Introduction
Cardiac ischemia/reperfusion (I/R) injury is a
serious disease that threatens human health (1). It may occur following various
procedures in which myocardial blood supply to an ischemic region
is reestablished, including treatment of thrombolysis, percutaneous
coronary angioplasty or coronary artery bypass grafting (2). The subsequent reperfusion that occurs
through the epicardial coronary arteries following these treatments
does not ensure achievement of complete myocardial reperfusion and
recovery of the normal internal environment, and cardiac I/R injury
may occur (3). Serious myocardial
damage-induced ventricular remodeling, deterioration of cardiac
function, development of congestive heart failure and mortality may
occur following cardiac I/R injury (4). Myocardial necrosis, apoptosis and
ventricular remodeling due to cardiac I/R injury are the
predominant pathological features of reperfusion-induced cardiac
dysfunction following treatment. Therefore, it is important to
prevent ventricular remodeling following cardiac I/R injury
(5). The expression of certain
genes may be dysregulated in cardiac cells following cardiac I/R
injury, thus resulting in the altered expression of corresponding
proteins; these effects may be the pathological basis of injury
remodeling in cardiac I/R injury. Therefore, studying ventricular
remodeling at the genetic level may provide novel strategies for
the clinical treatment of ventricular remodeling following I/R
injury (6).
MicroRNAs (miRNAs) are non-coding small eukaryotic
RNAs (~18–22 bp), which exert endogenous regulatory functions and
are highly conserved. miRNAs have a close association with several
types of heart disease (7).
Previous studies reported that, through the use of chip technology
on samples from a mouse model of myocardial hypertrophy and human
beings with heart disease, several miRNAs (miRNA-1, −21, −133,
−195, −214) are significantly upregulated compared with in control
samples; therefore, overexpression or inhibition of some miRNAs
(miRNA-1, −133, −195, −208 and −214) may exert a significant
influence on heart growth, myocardial hypertrophy and ventricular
remodeling (8,9).
Trimetazidine is a drug that directly stimulates the
myocardium and indirectly promotes myocardial glucose metabolism.
It reduces the demand for oxygen during high-energy phosphate
generation, decreases oxygen consumption during the production of
ATP, and increases oxygen utilization efficiency during myocardial
hypoxia (10). Compared with
traditional anti-ischemic drugs, trimetazidine is a drug with a
unique mechanism of action, since it exerts direct effects on
myocardial ischemia without inducing hemodynamic changes (11). Furthermore, the present study aimed
to investigate whether trimetazidine may protect against cardiac
I/R injury, and to determine whether its curative effects may be
associated with miRNA-21 expression, Akt, and the Bcl-2/Bax
pathway.
Materials and methods
Animals and experimental
protocols
Adult male Sprague-Dawley rats (age, 10–11 weeks;
weight, 250–300 g) were purchased from the Laboratory Animal Center
of Zhengzhou University (Zhengzhou, China) and were maintained in
cages at room temperature (23±2°C), under a 12 h light-dark cycle,
with constant humidity (55±5%). The rats had ad libitum
access to food and water. All animal protocols were performed in
accordance with the National Institutes of Health Guide for the
Care and Use of Laboratory Animals (2014). The present study was
approved by the Ethics Committee of Life Science of Zhengzhou
University. The rats were randomized into four groups: i) The
control group (n=24), in which normal rats were administered saline
alone (0.1 ml/100 g, i.p.) for 5 days; ii) the
control-trimetazidine group (n=25), in which normal rats received
trimetazidine (30 mg/kg; Sigma-Aldrich; Merck Millipore, Darmstadt,
Germany) for 5 days; iii) the cardiac I/R injury model group
(n=25), rats received saline following induction of cardiac I/R
injury (0.1 ml/100 g, i.p.) for 5 days; and iv) the trimetazidine
treatment group (n=25), rats received trimetazidine following
cardiac I/R injury (30 mg/kg) for 5 days.
Cardiac I/R injury model
establishment
Briefly, rats were anesthetized with pentobarbital
sodium (50 mg/kg, i.p.), placed in a supine position, intubated,
and artificially ventilated using a respirator. All surgical
procedures were executed under aseptic conditions. The hearts of
the rats were exposed following an incision into the fourth
intercostal space on the left side of the chest. The left anterior
descending coronary artery was ligated with 6–0 silk suture using a
snare occluder. Cardiac ischemia was confirmed by visual
observation and continuous electrocardiogram monitoring. Following
40 min of occlusion, the coronary artery was reperfused by
releasing the knot. The hearts of the rats were harvested following
180 min reperfusion. Rats in the control group underwent chest
incision without ligation. Subsequently, rats were anesthetized
with pentobarbital sodium (50 mg/kg, i.p.) and were sacrificed by
decapitation.
Measurement of casein kinase (CK) and
lactate dehydrogenase (LDH) levels
Briefly, whole blood samples were extracted from the
vena cava following treatment with trimetazidine or saline.
Subsequently, the serum samples were acquired following
centrifugation of the blood samples at 3,000 × g for 10 min
at 4°C. The supernatant was considered the serum, which was
maintained at −80°C until further use. CK (cat. no. A032) and LDH
(cat. no. A020-1) activities were determined using a series of
commercial kits, according to the manufacture's protocols (Sangon
Biotech Co., Ltd., Shanghai, China).
Determination of infarct size
Briefly, the rat hearts were injected with Evans
Blue solution (1.5%; Sigma-Aldrich; Merck Millipore) and were
immediately separated following treatment with trimetazidine or
saline. Subsequently, the hearts were sliced into 2 mm sections for
infarct size measurement. Infarct size was determined following
staining with 2,3,5-triphenyltetrazolium chloride (1.5%) at 37°C
for 30 min in the dark (12,13).
After staining, the sections were fixed by immersion in 4%
paraformaldehyde solution. The area of the heart without color was
regarded as ischemic myocardium, whereas the area stained brick red
was regarded as normal myocardium.
Cell culture
H9c2 adult rat ventricular myocyte cells (Shanghai
Cell Bank of Chinese Academy of Sciences, Shanghai, China) were
cultured in Dulbecco's modified Eagle's medium (DMEM) medium
(Sigma-Aldrich; Merck Millipore) supplemented with 10% fetal bovine
serum (FBS; Invitrogen; Thermo Fisher Scientific, Inc.) at 37°C in
a humidified atmosphere containing 5% CO2. Medium was refreshed
every 2–3 days. For oxygen-glucose deprivation-treated cells, H9c2
cells were incubated with DMEM without glucose in a humidified
atmosphere containing 5% CO2 and 95% N2 (v/v) at 37°C for 10 h.
Following hypoxia, the culture medium was removed, and fresh high
glucose DMEM containing 10% FBS was added to H9c2 cells in a
regular 5% CO2 incubator.
Transfection and treatment
Negative control miRNA (miR-NC) and anti-miR-21
plasmids were designed and purchased by Sangon Biotech Co., Ltd.
