Introduction
Inonotus obliquus has been commonly used in
Asian and Russian folk medicine for thousands of years due its
health-promoting properties and relatively low toxicity, it has
been widely studied in recent years. It has been demonstrated that
I. obliquus, a fungus in the Hymenochaetaceae family,
contain numerous steroids, phenolic compounds and polysaccharides
that have various biological activities (1). Numerous mushrooms have biologically
active polysaccharides in their fruiting bodies, cultured mycelium,
and culture broth. I. obliquus has been reported to exhibit
various beneficial biological properties, including anti-tumor,
anti-metastatic, anti-oxidative, anti-viral, anti-diabetes, and
immunomodulatory activities (2–6). The
fruiting bodies of wild I. obliquus are expensive due to
host specificity and rarity in nature. Thus, the production of
adequate amounts of the fruiting bodies of wild I. obliquus
for use as a different chemotherapeutic option is currently
impractical. Liquid cultures of mushrooms, in addition to the
culture broth and mycelia, are a promising alternative to wild
resources for the efficient production of polysaccharides, as this
method has high productivity and low cost. It is required to
demonstrate that fermented polysaccharides exhibit medicinal and
nutritional values that are comparable to those from medicinal
mushroom fruiting bodies (7), as
previous studies have reported that the mycelial biomass of
medicinal mushrooms possesses different pharmacologic properties
from those of the mushroom fruiting bodies (8,9).
Thus, the present study aimed to investigate the biological
activities of polysaccharides from liquid culture with mushroom
mycelia.
Cancer is an important cause of human mortality
worldwide, and numerous cancer treatments, including cancer
chemotherapeutic agents, are known to result in adverse side
effects (10,11). Metastasis is a characteristic of
highly malignant tumor cells leading to poor clinical outcomes. The
excessive degradation of extracellular matrix (ECM) is a
characteristic of tumor metastasis and invasion (12). Furthermore, matrix
metalloproteinases (MMPs) in humans have been identified as key
factors involved in these processes. MMPs are a family of
zinc-dependent endopeptidases that facilitate proteolytic cleavage
of ECM components, including proteoglycan, collagen, laminin,
elastin, and fibronectin (13). It
has been demonstrated that the phosphatidylinositol 3-kinase
(PI3K)/AKT and mitogen-activated protein kinase (MAPK) signaling
pathways regulate metastasis in various types of cancer cell
(14). Activation of the PI3K-AKT
pathway may increase the expression levels of MMP directly,
phosphorylate IκB kinases and activate nuclear factor-κB (NF-κB)
signaling pathways, which promote NF-κB translocation to the
nucleus and then regulates NF-κB-dependent MMP transcription
(15). NF-κB is a key
transcription factor in cancer cells, which has been associated
with cancer development and maintenance, including preventing
apoptosis, growth factor-independent proliferation, and tissue
invasion and metastasis (16).
Our previous studies have demonstrated that
polysaccharides from the I. obliquus fruit body exhibit
anti-metastatic activities in B16-F10 murine melanoma cells and
A549 human non-small cell lung carcinoma cells (17,18).
However, the activities of polysaccharides isolated from liquid
cultures of I. obliquus (PLIO) remain to be elucidated.
Thus, the present study aimed to investigate the anti-metastatic
effects and the potential underlying signaling pathways involved in
PLIO treatment of the highly metastatic B16-F10 murine melanoma
cells in vitro.
Materials and methods
Materials
Streptomycin, fetal bovine serum (FBS), and
penicillin G and were purchased from Gibco (Thermo Fisher
Scientific, Inc., Waltham, MA, USA). Dulbecco's modified Eagle's
medium (DMEM) was obtained from Lonza Group, Ltd. (Basel,
Switzerland). Isopropyl alcohol and
3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT)
were obtained from Sigma-Aldrich. (St. Louis, MO, USA). Antibodies
against extracellular signal-regulated kinase (ERK; 1:1,000
dilution; rabbit monoclonal antibody; cat. no. 4695),
phosphorylated (p)-ERK (1:1,000 dilution; Thr202/Tyr204 rabbit
polyclonal antibody; cat. no. 9101S), stress-activated protein
kinase/c-Jun N-terminal kinase (SAPK/JNK; 1:1,000 dilution; rabbit
polyclonal antibody; cat. no. 9252), p-SAPK/JNK (1:1,000 dilution;
Thr183/Tyr185 mouse monoclonal antibody; cat. no. 9255S), p38 MAPK
(1:1,000 dilution; rabbit polyclonal antibody; cat. no. 9212),
p-p38MAPK (1:1,000 dilution; Thr180/Tyr182 rabbit monoclonal
antibody; cat. no. 4631S) and NF-κB p65 (1:1,000 dilution; rabbit
polyclonal antibody; cat. no. 3034), and horseradish peroxidase
(HRP)-conjugated anti-rabbit IgG (1:2,000 dilution; cat. no. 7074)
were purchased from Cell Signaling Technology, Inc. (Danvers, MA,
USA). MMP-2 (1:1,000 dilution; rabbit polyclonal antibody; cat. no.
