation is reciprocally
inhibited (9); therefore,
increased Th1 polarization is important for the control of
asthma.
Dendritic cells (DCs) are the most important
antigen-presenting cells; they are able to present antigens to
naïve CD4+ T cells, and mediate their activation and
differentiation (10). In the
pulmonary system, DCs predominantly exist in the bronchial
epithelium, subepithelial tissue and bronchial lymph nodes, where
they exhibit a grid-like distribution. DCs cluster with T cells in
the subepithelial tissue and finely orchestrate the polarization of
Th cells, which serves a key role in the regulation of inflammation
and airway tolerance (11,12). It has previously been reported that
following injection of ovalbumin (OVA) into the airway, DCs can
activate naïve CD4+ T cells surrounding the pulmonary
lymph nodes to a Th2 phenotype, thus inducing asthma (13). The release of IL-12 from DCs has a
central role in the induction of Th1 cell differentiation and
IL-12-dependent DC-induced Th1 cell differentiation may suppress
murine asthma (14,15). Decreased IL-12 levels are involved
in Th2 polarization and are associated with the severity of asthma
(16). CD38, which consists of 304
amino acids, is a single chain type 2 transmembrane glycoprotein
that belongs to the multifunctional ectoenzyme family (17,18).
CD38 is expressed in several types of cells, including T cells, B
cells and monocytes (19,20). Furthermore, as a multifunctional
ectoenzyme, CD38 possesses numerous immunologically relevant
functions. For example, CD38 participates in T-cell activation,
B-cell growth and prevents apoptosis of tonsillar germinal center B
cells (21). Compared with wild
type mice, the splenocytes from CD38-knockout mice were shown to
secrete reduced interferon (IFN)-γ and increased IL-4, thus
suggesting that CD38 has a role in Th1 polarization (22). In addition, it has been reported
that CD38 expression fluctuates during differentiation of human
monocyte-derived DCs. CD38 was downregulated during differentiation
into immature monocyte-derived DCs, whereas expression was restored
upon maturation. When CD38 signaling was suppressed,
monocyte-derived DCs exhibited a more immature phenotype and a
reduced ability to present antigens and produce IL-12 (23).
Due to the key role of IL-12 in the polarization of
Th1 cells, the present study aimed to determine whether
overexpression of CD38 in DCs was able to improve IL-12 production
and Th1 cell polarization. Furthermore, the effects of CD38
overexpression in DCs on asthma development were determined.
Materials and methods
Reagents
Recombinant mouse granulocyte macrophage
colony-stimulating factor (rmGM-CSF) and IL-4 were purchased from
PeproTech (Rocky Hill, NJ, USA). Mouse Naïve CD4+ T cell Isolation
kit was obtained from Miltenyi Biotec GmbH (Bergisch Gladbach,
Germany). Anti-mouse IL-4 (BE0045) antibodies were purchased from
Bio X Cell (West Lebanon, NH, USA). Anti-mouse major
histocompatibility complex class II (MHC-II) (107607), anti-mouse
CD80 (104707), anti-mouse CD86 (105007) and anti-mouse CD40
(124609) antibodies were purchased from Biolegend (San Diego, CA,
USA). Anti-mouse CD38 (90), anti-mouse CD4 (17–0041), anti-mouse
IFN-γ (11–7311) antibodies; mouse IL-4 (80-7044-22), IL-5
(80-7054-22), IL-13 (88-7137-22), IFN-γ (88-7314-22) and
immunoglobulin (Ig)E (88-50460-22) enzyme-linked immunosorbent
assay (ELISA) kits; and the Intracellular Fixation &
Permeabilization Buffer Set were purchased from eBioscience (San
Diego, CA, USA). Anti-phosphorylated (p)-p38 (sc-7973) and p38
(sc-7972) antibodies, and the p38-specific inhibitor SB203580 (10
µg/ml at 37°C for 30 min) were purchased from Santa Cruz
Biotechnology, Inc. (Dallas, TX, USA). Lipopolysaccharide (LPS)
from Escherichia coli and OVA were purchased from
Sigma-Aldrich (Merck Millipore, Darmstadt, Germany).
Mice
For all experiments, female mice were used. A total
of 15 mice were used to induce BMDCs, 25 to isolate splenocytes and
72 to establish the asthma model. Female C57BL/6J mice (age, 6–8
weeks; weight, 18–20 g) were purchased from Joint Ventures Sipper
BK Experimental Animal Co., Ltd. (Shanghai, China). Mice were
maintained in specific pathogen-free facilities with temperature
ranging from 22–24°C, humidity ranging from 50–60% and 12 h of
light/dark cycle at Zhejiang University (Hangzhou, China). Mice had
free access to food and water and were sacrificed by
intraperitoneal injection with nembutal (160 mg/kg; Sigma-Aldrich;
Merck Millipore). All experiments using mice were approved by and
performed according to the guidelines of the Animal Ethics
Committee of Zhejiang University.
Construction of recombinant mouse CD38
adenovirus (Ad-CD38)
Ad-CD38 was constructed using the AdMax™ system
(Microbix Biosystems, Inc., Mississauga, OR, Canada). Briefly, the
DNA fragment for murine CD38 was amplified from the splenocytes
(1×106 cells) of C57BL/6 mice by polymerase chain
reaction (PCR) using the following specific primers: Sense,
5′-GGGGTACCTTCGGGAGCCCAATGGCTAA-3′ and antisense,
5′-GCTCTAGAAGTCCAGGCTACAGGTGATCTAA-3′. The primers were synthesized
by Sangon Biotech (Shanghai) Co., Ltd. (Shanghai, China). The PCR
was performed under the following conditions: 5 min at 94°C; 30
cycles for 45 sec at 94°C, 45 sec at 55°C, and 90 sec at 72°C, and
ended with 10 min at 72°C. The CD38 sequence was subsequently
inserted into a pDC315 shuttle vector (BioVector, Beijing, China)
and then 5 µg pDC315 vector was mixed with 5 µg pBHGloxΔE1, 3Cre Ad
backbone plasmid (BioVector), 10 µl Lipofectamine 2000 (Thermo
Fisher Scientific, Inc.). The mixture was transfected into HEK293
cells (American Type Culture Collection, Manassas, VA, USA).