The sequences were as follows: Anti-miR-21,
5′-UCAACAUCAGUCUGAUAAGCUA-3′; and miR-NC,
5′-CAGUACUUUUGUGUAGUACAA-3′. The H9c2 cells were divided into three
groups: Control group, in which cells were transfected with miR-NC
plasmids for 48 h; trimetazidine group, in which cells were treated
with 10 µM trimetazidine for 48 h; and anti-miR-21 group, in which
cells were transfected with anti-miR-21 plasmids for 24 h and were
then treated with 10 µM trimetazidine for 48 h. The plasmids were
transfected into H9c2 cells using Lipofectamine 2000 (Invitrogen;
Thermo Fisher Scientific, Inc.) according to the manufacturer's
protocol.
Quantitative polymerase chain reaction
(qPCR) assay of miRNA-21
Rats were anesthetized with pentobarbital sodium (50
mg/kg, i.p.) and were sacrificed by decapitation. Subsequently,
heart tissues were obtained from the pericardium and were washed
with phosphate-buffered saline. miRNA-21 expression was detected in
heart tissue samples or H9c2 cells following RNA extraction using
TRIzol reagent (Invitrogen; Thermo Fisher Scientific, Inc.,
Waltham, MA, USA), according to the manufacturer's protocol. cDNA
was generated from total RNA (1 µg) using the Superscript
First-Strand Synthesis system (Invitrogen; Thermo Fisher
Scientific, Inc.) at 37°C for 60 min and 85°C for 60 sec. The
primers used in the present study were as follows: miR-21, forward
5′-GGGGGTACCCTTCAGGAAGCTGGTTTC-3′, reverse
5′-GGGGATATCTACATGTGAGGCAGGTTCTCAC-3′; and U6, forward
5′-CGCTTCGGCACATATACTA-3′ and reverse 5′-CGCTTCACGAATTTGCGTGTCA-3′
(Sangon Biotech Co., Ltd.). qPCR detection of miR-21 expression was
performed on a 7300 Sequence Detection system (Applied Biosystems;
Thermo Fisher Scientific, Inc.) using the TaqMan MicroRNA Assay kit
(Invitrogen; Thermo Fisher Scientific, Inc.), according to the
manufacturer's protocols. The PCR cycling conditions were as
follows: 94°C for 60 sec, followed by 40 cycles at 95°C for 15 sec,
60°C for 30 sec and 72°C for 15 sec. The expression levels of
miR-21 were normalized to U6, and the levels were quantified using
the 2−ΔΔCq method (14).
Western blot analysis
Briefly, the hearts were rapidly removed and
homogenized in 1 ml modified tonic sucrose solution, following
treatment with trimetazidine or saline. Total protein was extracted
from heart tissues and cells using radioimmunoprecipitation assay
buffer (Beyotime Institute of Biotechnology, Shanghai, China).
Following centrifugation at 12,000 × g for 10 min at 4°C,
the protein concentration was determined using a Bicinchoninic Acid
protein assay (Beyotime Institute of Biotechnology). Protein
samples (50–60 µg) were separated by 8–12% sodium dodecyl
sulfate-polyacrylamide gel electrophoresis and were
electrophoretically transferred to polyvinylidene fluoride
membranes (EMD Millipore, Billerica, MA, USA, 0.22 mm).
Subsequently, the membranes were washed with Tris-buffered saline
containing 5% nonfat milk for 1 h at 37°C. The blocked membranes
were then incubated with the following antibodies:
Anti-phosphorylated (p)-Akt (cat. no. sc-135650; 1:2,000; Santa
Cruz Biotechnology, Inc., Dallas, TX, USA), anti-B-cell lymphoma 2
(Bcl-2; cat. no. sc-492; 1:1,000; Santa Cruz Biotechnology, Inc.),
anti-Bcl-2-associated X protein (Bax; cat. no. sc-6236; 1:1,000;
Santa Cruz Biotechnology, Inc.) and anti-β-actin (1:500; cat. no.
D110007; Sangon Biotech Co., Ltd.) overnight at 4°C. The membranes
were then probed with an anti-rabbit horseradish
peroxidase-conjugated immunoglobulin G secondary antibody (1:5,000;
cat. no. sc-2054; Santa Cruz Biotechnology, Inc.) at 37°C for 1 h.
Protein bands were visualized using a highly sensitive enhanced
chemiluminescence reagent (cat. no. C500044, Sangon Biotech Co.,
Ltd.) and were quantified by Odyssey v1.2 software (LI-COR
Biosciences, Lincoln, NE, USA).
Statistical analysis
Results were analyzed using SPSS 19.0 statistical
software (IBM SPSS, Armonk, NY, USA) and are presented as the mean
± standard error of the mean. The differences between more than two
groups were analyzed using analysis of variance followed by Tukey's
honest significant difference test. All experiments were repeated
three times. P<0.05 was considered to indicate a statistically
significant difference.
Results
Trimetazidine protects cardiac
function
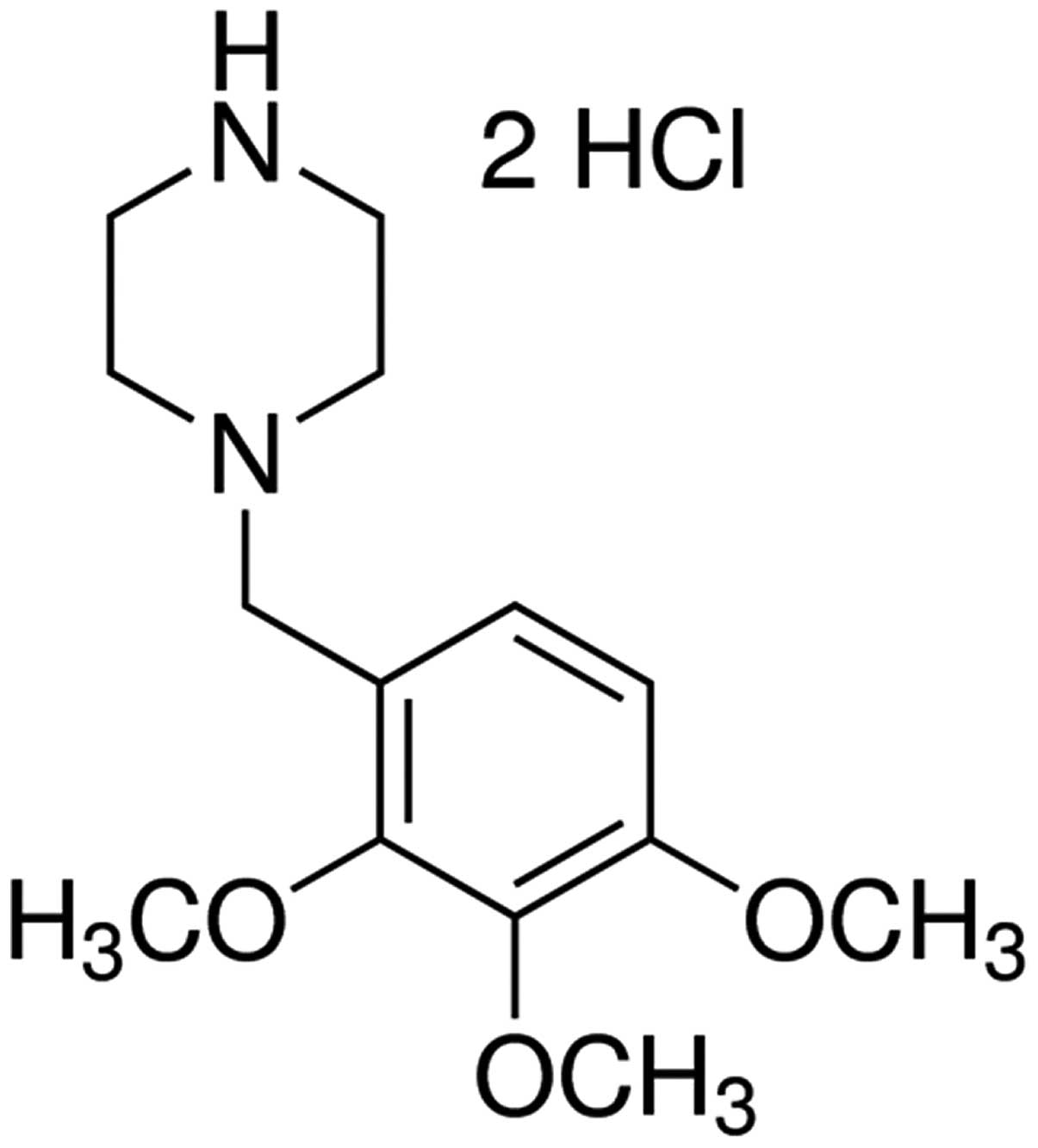
The chemical structure of trimetazidine is presented
in Fig. 1. In the present study
trimetazidine (97% purity) was dissolved in physiological saline.
