Introduction
Myelodysplastic syndromes (MDS) are clonal stem cell
disorders characterized by peripheral cytopenias with dysplasia in
one or more cell lineages, including erythrocytic, granulocytic and
megakaryocytic lineages, leading to the progression to acute
myelogenous leukemia (AML) with a poor prognosis (1–4). At
present, allogeneic hematopoietic stem-cell transplantation is the
only treatment option, which can induce long-term remission
(5,6). However, its use is only possible in a
minority of patients with MDS due to the advanced age of
presentation, limited availability of donor sources, high rate of
treatment-associated mortality (~39% at 1 year), suboptimal
disease-free survival rates (~29% at 5 years) and chronic
graft-versus-host disease (~15% at 1 year) (6). Aberrant DNA methylation is frequently
associated with MDS; therefore, demethylating agents, including as
azacytidine and decitabine, are used to treat patients with MDS.
However, treatment of patients with a higher risk of MDS with
azacitidine (7,8) only increases the overall survival
rate to 24.5 months, compared with 15.0 months with conventional
care, supportive care, treatment with low-dose cytarabine or
intensive chemotherapy. In addition, treatment with decitabine
(9) prolongs the median duration
of the progression of AML or associated mortality rates to 12
months, compared with 6.8 months following supportive care alone.
In addition, the rates of complete remission (9–17%) following
treatment with demethylating agents (7–9) are
similar to those following conventional care with low-dose
cytarabine (11–18%) (10), and
substantially lower, compared with those following induction
chemotherapy in patients with AML (>50%) (11). Lenalidomide, a derivative of
thalidomide, reduces transfusion requirements, and reverses
cytologic and cytogenetic abnormalities in patients who have MDS
with the 5q31 deletion (12).
However, lenalidomide increases the risk of developing other
malignancies, including AML and B-cell lymphoma (13). Thus, a more effective treatment
option for MDS is urgently required.
Arsenic trioxide (As2O3) is a
traditional Chinese medicine, which is effective in the clinical
management of patients with acute promyelocytic leukemia (APL)
(14,15). However, in two-phase II multicenter
trials, rates of hematological improvement with
As2O3 were 20–29%, with moderate toxicity
reported (16,17). As2O3 induces
the apoptosis of nonpromyelocytic leukemia and other types of
malignant tumor cells (18–20)
through the inhibition of B cell lymphoma-2 (Bcl-2) (21), and the upregulation of
Bcl-2-associated X protein (Bax) (22) and caspase-3 (23).
Extracts of the Chinese herb, Tripterygium
wilfordii Hook F are used to treat autoimmune and/or
inflammatory diseases, and triptolide (TL) is the active substance
of these extracts in vitro and in vivo (24). Several studies have demonstrated
that TL may be an effective therapeutic agent for the treatment of
MDS (25), several types of human
pancreatic (26) and adrenal
(27) cancer, and T cell
lymphocytic leukemia (28) via
inducing cell apoptosis through the activation of caspase-3 and
generation of reactive oxygen species (ROS) (25–27).
Although certain combination therapies involving
As2O3 and other agents, are ongoing for
several types of human cancer, few As2O3
combination therapies are clinically effective. These include
combination therapy of As2O3 with ascorbic
acid in nonrefractory APL hematologic malignancies and multiple
myeloma (18), but not in other
AML except nonrefractory APL, acute lymphoid leukemia (18), chronic myeloid leukemia and chronic
lymphoid leukemia (18). The use
of phase 2 combination therapy with As2O3 and
gemtuzumab ozogamicin for the treatment of MDS and secondary AML
has been found to have acceptable response rates and toxicity,
however, the median overall survival rate was only 9.7 months
(29).
The aim of the present study was to investigate the
effect of As2O3 in combination with TL on the
apoptosis of MDS SKM-1 cells by evaluating the gene expression
levels of Bcl-2, Bax and caspase-3, and the generation of ROS.
Materials and methods
Reagents and cell culture
TL (purity >99.0%; Chinese Academy of Medical
Sciences, Nanjing, China) was dissolved in dimethyl sulfoxide
(DMSO; Sigma-Aldrich; Thermo Fisher Scientific, Inc., Waltham, MA,
USA) to form a 1 mM stock solution. As2O3
powder (Beijing Double-Crane Pharmaceutical Co., Ltd., Beijing,
China) was dissolved in phosphate-buffered saline (PBS). The MDS
SKM-1 cell line was obtained from the Cell Bank of the Japanese
Collection of Research Bioresources (Osaka, Japan). The SKM-1 cells
were cultured in RPMI 1640 medium (Life Technologies; Thermo Fisher
Scientific, Inc.) supplemented with 10% fetal calf serum and 1%
penicillin/streptomycin at 37°C in a humidified incubator with 5%
CO2. Cells in the second to fourth passages and
logarithmic growth phase, with >95% viability on trypan blue
staining, were used for the following experiments.
