Introduction
Arthritis, which includes osteoarthritis (OA) and
rheumatoid arthritis, is a chronic inflammatory disease
characterized by pain and inflammation, progressive joint
destruction, and disability (1).
The proinflammatory cytokine interleukin (IL)-1β has a key role in
arthritis (2); IL-1β is able to
stimulate the production of matrix metalloproteinases (MMPs), which
are enzymes that degrade all components of the extracellular matrix
(3). It has also been suggested
that IL-1β may affect chondrocyte apoptosis (4), which is correlated with the severity
of cartilage damage and is associated with the pathogenesis of
arthritis (5).
Therefore, inhibition of IL-1β-dependent apoptosis
and the function of MMPs may be considered potential therapeutic
strategies. Platelet-rich plasma (PRP) consists of blood plasma
enriched with platelets, and is a rich source of autologous growth
factors, including platelet-derived growth factor (PDGF) and
transforming growth factor (TGF)-β1 (6). PRP is able to promote cell
proliferation, angiogenesis and collagen synthesis, thus
contributing to healing of skeletal muscle, tendon, bone and
ligaments (7,8). PRP (9–11) is
widely used in clinical practice, particularly during aesthetic
plastic surgery and the treatment of soft-tissue ulcers (12–14),
and may improve the survival of surgically implanted adipose tissue
in patients (15,16). Notably, it has been reported that
treatment of chondrocytes with PRP may enhance cell division and
collagen synthesis (17). In light
of the role of PRP in arthritis therapy, determination of its
effects on chondrocyte apoptosis may provide evidence for future
clinical applications.
The present study aimed to determine the effects of
PRP on IL-1β-dependent chondrocyte apoptosis and extracellular
matrix anabolism. The results demonstrated that PRP was able to
inhibit chondrocyte apoptosis induced by IL-1β and promote matrix
anabolism. These results suggested that PRP may protect
chondrocytes from apoptosis, thus indicating a potential
therapeutic strategy for the treatment of arthritis.
Materials and methods
Ethics approval
The present study was approved by the ethics
committee of Beijing Friendship Hospital of the Capital Medical
University of China.
Cell culture
Chondrocytes were isolated from cartilage tissue in
the knee joints of Sprague-Dawley rat neonates. Cartilage samples
from three rat neonates were harvested and pooled for use in the
present study. The animals used were 4-week-old male Sprague-Dawley
rats (70–100 g; obtained from Charles River Laboratories, Inc.,
Wilmington, DE, USA), and they were housed in a wire mesh cage in
an animal room under controlled conditions (temperature, 23±3°C;
relative humidity, 50±20%, 12-h light/dark cycle). Male
Sprague-Dawley rats, on reaching a weight of 180–250 g, were
sacrificed by cervical dislocation. Briefly, cartilage was isolated
and digested in 1 mg/ml collagenase (cat. no. C9263; Sigma-Aldrich,
St. Louis, MO, USA) in Dulbecco's modified Eagle's medium (DMEM;
cat. no. 11965092; Gibco; Thermo Fisher Scientific, Inc., Waltham,
MA, USA) supplemented with 10% Gibco™ fetal bovine serum (FBS) for
16 h at 37°C. Undigested tissue were removed using a 70 µm cell
strainer. Chondrocytes were washed with phosphate-buffered saline
(PBS) and were resuspended in basic medium (DMEM supplemented with
10% FBS, 100 U/ml penicillin and 100 mg/ml streptomycin).
Chondrocytes were subsequently cultured in basic medium in culture
flasks at 37°C in an incubator containing 5% CO2 (BPX-82 incubator;
Shanghai Boxun Medical Biological Instrument Corp., Shanghai,
China).
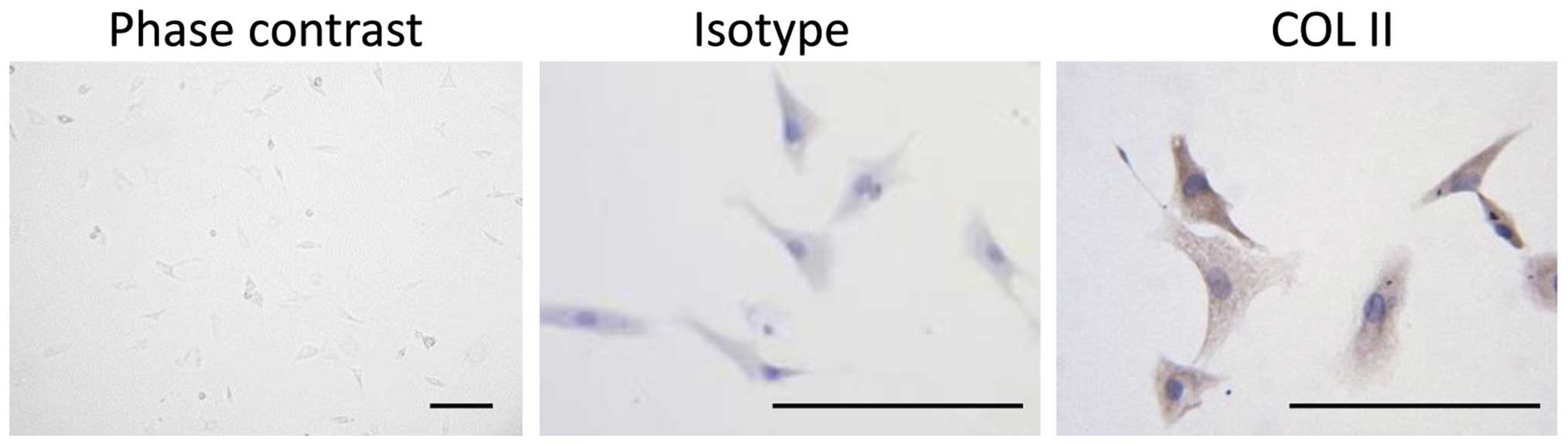
Cultured chondrocytes were characterized by
immunohistochemical staining of collagen type II (COL2). Briefly,
chondrocytes (1×106) were plated on glass cover slips in
six-well plates. After 24 h, the cells were washed with PBS and
were fixed in 4% paraformaldehyde for 30 min at room temperature.
