Introduction
The kidney is crucial in maintaining homeostasis
within the body by regulating the excretion of water and
electrolytes according to the requirements of the body. The
maintenance of homeostasis by the kidney is controlled at the
neural and humoral levels, however, it is also mediated through
autoregulation, which has a critical effect on function. The kidney
can produce a multitude of local, active substances, including
adenosine triphosphate, adenosine and angiotensin II (1–7).
Previous studies have shown that, in addition to
cardiac and skeletal muscle undergoing autoregulation by adenosine,
kidney function may be controlled in the same manner (8–10).
Under physiological conditions, adenosine has been shown to control
the release of renin (11–13), renal blood flow, glomerular
filtration rate (14),
glomerulotubular balance and tubuloglomerular feedback (15,16).
It is also involved in regulating ion transport in several nephron
segments, including the proximal tubule and the thick ascending
limb (TAL) (6,14,17–19).
The degree of NaCl transport has a direct effect on
the formation of the renal medulla hypertonic gradient, which
ultimately affects urine dilution and concentration. Furthermore,
NaCl transport is affected significantly by chloride channel
activity. Our previous studies showed that arachidonic acid (AA), a
local and active substance, inhibits the activities of potassium
and chloride channels. Additionally, adenosine was found to affect
the activity of potassium channels in the medullary TAL (mTAL).
Thus, elucidating the regulatory mechanism of adenosine on chloride
channels has the potential not only to improve current
understanding of Na+, K+ and Cl−
transport in the mTAL, but also offers novel insights for
developing high performance diuretics and therapies for the
clinical treatment of hypertension. The current study investigated
the effects of adenosine stimulation on the expression of
CLCNKB mRNA in the basolateral mTAL of the rat kidney, and
the pathways involved in th effects. This study provides insight
into the regulation and mechanisms of kidney function, and a
potential new target for the clinical treatment of kidney
disease.
Materials and methods
Animals
Pathogen-free Sprague-Dawley rats (male and female;
50–60 g; n=20) were obtained from the Animal Center of the Second
Affiliated Hospital of Harbin Medical University (Harbin, China)
and were maintained with standard rat chow and access to tap water
ad libitum. The study was approved by the ethics committee
of Harbin Medical University.
Isolation of mTAL tubules and
cells
The method for the preparation of mTAL suspensions
was established on the basis of the methods described in several
previous studies (20–22). The rats were sacrificed by cervical
dislocation and the kidneys were removed immediately for future
dissection of the mTAL cells. The inner stripe of the outer medulla
was carefully excised and minced with a sterile blade. It was then
sequentially incubated and shaken at 37°C in HEPES buffer solution
containing 0.01% collagenase type IA (1 mg/ml; Sigma-Aldrich;
Thermo Fisher Scientific, Inc., Walthlam, MA, USA) and 140 mM NaCl,
5 mM KCl, 1.8 mM MgCl2, 1.8 mM CaCl2 and 10 mM HEPES (pH
7.4) for 5 min. The suspension was precipitated on ice, and then
mixed with HEPES buffer solution again. Finally, and the
supernatant containing the crude suspension of tubules was
collected. The undigested tissues were subjected to three
treatments with collagenase (5 min each), and the combined
supernatants were suspended in HEPES buffer solution and then
filtered through 180 and 50 µm nylon mesh membranes. The tubules
retained on the 50 µm mesh were collected with centrifugation
(1,000 × g, 5 min, 4°C) and suspended in HEPES buffer
solution. The suspension, which contained the mTAL tubules, was
used to establish primary cultures of mTAL cells. The mTAL tubules
(5×105/ml) were cultured at 37°C in 90-mm-diameter Petri
dishes using Renal Epithelial Cell culture medium (Dulbecco's
modified Eagle's medium/F12; 1:1; Hyclone; GE Healthcare Life
Sciences, Logan, UT, USA), which contained 10% fetal bovine serum
(Gibco; Thermo Fisher Scientific, Inc.), 1% streptomycin-penicillin
(100 U/ml), rhEGF (Peprotech, Inc., Rocky Hill, NJ, USA), insulin,
hydrocortisone, amphotericin B and epinephrine (Nanjing KGI
Biological Technology Development Co., Ltd., Nanjing, China). After
6–7 days, the monolayer of cells was 80–90% confluent, and used in
the subsequent experiments.
Cell treatments
When the cells were in the logarithmic growth phase,
the medium was changed to serum-free medium. Initially, 25.6 µM
adenosine was added to the cells and incubated for 6, 12, 24 and 48
h to find the greatest effect time. Subsequently, different
concentrations of adenosine (1.6, 3.2, 6.4, 12.8, 25.6 and 51.2 µM)
were added to indicate the best concentration to use. The mRNA
expression of CLCNKB was analyzed using the chosen duration
and concentration of adenosine treatment. Finally, 5 µM AACOCF3 or
5 µM H8 were used to treat cells for 6 h, then 25.6 µM adenosine
was added 24 h to determine whether the cAMP-PKA and PLA2-AA
pathways affected the mRNA expression of CLCNKB in the
presence of adenosine.