4022), MMP-7 (1:1,000 dilution; rabbit monoclonal antibody; cat.
no. 3801), MMP-9 (1:1,000 dilution; rabbit polyclonal antibody;
cat. no. 3852), β-actin (1:1,000 dilution; mouse monoclonal
antibody; cat. no. sc-47778), and HRP-conjugated goat anti-mouse
IgG (1:2,000 dilution; cat. no. sc-2005) were obtained from Santa
Cruz Biotechnology, Inc. (Dallas, TX, USA) or BD Biosciences
(Franklin Lakes, NJ, USA). All other chemicals were of analytical
grade.
Preparation of polysaccharides from
the broth of I. obliquus culture
Exopolysaccharide from I. obliquus liquid
culture broth was isolated using previously described methods
(19). Briefly, I. obliquus
KCTC 26147 was inoculated at 5% (v/v) and cultivated for 7 days at
25°C, 600 × g, with an uncontrolled pH in a modified medium
containing 40 g/l glucose, 5 g/l yeast extract, 1 g/l MgSO4·7H2O,
and 2 g/l KH2PO4. After 7 days of cultivation, the culture broth
was centrifuged at 12,000 × g for 20 min at 4°C. Polysaccharides
were precipitated from the liquid culture broth using 75% ethanol
and centrifuged at 8,000 rpm for 20 min. The precipitated
polysaccharides were resuspended, dialyzed against distilled water
for 3 days to remove low-molecular-weight compounds, and then
freeze-dried.
Cell culture
The B16-F10 murine melanoma cell line was obtained
from the Korean Cell Line Bank (Seoul, South Korea). Cells were
grown in complete DMEM medium supplemented with 10%
heat-inactivated FBS, 100 µg/ml streptomycin, and 100 U/ml
penicillin. Cells were maintained at 37°C in a humidified 5% CO2
incubator.
Cell viability
Cell viability was assessed using the MTT
colorimetric assay, as previously described (20). Cells were pre-incubated in 12-well
plates for 24 h at 37°C in a humidified 5% CO2 incubator. PLIO at
different concentrations (1–1,000 µg/ml) was incubated with the
cells for 24 h. Following incubation, cells were washed with 1X
phosphate-buffered saline (PBS) in order to remove dead cells and
0.5 mg/ml of MTT solution was then added to each well. After 2 h
incubation, formazan crystals in each well were dissolved in
isopropyl alcohol to solubilize the formazan salt formed. The
absorbance was determined at a wavelength of 595 nm using a
microplate reader.
Flow cytometry
A fluorescein isothiocyanate (FITC)-labeled Annexin
V/propidium iodide (PI) apoptosis detection kit (Molecular Probes;
Thermo Fisher Scientific, Inc.) was used to determine the level of
apoptosis in tumor cells, according to the manufacturer's
protocols. Briefly, cells were harvested using trypsin/EDTA
solution, washed with PBS, and centrifuged at 600 × g for 5 min at
room temperature to pellet the cells. Cell concentration was
adjusted to 1×106 cells/ml and the cells were resuspended in
binding buffer (10 mM HEPES, 140 mM NaCl, and 2.5 mM
CaCl2, at pH 7.4) prior to staining with FITC-labeled
Annexin V and PI for 15 min at room temperature in light-protected
conditions. Flow cytometric analysis was performed using a
FACSCalibur flow cytometer (BD Biosciences) within 1 h after
staining. The percentage of apoptotic cells was calculated using
the CellQuest software program (version 4.0.4; BD Biosciences). The
apoptotic cell rate was calculated as the sum of cells in the early
and late phase of apoptosis divided by the total number of events
recorded by the flow cytometer.