Permeabilization and blocking were performed by incubating the
cells with 1% Triton-X 100 and 1% bovine serum albumin for 15 min.
Cover slips were then incubated overnight at 4°C with rabbit
polyclonal anti-COL2 primary antibodies (cat. no. Bs-11929R;
dilution, 1:500; Bioss, Inc., Woburn, MA, USA). Sequentially,
primary antibodies were visualized using an EnVision Detection
system (cat. no. GK400315; Dako Belgium Nv, Leuven, Belgium) and a
DMLP-MP30 microscope (Leica, Heidelberg, Germany).
PRP preparation
A total of 10 ml blood was collected in a syringe
containing 1 ml 3.8% sodium citrate from the artery of one male rat
(age, 10 weeks). In addition, blood samples were collected from
three rats and processed separately. Blood samples were gently
agitated to thoroughly mix the sodium citrate with the blood.
Subsequently, the samples were centrifuged at 1,500 rpm for 10 min
at 4°C. The plasma and buffy coat layer were then transferred to
another centrifuge tube, and were centrifuged at 3,000 rpm for 10
min at 4°C. PRP was obtained after discarding 3/4 of the upper
layer of plasma. The concentration of platelets in PRP was
1.0–1.5×1012/l. To release growth factors from alpha granules, 8 ml
PRP was mixed with 10% CaCl2 (containing 1,000 U/ml
thrombin) with a ratio of 9:1 (volume/volume). The mixture was
centrifuged at 4,000 rpm for 10 min at 4°C. Approximately 7.5 ml of
supernatant was obtained from one preparation. The supernatant was
then mixed with DMEM (high-glucose) supplemented with 10% FBS to
prepare medium with various concentrations of PRP.
Cell counting kit-8 (CCK8) assay
Cell proliferation was monitored using the
colorimetric water-soluble tetrazolium salt (CCK8) assay. Briefly,
100 µl cell mixtures (2×105cells) were seeded onto
96-well plates. After adding various concentrations of PRP (1, 2,
5, 10 and 25%, volume/volume), all samples were incubated for 24,
48 or 72 h (37°C, 5% CO2). Following incubation with 10 µl CCK
solution for 2 h, the number of viable cells was assessed by
measuring the absorbance at 450 nm. Cells cultured in basic medium
(DMEM supplemented with 10% FBS, 100 U/ml penicillin and 100 mg/ml
streptomycin) were used as a control group.
PRP was prepared and diluted with PBS to obtain five
different concentrations (1, 2, 5, 10 and 25%, volume/volume),
which was added to the chondrocytes and incubated for 24, 48 and 72
h. Chondrocytes were incubated with three concentrations of IL-1β
(5, 10 and 50 ng/ml) for 24, 48 and 72 h.
Quantitative polymerase chain reaction
(qPCR)
Total RNA was isolated from the cells using
TRIzol® reagent (1 ml per 107 cells; Invitrogen; Thermo
Fisher Scientific, Inc.). Reverse transcription was conducted using
the Takara PrimeScript II 1st Strand cDNA Synthesis kit (Takara
Biotechnology Co., Ltd., Dalian, China), according to the
manufacturer's protocol.
All primers used in the present study were designed
using Primer 3.0 software (Premier Biosoft, Palo Alto, CA, USA).
The primer sequences used were as follows: B-cell lymphoma 2
(Bcl-2), forward 5′CTGGTGGACAACATCGCTCT3′, reverse
5′GCATGCTGGGGCCATATAGT3′; Bcl-2-associated X protein (Bax), forward
5′AGGACGCATCCACCAAGAAG3′, reverse 5′CAGTTGAAGTTGCCGTCTGC3′;
caspase-3, forward 5′GGAGCTTGGAACGCGAAGAA3′, reverse
5′ACACAAGCCCATTTCAGGGT3′; poly (ADP-ribose) polymerase PARP,
forward 5′ACCACGCACAATGCCTATGA3′, reverse 5′AGTCTCCGGTTGTGAAGCTG3′;
MMP1, forward 5′CACCAATCAGTTCAACGCAGA3′, reverse 5′
TGACTTGGTAATGGGTTGCC3′; MMP3, forward 5′TTTGGCCGTCTCTTCCATCC3′,
reverse 5′GCATCGATCTTCTGGACGGT3′; MMP9, forward
5′GATCCCCAGAGCGTTACTCG3′, reverse 5′GTTGTGGAAACTCACACGCC3′; MMP13,
forward 5′TGCATACGAGCATCCATCCC3′, reverse 5′CTCAAAGTGAACCGCAGCAC3′;
tissue inhibitor of metalloproteinases (TIMP), forward
5′TCCCCAGAAATCATCGAGAC3′, reverse 5′GATGTGCAAATTTCCGTTCC3′; SRY-box
9 (SOX9), forward 5′CCAGAGAACGCACATCAAGA3′, reverse
5′TCTGGTGGTCGGTGTAGTCA3′; COL2, forward 5′GGCCAGGATGCCCGAAAATTA3′,
reverse 5′ACCCCTCTCTCCCTTGTCAC3′; hypoxia-inducible factor-1α
(HIF-1α), forward 5′TGCTCATCAGTTGCCACTTC3′, reverse
5′CCATCCAGGGCTTTCAGATA3′; and glyceraldehyde 3-phosphate
dehydrogenase (GAPDH), forward 5′TGCCACTCAGAAGACTGTGG3′, reverse
5′TTCAGCTCTGGGATGACCTT3′.