Western blot analysis
When the cells were in logarithmic growth phase, the
medium was completely removed, the cells were washed twice in
ice-cold PBS and lysed with 400 µl cell lysis buffer (1% sodium
deoxycholate; 10 mM Tris-Cl, pH 8; 1 nM EDTA-Na, pH 8; 0.5 mM PMSF;
1.5 µM aprotinin; 154 nM pepstatin; 50 µM phloroglucinol) per dish.
Cells were scraped off the dish and transferred into eppendorf
tubes, and maintained on ice for 15 min. The samples were sonicated
for 3–5 sec. Protein concentrations were determined by
bicinchoninic acid assay (Thermo Fisher Scientific, Inc.) The
protein samples (50 µg) from the primary cultures of mTAL cells
were separated by electrophoresis using 10% SDS-PAGE and
transferred onto nitrocellulose membranes (Pall Life Sciences, Port
Washington, NY, USA). The membranes were blocked with 5% nonfat dry
milk in 0.1% Tween-Tris-buffered saline (TBS-T), and then washed
with 0.1% TBS-T. The membranes were incubated with primary antibody
overnight at 4°C (rabbit anti-rat cPLA2, 1:1,000; rabbit
anti-rat PKA, 1:1,000; rabbit anti-rat β-actin, 1:1,000). Following
incubation, the membranes were washed four times (10 min per wash)
with 0.1% TBS-T, following which the membranes were incubated with
horseradish peroxidase-conjugated goat anti-rabbit secondary
antibody (1:10,000; OriGene Technologies, Inc., Rockville, MD, USA)
for 1 h at room temperature. SuperSignal West Pico Chemiluminescent
Substrate (Pierce; Thermo Fisher Scientific, Inc.) was used to
detect the protein bands. The protein bands were quantified by
densitometry using Quantity One software (Bio-Rad Laboratories,
Inc., Hercules, CA, USA).
Reverse transcription-polymerase chain
reaction (RT-PCR) analysis
Total RNA was purified from the primary cultures of
the mTAL cells in the logarithmic growth phase using a Protein and
RNA Extraction kit for mammalian cells (Takara Biotechnology Co.,
Ltd., Dalian, China). RNA was then quantified using
spectrophotometry and reverse transcribed into cDNA using RT-PCR
kit (Takara Biotechnology Co., Ltd.). The cDNA was amplified using
a master mix containing Taq polymerase (Takara Biotechnology Co.,
Ltd.) and the following primers from AuGCT DNA-SYN Biotechnology
Co., Ltd. (Beijing, China): CLCNKB, sense
5′-CTGTTCCGTGTGGGTGAG-3′ and antisense 5′-GGGTACACGGTCCAAGAG-3′;
β-actin, sense 5′ 3′, antisense 5′-CCAGGGAGGAAGAGGATGCG-3′. Each
primer was used at a final concentration of 20 µM. The
thermocycling steps were as follows: β-actin; 94°C for 5
min, then 94°C for 30 sec, 56°C annealing for 30 sec, 72°C
extension for 25 sec (25 cycles), and 72°C for 7 min;
CLCNKB: 94°C for 5 min, then 94°C for 30 sec, 56°C annealing
for 30 sec, 72°C extension for 25 sec (40 cycles), and 72°C for 7
min The PCR products were separated on a 3% agarose gel, stained
with ethidium bromide to identify fragments of CLCNKB,
β-actin. The gene fragments were quantified by densitometry using
Quantity One software (Bio-Rad Laboratories, Inc.).
Chemicals
The antibodies for β-actin (cat. no. sc-7210), PKA
(cat. no. sc-28892) and cPLA2 (cat. no. sc-438) were obtained from
Santa Cruz Biotechnology, Inc. (Dallas, TX, USA). Reagent-grade
chemicals, adenosine, AACOCF3 (an antagonist of PLA2) and H8 (an
antagonist of PKA) were obtained from Sigma-Aldrich; Thermo Fisher
Scienfiic, Inc. The prestained protein ladder was obtained from
Fermentas; Thermo Fisher Scientific, Inc.
Statistical analysis
Data are shown as the mean ± standard deviation.
Paired Student's t-tests were used to determine the
significance of differences between the control and experimental
groups. P<0.05 was considered to indicate a statistically
significant difference.
Results
Expression levels of PKA and
PLA2 in primary cultures of mTAL cells
Upon adenosine binding to its receptors, target
cells are typically affected through the activation or inhibition
of the cyclic adenosine monophosphate-protein kinase A (cAMP-PKA),
phospholipase A2-arachidonic acid (PLA2-AA) and phospholipase
C-protein kinase C pathways (23).