In vitro migration and invasion
assay
Six-well chambers with polycarbonate filters with a
pore size of 8.0 µm were used to perform the migration assays. The
filters (Corning Incorporated, Corning, NY, USA) were coated with
gelatin (Sigma-Aldrich). The cells were seeded to the upper part of
the chamber at a density of 1×106 cells/ml with or without PLIO (50
or 100 µg/ml). In the lower chamber, DMEM containing 10% FBS served
as a source of chemoattractants. Following incubtion for 24 h,
cells that had migrated through the gelatin were stained with 2%
crystal violet. The non-migrated cells in the upper chamber were
removed with a cotton swab. Images of the migrated cells were
captured and the cells were counted under a light microscope
(magnification, ×40). Cell invasion assays were performed using a
Matrigel-coated Transwell chamber. The cells (1×106 cells/ml) were
seeded to the upper chamber of the Transwell insert with or without
PLIO (50 or 100 µg/ml) in serum-free medium. In the lower chamber,
DMEM medium containing 10% FBS was used as a source of
chemoattractants. Following incubation, cells that had invaded
through the Matrigel were fixed with 4% formaldehyde in PBS,
stained with 2% crystal violet, images were captured and cells were
counted under a light microscope (magnification, ×40) (21).
Western blot analysis
Following PLIO treatment (25, 50 or 100 µg/ml), the
cells were rinsed with PBS twice and were lysed in lysis buffer [10
mM NaH2PO4/NaHPO4 (pH 7.5), 10 mM Tris-HCl (pH 7.5), 1% Triton
X-100, 130 mM NaCl, 10 mM NaPPi, 2 µg/ml pepstatin A, and 1 mM
phenylmethylsulfonyl fluoride] on ice for 30 min. The cell lysates
were centrifuged of 12,000 × g for 20 min at 4°C to remove cell
debris and the supernatant was collected. Nuclear extracts were
prepared using a nuclear extraction kit (Panomics Inc., Fremont,
CA, USA) according to the manufacturer's protocol. Protein content
was determined using a Bio-Rad Protein assay kit (Bio-Rad
Laboratories, Inc., Hercules, CA, USA). Equal quantities of nuclear
and cytosolic protein samples (50 µg per lane) were loaded on
10–15% SDS-PAGE for separation, and transferred onto 0.2 mm
Immun-Blot nitrocellulose membranes (Bio-Rad Laboratories, Inc.) by
electroblotting. The blot was blocked with 1.5% non-fat milk in 1X
Tris-buffered saline containing 0.1% Tween-20 (TBST) for 1 h,
followed by incubation with the specific primary antibodies at 4°C
overnight. The blot was finally incubated with HRP-conjugated
secondary antibodies. The membranes were washed with TBS-T after
each antibody binding reaction. Detection of protein-antibody
complexes was conducted using an enhanced chemiluminescence kit
(EMD Millipore, Billerica, MA, USA) followed by exposure to X-ray
film.
Statistical analysis
All measurements were from at least three