Approximately 10–15 days later, the cells floated and were
collected in 1 ml phosphate-buffered saline (PBS). Then the cells
were subjected to 3 cycles of freeze-thaw and the cell debris was
removed by centrifugation (12,000 × g for 15 min). The supernatants
were then rich in Ad-CD38, and were stored at −80°C until further
use.
Generation of bone marrow-derived DCs
(BMDCs) and Ad infection
After removing all muscle tissues with gauze from
the femurs and tibias, the bones were placed in a 60-mm dish with
70% alcohol for 1 min, washed twice with PBS, and transferred into
a fresh dish with RPMI 1640. Both ends of the bones were cut with
scissors in the dish, and then the marrow was flushed out using 2
ml RPMI 1640 with a syringe and 25-gauge needle. The tissue was
suspended, passed through nylon mesh to remove small pieces of bone
and debris, and red cells were lysed with ammonium chloride. Then
the bone marrow mononuclear cells were cultured at a density of
2×106 cells/ml in RPMI 1640 medium supplemented with 10%
fetal calf serum (Thermo Fisher Scientific, Inc., Waltham, MA,
USA), 10 ng/ml rmGM-CSF and 1 ng/ml IL-4 at 37°C. Non-adherent
cells were gently washed away after 48 h of culture; the remaining
loosely adherent clusters were cultured for a further 48 h and were
harvested for Ad transduction. For Ad infection, 1×106 BMDCs were
mixed with 5×107 PFU Ad in a total volume of 1 ml serum-free medium
for 24 h.
Fluorescence-activated cell sorting
(FACS) analysis
To confirm CD38 expression, Ad-infected BMDCs were
stained with anti-CD38 at 4°C for 20 min. Ad-infected BMDCs were
stimulated with or without 100 ng/ml LPS for 24 h at 37°C. The
BMDCs were then collected and stained with MHC-II, CD80, CD86 and
CD40 antibodies at 4°C for 20 min. The non-specific binding of
BMDCs and antibodies was determined by isotype control antibodies
staining at 4°C for 20 min. For the induction of Th1 cell
differentiation, CD4+ naïve T cells from splenocytes were purified
using the Mouse Naïve CD4+ T cell Isolation kit according to the
manufacturers instructions. Subsequently, 2×106/ml naïve CD4+ T
cells were seeded into 1 µg/ml anti-CD3 (BE0002; Bio X Cell) and 1
µg/ml anti-CD28 (BE0015-1; Bio X Cell) pre-coated 96-well plates in
the presence of 10 µg/ml anti-IL-4. Ad-CD38-infected BMDCs
(Ad-CD38/BMDCs), Ad-LacZ-infected BMDCs (Ad-LacZ/BMDCs) or equal
volume PBS-treated BMDCs (Control/BMDCs) were added at a ratio of
10:1. Ad-LacZ was donated by Dr Zhijian Cai (Zhejiang University).
CD4+ naïve T cells co-cultured with each group of BMDCs
without anti-CD3 and anti-CD28 stimulation were designated as Th0
cells. After 3 days of induction at 37°C, the cells were collected
and stimulated with phorbol 12-myristate 13-acetate/ionomycin for 5
h. The cells were then stained with anti-CD4 antibody at 4°C for 20
min, were fixed and permeabilized using the Intracellular Fixation
& Permeabilization Buffer set, and were stained with anti-IFN-γ
antibody at 4°C for 20 min. All cells were examined by flow
cytometry, and data were analyzed using the FlowJo software,
version 7.6 (FlowJo, LLC, Ashland, OR, USA).
Quantitative (q)PCR
Th1 cells were induced as aforementioned. After 3
days of coculture with BMDCs, CD4+ T cells were sorted and total
RNA was extracted from the CD4+ T cells using TRIzol®
reagent (Invitrogen; Thermo Fisher Scientific, Inc.) according to
the manufacturer's protocol. The RNA was then reverse transcribed
into cDNA using ReverTra Ace qPCR RT Kit (FSQ-101; Toyobo Co.,
Ltd., Osaka, Japan) according to the manufacturer's protocol and
the qPCR was performed using SYBR Premix Ex Taq (RR420A; Takara
Bio, Inc., Kusatsu, Japan) in a final volume of 20 µl according to
the manufacturer's protocol. The specific primers synthesized by
Sangon Biotech (Shanghai) Co., Ltd. used for qPCR were: β-actin,
sense 5′-CGTTGACATCCGTAAAGACC-3′, antisense
5′-AACAGTCCGCCTAGAAGCAC-3′; T-bet, sense 5′-AGCAAGGACGGCGAATGTT-3′,
antisense 5′-GGGTGGACATATAAGCGGTTC-3′; and IFN-γ, sense
5′-AGCGGCTGACTGAACTCAGATTGTAG-3′ and antisense
5′-GTCACAGTTTTCAGCTGTATAGGG-3′. The following PCR conditions were
used: 1 cycle at 95°C for 30 sec, followed by 40 cycles at 95°C for
5 sec and 60°C for 34 sec. qPCR was performed using an Applied
Biosystems 7500 Real Time PCR system (Applied Biosystems; Thermo
Fisher Scientific, Inc.). The relative mRNA levels of the target
gene were calculated by the following formula: 2(Ct of target gene
- Ct of β-actin). The relative mRNA levels were normalized by the
relative mRNA level of one sample from the Control/BMDCs + LPS
group.