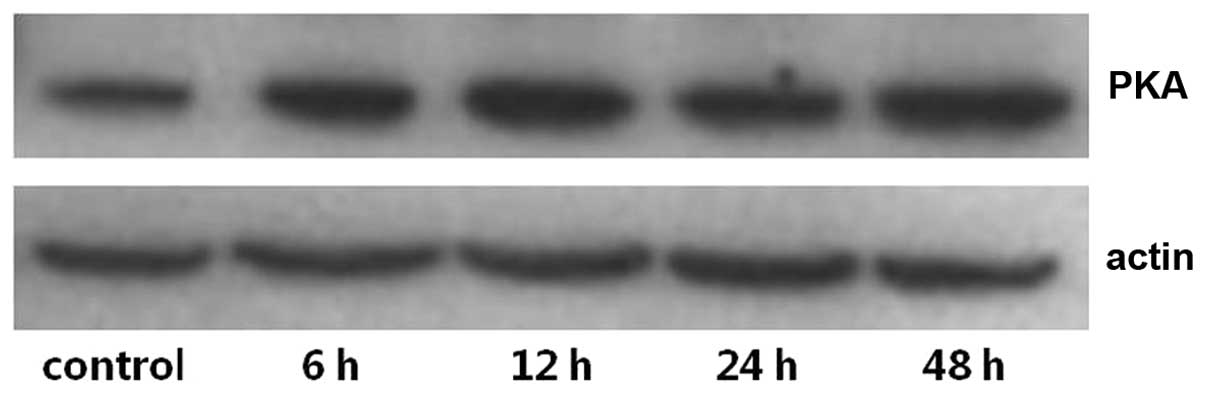
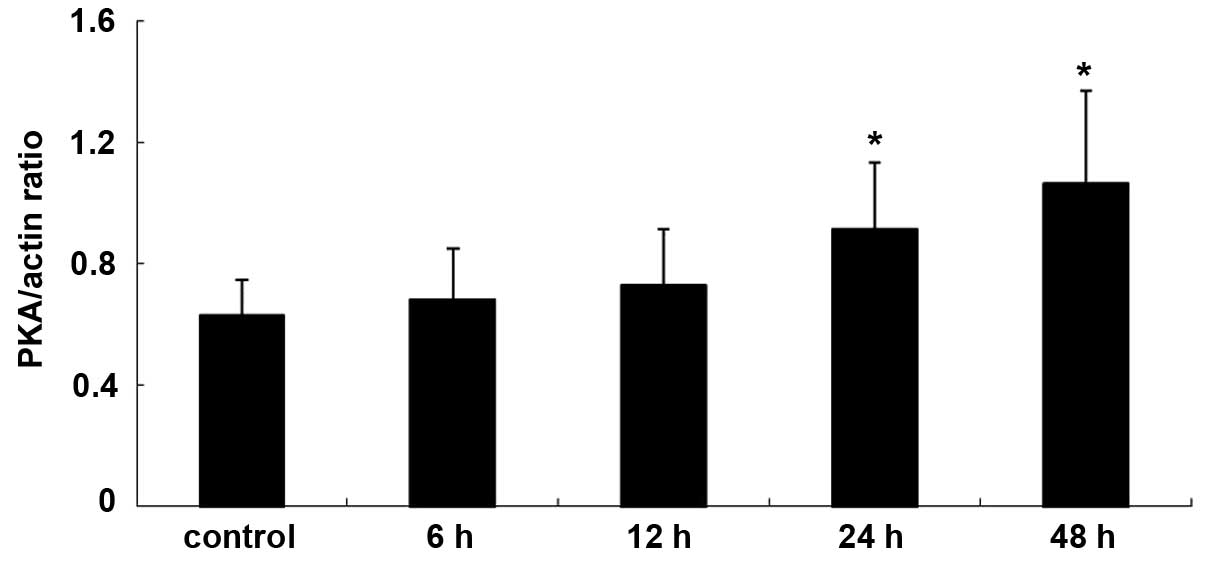
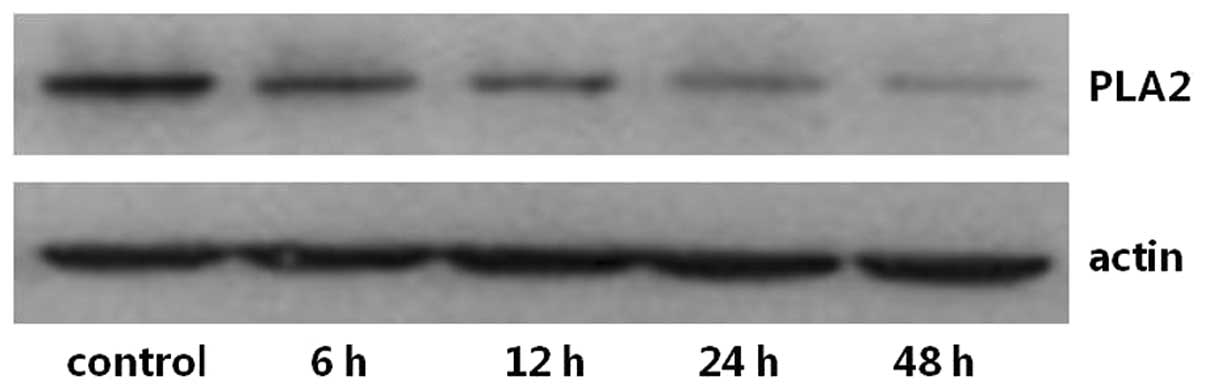
The expression levels of PKA and PLA2 in primary cultures of mTAL
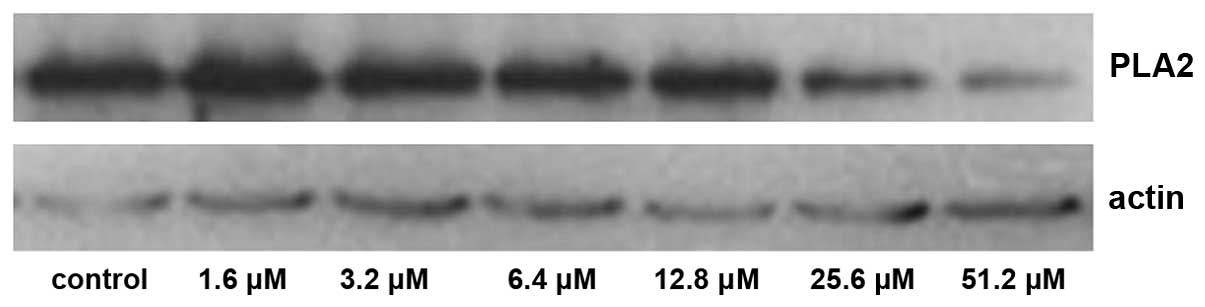
cells were determined using western blot analysis. The expression
levels of PKA and PLA2 were detected over time and in the presence
of increasing concentrations of adenosine. Compared with the
control group, the expression of PKA increased significantly at 24
and 48 h post-adenosine treatment (P<0.05; Figs. 1 and 2), whereas the expression of PLA2
decreased significantly at 24 and 48 h post-adenosine treatment
(P<0.05; Figs. 3 and 4). No change was observed at 6 or 12
h.