The reaction mixture was prepared according to the
instructions provided with Takara SYBR Premix Ex Taq II
(Takara Biotechnology Co., Ltd.). The amplification curve and
melting curve were produced using the Real-Time PCR system (CFX96;
Bio-Rad Laboratories, Inc., Hercules, CA, USA). PCR primers were
obtained from GenePharma Co., Ltd. (Shanghai, China). Amplification
conditions were as follows: 10 min at 95°C for one cycle, followed
by 45 cycles at 95°C for 10 sec, 60°C for 20 sec and 70°C for 30
sec, and a final extension step at 70°C for 5 min. Blank controls
were included to determine the amplification efficiency within each
experiment. Quantification was conducted using the
2−ΔΔCq method (18).
GAPDH was used for normalization.
Western blotting
Cells were harvested and washed with PBS (pH 7.4)
three times. The pellets were incubated with 100 µl lysis buffer
(P0013C; Beyotime Institute of Biotechnology, Shanghai, China) for
30 min, and were centrifuged at 12,000 rpm for 15 min at 4°C. The
supernatants were used for western blotting. Sample loading was
adjusted according to protein concentration, which was determined
using a bicinchoninic acid kit (Pierce Biotechnology; Thermo Fisher
Scientific, Inc.). The protein samples (3 µg/µl) were separated by
sodium dodecyl sulfate-polyacrylamide gel electrophoresis (10%
gels). Following electrophoresis, the proteins were transferred to
polyvinylidene fluoride membranes at 70 V for 20–70 min at 4°C. The
membranes were blocked with Tris-buffered saline (TBS) wash
solution containing 5% nonfat milk and 0.1% Tween 20 for 1 h. The
membranes were then incubated with the primary antibodies (1:500),
with agitation overnight at 4°C, followed by incubation with the
horseradish peroxidase-conjugated secondary antibody (1:2,500) for
1 h at room temperature. Primary antibodies used in the present
study are listed in Table I. The
secondary antibody was goat-anti-rabbit immunoglobulin G
(IgG)-horseradish peroxidase (HRP)-conjugated secondary antibody
(cat. no. sc-2004; Santa Cruz Biotechnology, Inc., CA, USA). The
membranes were washed three times (15 min each wash) between
antibody incubations with TBS containing 0.1% Tween 20, and the
blots were developed using ECL Plus Western Blotting Substrate
(Pierce Biotechnology; Thermo Fisher Scientific, Inc.). Blots were
semi-quantified using ImageJ 2x software (Rawak Software, Inc.,
Stuttgart, Germany).
 | Table I.List of antibodies used in western
blotting. |
Table I.
List of antibodies used in western
blotting.
| Antibody | Manufacturer | Catalog no. |
|---|
| Anti-MMP1 | Bioss | Bs-0424R |
| Anti-MMP3 | Bioss | Bs-0413R |
| Anti-MMP13 | Bioss | Bs-0575R |
| Anti-MMP9 | Bioss | Bs-4593R |
| Anti-TIMP | Bioss | Bs-4600R |
| Anti-Bax | Bioss | Bs-0127R |
| Anti-caspase-3 | Bioss | Bs-0081R |
| Anti-PARP | Bioss | Bs-2138R |
| Anti-SOX9 | Bioss | Bs-4177R |
| Anti-COL2 | Bioss | Bs-11929R |
| Anti-HIF-1α | Bioss | Bs-0737R |
| Anti-Bcl-2 | Bioss | Bs-0032R |
| Anti-GAPDH | Abcam | ab9485 |
Flow cytometry
The cell suspension and culture medium were mixed in
a six-well plate, and were incubated with various concentrations
(5, 10 and 50 ng/ml) of IL-1β (cat. no. SRP3083; Sigma-Aldrich) for
1 h (37°C, 5% CO2). Subsequently, the cells were digested with
trypsin (without EDTA) for 5 min, and centrifuged at 300 × g
for 5 min (4°C). PBS was then used to wash the mixture twice, and
the cells were further centrifuged at 300 × g for 5 min
(4°C). The cells were then resuspended with 100 µl binding buffer,
and were incubated with 5 µl Annexin V-fluorescein isothiocyanate
and 5 µl propidium iodide staining solution for 10 min (using an
Annexin V-FITC and PI kit; cat. no. 556547, BD Biosciences,
Mountain View, CA, USA). After mixing with 400 µl binding buffer,
the samples were examined using a flow cytometer (BD FACSVerse; BD
Biosciences, San Jose, CA, USA).
Statistical analysis
For all experiments, three mice donors of
chondrocytes were used. For each donor, three technical replicates
were tested. Data are presented as the mean ± standard deviation.
For evaluation of differences between two groups, Student's t-test
was applied. Analysis of variance (ANOVA) tests were used for
multiple comparisons. P<0.05 was considered to indicate a
statistically significant difference. Data analysis was conducted
using SAS 9.3 software (SAS Institute, Inc., Cary, NC, USA).