Cell treatment and cell viability
assessment using an MTT assay
The cells were seeded at a density of
4–6×104 cells/well in 96-well plates, cultured RPMI 1640
medium (Gibco; Thermo Fisher Scientific, Inc.) supplemented with
10% fetal calf serum and 1% penicillin/streptomycin mixture at 37°C
in humidified incubator with 5% CO2 for 48 h and treated
with various concentrations of As2O3 (0.25,
0.5, 2, 8 or 32 µM), TL (10, 20, 40, 80 or 160 ng/ml) or
As2O3+TL (0.25+10 ng/ml, 0.5+20 ng/ml, 2.0+40
ng/ml, 8+80 ng/ml or 32+160 ng/ml), or were mock-treated with
RPMI-1640 medium containing 0.002% DMSO. Following treatment for 48
h, cell viability was assessed using a CellTiter 96 AQueous One
Solution Cell Proliferation Assay kit (Promega, Nanjing, China),
according to the manufacturer's protocol. The absorbance at 490 nm
was measured using a SpectraMAX M5 spectrophotometer (Molecular
Devices, LLC, Sunnyvale, CA, USA).
Flow cytometric analysis of MDS SKM-1
cell apoptosis
Following treatment of the cells for 48 h with
As2O3, TL, As2O3 and
TL, or mock treatment with RPMI-1640 media, the cells were
collected by centrifugation at 1,300 × g for 3 min at room
temperature, washed twice with PBS (BD Biosciences, Beijing,
China), and resuspended in binding buffer (Novagen; EMD Millipore,
Billerica, MA, USA) at 1×106 cells/ml. Subsequently, the
cells were stained with 5 µl of annexin V-fluorescein
isothiocyanate (FITC) and 5 µl of propidium iodide (PI), incubated
in the dark at room temperature for 15 min, and mixed with binding
buffer (400 µl). Analysis of apoptosis was then performed on a
Calibur flow cytometer (BD Biosciences). Early and late apoptotic
cells were calculated based on annexin V-positivity/PI-negativity
and annexin V-positivity/PI-positivity, respectively.
Intracellular ROS
The cells (3×105/well) in 6-well plates
were treated with As2O3, TL,
As2O3 and TL or mock treatment, cultured in
RPMI 1640 medium, supplemented with 10% FCS and 1%
penicillin/streptomycin mixture at 37°C in humidified incubator
with 5% CO2 for 48 h. Following treatment, the cells
were washed once with PBS and treated with 100 nM
2′,7′-dichlorodihydrofluorescein diacetate in a cell culture
incubator for 30 min at 37°C with 5% CO2. Following
trypsinization, the cells were washed once with PBS and centrifuged
at 1,300 × g for 3 min. The cell pellets were then resuspended in 1
ml PBS and analyzed on a Calibur flow cytometer (BD
Biosciences).
Reverse transcription-quantitative
polymerase chain reaction (RT-qPCR) analysis
Following the treatment of the cells for 48 h, total
RNA was extracted using TRIzol reagent (Invitrogen; Thermo Fisher
Scientific, Inc.), according to the manufacturer's protocol.
RT-qPCR analysis was performed on an ABI 7900 sequence detection
system (Applied Biosystems; Thermo Fisher Scientific, Inc.) using a
qRT-PCR kit (Qiagen, Beijing, China), according to the
manufacturer's protocol. The Abl gene was used as an internal
control. The primer sequences were as follows: Bcl-2, forward (F)
5′-AGGATCATGCTGTACTTAA-3′ and reverse (R)
5′-ATGAGGCACGTTATTATTAG-3′; Bax, F 5′-CGAACTGGACAGTAACAT-3′ and R
5′-CTCGGAAAAAGACCTCTC-3′; caspase-3, F 5′-TTGTAGAAGTCTAACTGGAA-3′
and R 5′-CCATGTCATCATCAACAC-3′; Abl, F 5′-GATACGAAGGGAGGGTGTACCA-3′
and R 5′-CTCGGCCAGGGTGTTGAA-3′. The 25 µl PCR reaction system
included PCR mix 12.5 µl, F primer 0.5 µl, R primer 0.5 µl, probe
0.3 µl, ddH2O 7.2 µl, cDNA 4 µl. The reaction parameters
of Bcl-2, Bax and caspase-3 were as follows: 94°C 5 min, 94°C 40
sec, 56°C 55 sec, 72°C 1 min for 45 cycles, 72°C extension 7 min;
94°C 5 min, 94°C 40 sec, 58°C 55 sec, 72°C 1 min for 45 cycles,
72°C extension 7 min; 94°C 5 min, 94°C 40 sec, 50°C 55 sec, 72°C 1
min for 45 cycles, 72°C extension 7 min. The results were reported
as 2−∆∆Cq relative to the gene expression of Abl
(30).
Statistical analysis
Statistical analysis was performed with SPSS version
16.0 (SPSS, Inc., Chicago, IL, USA). Data are expressed as the mean
± standard error of the mean. Statistical analysis was performed
using one-way analysis of variance followed by the least
significant difference post-hoc test and Student's t-test.
Factorial design analysis of variance was used to determine
additive or synergistic effects. P<0.05 was considered to
indicate a statistically significant difference.