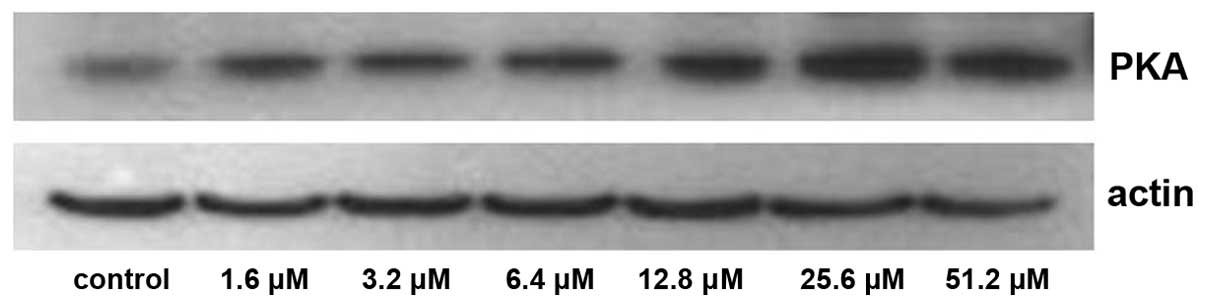
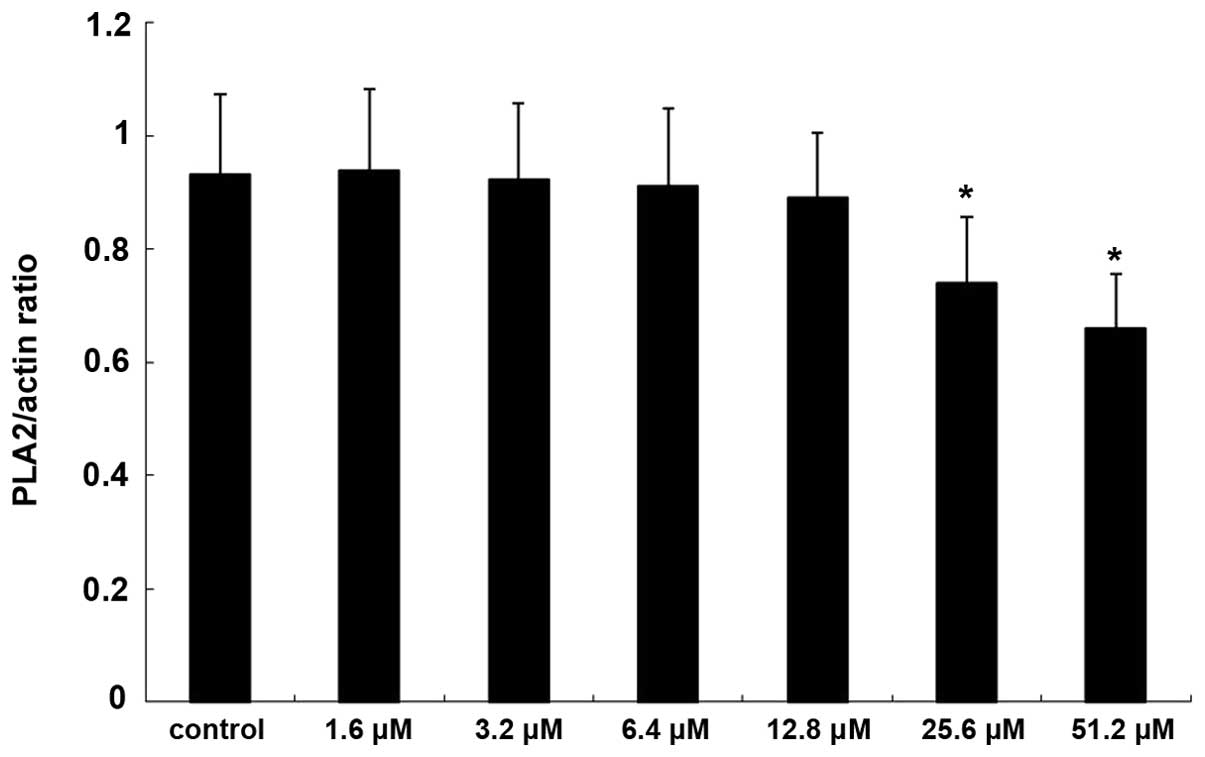
The expression of PKA increased significantly
following treatment with 25.6 and 51.2 µM adenosine (P<0.05;
Figs. 5 and 6), whereas the expression of
PLA2 decreased significantly following treatment with
25.6 and 51.2 µM adenosine (P<0.05; Figs. 7 and 8).