independent experiments and all results are presented as the mean ±
standard error of the mean. The data were analyzed using Student's
t-test or nonparametric analysis of variance Duncan's multiple
range tests were performed to compare multiple groups when
appropriate. P<0.05 was considered to indicate a statistically
significant difference.
Results
Treatment with PLIO at low
concentrations had no effect on cell viability in melanoma
cells
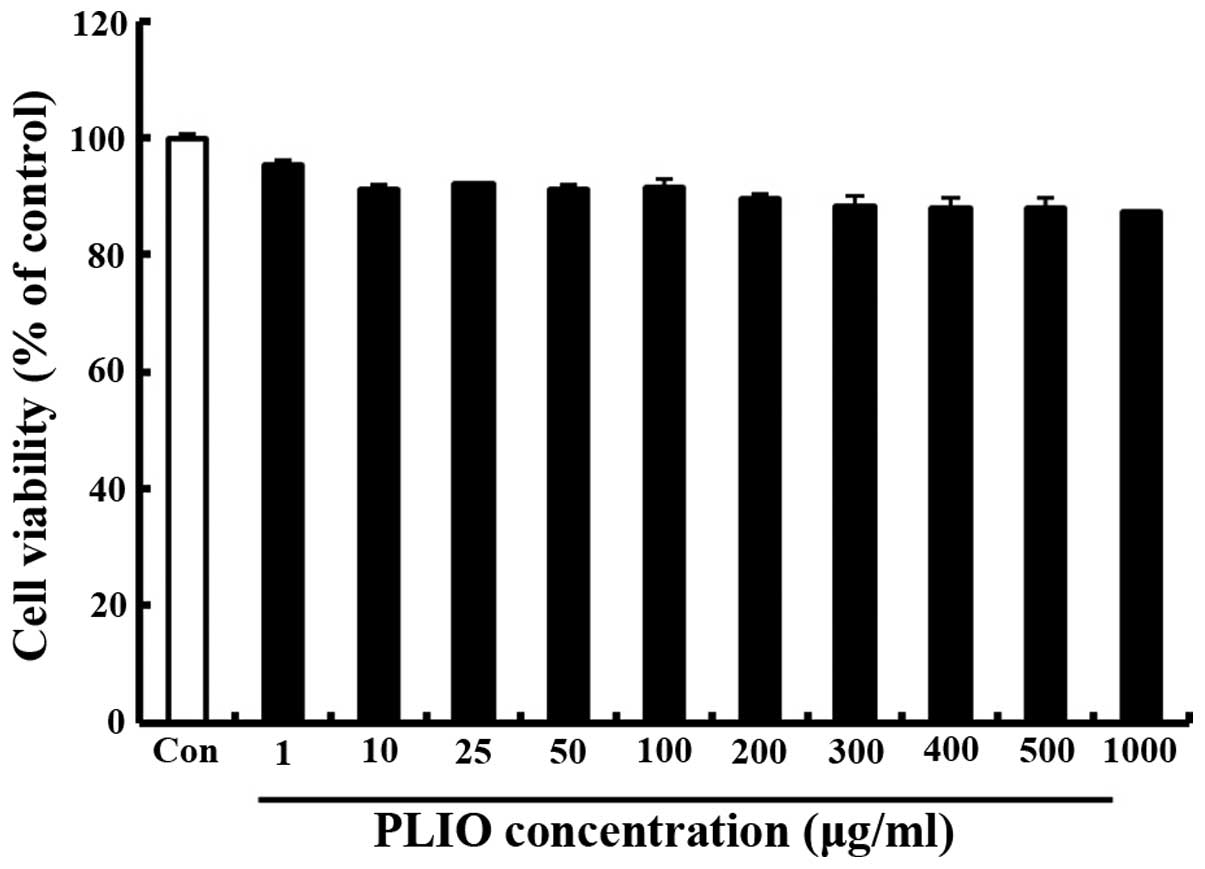
The effect of PLIO on the viability of murine
melanoma cell B16-F10 was determined using the MTT assay. Various
concentrations (0–1,000 µg/ml) of PLIO were added to the cells
followed by incubation for 24 h. Cell viability was determined to
be 89% at 200 µg/ml PLIO compared with the control (Fig. 1). These results indicated that PLIO
at lower concentrations (0 to 200 µg/ml) did not affect cell
viability.
Low concentrations of PLIO did not
induce apoptosis of melanoma cells
To determine whether PLIO induces cellular apoptosis
in melanoma cells, FITC-labeled Annexin V and PI nucleic acid
binding dye were used. Following staining of the cell population
with the double staining method, apoptotic cells exhibit green
fluorescence, dead cells exhibit red and green fluorescence, and
live cells exhbit little or no fluorescence (22). Apoptotic cells were detected by
flow cytometry. Low concentrations of PLIO (25, 50, or 100 µg/ml)
did not increase apoptosis (Fig. 2A
and B), suggesting that PLIO, at ≤100 µg/ml, did not induce
cell death or apoptosis in B16-F10 cells. This concentration range
was then used in all subsequent experiments.
PLIO did not affect migration of
B16-F10 cells, however, invasion of the melanoma cells was
inhibited
To investigate whether PLIO had in vitro
anti-metastatic activity, the present study evaluated B16-F10 cell
migration and invasion in the presence of PLIO using gelatin- or
Matrigel-coated Transwell assays with polycarbonate filters (pore
size, 8-µm). It was observed that B16-F10 cells migrated from the
upper chamber to the lower chamber in the untreated control,
suggesting that the cells are able to migrate through a
gelatin-coated Transwell insert. PLIO at concentrations of 50 and
100 µg/ml did not inhibit B16-F10 cell migration, which was 97 and
92% of the control level, respectively (Fig. 3). In the results of the invasion
assay, untreated B16-F10 cells moved from the upper chamber to the
lower chamber, indicating that the melanoma cells can invade
through the Transwell insert pre-coated with Matrigel (Fig. 4). However, the presence of PLIO had
an inhibitory effect on the invasion of B16-F10 cells in a
concentration-dependent manner. As presented in Fig. 4B, 100 µg/ml of PLIO significantly
inhibited invasion of B16-F10 melanoma cell to 35% (P<0.05).
Thus, PLIO could inhibit invasion of melanoma cells.