Protocol for the model of
allergen-challenged mice
Mice were sensitized on days 0 and 14 by
intraperitoneal injection of 0.08 mg OVA and 0.1 ml aluminum
hydroxide in 0.1 ml PBS or PBS alone. A total of 1×106 Ad-infected
BMDCs were intravenously injected into the OVA-sensitized mice on
day 23. Subsequently, OVA- or PBS-sensitized mice were exposed to
aerosolized 1% OVA/0.01% LPS/PBS or normal saline for 40 min,
respectively, once per day for 3 consecutive days (days 24–26). On
day 27, the mice were sacrificed by intraperitoneal injection with
nembutal (160 mg/kg) and the lungs were divided into two groups for
analysis: The left lung lobes were lavaged three times with 1 ml
PBS containing 1% fetal calf serum and 5 U/ml heparin, and the
right lung lobes were fixed with 4% paraformaldehyde for
hematoxylin (0.5%; 5 min) and eosin (1%; 30 sec) and Periodic acid
(0.1%; 10 min) and Schiff (0.5%; 10 min) Schiff staining. Imaging
was conducted using the BX53 microscope (Olympus Corporation,
Tokyo, Japan).
Cytokine assays
Ad-infected BMDCs were stimulated with or without
100 ng/ml LPS for 24 h. To collect sera, mice were anesthetised by
intraperitoneal injection with nembutal (80 mg/kg). Blood was
exsanguinated from the heart with a syringe and 25-gauge needle and
sera were collected after incubation at room temperature for 30
min. IL-12 levels in the supernatant were detected by ELISA. The
levels of IL-4, IL-5, IL-13 and IFN-γ in the bronchoalveolar lavage
fluid (BALF), and the levels of IgE in the sera were also detected
by ELISA. Th1 cells were induced as aforementioned. After 3 days of
coculture with BMDCs, CD4+ T cells were sorted and re-stimulated
with 1 µg/ml anti-CD3 and 1 µg/ml anti-CD28 (pre-coated onto
plates) for 24 h. Subsequently, the levels of IFN-γ in the
supernatant were measured by ELISA. All ELISA assays were conducted
according to manufacturer's instructions.
Western blot analysis
For the detection of p-p38, Ad-infected BMDCs were
stimulated with 100 ng/ml LPS for the indicated duration.
Subsequently, the cells were washed and lysed by RIPA lysis buffer
(Beyotime Institute of Biotechnology, Haimen, China). The protein
concentrations of lysates were measured by the Bradford assay kit
(Thermo Fisher Scientific, Inc.). Cell proteins (20 µg) were
separated by 10% sodium dodecyl sulfate-polyacrylamide gel
electrophoresis, and were transferred onto polyvinylidene
difluoride membranes. Membranes were blocked by Tris-buffered
saline with Tween-20 containing 5% bovine serum albumin
(Sigma-Aldrich; Merck Millipore) at room temperature for 2 h and
then incubated with p-p38 (1:1,000) and p38 (1:1,000) primary
antibodies at room temperature for 1 h, followed by horseradish
peroxidase-coupled secondary antibody (sc-2005; 1:500) at room
temperature for 1 h. Specific bands on the membrane were then
visualized using an enhanced chemiluminescence (ECL) kit (ECL
Detection kit; Amersham Biosciences; GE Healthcare Life Sciences,
Little Chalfont, UK).
Statistical analysis
Data are presented as the mean ± standard error of
the mean. The significance of differences between groups was
estimated using the unpaired Student's t-test for two groups, or
one-way analysis of variance followed by the Newman-Keuls test for
multiple group comparisons. GraphPad Prism softtware, version 5
(GraphPad, Inc., La Jolla, CA, USA) was used for statistical
analysis. P<0.05 was considered to indicate a statistically
significant difference.
Results
CD38 promotes LPS-induced maturation
of DCs and IL-12 secretion
The number of natural DCs is very limited,
therefore, BMDCs are widely used for study of DC function in
vitro and in vivo. CD38 was overexpressed in BMDCs
following Ad-CD38 infection. To confirm the optimal multiplicity of
infection (MOI), BMDCs were infected with Ad-CD38 at the following
MOI: 10, 25, 50 and 100. A total of 24 hours after infection, CD38
expression was detected in BMDCs by FACS. As MOI increased, CD38
expression in BMDCs was elevated. However, there was no difference
in CD38 expression between BMDCs infected with an MOI of 50 or 100
(Fig. 1A). Therefore, for
subsequent experiments, MOI 50 was selected for BMDC infection.
Subsequently, CD38 expression was compared between Ad-CD38/BMDCs,
Ad-LacZ/BMDCs or Control/BMDCs. The results demonstrated that
Ad-CD38/BMDCs, but not Ad-LacZ/BMDCs or Control/BMDCs, exhibited
increased CD38 expression (Fig.
1B). By detecting the expression levels of MHC-II, CD80, CD86
and CD40 molecules, overexpression of CD38 was not able to affect
DC maturation. However, following LPS stimulation, a more mature
phenotype of Ad-CD38/BMDCs was detected compared with the
Ad-LacZ/BMDCs and Control/BMDCs (Fig.