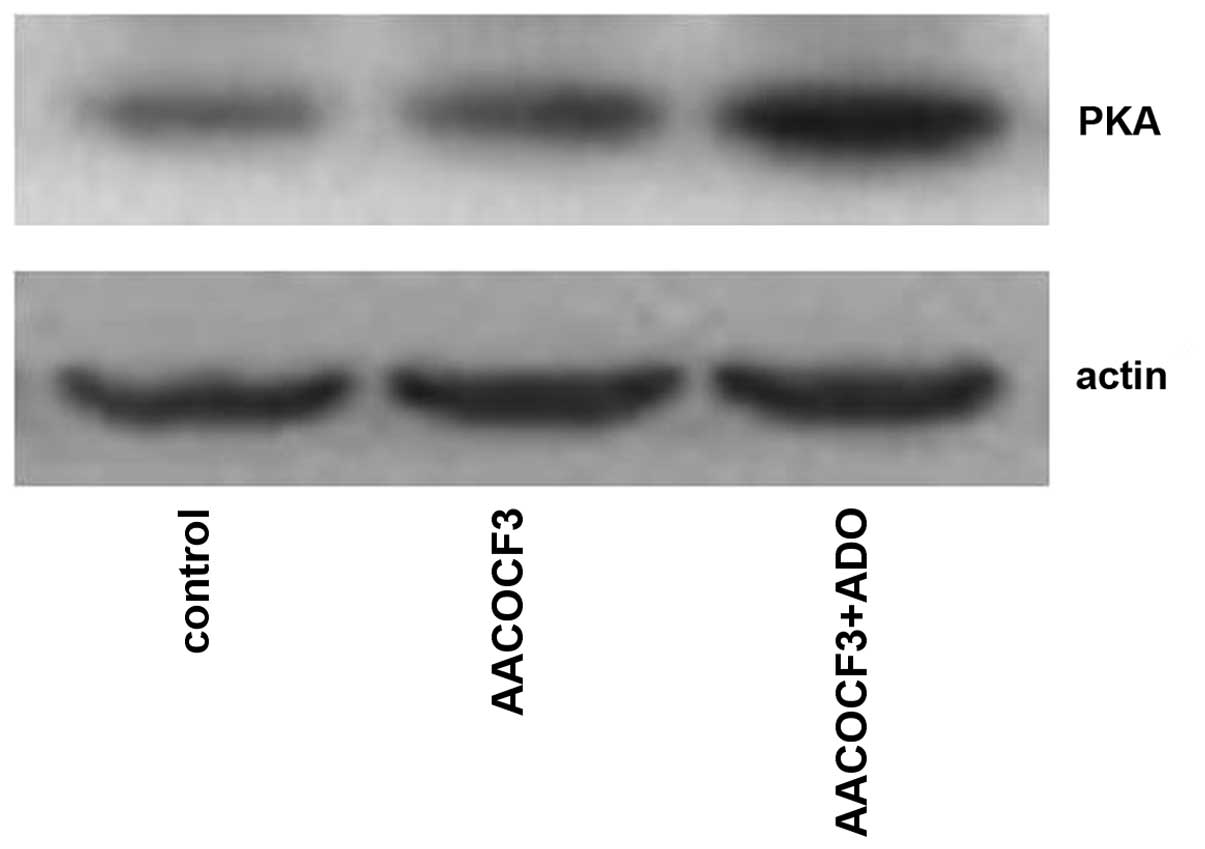
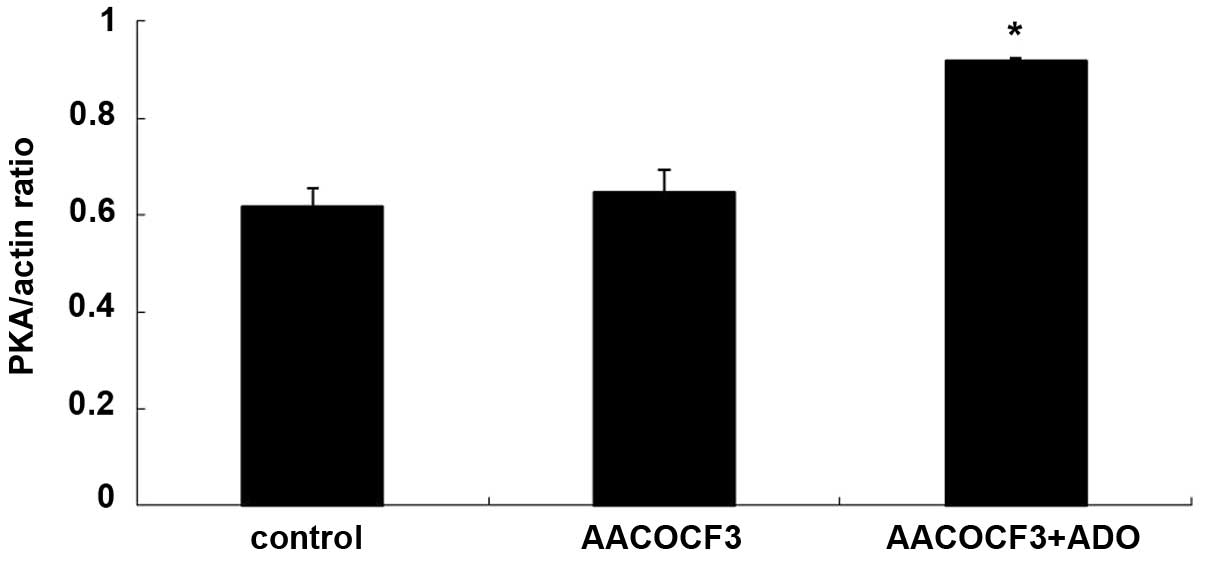
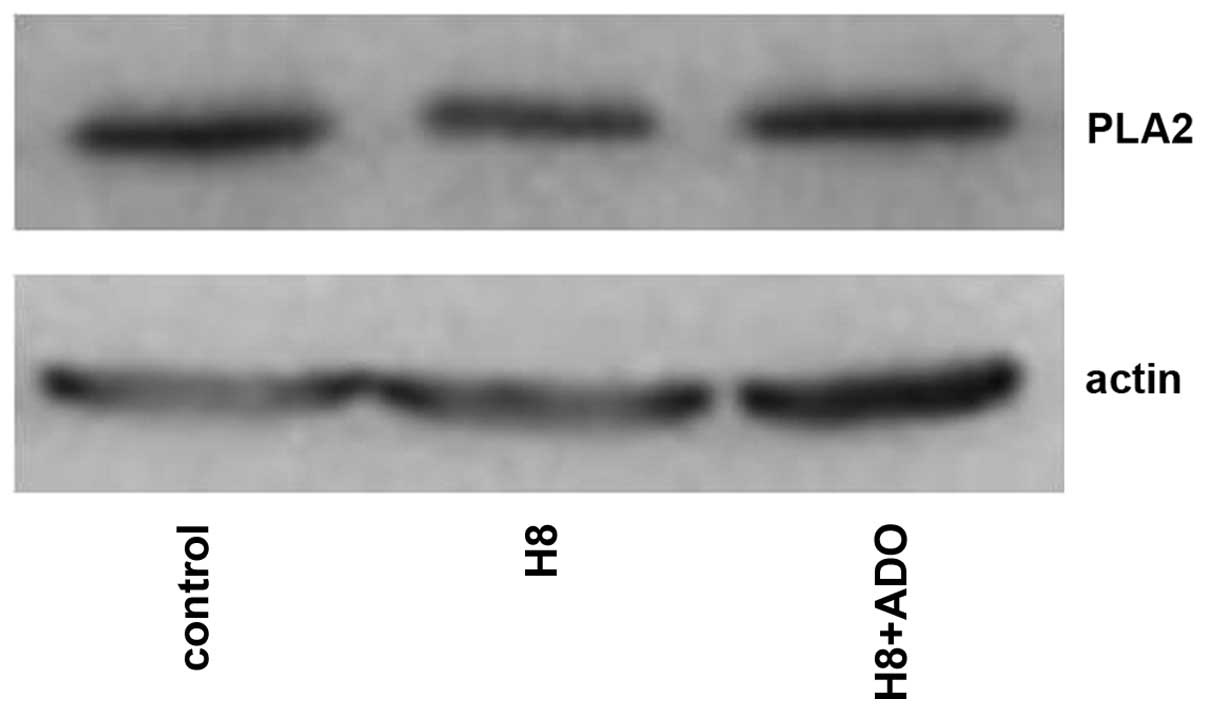
Interaction of the cAMP-PKA and
PLA2-AA pathways
As shown in Figs. 9
and 10, adenosine treatment
significantly increased the expression of PKA in primary cultures
of mTAL cells (P<0.05), which were pretreated with AACOCF3 for 6
h prior to treatment with adenosine for 24 h. However, as shown in
Figs. 11 and 12, adenosine treatment did not alter the
expression of PLA2 in the primary cultures of the mTAL cells, which
had been pretreated with H8 for 6 h prior to treatment with
adenosine for 24 h.