Results
As2O3 and TL
synergistically inhibit the growth of MDS SKM-1 cells
To examine whether TL enhances the chemosensitivity
of MDS SKM-1 cells to As2O3, the present
study examined the growth of MDS SKM-1 cells following treatment
with As2O3 in combination with TL. The
combination treatment of As2O3+TL
substantially suppressed SKM-1 cell growth, compared with the cells
treated with As2O3 or TL alone (Fig. 1A). To evaluate whether the cell
growth inhibition induced by the combination of
TL+As2O3 was additive or synergistic, the CI
values were determined according to the Chou-Talalay combination
index equation CI=(C)1/(CX)1+(C)2/(CX)2+(C)1(C)2/(CX)1(CX)2
(31), where CI <1 defines
synergism. The CI analysis revealed that the CI values ranged
between 0.70 and 0.87 (Fig. 1B).
These results indicated that As2O3 and TL
synergistically inhibited MDS SKM-1 cell growth.
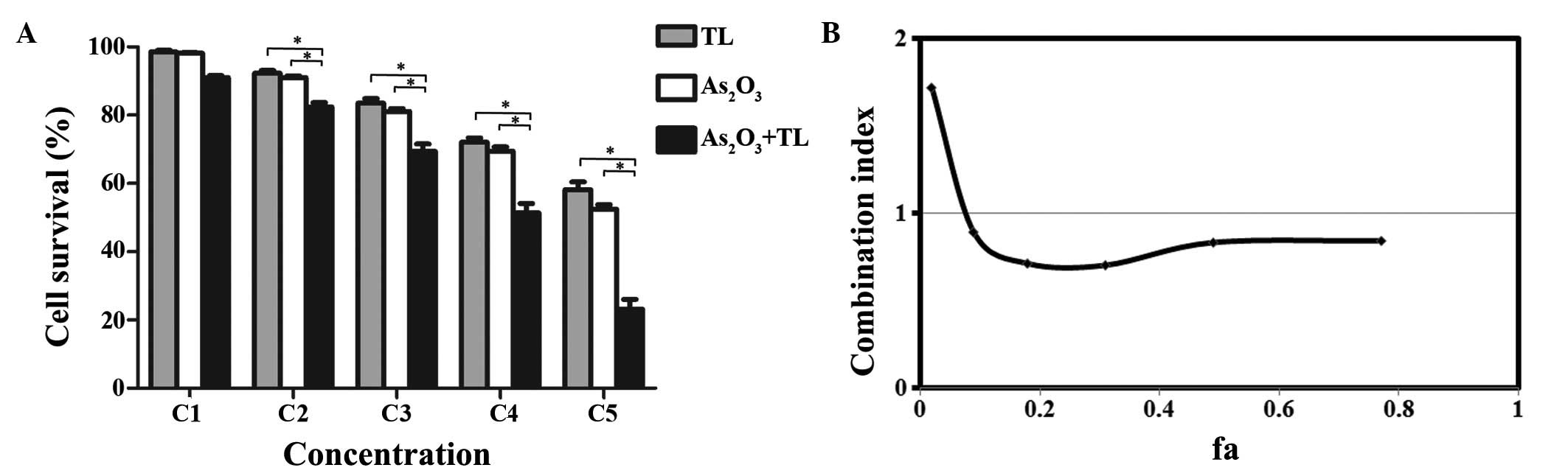 | Figure 1.Synergistic effect of
As2O3 and TL on the inhibition of MDS SKM-1
cell growth. (A) MDS SKM-1 cells treated with different
concentrations of As2O3 (0.25, 0.50, 2, 8 or
32 µM as C1-C5) and/or TL (10, 20, 40, 80 or 160 ng/ml as C1-C5).
Cell growth was measured using an MTT assay. Data are expressed as
the mean ± standard error of the mean (*P<0.01; n=5). (B)
Combination index of As2O3 with TL. The ‘fa’
on the x-axis denotes the fraction affected (i.e., a value of 0.2
is equivalent to a 20% reduction in cell growth).
As2O3, arsenic trioxide; TL, triptolide; MDS,
myelodysplastic syndrome. |
As2O3 and TL
synergistically induce apoptosis in MDS SKM-1 cells
To examine whether As2O3 and
TL synergistically inhibit MDS SKM-1 cell growth through the
induction of cell apoptosis by treatment with
As2O3 in combination with TL, cell apoptosis
was assessed using flow cytometry with annexin V-FITC/PI double
staining. The combination treatment of
As2O3+TL substantially induced SKM-1 cell
apoptosis, compared with either As2O3 or TL
alone (Fig. 2A). CI analysis
revealed that the CI values ranged between 0.65 and 0.85 (Fig. 2B). The results indicated that
As2O3 and TL synergistically induced MDS
SKM-1 cell apoptosis.