The present study aimed to determine the potential protective
effects of trimetazidine on cardiac function following cardiac I/R
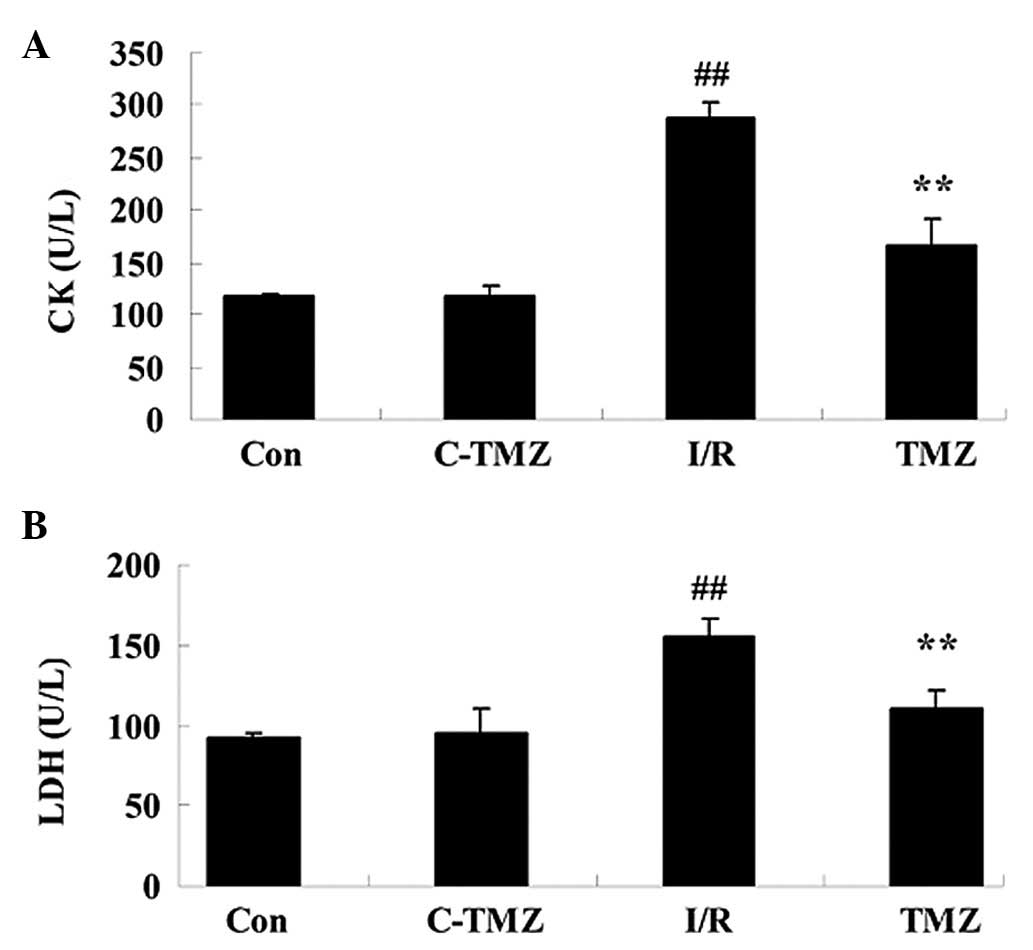
injury. I/R injury effectively increased CK and LDH activities
compared with the control group (Fig.
2; P=0.0023 and P=0.0043). However, trimetazidine markedly
reduced CK and LDH activities in rats following I/R injury
(Fig. 2; P=0.0031 and
P=0.0059).