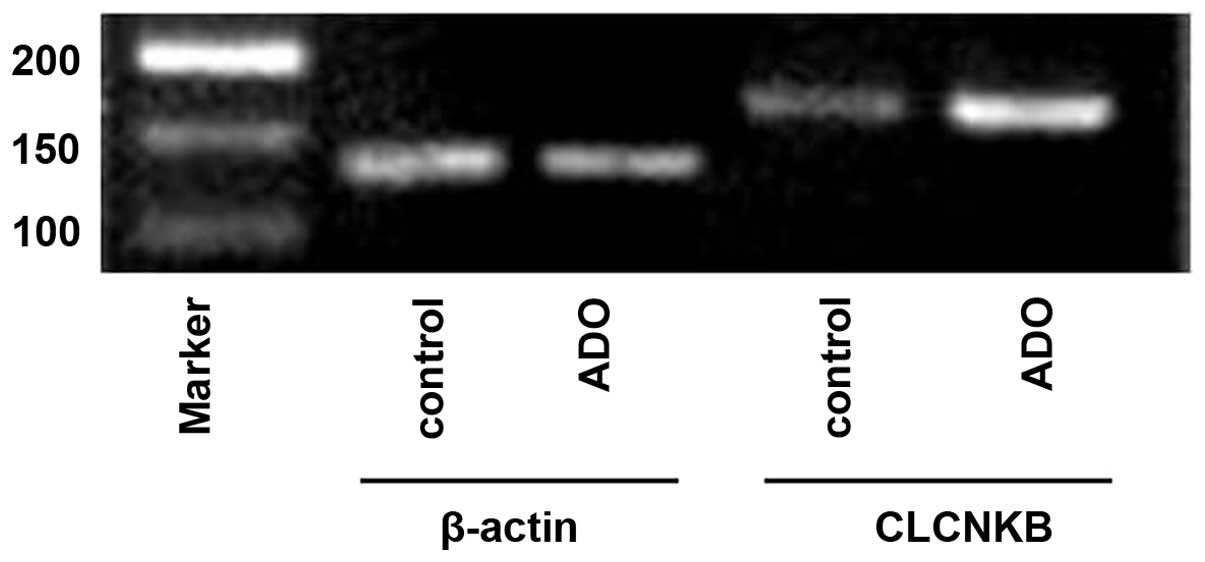
mRNA expression levels of CLCNKB in
primary cultures of mTAL cells
Several studies have confirmed that the mRNA
expression of CLCNKB is highest in the TAL of the loop of
Henle and the distal convoluted tubule (24–26).
In the present study, and total RNA was isolated from primary
cultures of mTAL cells, and the mRNA expression level of
CLCNKB was detected using RT-qPCR analysis.
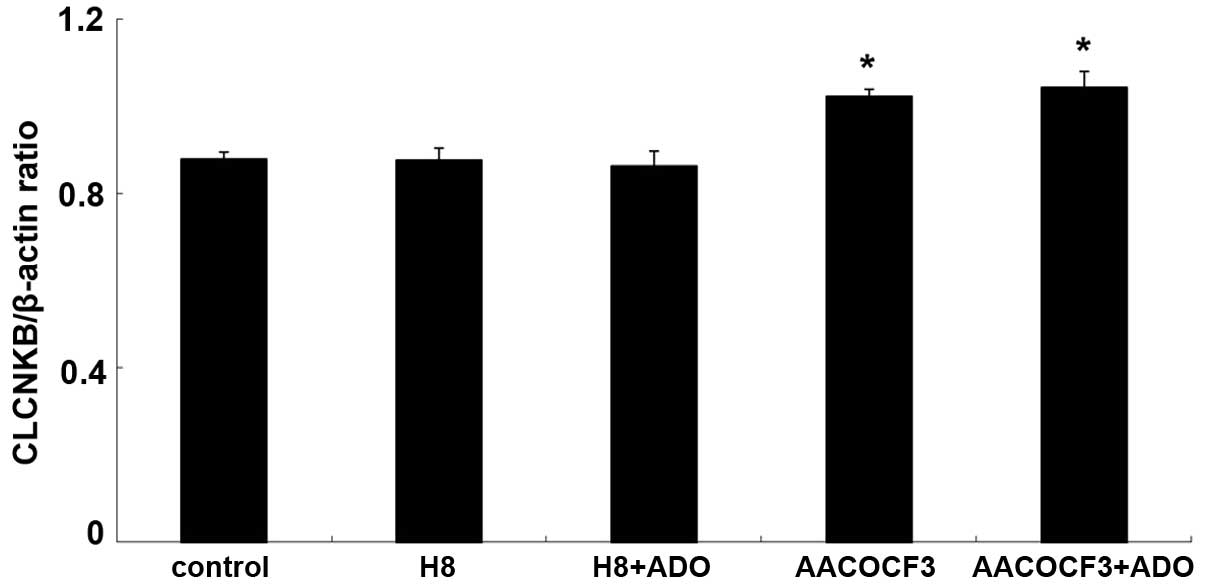
The mRNA expression of CLCNKB increased
significantly following treatment with adenosine (P<0.05;
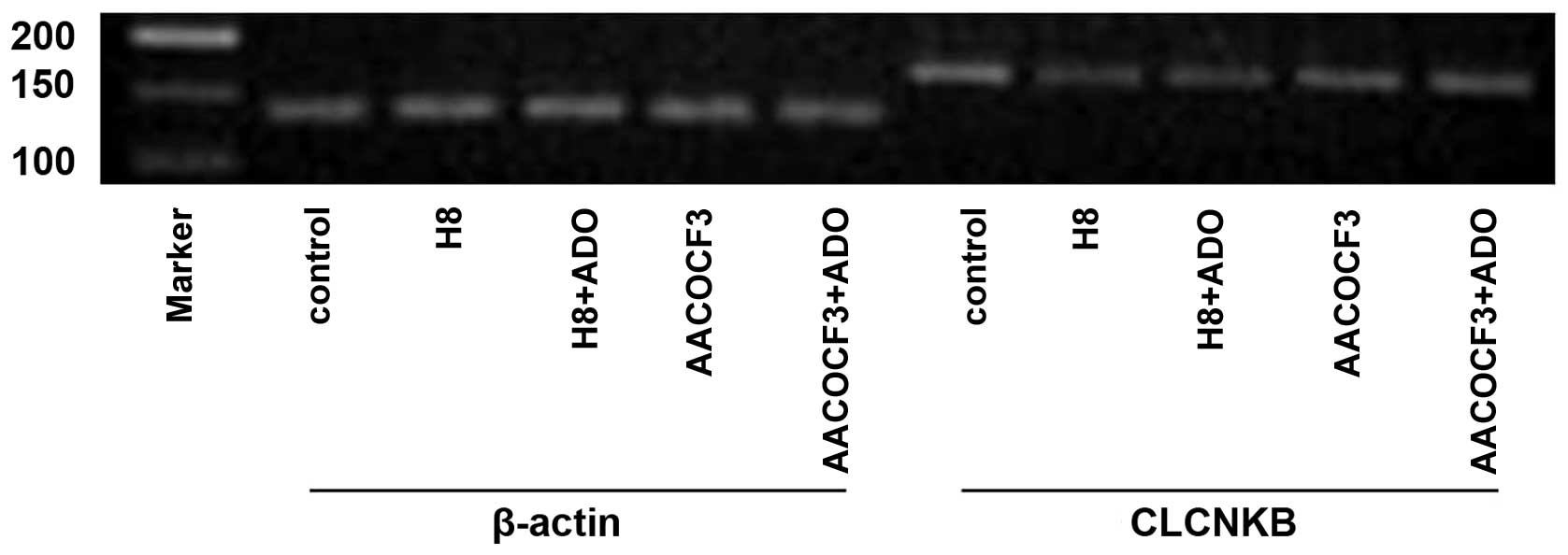
Figs. 13 and 14). To determine whether the cAMP-PKA
and PLA2-AA pathways affected the mRNA expression of
CLCNKB in the presence of adenosine, the cells were
pretreated with H8 and AACOCF3 for 6 h and then stimulated with
adenosine for 24 h. It was found that H8 and AACOCF3 treatment
inhibited the adenosine-induced changes in the mRNA expression of
CLCNKB (Figs. 15 and
16). Thus, it was concluded that
adenosine affected the mRNA expression of CLCNKB through the
cAMP-PKA and PLA2-AA pathways.
Discussion
The findings of the present study demonstrated that
adenosine treatment increased the mRNA expression of CLCNKB
in the primary cultures of mTAL cells. Furthermore, it was found
that the stimulatory effect of adenosine was mediated by the
cAMP-PKA and PLA2-AA pathways. This was supported by the
observation that the inhibition of PKA and PLA2
eliminated the stimulatory effect of adenosine on the mRNA
expression of CLCNKB. The present study also found that
adenosine treatment resulted in increased expression of PKA and
decreased expression of PLA2. Sequential crosstalk was
observed between these two pathways, in that adenosine first