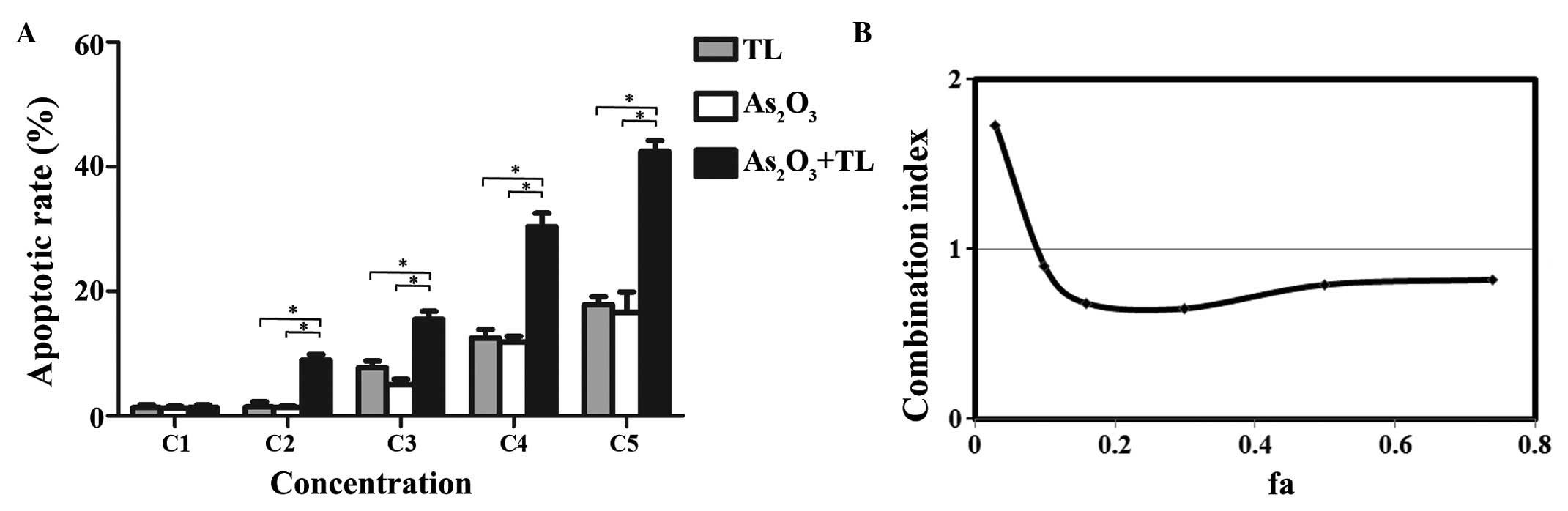 | Figure 2.As2O3+TL
treatment induces MDS SKM-1 cell apoptosis. The MDS SKM-1 cells
were treated with different concentrations of
As2O3 (0.25, 0.50, 2, 8 or 32 µM as C1-C5)
and/or TL (10, 20, 40, 80 or 160 ng/ml as C1-C5) for 48 h. (A) Flow
cytometric analysis of MDS SKM-1 cell apoptosis by double staining
with annexin V/PI. Data are expressed as the mean ± standard error
of the mean (*P<0.01; n=5). (B) Combination index of
As2O3+TL. The ‘fa’ on the x-axis denotes the
fraction affected (i.e., a value of 0.2 is equivalent to a 20%
increase in apoptosis). As2O3, arsenic
trioxide; TL, triptolide; MDS, myelodysplastic syndrome. |
As2O3 and TL
synergistically induce apoptosis via the generation of ROS in MDS
SKM-1 cells
Treatment with As2O3 in
combination with TL substantially increased the intracellular ROS
levels, compared with either As2O3 or TL
alone (Fig. 3A; P<0.01). CI
analysis revealed that the CI values ranged between 0.60 and 0.86
(Fig. 3B). The results indicated
that As2O3 and TL synergistically induced MDS
SKM-1 cell apoptosis via the generation of ROS.
 | Figure 3.Analysis of ROS in SKM-1 cells using
flow cytometry. (A) MDS SKM-1 cells were treated with different
concentrations of As2O3 (0.25, 0.50, 2, 8 or
32 µM as C1-C5, respectively) and/or TL (10, 20, 40, 80 or 160
ng/ml as C1-C5, respectively) for 48 h. The ROS levels were then
determined by counting the cells with
2′,7′-dichlorodihydrofluorescein diacetate fluorescence using flow
cytometry. Data are expressed as the mean ± standard error of the
mean (*P<0.01; n=5). (B) Combination index of
As2O3 with TL. The ‘fa’ on the x-axis denotes
the fraction affected (i.e., a value of 0.2 is equivalent to a 20%
increase in intracellular ROS levels). As2O3,
arsenic trioxide; TL, triptolide; MDS, myelodysplastic
syndrome. |
As2O3 and TL
synergistically regulate the expression of apoptosis-associated
genes in MDS SKM-1 cells
To determine whether As2O3 in
combination with TL synergistically regulates the expression of
apoptosis-associated genes, the mRNA expression levels of Bax,
Bcl-2 and caspase-3 were measured in the cells treated with
As2O3, TL or As2O3+TL
for 48 h. As shown in Fig. 4,
treatment with As2O3+TL led to significant
increases in the expression levels of Bax and caspase-3, and a
significant decrease in the mRNA expression of Bcl-2, compared with
either As2O3 or TL alone (P<0.01; Fig. 4A-C). These results demonstrated
that the combination of As2O3 and TL
significantly induced apoptotic activity via inhibiting Bcl-2 and
promoting the expression of Bax and caspase-3. CI analysis revealed
that the CI values were 0.57–0.82 for Bax (Fig. 4A), 0.53–0.78 for Bcl-2 (Fig. 4B) and 0.56–0.82 for caspase-3
(Fig. 4C). These results indicated
that As2O3 and TL synergistically induced MDS
SKM-1 cell apoptosis via increasing the mRNA expression levels of
Bax and caspase-3 and decreasing the mRNA expression of Bcl-2.