1C). The levels of IL-12 in Ad-CD38/BMDCs, Ad-Lac Z/BMDCs and
Control/BMDCs were low and showed no difference without LPS
stimulation (data not shown). Similarly, a higher production of
IL-12 could only be detected in Ad-CD38/BMDCs following LPS
stimulation (Fig. 1D). These
results suggest that CD38 synergizes with LPS to promote DC
maturation and IL-12 secretion.
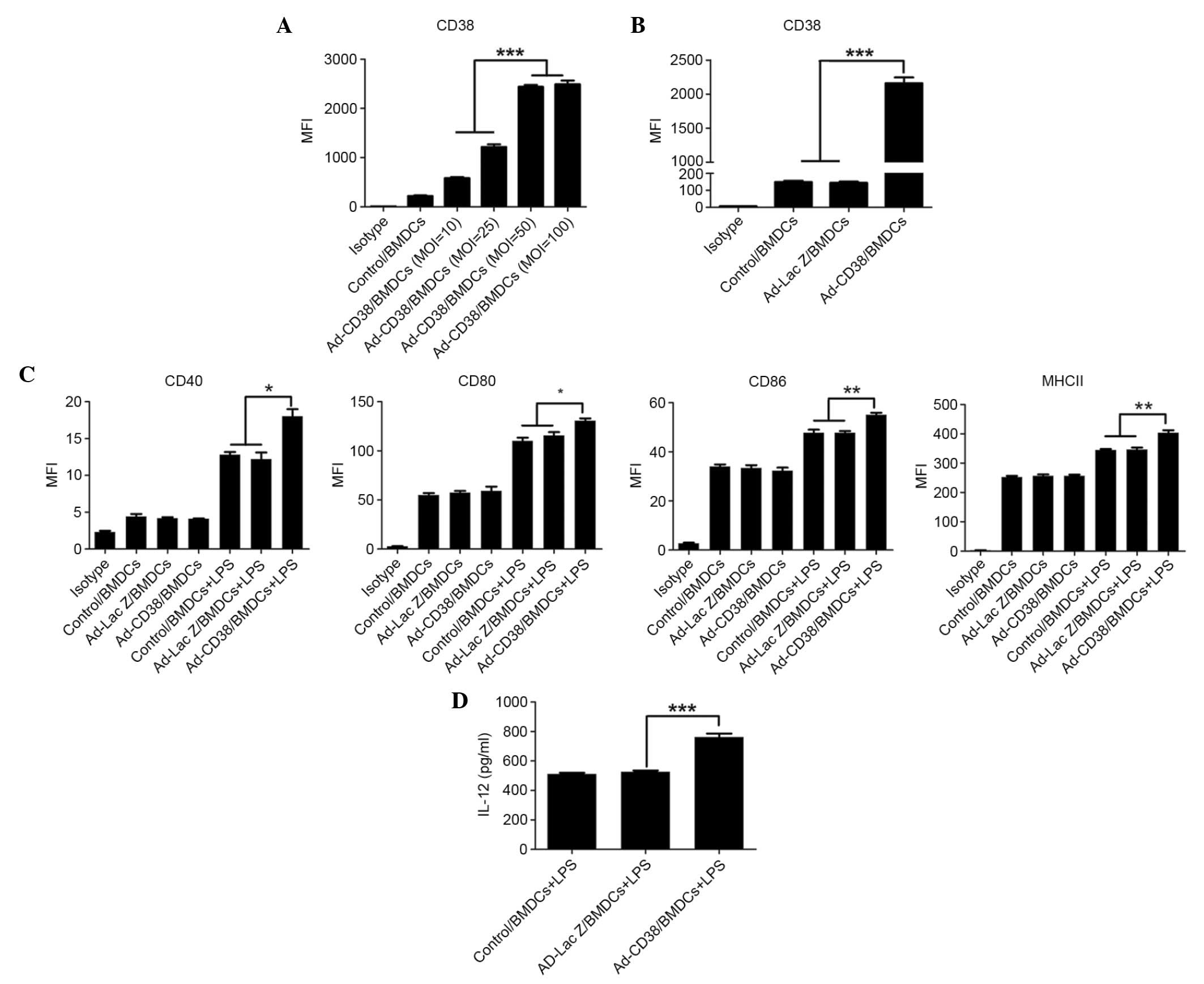 | Figure 1.CD38 promotes LPS-induced maturation
of BMDCs and IL-12 secretion. (A) BMDCs were infected with Ad-CD38
at an MOI of 10, 25, 50 or 100 for 24 h. CD38 expression in BMDCs
was detected by FACS. (B-D) BDMCs were infected with Ad-CD38 (MOI
50) Ad-LacZ or were mock infected for 24 h. (B) CD38 expression in
these BMDCs was detected by FACS. (C) Ad-infected BMDCs were
stimulated with or without 100 ng/ml LPS for 24 h. MHC-II, CD80,
CD86 and CD40 expression was detected by FACS (n=3). (D) IL-12
production in the supernatants was detected by enzyme-linked
immunosorbent assay (n=5). Data are representative of three
independent experiments. *P<0.05, **P<0.01, ***P<0.001.