Results
PRP increases chondrocyte
viability
Chondrocytes were isolated from rat joint cartilage,
and were verified by immunostaining with the cartilage-specific
matrix component, COL2 (Fig. 1).
PRP was prepared and diluted with PBS or basic medium to obtain
five different concentrations (1, 2, 5, 10 and 25%, volume/volume),
which was added to the culture medium for 24, 48 and 72 h. Cell
number increased in the PRP treatment groups in a dose-dependent
manner (Table II). However, 10%
PRP in the culture medium resulted in increased cell growth
compared with in the 25% PRP group at 24 and 48 h (P<0.05).
Therefore, 10% PRP treatment was used for subsequent
experiments.
 | Table II.Viability of chondrocytes treated with
various concentrations of PRP, as measured by Cell Counting kit-8
assay. Numbers indicate percentage of control groups. |
Table II.
Viability of chondrocytes treated with
various concentrations of PRP, as measured by Cell Counting kit-8
assay. Numbers indicate percentage of control groups.
| Group | 24 h | 48 h | 72 h |
|---|
| Control | 100±4.63 | 100±3.69 | 100±7.03 |
| 1% PRP | 91.63±2.31 | 116.77±1.88 |
123.09±3.78a |
| 2% PRP | 91.97±4.28 |
129.44±4.4a |
149.64±6.37a |
| 5% PRP |
131.81±19.28a |
155.1±3.78a |
176.62±17.06a |
| 10% PRP |
159.31±12.49a |
195.57±13.68a,b |
204.17±19.23a,b |
| 25% PRP |
132.05±10.91a |
171.4±7.65a |
235.39±8.5a |
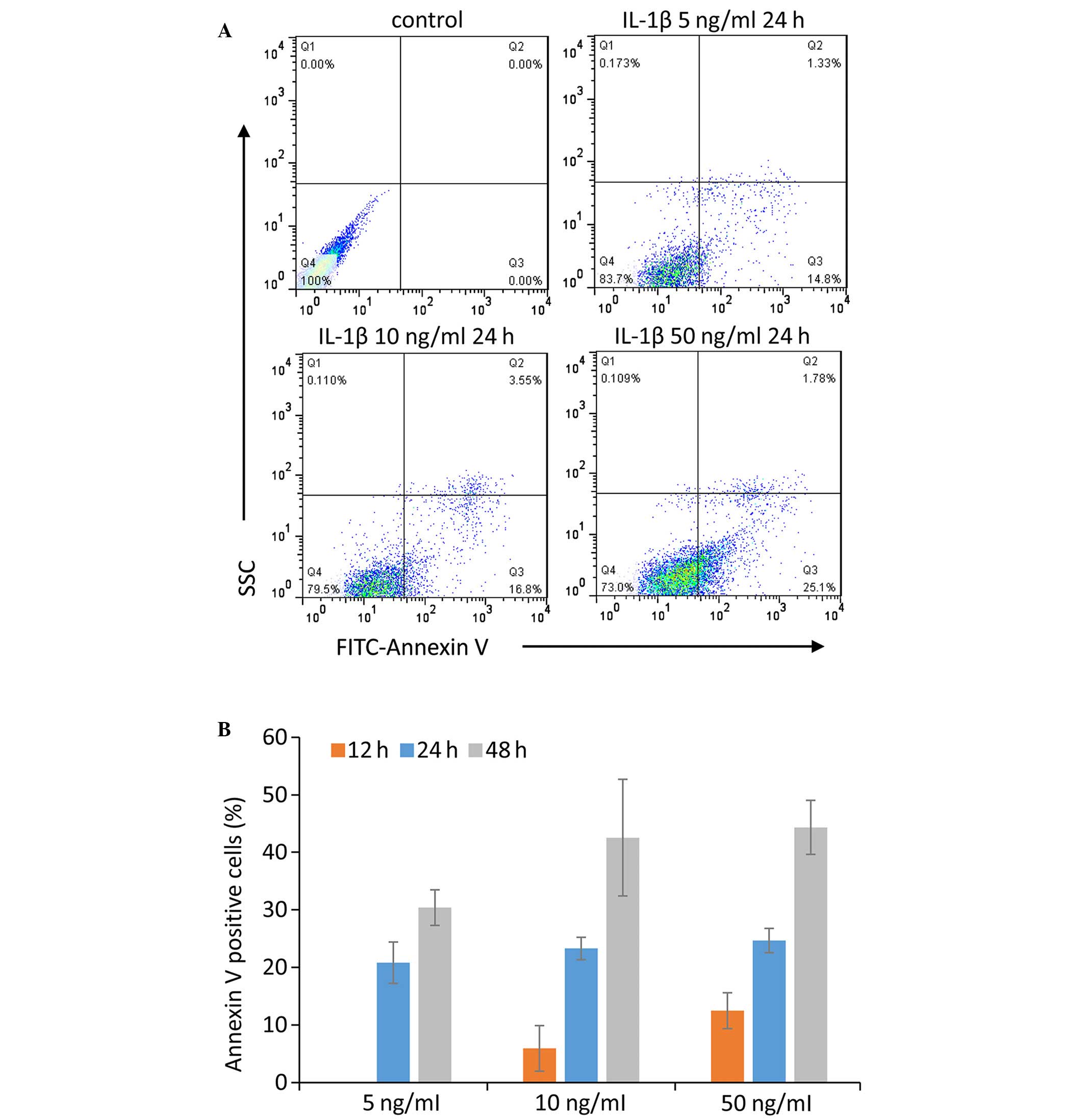
Model establishment of IL-1β-induced
chondrocyte apoptosis
Chondrocytes were treated with three concentrations
of IL-1β (5, 10 and 50 ng/ml) for 24, 48 and 72 h. Subsequently,
flow cytometric analysis was used to determine the percentage of
apoptotic cells. Apoptotic chondrocytes were defined as Annexin
V-positive cells (Fig. 2A), and
the apoptotic rate of the chondrocytes in various conditions are
presented in Fig. 2B. Treatment
with 10 ng/ml IL-1β for 24 h was selected for further experiments,
since it induced an increase in Annexin V-positive cells, saved
time, and used a relatively low concentration of IL-1β.