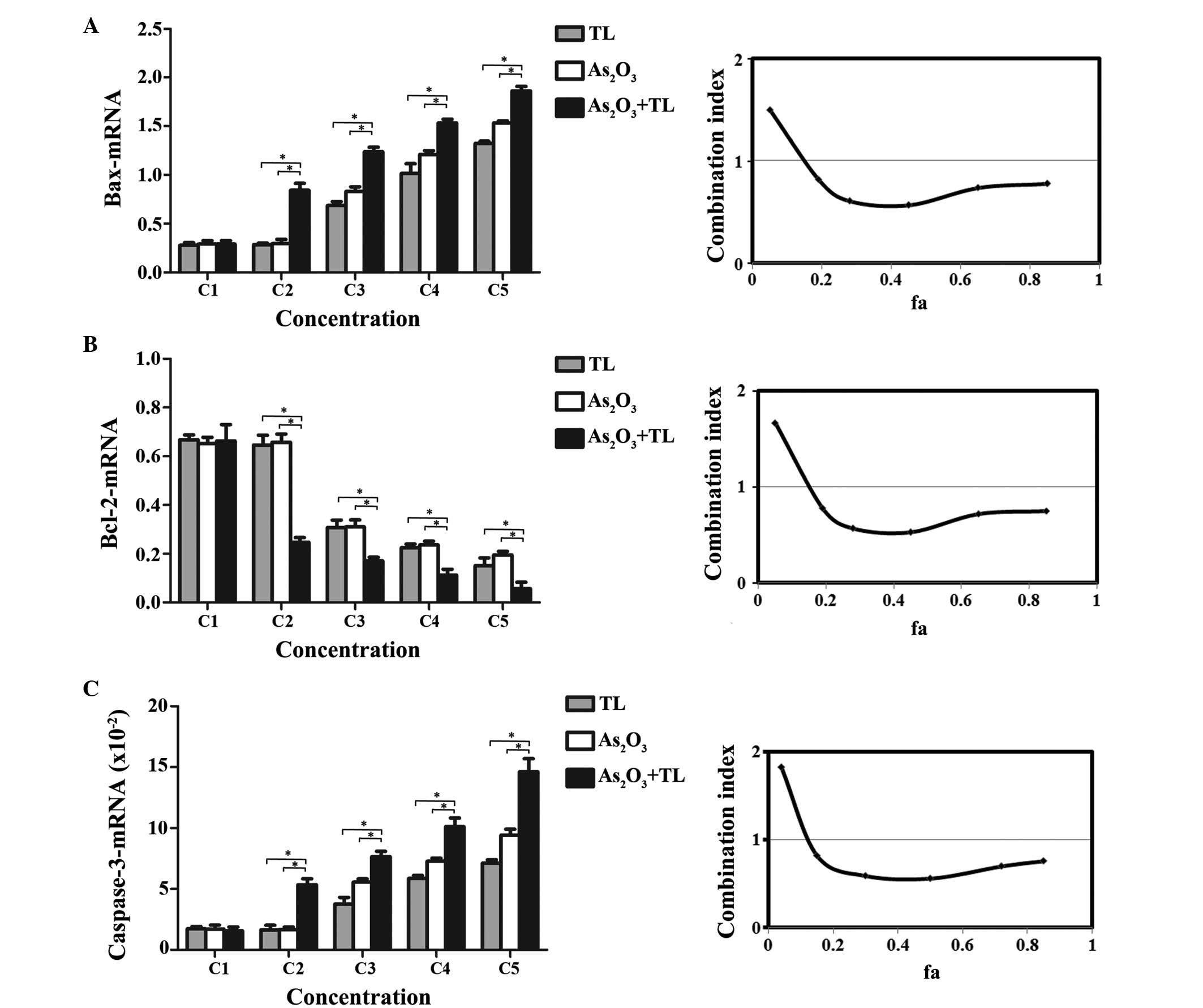 | Figure 4.mRNA levels of apoptosis-associated
genes in MDS SKM-1 cells. MDS SKM-1 cells were treated with
different concentrations of As2O3 (0.25,
0.50, 2, 8 or 32 µM as C1-C5) and/or TL (10, 20, 40, 80 or 160
ng/ml as C1-C5) for 48 h. The mRNA expression levels were
determined by reverse transcription-quantitative polymerase chain
reaction analysis, and quantified using the 2−∆∆Cq
method relative to Abl. Combination treatment led to a significant
(A) increase in the mRNA expression of Bax (*P<0.01), decrease
in the mRNA expression of (B) Bcl-2 (*P<0.01) and (C) increase
in the mRNA expression of caspase-3 (*P<0.01). Data are
expressed as the mean ± standard error of the mean (n=5;
*P<0.01). The graphs on the right show the combination index of
As2O3+TL. The ‘fa’ on the x-axis denotes the
fraction affected (i.e., 0.2 is equivalent to a 20% change in mRNA
expression). As2O3, arsenic trioxide; TL,
triptolide; MDS, myelodysplastic syndrome; Bcl-2, B cell
lymphoma-2; Bax, Bcl-2-associated X protein. |
Discussion
To investigate whether TL enhances the
chemosensitivity of MDS SKM-1 cells to As2O3,
the present study treated MDS SKM-1 cells with
As2O3, TL or the two in combination. It was
found that As2O3/TL synergistically inhibited
SKM-1 cell growth through upregulation of ROS levels and cell
apoptosis, as evidenced by synergistically increased expression
levels of Bax and caspase-3, and decreased mRNA expression of
Bcl-2.