Trimetazidine reduces infarct
size
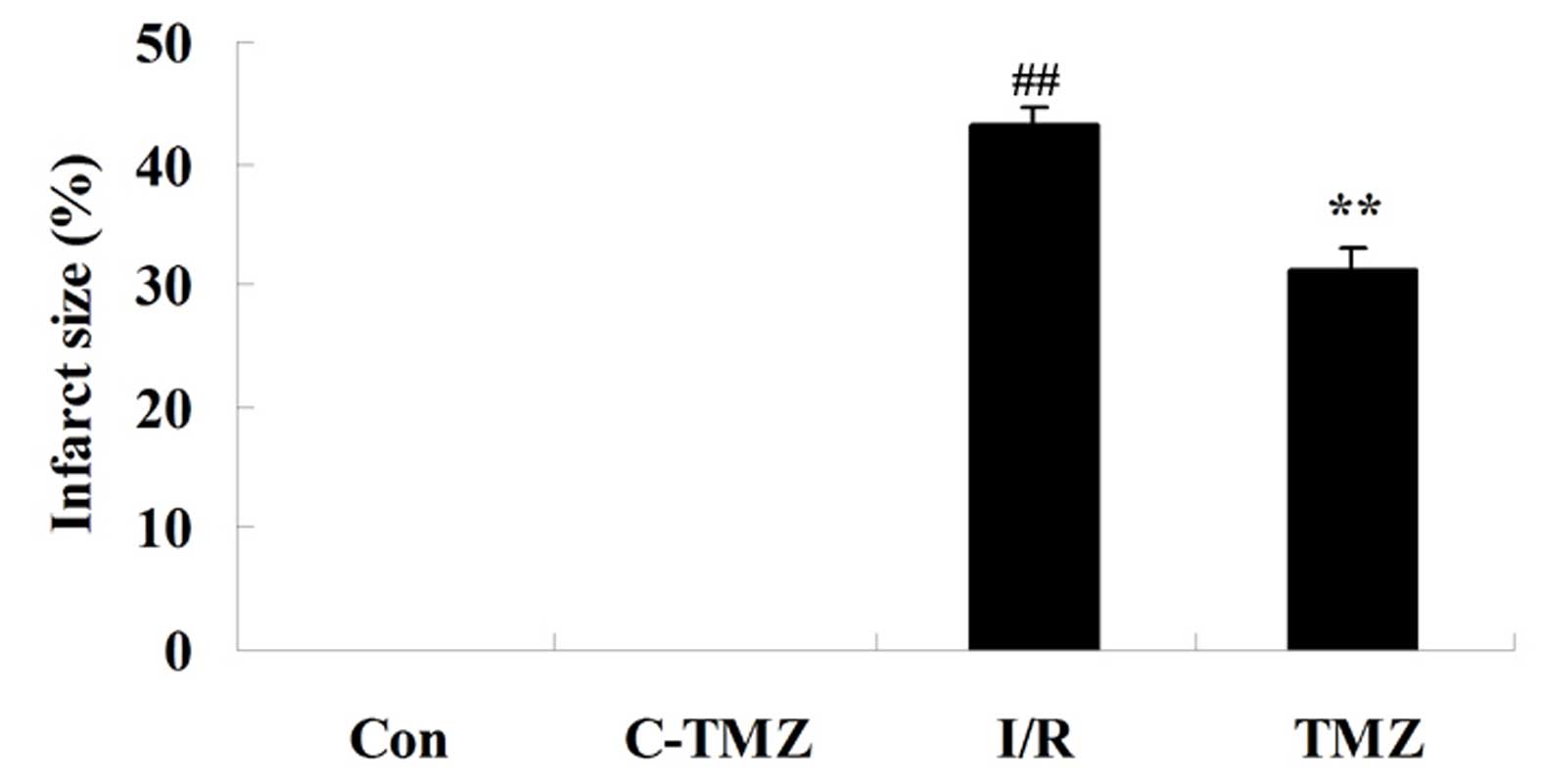
The present study aimed to determine whether the
potential protective effects of trimetazidine reduced infarct size
following cardiac I/R injury. As shown in Fig. 3, compared with the control group,
the infarct size was markedly increased in rats following cardiac
I/R injury (P=0.0056). However, treatment with trimetazidine
markedly reduced infarct size following cardiac I/R injury
(Fig. 3; P=0.0077).
Trimetazidine activates miRNA-21
expression
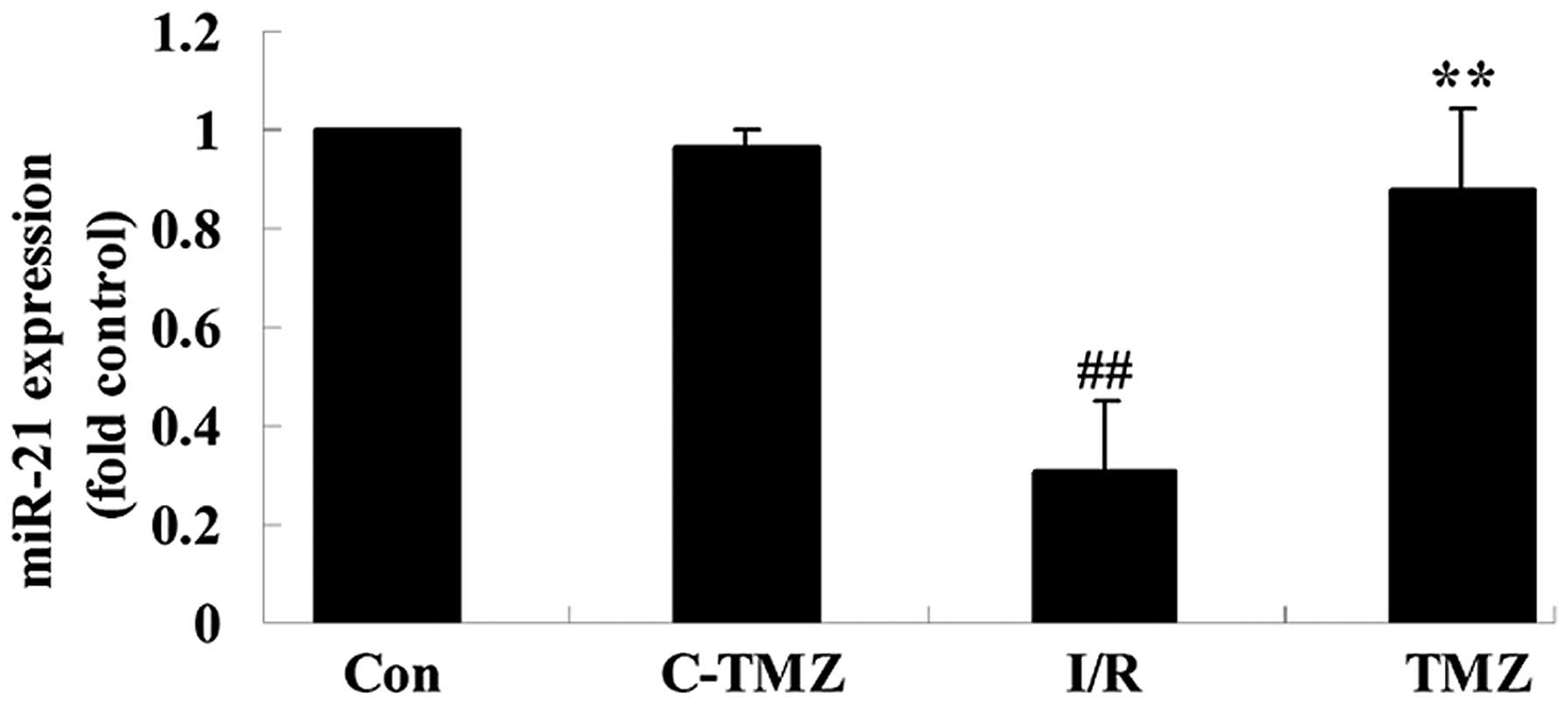
To investigate the potential effects of
trimetazidine on cardiac I/R injury, miRNA-21 expression was
detected using qPCR. As shown in Fig.
4, cardiac I/R injury markedly suppressed miRNA-21 expression
in rats, as compared with in the control group (Fig. 4; P=0.0009). Conversely, the
protective effects of trimetazidine markedly upregulated miRNA-21
expression following cardiac I/R injury (Fig. 4; P=0.0016).