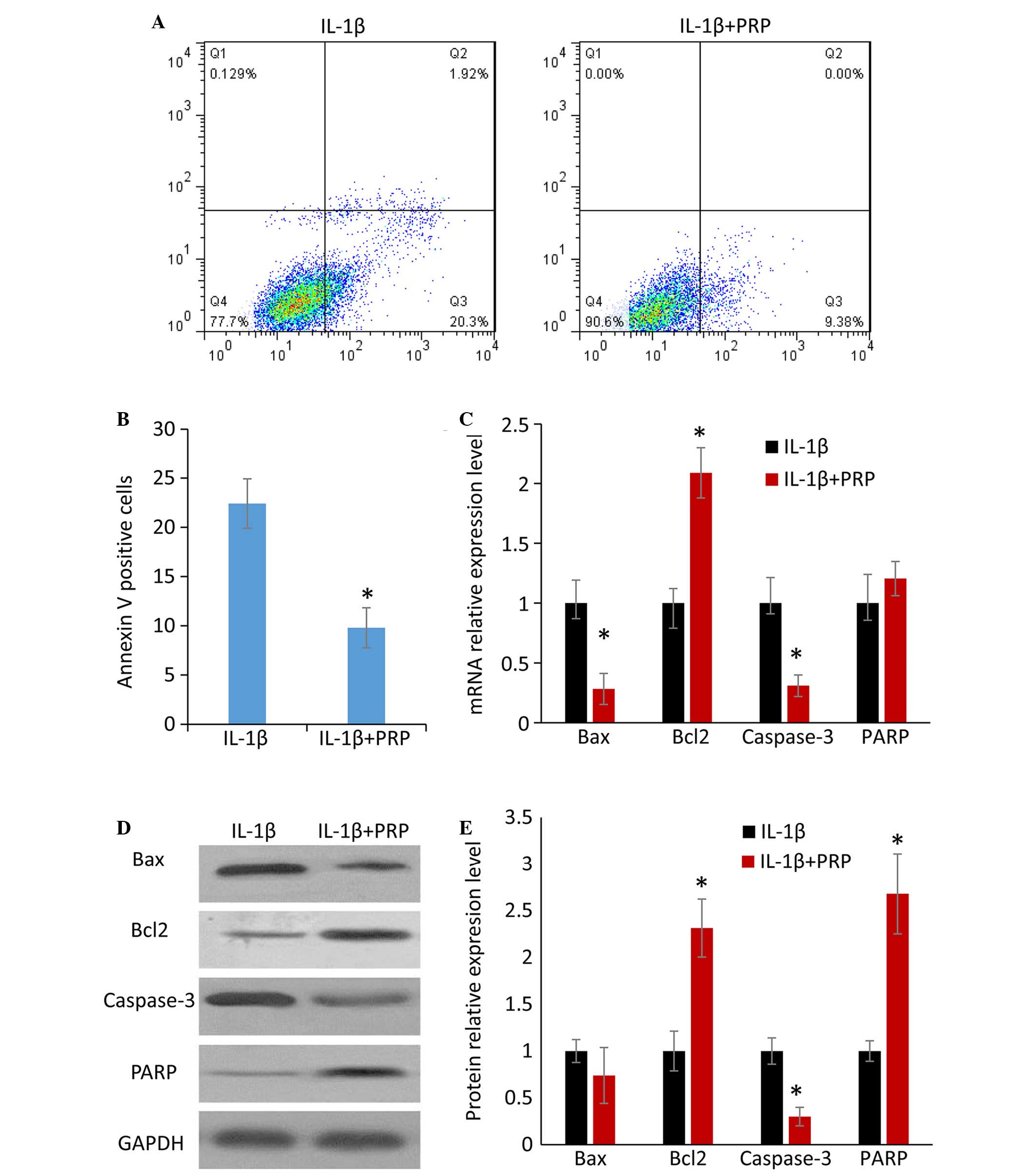
PRP inhibits IL-1β-induced chondrocyte
apoptosis
To evaluate the effects of PRP on IL-1β-induced
apoptosis, flow cytometry was performed to determine the number of
Annexin V-positive chondrocytes. Treatment with PRP reduced the
Annexin V-positive cell population (Fig. 3A). The difference between the IL-1β
group and the IL-1β + PRP group was statistically significant
(P<0.05; Fig. 3B). qPCR and
western blotting were conducted to detect the expression of
apoptosis-related factors at the mRNA and protein level,
respectively. In the IL-1β + PRP group, the mRNA and protein
expression levels of Bax and caspase-3 were markedly downregulated,
whereas the expression levels of Bcl-2 and PARP were markedly
upregulated (Fig. 3C-E). These
results indicate that PRP may inhibit IL-1β-induced chondrocyte
apoptosis.