The present study found that
As2O3+ TL synergistically induced MDS SKM-1
cell apoptosis, determined from analysis of annexin V-FITC/PI
double staining using flow cytometry. Of note,
As2O3, in combination with a
mitogen-activated protein kinase kinase or proteinase (32) inhibitor, has been shown
experimentally to have a synergistic effect on the induction of AML
cell apoptosis. The present study also found that the combination
treatment with As2O3 and TL resulted in a
significant increase in the mRNA expression levels of Bax and
caspase-3, and a significant decrease in the mRNA expression of
Bcl-2, compared with the cells treated with either
As2O3 or TL alone. To evaluate whether the
combination of TL and As2O3 increased the
mRNA expression levels of Bax and caspase-3 and decreased the mRNA
expression of Bcl-2 in an additive or synergistic manner, the CI
values were determined. The results indicated that
As2O3+TL synergistically induced MDS SKM-1
cell apoptosis via increasing the mRNA expression levels of Bax and
caspase-3 and decreasing the expression of Bcl-2 (CI<1). These
results suggested that the synergistic cell apoptosis induced by
the combination treatment resulted from inhibiting the mRNA
expression of Bcl-2 and promoting the mRNA expression levels of Bax
and caspase-3. It has been reported previously that
As2O3 induces cell apoptosis via the
upregulation of Bax (21) and the
Bax/Bcl-2 ratio (22), and the
downregulation of Bcl-2 (18).
Caspase-3 is a member of the cysteine-aspartic acid protease family
(33), and sequential activation
of caspase proteins is central to the apoptosis of a variety of
cancer cells (21–23,26).
TL induces human breast and prostate cancer cell apoptosis
(33), and TL in combination with
tumor-necrosis factor-related apoptosis-inducing ligand enhances
the apoptosis of cholangiocarcinoma cells by increasing the
activity caspase-3 (34). In
addition, the combination treatment of low-dose
1,25-dihydroxyvitamin D(3)
combined with As2O3 synergistically inhibits
AML cell proliferation via cell apoptosis mediated by the increased
expression levels of Bax and caspase-3, and decreased expression of
Bcl-2 (34).
The present study also found that the combination
treatment of As2O3 with TL synergistically
increased the generation of ROS in the cells. Therefore, it was
hypothesized that the induction of cell apoptosis by the
combination treatment in the present study was mediated by the
generation of ROS. It is well known that the presence of increased
intracellular ROS in the mitochondria is involved in the induction
of apoptosis in cancer cells, and that an increased intracellular
ROS concentration has been shown to cause an increase in the
Bax/Bcl-2 ratio and activation of caspase-3 (35,36).
In the present study, it was found that, compared with the cells
treated with either As2O3 or TL alone, the
generation of intracellular ROS was significantly increased
following exposure to As2O3 and TL in
combination. TL has been found to induce human adrenal cancer
NCI-H295 cell apoptosis through the ROS pathway (27), and treatments involving the
combination of As2O3 and sulindac (34) or phytosphingosine (37) have been shown to enhance apoptotic
cell death via increasing intracellular ROS.
In conclusion, the present study demonstrated that
treatment with As2O3 in combination with TL
synergistically induced MDS SKM-1 cell apoptosis via the induction
of intracellular ROS, which upregulated the expression of Bax,
downregulated the expression of Bcl-2 and upregulated the
expression of caspase-3 (Fig. 5).
These findings may provide a strategy to develop a novel
combination therapy against MDS.
Acknowledgements
The present study was supported by a grant (grant
no. LZ09103) from the Jiangsu Provincial Bureau of traditional
Chinese Medicine (Jaingsu, China).
References
|
1
|
Cogle CR, Craig BM, Rollison DE and List
AF: Incidence of the myelodysplastic syndromes using a novel
claims-based algorithm: High number of uncaptured cases by cancer
registries. Blood. 117:7121–7125. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Jädersten M and Hellström-Lindberg E:
Myelodysplastic syndromes: Biology and treatment. J Intern Med.