CD, cluster of differentiation; LPS, lipopolysaccharide; BMDCs,
bone marrow-derived dendritic cells; IL, interleukin; MOI,
multiplicity of infection; FACS, fluorescence-activated cell
sorting; Ad, adenovirus; MHC-II, major histocompatibility complex
class II; MFI, mean fluorescent intensity. |
LPS-stimulated Ad-CD38/BMDCs promote
Th1 differentiation
Since Ad-CD38/BMDCs stimulated with LPS produced
more IL-12, the present study aimed to determine whether
Ad-CD38/BMDCs were more effective at inducing Th1 cell
differentiation. Naïve CD4+ T cells were isolated using the Naïve
CD4+ T cell Isolation kit, and the purity of naïve CD4+ T cells was
~95% (Fig. 2A). Naïve CD4+ T cells
were cocultured with BMDCs at a ratio of 10:1 for 3 days in Th1
cell-skewing conditions; with the exception of IL-12. As expected,
the mRNA expression levels of IFN-γ and T-bet, and the protein
levels of IFN-γ were higher in CD4+ T cells cocultured with
LPS-stimulated Ad-CD38/BMDCs, as compared with in CD4+ T cells
cocultured with LPS-stimulated Ad-LacZ/BMDCs or Control/BMDCs
(Fig. 2B and C). To further
confirm these results, IFN-γ levels were measured by intracellular
staining of IFN-γ in CD4+ T cells. As shown in Fig. 2D, the highest percentage of IFN-γ+
CD4+ T cells was detected in CD4+ T cells cocultured with
LPS-stimulated Ad-CD38/BMDCs (Fig.
2D). These results indicate that LPS-stimulated Ad-CD38/BMDCs
exhibit a higher potential for inducing Th1 cell
differentiation.
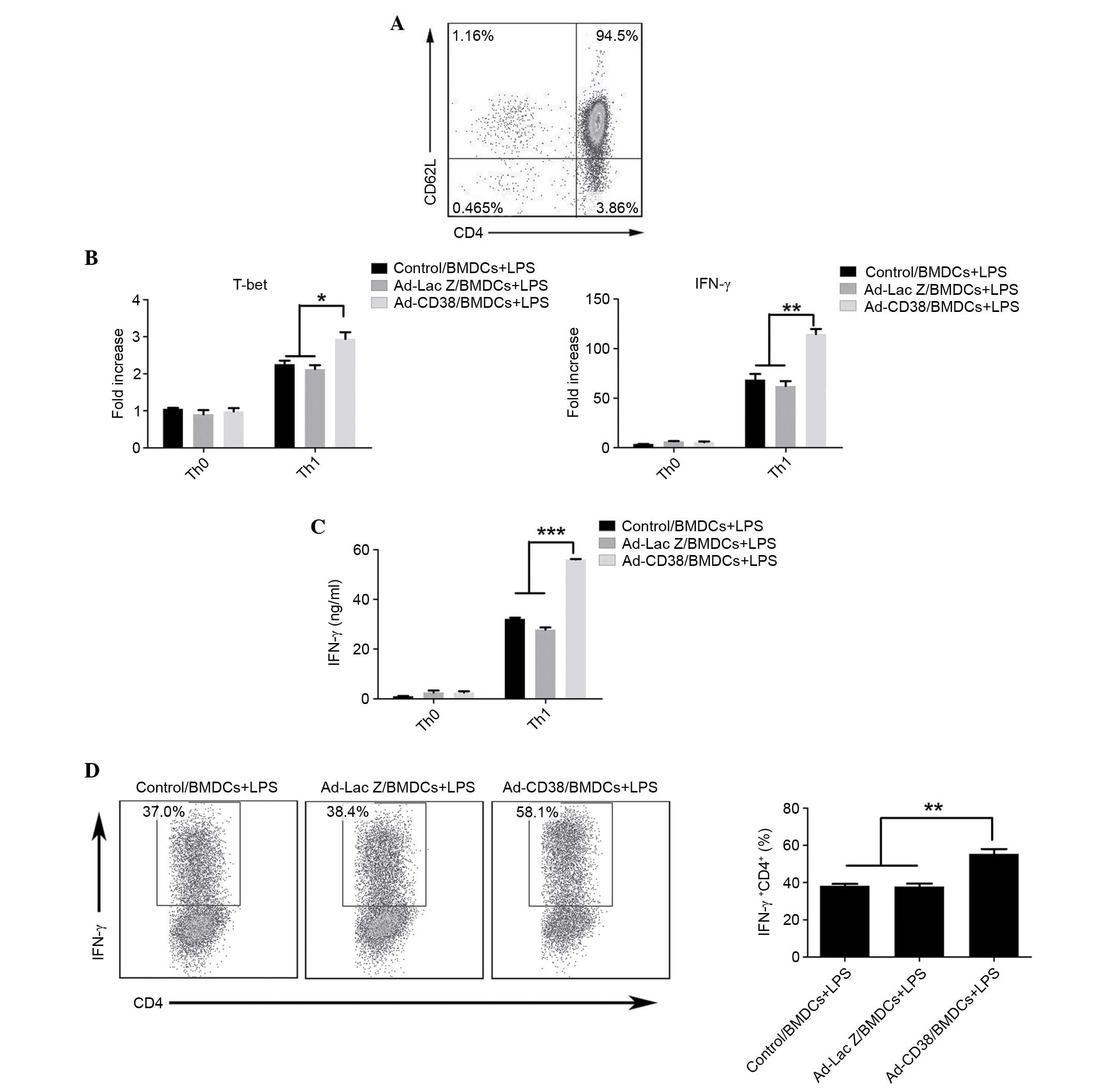 | Figure 2.LPS-stimulated Ad-CD38/BMDCs promote
Th1 differentiation. (A) Naïve CD4+ T cells were
isolated and purity was confirmed by FACS. (B-D) A total of
2×106/ml naïve CD4+ T cells were seeded into
1 µg/ml anti-CD3 and 1 µg/ml anti-CD28 pre-coated 96-well plates in
the presence of 10 µg/ml anti-IL-4. Meanwhile, each group of BMDCs
was added at a ratio of 10:1. After 3 days of induction,
CD4+ T cells were isolated. (B) The mRNA expression
levels of IFN-γ and T-bet were detected in CD4+ T cells
by quantitative polymerase chain reaction. (C) CD4+ T
cells were re-stimulated with 1 µg/ml anti-CD3 and 1 µg/ml
anti-CD28 (pre-coated onto plates) for 24 h and the protein
expression levels of IFN-γ were detected in the supernatant by
enzyme-linked immunosorbent assay. (D) After 3 days of induction,
the percentage of IFN-γ+ CD4+ T cells was
detected by FACS following intracellular staining with IFN-γ. Data
are representative of three independent experiments, each with n=3.