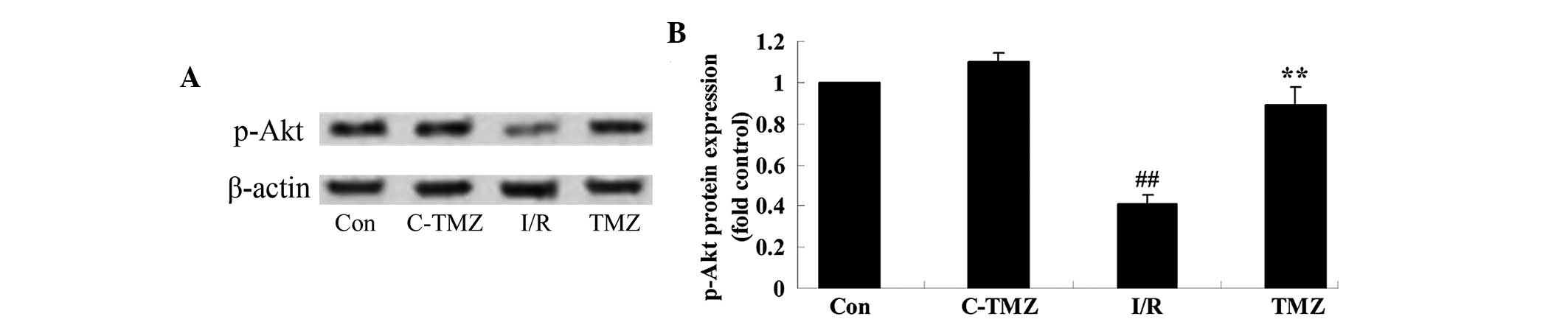
Trimetazidine activates Akt protein
expression
To determine the potential effects of trimetazidine
on cardiac I/R injury, p-Akt protein expression was detected using
western blot analysis. As shown in Fig. 5A and B, cardiac I/R injury
significantly suppressed p-Akt protein expression in rats compared
with the control group (P=0.0014). However, the protective effects
of trimetazidine significantly increased p-Akt protein expression
in rats following cardiac I/R injury (Fig. 5A and B; P=0.0031).
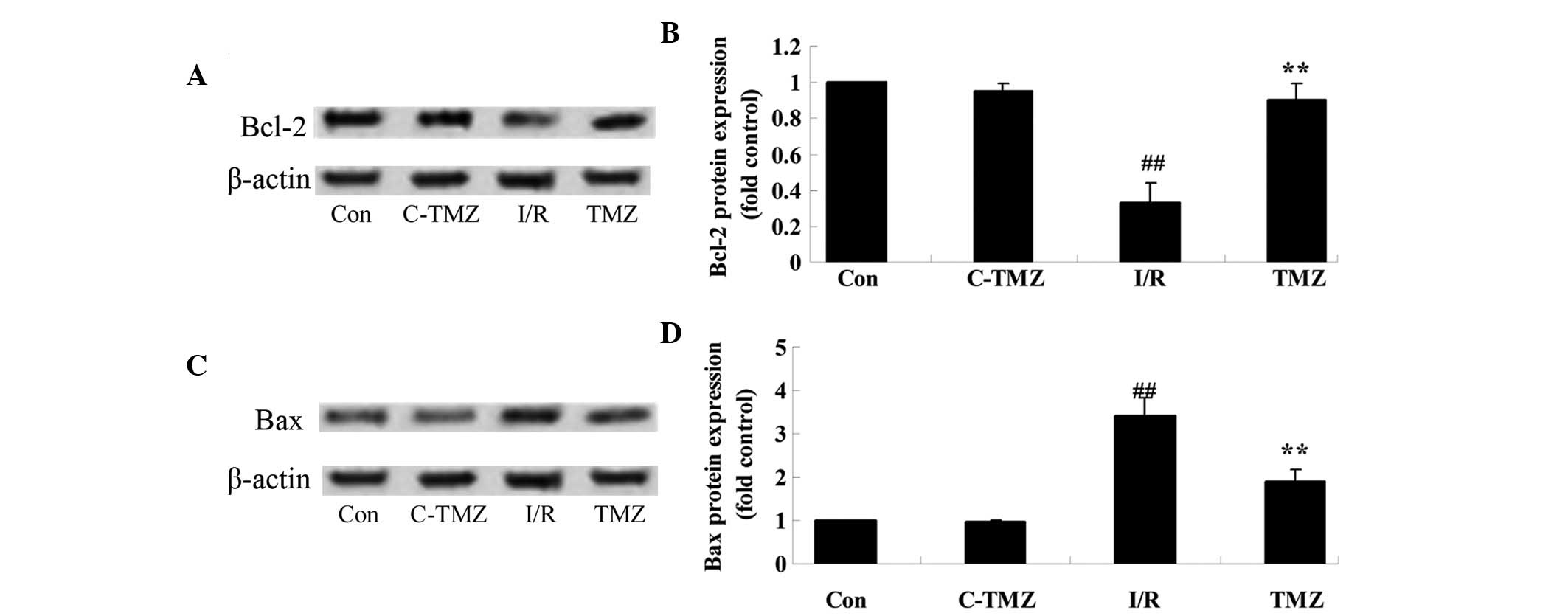
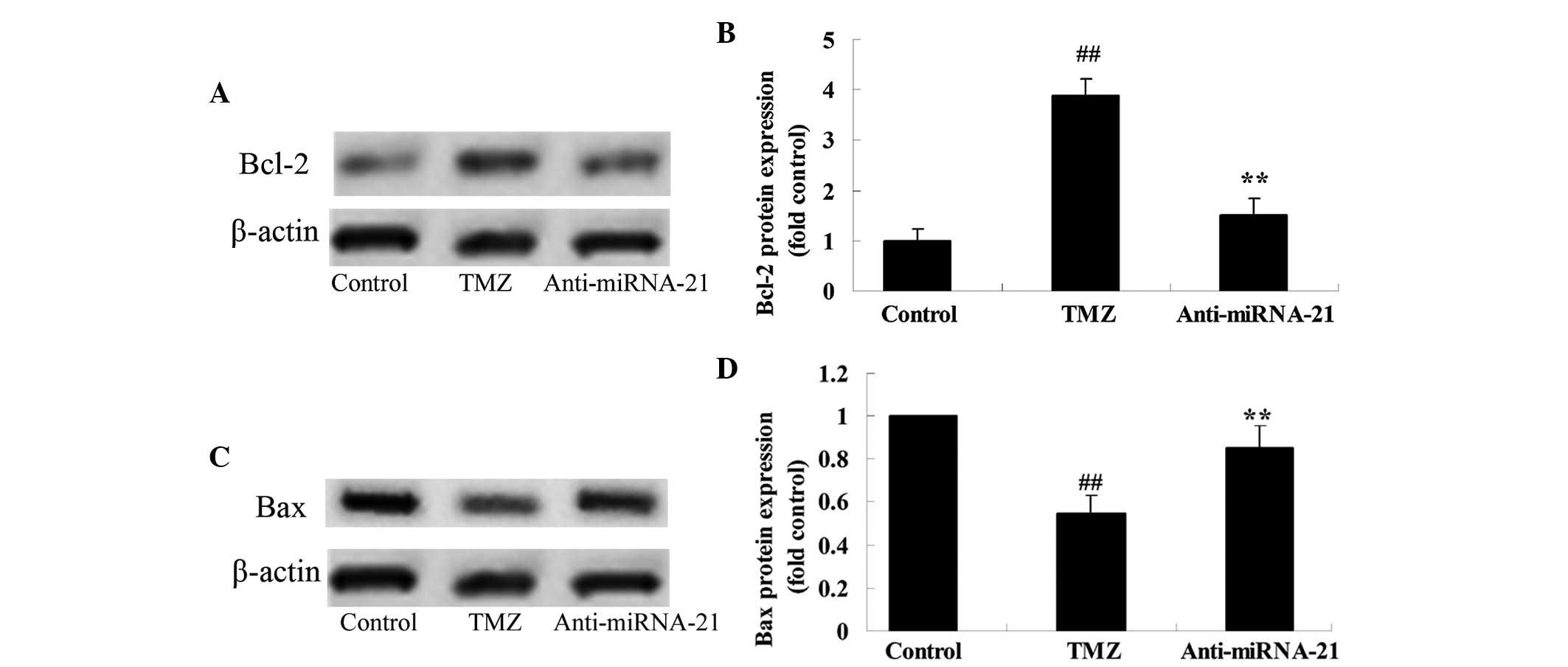
Trimetazidine suppresses Bcl-2/Bax
protein expressions
To explore the potential involvement of
trimetazidine in cardiac I/R injury, Bcl-2/Bax protein expression
was detected using western blot analysis. Cardiac I/R injury
significantly suppressed Bcl-2 protein expression and promoted Bax
protein expression in rats, as compared with in the control group
(Fig. 6A-D). Conversely, the
protective effects of trimetazidine significantly reversed Bcl-2
and Bax protein expression levels in rats following cardiac I/R
injury (Fig. 6A-D; P=0.0011).