PLIO regulated the expression of
MMP-2, MMP-7 and MMP-9 in melanoma cells
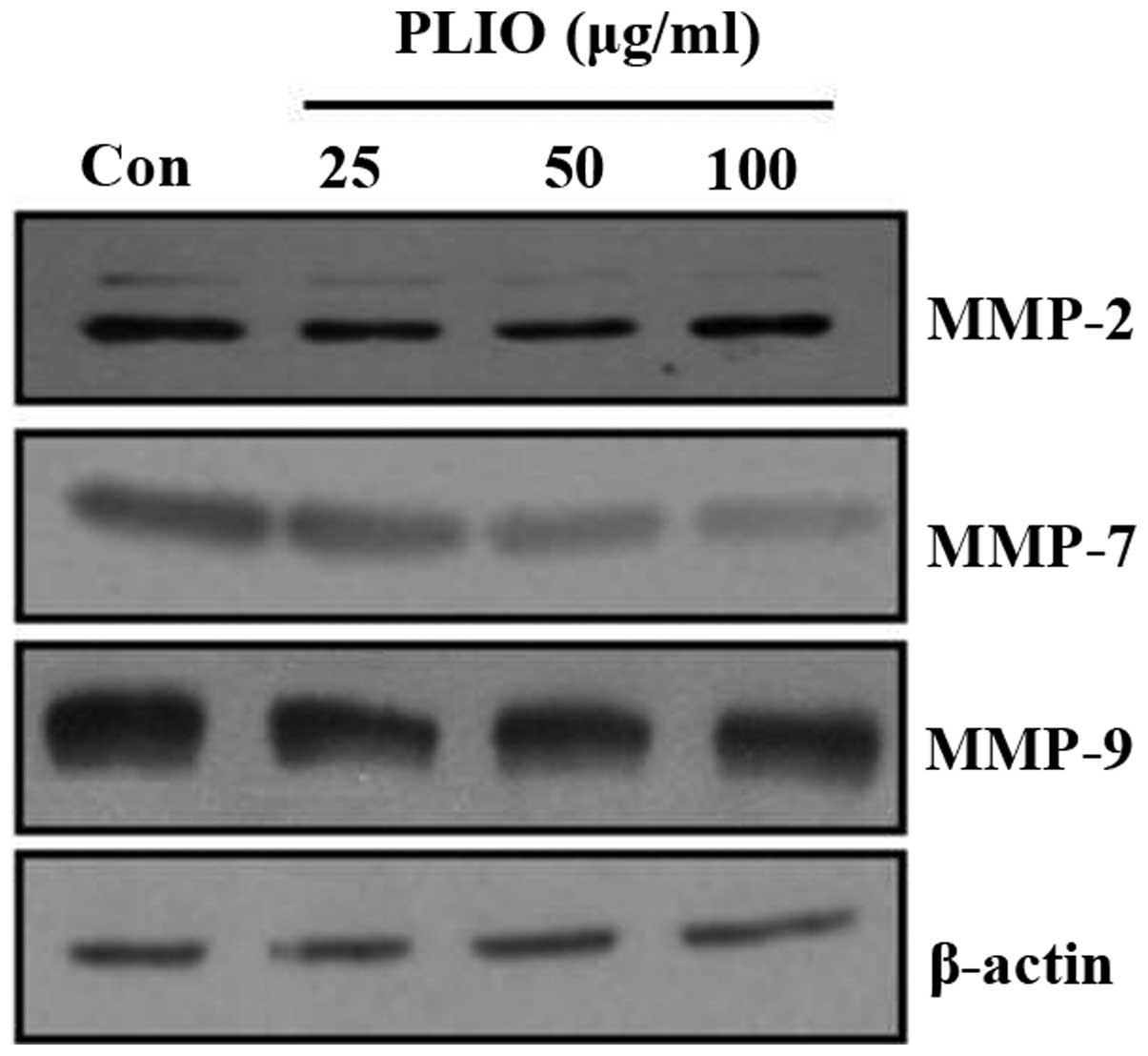
ECM degradation, which is key in cellular invasion,
involves matrix-degrading proteinases, including MMPs. To determine
whether PLIO suppressed MMP protein expression levels, western
blotting was used. PLIO treatment decreased the expression of MMP-2
and MMP-9 in B16-F10 cells. Particularly, PLIO was observed to
reduce the expression levels of MMP-7 (Fig. 5). These results suggest that PLIO
regulated the expression level of MMPs.