265:307–328. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Tefferi A and Vardiman JW: Myelodysplastic
syndromes. N Engl J Med. 361:1872–1885. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Gore SD and Hermes-DeSantis ER: Enhancing
survival outcomes in the management of patients with higher-risk
myelodysplastic syndromes. Cancer Control. 16:(Suppl). S2–S10.
2009.
|
|
5
|
Chang C, Storer BE, Scott BL, Bryant EM,
Shulman HM, Flowers ME, Sandmaier BM, Witherspoon RP, Nash RA,
Sanders JE, et al: Hematopoietic cell transplantation in patients
with myelodysplastic syndrome or acute myeloid leukemia arising
from myelodysplastic syndrome: Similar outcomes in patients with de
novo disease and disease following prior therapy or antecedent
hematologic disorders. Blood. 110:1379–1387. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Warlick ED, Cioc A, Defor T, Dolan M and
Weisdorf D: Allogeneic stem cell transplantation for adults with
myelodysplastic syndromes: Importance of pretransplant disease
burden. Biol Blood Marrow Transplant. 15:30–38. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Fenaux P, Mufti GJ, Hellstrom-Lindberg E,
Santini V, Finelli C, Giagounidis A, Schoch R, Gattermann N, Sanz
G, List A, et al: Efficacy of azacitidine compared with that of
conventional care regimens in the treatment of higher-risk
myelodysplastic syndromes: A randomised, open-label, phase III
study. Lancet Oncol. 10:223–232. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Gore SD, Fenaux P, Santini V, Bennett JM,
Silverman LR, Seymour JF, Hellström-Lindberg E, Swern AS, Beach CL
and List AF: A multivariate analysis of the relationship between
response and survival among patients with higher-risk
myelodysplastic syndromes treated within azacitidine or
conventional care regimens in the randomized AZA-001 trial.
Haematologica. 98:1067–1072. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Kantarjian H, Issa JP, Rosenfeld CS,
Bennett JM, Albitar M, DiPersio J, Klimek V, Slack J, de Castro C,
Ravandi F, et al: Decitabine improves patient outcomes in
myelodysplastic syndromes: Results of a phase III randomized study.
Cancer. 106:1794–1803. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Zwierzina H, Suciu S, Loeffler-Ragg J,
Neuwirtova R, Fenaux P, Beksac M, Harousseau J, Nuessler V, Cermak
J, Solbu G, et al: Low-dose cytosine arabinoside (LD-AraC) vs.
LD-AraC plus granulocyte/macrophage colony stimulating factor vs.
LD-AraC plus Interleukin-3 for myelodysplastic syndrome patients
with a high risk of developing acute leukemia: Final results of a
randomized phase III study (06903) of the EORTC Leukemia
Cooperative Group. Leukemia. 19:1929–1933. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Beran M, Shen Y, Kantarjian H, O'Brien S,
Koller CA, Giles FJ, Cortes J, Thomas DA, Faderl S, Despa S and
Estey EH: High-dose chemotherapy in high-risk myelodysplastic
syndrome: Covariate-adjusted comparison of five regimens. Cancer.
92:1999–2015. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
List A, Dewald G, Bennett J, Giagounidis
A, Raza A, Feldman E, Powell B, Greenberg P, Thomas D, Stone R, et
al: Lenalidomide in the myelodysplastic syndrome with chromosome 5q
deletion. N Engl J Med. 355:1456–1465. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Badros AZ: Lenalidomide in myeloma-a
high-maintenance friend. N Engl J Med. 366:1836–1838. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Lallemand-Breitenbach V, Zhu J, Chen Z and
de Thé H: Curing APL through PML/RARA degradation by As2O3. Trends
Mol Med. 18:36–42. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Shen ZX, Chen GQ, Ni JH, Li XS, Xiong SM,
Qiu QY, Zhu J, Tang W, Sun GL, Yang KQ, et al: Use of arsenic
trioxide (As2O3) in the treatment of acute promyelocytic leukemia
(APL): II. Clinical efficacy and pharmacokinetics in relapsed
patients. Blood. 89:3354–3360. 1997.PubMed/NCBI
|
|
16
|
Schiller GJ, Slack J, Hainsworth JD, Mason
J, Saleh M, Rizzieri D, Douer D and List AF: Phase II multicenter
study of arsenic trioxide in patients with myelodysplastic
syndromes. J Clin Oncol. 24:2456–2464. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Vey N, Bosly A, Guerci A, Feremans W,
Dombret H, Dreyfus F, Bowen D, Burnett A, Dennis M, Ribrag V, et
al: Arsenic trioxide in patients with myelodysplastic syndromes: A
phase II multicenter study. J Clin Oncol. 24:2465–2471. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Takahashi S: Combination therapy with
arsenic trioxide for hematological malignancies. Anticancer Agents
Med Chem. 10:504–510. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Xia J, Li Y, Yang Q, Mei C, Chen Z, Bao B,
Ahmad A, Miele L, Sarkar FH and Wang Z: Arsenic trioxide inhibits
cell growth and induces apoptosis through inactivation of notch
signaling pathway in breast cancer. Int J Mol Sci. 13:9627–9641.