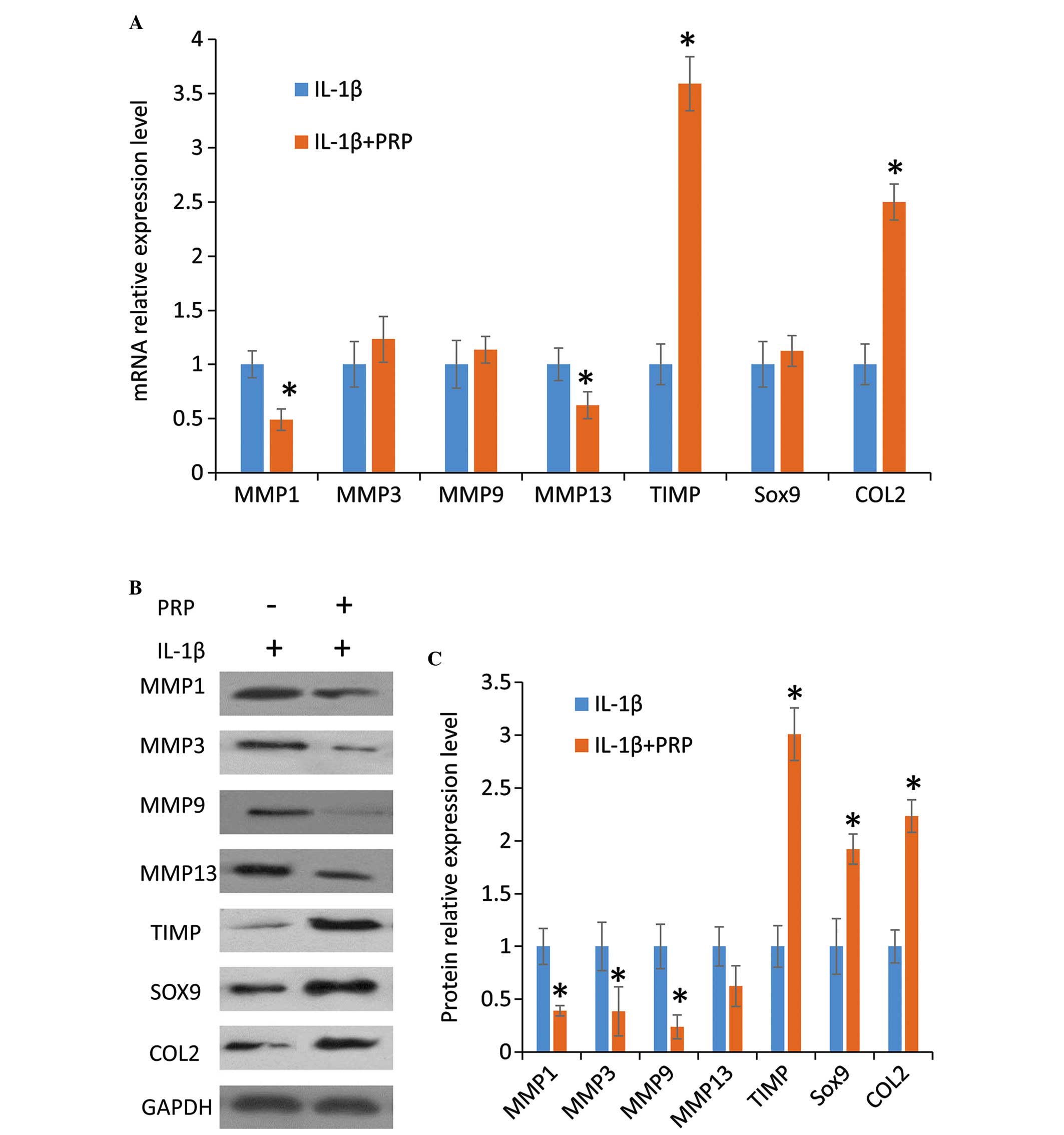
PRP protects the chondrocyte
extracellular matrix
To evaluate the effects of PRP on synthesis of the
chondrocyte extracellular matrix in the presence of IL-1β, qPCR and
western blotting were conducted to determine the mRNA and protein
expression levels of the relevant factors, respectively. Results
from the qPCR indicated that compared with the IL-1β group, the
addition of PRP significantly downregulated the expression levels
of the MMPs, MMP1 and MMP13, and modestly increased the expression
levels of MMP3 and MMP9; and upregulated the expression of the
cartilage matrix-producing proteins, SOX9 and COL2, and TIMP
(Fig. 4A). Western blotting
results exhibited a similar pattern (Fig. 4B and C). These results indicate
that PRP reduced the expression of catabolic genes and increased
the expression of anabolic genes.
Discussion
Cartilage degradation and loss are the major
characteristics of arthritis. For the treatment of cartilage damage
and to prevent cartilage loss, PRP is considered a promising
treatment strategy, due to its high content of growth factors,
which may act as a regenerative stimulus for cartilage tissues and
cells (19). Treatment of
chondrocytes from human osteoarthritic cartilage with PRP resulted
in an inhibitory effect on matrix loss (20). However, the effects of PRP on cell
apoptosis remain unknown. The results of the present study
demonstrated that PRP promoted chondrocyte survival by inhibiting
IL-1β-induced chondrocyte apoptosis. This result provides
scientific evidence for the beneficial effects of PRP at the
cellular level. In addition, the present study revealed that the
rate of matrix degradation was inhibited by PRP.