PLIO inhibited NF-κB nuclear
translocation in melanoma cells
To investigate whether PLIO inhibits the activation
of the NF-κB signaling pathway in melanoma cells, the current
investigated the effects of PLIO on translocation of the NF-κB
protein from the cytoplasm to the nucleus. Western blotting was
used to determine the levels of NF-κB translocation. Cytosolic
protein levels of NF-κB in B16-F10 cells were higher in
PLIO-treated cells than those in untreated cells (Fig. 6). By contrast, PLIO treatment
markedly decreased nuclear protein levels of NF-κB compared with
the levels in the untreated control. These results suggest that
PLIO inhibited the activation of NF-κB in melanoma cells.
PLIO inhibited phosphorylation of JNK
and AKT in melanoma cells
A previous study demonstrated that the activation of
the PI3K/AKT and MAPK signaling pathways promotes cancer cell
invasion and migration (23). It
was demonstrated that PI3K/AKT and MAPK signaling pathways in
different tumor cell types may be partially responsible for
induction of MMP expression (14,16,24).
To investigate whether PLIO regulates PI3K/AKT and MAPK signaling
pathways in melanoma cells, the levels of p-ERK, p-p38 MAPK, p-JNK,
and p-AKT protein in B16-F10 cells were evaluated by western blot
analysis following PLIO treatment. The western blotting
demonstrated that the protein expression levels of PI3K/AKT and
MAPK was not affected by PLIO treatment, however, the
phosphorylation levels of JNK and AKT were inhibited by the
addition of PLIO at 50 and 100 µg/ml (Fig. 7). This suggests PLIO inhibited the
phosphorylation of JNK and AKT in B16-F10 cells.
Discussion
Research has recently focused on the anti-tumor
properties of natural components for their potential
chemotherapeutic applications. Polysaccharides are often associated
with notable pharmacological activities. For example,
polysaccharides extracted from mushrooms, including Agaricus
blazei, Phellinus linteus, Hericium erinaceus,
and I. obliquus exhibit important pharmacological
properties. Tumor metastasis is a multi-step process, with complex
regulation, which includes angiogenesis, cell attachment, adhesion,
migration, invasion, and cell proliferation (25,26).
Developing therapeutic agents that inhibit metastasis is considered
key, however, effective anti-metastatic agents require further
research. The extracellular matrix and basement membrane are stable
structures that provide organizational structure. MMPs, which are
important for the degradation of extracellular matrix and basement
membrane, have been extensively studied and their expression
demonstrated to be markedly increased in a variety of types of
cancer. In addition, MMPs promote cancer growth by activating
cancer tissue growth factors and inhibiting the apoptosis of cancer
cells. MMPs are key in physiological and pathological matrix
turnover. Numerous reports have indicated that the expression
levels of MMP-1, −2, −7, −9, and −10 are notably increased during
cancer cell invasion, and may serve as independent prognostic
factors for unfavorable prognosis (14,24).
MMP-7 expression in primary melanomas and in metastatic melanoma
has been associated with melanoma progression and cell invasion
(27). The present study
investigated whether PLIO suppressed melanoma cell migration and
invasion in vitro. The results demonstrated that PLIO
suppressed the invasive ability of B16-F10 melanoma cells and
suppressed the expression of MMPs in B16-F10 cells. Previous
reports have demonstrated that the activation of activator protein
1 and NF-κB via multiple signaling pathways may induce
transcription of MMPs and enhance the invasion of tumor cells
(28,29). In the present study, PLIO
suppressed the expression of MMPs and inhibited the translocation
of NF-κB from the cytosol to the nucleus in melanoma cells. These
results indicate that PLIO inhibits the metastasis of B16-F10 cells
by suppressing the expression of MMPs via inhibiting the NF-κB
signaling pathway. It was reported that COX-2, one of the
downstream targets of NF-κB, is important in angiogenesis, invasion
and migration. It has also been demonstrated to modulate the
expression of MMPs (30). The way
in which MMPs are regulated by upstream factors, including COX-2,
has been investigated in previous studies (31,32).
Although multiple genetic alterations are required in cancer
invasion and metastasis, COX-2 is involved in the progression of
cancer and may be useful in the development of targeted therapies
in cancer cells. The current study demonstrated that protein
expression levels of COX-2 in B16-F10 cells treated with PLIO were
lower than those in the untreated cells, although this difference
was not indicated to be statistically significant (data not shown).
PLIO may also regulate the invasion of B16-F10 melanoma cells via
different mechanisms, including PI3K/AKT and MAPK signaling
pathways. It is generally demonstrated that the PI3K/AKT and MAPK
signaling pathways regulate metastasis in a variety of cancer
cells.