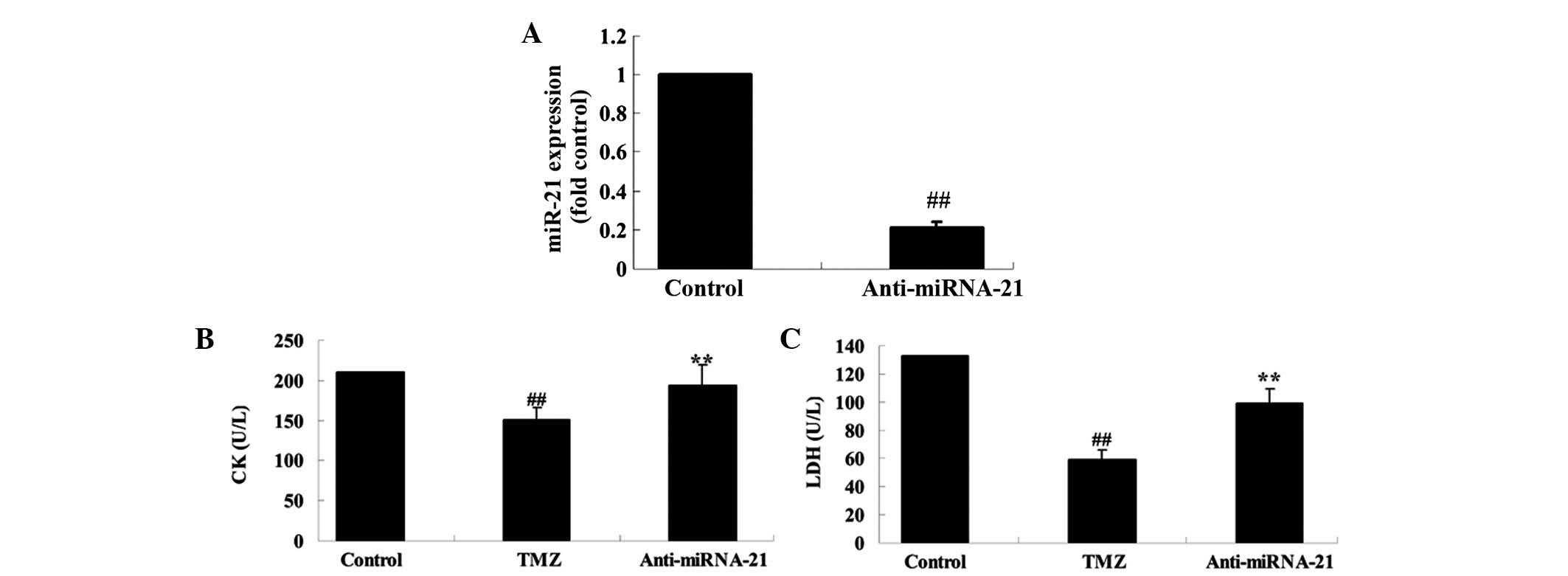
Knockdown of cardiac miRNA-21
expression affects the protective effect of trimetazidine
To further investigate the biological involvement of
miRNA-21 in trimetazidine-mediated cardiac protection, miR-NC and
anti-miR-21 plasmids were transfected into H9c2 cells using
Lipofectamine 2000. Transfection with anti-miR-21 plasmid
significantly decreased miRNA-21 expression in anoxia-induced H9c2
cells, as compared with in the control group (Fig. 7A; P=0.0032). Furthermore, the CK
and LDH activities of anoxia-induced H9c2 cells were markedly
suppressed following treatment with trimetazidine compared with the
control group (Fig. 7B and C;
P=0.0061 and P=0.0042). Conversely, transfection with the
anti-miR-21 plasmid markedly increased the CK and LDH activities of
anoxia-induced H9c2 cells (Fig. 7B and
C; P=0.0082 and P=0.0058).
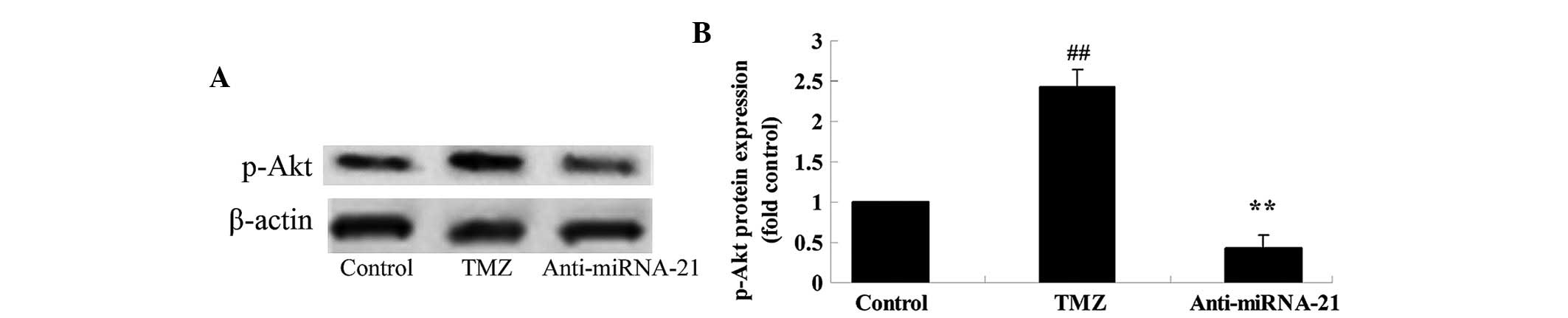
Knockdown of cardiac miRNA-21
expression affects p-Akt protein expression
To further explore the mechanism underlying
trimetazidine-induced miRNA-21-mediated cardiac protection in
vivo, the effects of cardiac miRNA-21 expression knockdown on
p-Akt protein expression were detected. The protein expression
levels of p-Akt in anoxia-induced H9c2 cells were markedly elevated
following treatment with trimetazidine compared with the control
group (Fig. 8A and B; P=0.0027).