IL-1β is considered a major proinflammatory cytokine
involved in arthritis. The levels of IL-1β are increased in the
synovial fluid of patients with OA. In addition, it is thought to
be associated with the degradation of cartilage extracellular
matrix (21). IL-1β can be
secreted by chondrocytes, mononuclear cells, osteoblasts and
synovial cells. Previous studies have reported that IL-1β may
stimulate apoptosis of chondrocytes and tendon cells (22,23)
by upregulating the expression of Bax and caspase-3 (24), and suppressing the expression of
Bcl-2 and PARP (25). Several
studies (21,26,27)
have demonstrated that IL-1β promotes catabolic activity of
chondrocytes through two major mechanisms: IL-1β suppresses the
synthesis of major extracellular matrix proteins, such as aggrecan
and COL2; and IL-1β stimulates the release of several proteolytic
enzymes, including MMPs such as MMP1, MMP3 and MMP13. Inhibition of
IL-1β may be beneficial in preventing the progression of OA
pathogenesis; therefore, IL-1β antagonists and their underlying
mechanisms are currently under active investigation (28,29).
However, simply inhibiting the IL-1β receptor does not appear to be
an effective solution. For example, an IL-1 receptor antagonist,
Anakinra™, was launched into clinical trials to treat OA; however,
improvements in symptoms were not detected compared with the
placebo group (30,31). Data from the present study
indicated that PRP treatment of rat chondrocytes downregulated the
expression of proapoptotic factors and upregulated the expression
of anti-apoptotic factors. Along with the well-known effects of PRP
on inhibiting the activity of catabolic enzymes, these
anti-apoptotic and anti-catabolic effects may partially explain the
clinical efficacy of PRP on treating cartilage injury.
It has previously been reported that high
concentrations of PDGF, endothelial growth factor and TGF, together
with the anti-inflammatory and proinflammatory cytokines IL-4,
IL-8, IL-13, IL-17, tumor necrosis factor-α and interferon-α, are
present in PRP (32). The present
study demonstrated that PRP was able to counteract the apoptotic
and catabolic effects of IL-1β in chondrocytes. The
anti-inflammatory factors present in PRP may induce several effects
on IL-1β-activated signaling pathways. However, the interaction
between PRP-derived anti-inflammatory factors and IL-1β requires a
more thorough investigation.
The expression levels of two catabolic enzymes, MMP3
and MMP9, were decreased at the protein level in the IL-1β +
PRP-treated chondrocytes, as compared with in the IL-1β-treated
chondrocytes. However, the expression of these two enzymes at the
mRNA level was not significantly different between the IL-1β and
IL-1β + PRP groups. This inconsistency between expression at the
mRNA and protein level may reflect the complexity of interactions
between IL-1β and growth factors present in PRP. Some factors in
PRP may be able to regulate the expression of catabolic enzymes by
inhibiting their translation from mRNA. In addition, 10% PRP in the
culture medium resulted in increased cell growth compared with in
the 25% PRP group at 24 and 48 h. These results indicated that the
beneficial effects of PRP on cell growth do not linearly increase
with higher PRP concentrations, at least in a short time period.
Considering all growth factors have best working concentrations,
better understanding of the concentrations of growth factors in PRP
is crucial to explain these observations.
In conclusion, the present study demonstrated that
PRP is able to protect chondrocytes from IL-1β-dependent apoptosis,
and can promote anabolism of chondrocyte extracellular matrix. The
effects of PRP on IL-1β-treated chondrocytes support the beneficial
effects of PRP application in the treatment of arthritis.
References
|
1
|
Pincus T: Long-term outcomes in rheumatoid
arthritis. Br J Rheumatol. 34:(Suppl 2). S59–S73. 1995. View Article : Google Scholar
|
|
2
|
Kay J and Calabrese L: The role of
interleukin-1 in the pathogenesis of rheumatoid arthritis.
Rheumatology (Oxford). 43:(Suppl 3). iii2–iii9. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Burrage PS, Mix KS and Brinckerhoff CE:
Matrix metalloproteinases: Role in arthritis. Front Biosci.
11:529–543. 2006. View
Article : Google Scholar : PubMed/NCBI
|
|
4
|
Hogquist KA, Nett MA, Unanue ER and
Chaplin DD: Interleukin 1 is processed and released during
apoptosis. Proc Natl Acad Sci USA. 88:8485–8489. 1991. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Vignon E, Arlot M and Vignon G: Cellular
density of the femur head cartilage in relation to age. Rev Rhum
Mal Osteoartic. 43:403–405. 1976.PubMed/NCBI
|
|
6
|
Marx RE: Platelet-rich plasma: Evidence to
support its use. J Oral Maxillofac Surg. 62:489–496. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Hall MP, Band PA, Meislin RJ, Jazrawi LM
and Cardone DA: Platelet-rich plasma: Current concepts and
application in sports medicine. J Am Acad Orthop Surg. 17:602–608.
2009. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Sánchez M, Anitua E, Orive G, Mujika I and
Andia I: Platelet-rich therapies in the treatment of orthopaedic
sport injuries. Sports Med. 39:345–354. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Eppley BL, Pietrzak WS and Blanton M:
Platelet-rich plasma: A review of biology and applications in
plastic surgery. Plast Reconstr Surg. 118:147e–159e. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Foster TE, Puskas BL, Mandelbaum BR,
Gerhardt MB and Rodeo SA: Platelet-rich plasma: From basic science
to clinical applications. Am J Sports Med. 37:2259–2272. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Redler LH, Thompson SA, Hsu SH, Ahmad CS
and Levine WN: Platelet-rich plasma therapy: A systematic
literature review and evidence for clinical use. Phys Sportsmed.