In conclusion, inhibition of metastasis is a key
issue in cancer research. PLIO may inhibit the invasion of highly
invasive melanoma cells by inhibiting MMPs expression via
downregulation of the NF-κB, AKT, and/or MAPK signaling pathways.
Based on these findings, the exact underlying anti-metastatic
mechanism of PLIO is remains unclear, however, it is concluded that
PLIO exhibits potent anti-metastatic effects.
Acknowledgements
The present study was supported by a grant from the
National Institute of Biological Resources, funded by the Ministry
of Environment of the Republic of Korea (grant no.
NIBR201528101).
References
|
1
|
Zheng W, Miao K, Liu Y, Zhao Y, Zhang M,
Pan S and Dai Y: Chemical diversity of biologically active
metabolites in the sclerotia of Inonotus obliquus and submerged
culture strategies for up-regulating their production. Appl
Microbiol Biotechnol. 87:1237–1254. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Ma L, Chen H, Dong P and Lu X:
Anti-inflammatory and anticancer activities of extracts and
compounds from the mushroom Inonotus obliquus. Food Chem.
139:503–508. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Mu H, Zhang A, Zhang W, Cui G, Wang S and
Duan J: Antioxidative properties of crude polysaccharides from
Inonotus obliquus. Int J Mol Sci. 13:9194–9206. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Fan L, Ding S, Ai L and Deng K: Antitumor
and immunomodulatory activity of water-soluble polysaccharide from
Inonotus obliquus. Carbohydr Polym. 90:870–874. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Shibnev VA, Mishin DV, Garaev TM,
Finogenova NP, Botikov AG and Deryabin PG: Antiviral activity of
Inonotus obliquus fungus extract towards infection caused by
hepatitis C virus in cell cultures. Bull Exp Biol Med. 151:612–614.
2011. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Xu HY, Sun JE, Lu ZM, Zhang XM, Dou WF and
Xu ZH: Beneficial effects of the ethanol extract from the dry
matter of a culture broth of Inonotus obliquus in submerged culture
on the antioxidant defence system and regeneration of pancreatic
beta-cells in experimental diabetes in mice. Nat Prod Res.
24:542–553. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Xu X, Hu Y and Quan L: Production of
bioactive polysaccharides by Inonotus obliquus under submerged
fermentation supplemented with lignocellulosic biomass and their
antioxidant activity. Bioprocess Biosyst Eng. 37:2483–2492. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Kabbaj W, Brecheret S, Guimberteau J,
Talou T, Oliver JM, Bensoussan M, Sobal M and Roussos S: Comparison
of volatile compound production in fruit body and in mycelium of
Pleurotus ostreatus identified by submerged and solid-state
cultures. Appl Biochem Biotechnol 102–103. 463–469. 2002.
View Article : Google Scholar
|
|
9
|
Xu X, Wu Y and Chen H: Comparative
antioxidative characteristics of polysaccharide-enriched extracts
from natural sclerotia and cultured mycelia in submerged
fermentation of Inonotus obliquus. Food Chem. 127:74–79. 2011.
View Article : Google Scholar
|
|
10
|
Monks NR, Biswas DK and Pardee AB:
Blocking anti-apoptosis as a strategy for cancer chemotherapy:
NF-kappaB as a target. J Cell Biochem. 92:646–650. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Walsh V and Goodman J: Cancer
chemotherapy, biodiversity, public and private property: The case
of the anti-cancer drug taxol. Soc Sci Med. 49:1215–1225. 1999.
View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Han JY, Kim HS, Lee SH, Park WS, Lee JY
and Yoo NJ: Immunohistochemical expression of integrins and
extracellular matrix proteins in non-small cell lung cancer:
Correlation with lymph node metastasis. Lung Cancer. 41:65–70.
2003. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Gacko M: Matrix metalloproteases (MMPS).
Postepy Hig Med Dosw. 51:577–589. 1997.(In Polish). PubMed/NCBI
|
|
14
|
Vivanco I and Sawyers CL: The
phosphatidylinositol 3-kinase AKT pathway in human cancer. Nat Rev
Cancer. 2:489–501. 2002. View
Article : Google Scholar : PubMed/NCBI
|
|
15
|
Kim D, Kim S, Koh H, Yoon SO, Chung AS,
Cho KS and Chung J: Akt/PKB promotes cancer cell invasion via
increased motility and metalloproteinase production. FASEB J.
15:1953–1962. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Naugler WE and Karin M: NF-kappaB and
cancer-identifying targets and mechanisms. Curr Opin Genet Dev.