*P<0.05, **P<0.01, ***P<0.001. LPS, lipopolysaccharide;
Ad, adenovirus; CD, cluster of differentiation; BMDCs, bone
marrow-derived dendritic cells; Th1, T helper 1; FACS,
fluorescence-activated cell sorting; IL, interleukin. |
Ad-CD38/BMDCs can alleviate the
severity of murine asthma
To determine whether Ad-CD38/BMDCs exert protective
effects on murine asthma, 1×106 Ad-infected BMDCs were
intravenously injected into OVA antigen-immunized mice 24 h prior
to the first OVA antigen challenge. After three OVA challenges, the
recipients of Control/BMDCs, Ad-LacZ/BMDCs and Ad-CD38/BMDCs all
developed airway inflammation, which was characterized by goblet
cell hyperplasia, peribronchovascular eosinophilic infiltration,
increased production of mucus, and a large number of total cells
and eosinophil fractions in the BALF (Fig. 3A and B). However, the recipients of
Ad-CD38/BMDCs exhibited the mildest symptoms of asthma (Fig. 3A and B). In addition, production of
IL-4, IL-5 and IL-13 was increased in the BALF, increased IgE was
detected in the sera, and production of IFN-γ was reduced in the
BALF of recipients of Control/BMDCs and Ad-LacZ/BMDCs (Fig. 3C). These results suggest that
Ad-CD38/BMDCs may inhibit asthma development in vivo,
probably through regulating the Th1/Th2 cell balance.
 | Figure 3.Administration of Ad-CD38/BMDCs can
alleviate the severity of murine asthma. Mice were sensitized with
PBS or OVA plus aluminium hydroxide on days 0 and 14. On day 23,
1×106 Control/BMDCs, Ad-LacZ/BMDCs or Ad-CD38/BMDCs were
intravenously injected into OVA-sensitized mice (6 mice/group). The
mice were then challenged with aerosolized PBS containing 1% OVA
and 0.01% LPS (OVA-sensitized mice) or challenged with aerosolized
NS (PBS-sensitized mice) for 3 consecutive days (days 24–26). Mice
were sacrificed 24 h after the last challenge and the lungs were
isolated. (A) Formalin-fixed lung tissue sections were stained with
H&E or PAS (magnification, ×400). (B) Total cells and
eosinophils in the BALF were enumerated. (C) Levels of IL-4, IL-5,
IL-13 and IFN-γ in the BALF, and IgE in the sera were measured by
enzyme-linked immunosorbent assay. Data are representative of three
independent experiments, each with n=5. *P<0.05, **P<0.01.
Ad, adenovirus; CD, cluster of differentiation; BMDCs, bone
marrow-derived dendritic cells; PBS, phosphate-buffered saline;
LPS, lipopolysaccharide; NS, normal saline; OVA, ovalbumin; BALF,
bronchoalveolar lavage fluid; IL, interleukin; IFN-γ, interferon-γ;
IgE, immunoglobulin E; H&E, hematoxylin and eosin; PAS,
Periodic acid-Schiff; OD, optical density. |
CD38 is dependent on the p38 signaling
pathway to promote LPS-induced BMDCs to release IL-12 and induce
Th1 differentiation
It has previously been reported that activation of
the p38 signaling pathway is involved in IL-12 secretion by
macrophages (24). The present
study determined whether CD38 promoted LPS-induced BMDCs to secrete
IL-12 via the p38 signaling pathway. Following LPS stimulation, the
expression levels of p-p38 were increased in Control/BMDCs,
Ad-LacZ/BMDCs and Ad-CD38/BMDCs; the biggest increase was detected
in Ad-CD38/BMDCs (Fig. 4A). To
further confirm the p38 signaling pathway was associated with
increased IL-12 production in LPS-stimulated Ad-CD38/BMDCs, BMDCs
were pre-treated with a p38-specific inhibitor, SB203580. The
results demonstrated that there was no significant difference in
IL-12 production between the Control/BMDCs, Ad-LacZ/BMDCs and
Ad-CD38/BMDCs following LPS stimulation (Fig. 4B). However, following pretreatment
of BMDCs with SB203580, IL-12 secretion was decreased and the
increased induction of Th1 cell differentiation by Ad-CD38/BMDCs
was abrogated (Fig. 4B and C).
These results suggest that LPS-stimulated Ad-CD38/BMDCs depend on
the p38 signaling pathway to promote IL-12 production and Th1 cell
differentiation.
 | Figure 4.CD38 promotes LPS-induced BMDCs to
release IL-12 and induce Th1 differentiation in a p38-dependent
manner. BMDCs were transduced with Ad-CD38 (multiplicity of
infection 50) or Ad-LacZ, or were mock-infected for 24 h. (A) These
BMDCs were stimulated with or without 100 ng/ml LPS for the
indicated time. The expression levels of p-p38 were detected by
western blotting. (B and C) BMDCs were then pre-treated with or
without 50 nM SB203580 for 30 min and were stimulated with 100
ng/ml LPS for 24 h. (B) IL-12p70 production in the supernatants was
detected by enzyme-linked immunosorbent assay (n=5). (C) Th1 cell
differentiation was induced. After 3 days of induction, the
percentage of IFN-γ+ CD4+ T cells was
detected by FACS following intracellular staining of IFN-γ (n=3).