39:42–51. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Margolis DJ, Kantor J, Santanna J, Strom
BL and Berlin JA: Effectiveness of platelet releasate for the
treatment of diabetic neuropathic foot ulcers. Diabetes Care.
24:483–488. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Martínez-Zapata MJ, Martí-Carvajal A, Solà
I, Bolibar I, Angel Expósito J, Rodriguez L and García J: Efficacy
and safety of the use of autologous plasma rich in platelets for
tissue regeneration: A systematic review. Transfusion. 49:44–56.
2009. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Sclafani AP: Applications of platelet-rich
fibrin matrix in facial plastic surgery. Facial Plast Surg.
25:270–276. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Albano JJ and Alexander RW: Autologous fat
grafting as a mesenchymal stem cell source and living bioscaffold
in a patellar tendon tear. Clin J Sport Med. 21:359–361. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Cervelli V, Gentile P, Scioli MG, Grimaldi
M, Casciani CU, Spagnoli LG and Orlandi A: Application of
platelet-rich plasma in plastic surgery: Clinical and in vitro
evaluation. Tissue Eng Part C Methods. 15:625–634. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Akeda K, An HS, Okuma M, Attawia M,
Miyamoto K, Thonar EJ, Lenz ME, Sah RL and Masuda K: Platelet-rich
plasma stimulates porcine articular chondrocyte proliferation and
matrix biosynthesis. Osteoarthritis Cartilage. 14:1272–1280. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(−Delta Delta C(T)) Method. Methods. 25:402–408. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Xie X, Zhang C and Tuan RS: Biology of
platelet-rich plasma and its clinical application in cartilage
repair. Arthritis Res Ther. 16:2042014. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
van Buul GM, Koevoet WL, Kops N, Bos PK,
Verhaar JA, Weinans H, Bernsen MR and van Osch GJ: Platelet-rich
plasma releasate inhibits inflammatory processes in osteoarthritic
chondrocytes. Am J Sports Med. 39:2362–2370. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Assirelli E, Pulsatelli L, Dolzani P,
Platano D, Olivotto E, Filardo G, Trisolino G, Facchini A, Borzì RM
and Meliconi R: Human osteoarthritic cartilage shows reduced in
vivo expression of IL-4, a chondroprotective cytokine that
differentially modulates IL-1β-stimulated production of chemokines
and matrix-degrading enzymes in vitro. PLoS One. 9:e969252014.
View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Mobasheri A and Shakibaei M: Is tendinitis
an inflammatory disease initiated and driven by pro-inflammatory
cytokines such as interleukin 1β? Histol Histopathol. 28:955–964.
2013.PubMed/NCBI
|
|
23
|
Nikoletopoulou V, Markaki M, Palikaras K
and Tavernarakis N: Crosstalk between apoptosis, necrosis and
autophagy. Biochim Biophys Acta. 1833:3448–3459. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Zhang X, Xu X, Xu T and Qin S:
β-Ecdysterone suppresses interleukin-1β-induced apoptosis and
inflammation in rat chondrocytes via inhibition of NF-κB signaling
pathway. Drug Dev Res. 75:195–201. 2014.PubMed/NCBI
|
|
25
|
Sena P, Manfredini G, Benincasa M, Mariani
F, Smargiassi A, Catani F and Palumbo C: Up-regulation of the
chemo-attractive receptor ChemR23 and occurrence of apoptosis in
human chondrocytes isolated from fractured calcaneal osteochondral
fragments. J Anat. 224:659–668. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Minashima T, Campbell KA, Hadley SR, Zhang
Y and Kirsch T: The role of ANK interactions with MYBBP1a and SPHK1
in catabolic events of articular chondrocytes. Osteoarthritis
Cartilage. 22:852–861. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Williams A, Smith JR, Allaway D, Harris P,
Liddell S and Mobasheri A: Carprofen inhibits the release of matrix
metalloproteinases 1, 3 and 13 in the secretome of an explant model
of articular cartilage stimulated with interleukin 1β. Arthritis
Res Ther. 15:R2232013. View
Article : Google Scholar : PubMed/NCBI
|
|
28
|
Attur MG, Dave MN, Leung MY, Cipolletta C,
Meseck M, Woo SL and Amin AR: Functional genomic analysis of type
II IL-1beta decoy receptor: Potential for gene therapy in human
arthritis and inflammation. J Immunol. 168:2001–2010. 2002.
View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Tsutsumi R, Ito H, Hiramitsu T, Nishitani
K, Akiyoshi M, Kitaori T, Yasuda T and Nakamura T: Celecoxib
inhibits production of MMP and NO via down-regulation of NF-kappaB
and JNK in a PGE2 independent manner in human articular
chondrocytes. Rheumatol Int. 28:727–736. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Bacconnier L, Jorgensen C and Fabre S:
Erosive osteoarthritis of the hand: Clinical experience with
anakinra. Ann Rheum Dis. 68:1078–1079. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Chevalier X, Goupille P, Beaulieu AD,
Burch FX, Bensen WG, Conrozier T, Loeuille D, Kivitz AJ, Silver D
and Appleton BE: Intraarticular injection of anakinra in
osteoarthritis of the knee: A multicenter, randomized,
double-blind, placebo-controlled study. Arthritis Rheum.
61:344–352. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Amable PR, Carias RB, Teixeira MV, da Cruz
Pacheco I, Corrêa do Amaral RJ, Granjeiro JM and Borojevic R:
Platelet-rich plasma preparation for regenerative medicine:
Optimization and quantification of cytokines and growth factors.
Stem Cell Res Ther. 4:672013. View
Article : Google Scholar : PubMed/NCBI
|