18:19–26. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Lee KR, Lee JS, Song JE, Ha SJ and Hong
EK: Inonotus obliquus-derived polysaccharide inhibits the migration
and invasion of human non-small cell lung carcinoma cells via
suppression of MMP-2 and MMP-9. Int J Oncol. 45:2533–2540.
2014.PubMed/NCBI
|
|
18
|
Lee KR, Lee JS, Kim YR, Song IG and Hong
EK: Polysaccharide from Inonotus obliquus inhibits migration and
invasion in B16-F10 cells by suppressing MMP-2 and MMP-9 via
downregulation of NF-κB signaling pathway. Oncol Rep. 31:2447–2453.
2014.PubMed/NCBI
|
|
19
|
Kwon JS, Lee JS, Shin WC, Lee KE and Hong
EK: Optimization of culture conditions and medium components for
the production of mycelial biomass and exo-polysaccharides with
Cordyceps militaris in liquid culture. Biotechnol Bioprocess Eng.
14:756–762. 2009. View Article : Google Scholar
|
|
20
|
Plumb JA: Cell sensitivity assay: The MTT
assay. Methods Mol Med. 28:25–30. 1999.PubMed/NCBI
|
|
21
|
M D and Brooks SA: In vitro invasion assay
using matrigel®. Methods Mol Med. 58:61–70.
2001.PubMed/NCBI
|
|
22
|
Niu G and Chen X: Apoptosis imaging:
Beyond annexin V. J Nucl Med. 51:1659–1662. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Kang MH, Oh SC, Lee HJ, Kang HN, Kim JL,
Kim JS and Yoo YA: Metastatic function of BMP-2 in gastric cancer
cells: The role of PI3K/AKT, MAPK, the NF-κB pathway, and MMP-9
expression. Exp Cell Res. 317:1746–1762. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Shiomi T and Okada Y: MT1-MMP and MMP-7 in
invasion and metastasis of human cancers. Cancer Metastasis Rev.
22:145–152. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Bartolome RA, Barderas R, Torres S,
Fernandez-Aceñero MJ, Mendes M, García-Foncillas J, Lopez-Lucendo M
and Casal JI: Cadherin-17 interacts with α2β1 integrin to regulate
cell proliferation and adhesion in colorectal cancer cells causing
liver metastasis. Oncogene. 33:1658–1669. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Zanina N, Mora L, Othmane A, Bénard M,
Duncan A, Jouenne T, Vaudry D and Souiri M: Differences in Caco-2
cell attachment, migration on collagen and fibronectin coated
polyelectrolyte surfaces. Biotechnol Bioprocess Eng. 18:144–154.
2013. View Article : Google Scholar
|
|
27
|
Kawasaki K, Kawakami T, Watabe H, Itoh F,
Mizoquchi M and Soma Y: Expression of matrilysin (matrix
metalloproteinase-7) in primary cutaneous and metastatic melanoma.
Br J Dermatol. 156:613–619. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Bergman MR, Cheng S, Honbo N, Piacentini
L, Karliner JS and Lovett DH: A functional activating protein 1
(AP-1) site regulates matrix metalloproteinase 2 (MMP-2)
transcription by cardiac cells through interactions with JunB-Fra1
and JunB-FosB heterodimers. Biochem J. 369:485–496. 2003.
View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Ding D, Xi P, Zhou J, Wang M and Cong YS:
Human telomerase reverse transcriptase regulates MMP expression
independently of telomerase activity via NF-κB-dependent
transcription. FASEB J. 27:4375–4383. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Zhang J, Luo J, Ni J, Tang L, Zhang HP,
Zhang L, Xu JF and Zheng D: MMP-7 is upregulated by COX-2 and
promotes proliferation and invasion of lung adenocarcinoma cells.
Eur J Histochem. 58:22622014. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Scoditti E, Nestola A, Massaro M,
Calabriso N, Storelli C, De Caterina R and Carluccio MA:
Hydroxytyrosol suppresses MMP-9 and COX-2 activity and expression
in activated human monocytes via PKCα and PKCβ1 inhibition.
Atherosclerosis. 232:17–24. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Xue J, Hua YN, Xie ML and Gu ZL: Aspirin
inhibits MMP-9 mRNA expression and release via the PPARalpha/gamma
and COX-2/mPGES-1-mediated pathways in macrophages derived from
THP-1 cells. Biomed Pharmacother. 64:118–123. 2010. View Article : Google Scholar : PubMed/NCBI
|