Data are representative of three independent experiments.
**P<0.01. CD, cluster of differentiation; LPS,
lipopolysaccharide; BMDCs, bone marrow-derived dendritic cells; Ad,
adenovirus; p-, phosphorylated; Th1, T helper 1; FACS,
fluorescence-activated cell sorting; IFN-γ, interferon-γ. |
Discussion
It is well known that a dysregulated Th1/Th2 balance
is the predominant mechanism underlying asthma development. The
present study demonstrated that overexpression of CD38 synergized
with LPS to induce IL-12 secretion from BMDCs and Th1
differentiation. CD38-overexpressing BMDCs were effective at
alleviating the severity of asthma, which was accompanied by a
decrease in the Th2 master cytokine IL-4, and an increase in the
Th1 master cytokine IFN-γ in the BALF of asthmatic mice. These
results suggested that CD38 may be involved in the regulation of
asthma development via its effects on the Th1/Th2 balance.
Consistent with the results of a previous study,
CD38 is able to synergize with LPS to induce IL-12 secretion from
DCs (25). However in this
previous study, the authors did not elucidate whether activation of
the CD38 signal alone could promote IL-12 secretion from DCs.
According to the results of the present study, the CD38 signal
alone exhibited no effect on IL-12 secretion of DCs. The CD38
signal is more inclined to regulate LPS-induced signal activation
in DCs, particularly via the p38 signaling pathway. As LPS is the
natural ligand for Toll-like receptor (TLR) 4 (26), it is suggested that there is a
point of intersection between TLR4 signaling and the CD38 signal.
Unlike in vitro results, administration of Ad-CD38/BMDCs,
without LPS stimulation, was able to alleviate asthmatic symptoms
in vivo. The TLR4 signal in DCs has been reported to be
activated in asthmatic mice (27).
TLR4 signaling in Ad-CD38/BMDCs may also be activated in asthmatic
mice, and CD38 signaling may possibly further promote activation of
TLR4 signaling in Ad-CD38/BMDCs.
CD31 is a platelet endothelial cell adhesion
molecule, which is a member of the immunoglobulin superfamily and
is considered the counter-receptor of CD38. DCs express CD31 and
CD38 at the same time (data not shown), so they may activate CD38
signal by themselves. In addition, CD31 has been demonstrated to be
overexpressed in endothelial cells (28,29),
suggesting CD38 signals in DCs may also be activated by endothelial
cells. Therefore, it is reasonable to suggest that CD38 signaling
in DCs is easily activated in vivo, thus indicating the
important function of CD38 in DCs.
In conclusion, the present study demonstrated that
overexpression of CD38 in BMDCs was able to increase LPS-induced
IL-12 secretion and promote Th1 cell differentiation in a p38
signaling pathway-dependent manner. CD38-overexpressing BMDCs
exhibited protective effects on murine asthma, potentially via
restoration of the Th1/Th2 balance. Therefore, CD38 may be
considered a promising candidate for the treatment of asthma.
Acknowledgements
The present study was supported by the National
Natural Science Foundation of China (grant nos. 81200014 and
81300203), the Public Welfare Technology Application Research
Project of Zhejiang Province (grant no. 2013C33146) and the
Medicine and Health Foundation of the Health Bureau of Zhejiang
Province (grant no. 2012KYA152).
Glossary
Abbreviations
Abbreviations:
|
DCs
|
dendritic cells
|
|
BMDCs
|
bone marrow-derived DCs
|
|
Ad-CD38
|
CD38 adenovirus
|
|
Ad-LacZ
|
LacZ adenovirus
|
|
BALF
|
bronchoalveolar lavage fluid
|
|
FACS
|
fluorescence-activated cell
sorting
|
References
|
1
|
D'Amato G, Holgate ST, Pawankar R, Ledford
DK, Cecchi L, Al-Ahmad M, Al-Enezi F, Al-Muhsen S, Ansotegui I,
Baena-Cagnani CE, et al: Meteorological conditions, climate change,
new emerging factors and asthma and related allergic disorders. A
statement of the world allergy organization. World Allergy Organ J.
8:252015. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Sun R, Xu F, Wang C and Dong E: NSFC spurs
significant basic research progress of respiratory medicine in
China. Clin Respir J. 2015.(Epub ahead of print). View Article : Google Scholar
|
|
3
|
Barnes PJ: Glucocorticoids. Chem Immunol
Allergy. 100:311–316. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Fajt ML and Wenzel SE: Asthma phenotypes
and the use of biologic medications in asthma and allergic disease:
The next steps toward personalized care. J Allergy Clin Immunol.
135:299–310; quiz 311. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Shen HH, Xu F, Zhang GS, Wang SB and Xu
WH: CCR3 monoclonal antibody inhibits airway eosinophilic
inflammation and mucus overproduction in a mouse model of asthma.
Acta Pharmacol Sin. 27:1594–1599. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Wills-Karp M: Immunologic basis of
antigen-induced airway hyperresponsiveness. Annu Rev Immunol.
17:255–281. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Neurath MF, Finotto S and Glimcher LH: The
role of Th1/Th2 polarization in mucosal immunity. Nat Med.