2012. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Hoffman E and Mielicki WP: Arsenic
trioxide: Impact on the growth and differentiation of cancer cells
and possible use in cancer therapy. Postepy Hig Med Dosw (Online).
67:817–827. 2013.(In Polish). View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Zhao H, Guo W, Peng C, Ji T and Lu X:
Arsenic trioxide inhibits the growth of adriamycin resistant
osteosarcoma cells through inducing apoptosis. Mol Biol Rep.
37:2509–2515. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Li C, Qu X, Xu W, Qu N, Mei L, Liu Y, Wang
X, Yu X, Liu Z, Nie D, et al: Arsenic trioxide induces cardiac
fibroblast apoptosis in vitro and in vivo by up-regulating TGF-β1
expression. Toxicol Lett. 219:223–230. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Yedjou C, Tchounwou P, Jenkins J and
McMurray R: Basic mechanisms of arsenic trioxide (ATO)-induced
apoptosis in human leukemia (HL-60) cells. J Hematol Oncol.
3:282010. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Han R, Rostami-Yazdi M, Gerdes S and
Mrowietz U: Triptolide in the treatment of psoriasis and other
immune-mediated inflammatory diseases. Br J Clin Pharmacol.
74:424–436. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Deng J and Jin J: Study of
triptolide-induced apoptosis in MUTZ-1 cells and its allied
mechanism. Zhongguo Shi Yan Xue Ye Xue Za Zhi. 13:434–439. 2005.(In
Chinese). PubMed/NCBI
|
|
26
|
Mujumdar N, Mackenzie TN, Dudeja V, Chugh
R, Antonoff MB, Borja-Cacho D, Sangwan V, Dawra R, Vickers SM and
Saluja AK: Triptolide induces cell death in pancreatic cancer cells
by apoptotic and autophagic pathways. Gastroenterology.
139:598–608. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Wu PP, Liu KC, Huang WW, Ma CY, Lin H,
Yang JS and Chung JG: Triptolide induces apoptosis in human adrenal
cancer NCI-H295 cells through a mitochondrial-dependent pathway.
Oncol Rep. 25:551–557. 2011.PubMed/NCBI
|
|
28
|
Meng HT, Zhu L, Ni WM, You LS, Jin J and
Qian WB: Triptolide inhibits the proliferation of cells from
lymphocytic leukemic cell lines in association with downregulation
of NF-κB activity and miR-16-1*. Acta Pharmacol Sin. 32:503–511.
2011. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Sekeres MA, Maciejewski JP, Erba HP,
Afable M, Englehaupt R, Sobecks R, Advani A, Seel S, Chan J and
Kalaycio ME: A Phase 2 study of combination therapy with arsenic
trioxide and gemtuzumab ozogamicin in patients with myelodysplastic
syndromes or secondary acute myeloid leukemia. Cancer.
117:1253–1261. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(−Delta Delta C(T)) method. Methods. 25:402–408. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Chou TC and Talalay P: Quantitative
analysis of dose-effect relationships: The combined effects of
multiple drugs or enzyme inhibitors. Adv Enzym Regul. 22:27–55.
1984. View Article : Google Scholar
|
|
32
|
Takahashi S, Harigae H, Yokoyama H,
Ishikawa I, Abe S, Imaizumi M, Sasaki T and Kaku M: Synergistic
effect of arsenic trioxide and flt3 inhibition on cells with flt3
internal tandem duplication. Int J Hematol. 84:256–261. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Li M, Ding Y, Mu Y, Ao J and Chen X:
Molecular cloning and characterization of caspase-3 in large yellow
croaker (Pseudosciaena crocea). Fish Shellfish Immunol. 30:910–916.
2011. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Clawson KA, Borja-Cacho D, Antonoff MB,
Saluja AK and Vickers SM: Triptolide and TRAIL combination enhances
apoptosis in cholangiocarcinoma. J Surg Res. 163:244–249. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Spampanato C, De Maria S, Sarnataro M,
Giordano E, Zanfardino M, Baiano S, Cartenì M and Morelli F:
Simvastatin inhibits cancer cell growth by inducing apoptosis
correlated to activation of Bax and down-regulation of BCL-2 gene
expression. International J Onco. 40:935–941. 2012.
|
|
36
|
Abdelrahman IY, Helwa R, Elkashef H and
Hassan NH: Induction of P3NS1 myeloma cell death and cell cycle
arrest by simvastatin and/or γ-radiation. Asian Pac J Cancer Prev.
16:7103–7110. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Park MT, Kang YH, Park IC, Kim CH, Lee YS,
Chung HY and Lee SJ: Combination treatment with arsenic trioxide
and phytosphingosine enhances apoptotic cell death in arsenic
trioxide-resistant cancer cells. Mol Cancer Ther. 6:82–92. 2007.
View Article : Google Scholar : PubMed/NCBI
|



