Notably, knockdown of cardiac miRNA-21 expression significantly
reduced p-Akt protein expression in anoxia-induced H9c2 cells
(Fig. 8A and B; P=0.0047).
Knockdown of cardiac miRNA-21
expression affects Bcl-2/Bax protein expression
To further analyze the mechanism underlying
trimetazidine-induced miRNA-21-mediated cardiac protection in
vivo, the effects of cardiac miRNA-21 expression knockdown on
Bcl-2/Bax protein expression were detected. As shown in Fig. 9A and B, treatment with
trimetazidine markedly increased Bcl-2 protein expression in
anoxia-induced H9c2 cells compared with in the control group
(P=0.0013). However, knockdown of cardiac miRNA-21 expression
significantly inhibited Bcl-2 protein expression in anoxia-induced
H9c2 cells (Fig. 9A and B;
P=0.0026). Furthermore, treatment with trimetazidine markedly
reduced Bax protein expression in anoxia-induced H9c2 cells,
compared with the control group (Fig.
9C and D; P=0.0025). Conversely, knockdown of cardiac miRNA-21
expression significantly augmented Bax protein expression in
anoxia-induced H9c2 cells (Fig. 9C and
D; P=0.0047).
Discussion
Cardiac I/R injury refers to a series of myocardial
episodes that are caused by coronary recanalization and myocardial
reperfusion after myocardial ischemia. I/R injury induces complex
physiological and pathological alterations (15). Following myocardial ischemia, due
to cardiac ischemia, hypoxia and damage to the myocardial cell
membranes, cardiac cells exhibit a reduced energy supply, cell
membrane permeability is increased, dysfunction of the membrane
pump occurs, the ability of the cell to regulate intracellular
Ca2+ levels is lost, and the cells undergo degeneration
or necrosis. In addition, LDH, CK and other enzymes leak from the
cells resulting in increased serum concentrations (16). The increased degree of enzymatic
activity in the serum can reflect the extent of myocardial damage
(17). The results of the present
study indicated that the potential protective effects of
trimetazidine effectively reduced these alterations in rats
following I/R injury. Ussher et al reported that treatment
with trimetazidine may prevent obesity-induced cardiomyopathy in
mice (18). Furthermore, Allibardi
et al demonstrated that trimetazidine induces metabolic and
functional recovery in post-ischemic rat hearts (19).
Previous studies demonstrated that heat shock and
ischemic preconditioning can induce miRNA-1, −21 and −24 expression
in heart tissue, which affects the expression of endothelial nitric
oxide synthase and heat shock protein 70, resulting in decreased
I/R injury after 24 h (20–22).
miRNA-21 is an anti-apoptotic gene that has an important role in
the mechanism of anti-apoptosis and the regulation of programmed
cell death factor 4 (PDCD4). In addition, miRNA-21 is able to
reduce myocardial cell death after H2O2
stimulation via regulation of PDCD4 (23). Furthermore, myocardial ischemia may
induce damage to myocardial cells via miRNA expression (24). It is well-known that miRNA in
myocardial tissues has a role in the preliminary stage of
myocardial ischemia. It is of great significance to further study
the regulatory mechanisms of miRNA in cardiac I/R injury. In the
present study, the protective effects of trimetazidine
significantly promoted the expression of miRNA-21 in rats following
cardiac I/R injury. In a recent study, Liu et al reported
that trimetazidine improves right ventricular function via the
upregulation of miR-21 expression (8).
Apoptosis is a genetically programmed form of cell
death. Akt has a key role in preventing apoptosis, and the
regulation of glucose metabolism and protein synthesis (25). The isolated hearts from a rat model
of ischemic pretreatment exhibited significantly increased levels
of Akt phosphorylation and reduced myocardial infarction; however,
administration of the phosphoinositide 3-kinase suppressor
LY-294002 inhibited the myocardial protective effects of ischemic
preconditioning (26). In the
present study, the protective effects of trimetazidine
significantly increased the expression of p-Akt in rats following
cardiac I/R injury. It has previously been demonstrated that the
protective effects of trimetazidine significantly enhanced heart
function recovery via Akt activation (27).
Apoptosis is a genetically programmed form of cell
death. Bcl-2 exerts an inhibitory function on apoptosis. Bcl-2 and
Bax proteins are the two main members of the Bcl-2 multi-gene
family (28). Bcl-2 inhibits
apoptosis, whereas Bax exerts a proapoptotic effect. In rat cardiac
I/R injury, increased expression of the anti-apoptosis gene Bcl-2
in the myocardium and reduced expression of Bax may reduce cardiac
I/R injury (29). The present
study demonstrated that trimetazidine significantly augmented the
Bcl-2/Bax ratio in rats following cardiac I/R injury. Furthermore,
Khan et al demonstrated that trimetazidine ameliorates
myocardial dysfunction and injury via activation of Akt signaling
(30). To further analyze the
mechanism underlying trimetazidine-induced miRNA-21-mediated
cardiac protection in vivo, the effects of cardiac miRNA-21
expression knockdown on Bcl-2/Bax protein expression were detected.
The results confirmed that silencing miRNA-21 expression reversed
the protective effects of trimetazidine against cardiac I/R injury
via suppression of p-Akt and the Bcl-2/Bax pathway.
In conclusion, the present study demonstrated that
trimetazidine protects against cardiac I/R injury in vitro.
The findings indicated that miR-21 contributes to the protective
effect of trimetazidine against cardiac I/R injury via Akt and the
Bcl-2/Bax pathway. The results of the present study may help to
provide a rational novel drug for the treatment of cardiac I/R
injury.
References
|
1
|
Xiao J, Li J, Xu T, Lv D, Shen B, Song Y
and Xu J: Pregnancy-induced physiological hypertrophy protects
against cardiac ischemia-reperfusion injury. Int J Clin Exp Pathol.
7:229–235. 2013.PubMed/NCBI
|
|
2
|
Pisarenko OI, Lankin VZ, Konovalova GG,
Serebryakova LI, Shulzhenko VS, Timoshin AA, Tskitishvili OV,
Pelogeykina YA and Studneva IM: Apelin-12 and its structural analog
enhance antioxidant defense in experimental myocardial ischemia and
reperfusion. Mol Cell Biochem. 391:241–250. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Liu LF, Qin Q, Qian ZH, Shi M, Deng QC,
Zhu WP, Zhang H, Tao XM and Liu Y: Protective effects of melatonin
on ischemia-reperfusion induced myocardial damage and hemodynamic
recovery in rats. Eur Rev Med Pharmacol Sci. 18:3681–3686.