8:567–573. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Bosnjak B, Stelzmueller B, Erb KJ and
Epstein MM: Treatment of allergic asthma: Modulation of Th2 cells
and their responses. Respir Res. 12:1142011. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Zhang Y, Zhang Y, Gu W, He L and Sun B:
Th1/Th2 cell's function in immune system. Adv Exp Med Biol.
841:45–65. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Joffre O, Nolte MA, Spörri R and e Sousa C
Reis: Inflammatory signals in dendritic cell activation and the
induction of adaptive immunity. Immunol Rev. 227:234–247. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Hammad H and Lambrecht BN: Dendritic cells
and airway epithelial cells at the interface between innate and
adaptive immune responses. Allergy. 66:579–587. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Wu X and Xu F: Dendritic cells during
Staphylococcus aureus infection: Subsets and roles. J Transl Med.
12:3582014. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Sung S, Rose CE and Fu SM: Intratracheal
priming with ovalbumin- and ovalbumin 323–339 peptide-pulsed
dendritic cells induces airway hyperresponsiveness, lung
eosinophilia, goblet cell hyperplasia and inflammation. J Immunol.
166:1261–1271. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Heufler C, Koch F, Stanzl U, Topar G,
Wysocka M, Trinchieri G, Enk A, Steinman RM, Romani N and Schuler
G: Interleukin-12 is produced by dendritic cells and mediates T
helper 1 development as well as interferon-gamma production by T
helper 1 cells. Eur J Immunol. 26:659–668. 1996. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Koltsida O, Hausding M, Stavropoulos A,
Koch S, Tzelepis G, Ubel C, Kotenko SV, Sideras P, Lehr HA, Tepe M,
et al: IL-28A (IFN-λ2) modulates lung DC function to promote Th1
immune skewing and suppress allergic airway disease. EMBO Mol Med.
3:348–361. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Chen XQ, Yang J, Hu SP, Nie HX, Mao GY and
Chen HB: Increased expression of CD86 and reduced production of
IL-12 and IL-10 by monocyte-derived dendritic cells from allergic
asthmatics and their effects on Th1- and Th2-type cytokine balance.
Respiration. 73:34–40. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Malavasi F, Funaro A, Roggero S,
Horenstein A, Calosso L and Mehta K: Human CD38: A glycoprotein in
search of a function. Immunol Today. 15:95–97. 1994. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Jackson DG and Bell JI: Isolation of a
cDNA encoding the human CD38 (T10) molecule, a cell surface
glycoprotein with an unusual discontinuous pattern of expression
during lymphocyte differentiation. J Immunol. 144:2811–2815.
1990.PubMed/NCBI
|
|
19
|
Deterre P, Berthelier V, Bauvois B,
Dalloul A, Schuber F and Lund F: CD38 in T- and B-cell functions.
Chem Immunol. 75:146–168. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Lande R, Urbani F, Di Carlo B, Sconocchia
G, Deaglio S, Funaro A, Malavasi F and Ausiello CM: CD38 ligation
plays a direct role in the induction of IL-1beta, IL-6, and IL-10
secretion in resting human monocytes. Cell Immunol. 220:30–38.
2002. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Zupo S, Rugari E, Dono M, Taborelli G,
Malavasi F and Ferrarini M: CD38 signaling by agonistic monoclonal
antibody prevents apoptosis of human germinal center B cells. Eur J
Immunol. 24:1218–1222. 1994. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Viegas MS, do Carmo A, Silva T, Seco F,
Serra V, Lacerda M and Martins TC: CD38 plays a role in effective
containment of mycobacteria within granulomata and polarization of
Th1 immune responses against Mycobacterium avium. Microbes Infect.
9:847–854. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Fedele G, Frasca L, Palazzo R, Ferrero E,
Malavasi F and Ausiello CM: CD38 is expressed on human mature
monocyte-derived dendritic cells and is functionally involved in
CD83 expression and IL-12 induction. Eur J Immunol. 34:1342–1350.
2004. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Mathur RK, Awasthi A, Wadhone P,
Ramanamurthy B and Saha B: Reciprocal CD40 signals through p38MAPK
and ERK-1/2 induce counteracting immune responses. Nat Med.
10:540–544. 2004. View
Article : Google Scholar : PubMed/NCBI
|
|
25
|
Frasca L, Fedele G, Deaglio S, Capuano C,
Palazzo R, Vaisitti T, Malavasi F and Ausiello CM: CD38
orchestrates migration, survival and Th1 immune response of human
mature dendritic cells. Blood. 107:2392–2399. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Takeda K, Kaisho T and Akira S: Toll-like
receptors. Annu Rev Immunol. 21:335–376. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Duez C, Gosset P and Tonnel AB: Dendritic
cells and toll-like receptors in allergy and asthma. Eur J
Dermatol. 16:12–16. 2006.PubMed/NCBI
|
|
28
|
Deaglio S, Morra M, Mallone R, Ausiello
CM, Prager E, Garbarino G, Dianzani U, Stockinger H and Malavasi F:
Human CD38 (ADP-ribosyl cyclase) is a counter-receptor of CD31, an
Ig superfamily member. J Immunol. 160:395–402. 1998.PubMed/NCBI
|
|
29
|
Deaglio S, Dianzani U, Horenstein AL,
Fernández JE, van Kooten C, Bragardo M, Funaro A, Garbarino G, Di
Virgilio F, Banchereau J and Malavasi F: Human CD38 ligand. A
120-KDA protein predominantly expressed on endothelial cells. J
Immunol. 156:727–734. 1996.PubMed/NCBI
|


