2014.PubMed/NCBI
|
|
4
|
Lenčová-Popelová O, Jirkovský E, Mazurová
Y, Lenčo J, Adamcová M, Šimůnek T, Geršl V and Štěrba M: Molecular
remodeling of left and right ventricular myocardium in chronic
anthracycline cardiotoxicity and post-treatment follow up. PLoS
One. 9:e960552014. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Chua CC, Gao J, Ho YS, Xu X, Kuo IC, Chua
KY, Wang H, Hamdy RC, Reed JC and Chua BH: Over-expression of a
modified bifunctional apoptosis regulator protects against cardiac
injury and doxorubicin-induced cardiotoxicity in transgenic mice.
Cardiovasc Res. 81:20–27. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Wang Y, Zhang ZZ, Wu Y, Zhan J, He XH and
Wang YL: Honokiol protects rat hearts against myocardial ischemia
reperfusion injury by reducing oxidative stress and inflammation.
Exp Ther Med. 5:315–319. 2013.PubMed/NCBI
|
|
7
|
Mishra PK, Tyagi N, Kundu S and Tyagi SC:
MicroRNAs are involved in homocysteine-induced cardiac remodeling.
Cell Biochem Biophys. 55:153–162. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Liu F, Yin L, Zhang L, Liu W, Liu J, Wang
Y and Yu B: Trimetazidine improves right ventricular function by
increasing miR-21 expression. Int J Mol Med. 30:849–855.
2012.PubMed/NCBI
|
|
9
|
Ikeda S and Pu WT: Expression and function
of microRNAs in heart disease. Curr Drug Targets. 11:913–925. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Opie LH and Boucher F: Trimetazidine and
myocardial ischemic contracture in isolated rat heart. Am J
Cardiol. 76:38B–40B. 1995. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Kim JS, Kim CH, Chun KJ, Kim JH, Park YH,
Kim J, Choi JH, Lee SH, Kim EJ, Yu DG, et al: Effects of
trimetazidine in patients with acute myocardial infarction: Data
from the Korean acute myocardial infarction registry. Clin Res
Cardiol. 102:915–922. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Hoda MN, Li W, Ahmad A, Ogbi S, Zemskova
MA, Johnson MH, Ergul A, Hill WD, Hess DC and Sazonova IY:
Sex-independent neuroprotection with minocycline after experimental
thromboembolic stroke. Exp Transl Stroke Med. 3:162011. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Hoda MN, Siddiqui S, Herberg S,
Periyasamy-Thandavan S, Bhatia K, Hafez SS, Johnson MH, Hill WD,
Ergul A, Fagan SC and Hess DC: Remote ischemic perconditioning is
effective alone and in combination with intravenous tissue-type
plasminogen activator in murine model of embolic stroke. Stroke.
43:2794–2799. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(−Delta Delta C(T)) Method. Methods. 25:402–408. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Li X, Liu J, Lin L, Guo Y, Lin C, Zhang C
and Yang B: Traditional Chinese medicine shuang shen ning xin
attenuates myocardial ischemia/reperfusion injury by preserving of
mitochondrial function. Evid Based Complement Alternat Med.
2014:1809652014. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Qiao Z, Ma J and Liu H: Evaluation of the
antioxidant potential of Salvia miltiorrhiza ethanol extract in a
rat model of ischemia-reperfusion injury. Molecules.
16:10002–10012. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Shen B, Li J, Gao L, Zhang J and Yang B:
Role of CC-chemokine receptor 5 on myocardial ischemia-reperfusion
injury in rats. Mol Cell Biochem. 378:137–144. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Ussher JR, Fillmore N, Keung W, Mori J,
Beker DL, Wagg CS, Jaswal JS and Lopaschuk GD: Trimetazidine
therapy prevents obesity-induced cardiomyopathy in mice. Can J
Cardiol. 30:940–944. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Allibardi S, Chierchia SL, Margonato V,
Merati G, Neri G, Dell'Antonio G and Samaja M: Effects of
trimetazidine on metabolic and functional recovery of postischemic
rat hearts. Cardiovasc Drugs Ther. 12:543–549. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Zhou J and Zhang J: Identification of
miRNA-21 and miRNA-24 in plasma as potential early stage markers of
acute cerebral infarction. Mol Med Rep. 10:971–976. 2014.PubMed/NCBI
|
|
21
|
Duan X, Ji B, Wang X, Liu J, Zheng Z, Long
C, Tang Y and Hu S: Expression of microRNA-1 and microRNA-21 in
different protocols of ischemic conditioning in an isolated rat
heart model. Cardiology. 122:36–43. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Yin C, Wang X and Kukreja RC: Endogenous
microRNAs induced by heat-shock reduce myocardial infarction
following ischemia-reperfusion in mice. FEBS Lett. 582:4137–4142.
2008. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Damania P, Sen B, Dar SB, Kumar S, Kumari
A, Gupta E, Sarin SK and Venugopal SK: Hepatitis B virus induces
cell proliferation via HBx-induced microRNA-21 in hepatocellular
carcinoma by targeting programmed cell death protein4 (PDCD4) and
phosphatase and tensin homologue (PTEN). PLoS One. 9:e917452014.
View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Jansen F, Yang X, Proebsting S, Hoelscher
M, Przybilla D, Baumann K, Schmitz T, Dolf A, Endl E, Franklin BS,
et al: MicroRNA expression in circulating microvesicles predicts
cardiovascular events in patients with coronary artery disease. J
Am Heart Assoc. 3:e0012492014. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Song JQ, Teng X, Cai Y, Tang CS and Qi YF:
Activation of Akt/GSK-3beta signaling pathway is involved in
intermedin(1–53) protection against myocardial apoptosis induced by
ischemia/reperfusion. Apoptosis. 14:1299–1307. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Zhang XJ, Xiong ZB, Tang AL, Ma H, Ma YD,
Wu JG and Dong YG: Rosiglitazone-induced myocardial protection
against ischaemia-reperfusion injury is mediated via a
phosphatidylinositol 3-kinase/Akt-dependent pathway. Clin Exp
Pharmacol Physiol. 37:156–161. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Kutala VK, Khan M, Mandal R, Ganesan LP,
Tridandapani S, Kalai T, Hideg K and Kuppusamy P: Attenuation of
myocardial ischemia-reperfusion injury by trimetazidine derivatives
functionalized with antioxidant properties. J Pharmacol Exp Ther.
317:921–928. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Liu Z, Li Z and Liu X: Effect of
ginsenoside Re on cardiomyocyte apoptosis and expression of
Bcl-2/Bax gene after ischemia and reperfusion in rats. J Huazhong
Univ Sci Technolog Med Sci. 22:305–309. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Tian Y, Zhang W, Xia D, Modi P, Liang D
and Wei M: Postconditioning inhibits myocardial apoptosis during
prolonged reperfusion via a JAK2-STAT3-Bcl-2 pathway. J Biomed Sci.
18:532011. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Khan M, Meduru S, Mostafa M, Khan S, Hideg
K and Kuppusamy P: Trimetazidine, administered at the onset of
reperfusion, ameliorates myocardial dysfunction and injury by
activation of p38 mitogen-activated protein kinase and Akt
signaling. J Pharmacol Exp Ther. 333:421–429. 2010. View Article : Google Scholar : PubMed/NCBI
|