affected the mRNA expression of CLCNKB through the cAMP-PKA
pathway, followed by the PLA2-AA pathway. These results
led to the conclusion that the cAMP-PKA pathway was upstream of the
PLA2-AA pathway. This was supported by the observation
that adenosine stimulated the expression of PKA following
inhibition of PLA2 with AACOCF3, but failed to affect
the expression of PLA2 following inhibition of PKA with
H8.
In the kidney, ~20 to 25% of the Na+ and
Cl− in the renal filtrate are actively reabsorbed. This
indicates that the TAL is key in mediating the renal medulla
hypertonic gradient, the concentration and dilution of urine, and
salt capacity. Chloride channels are important anion channels in
the human body (27), and
Cl− transport is a crucial moderator of
Na+-2Cl−-K+ cotransport. Utilizing
patch-clamp and molecular biology techniques, six categories of
chloride channels have been identified, and are encoded by several
unrelated gene families (28).
These include the CLC family, cystic fibrosis transmembrane
conductance regulators (CFTRs), intracellular chloride channels
(CLICs), calcium activated channels (CaCCs), volume-regulated anion
channels (VRACs) and glycine or γ-GABA-activated chloride channels.
CLCs, CFTRs, CaCCs and VRACs are chloride channels specific to the
kidney (29). Using in situ
hybridization and immunohistochemical staining, studies have
confirmed that CLC-K2 is predominantly expressed in the basolateral
mTAL of the rat kidney (24–26,30).
The human ortholog, ClC-Kb, which has ~90% sequence homology and
~80% homology with the rat ortholog (31), is also located in the basolateral
mTAL, and is encoded for by CLCNKB, which has been listed as
one of the candidate genes involved in hypertension (32,33).
ClC-Kb is crucial, not only for the reabsorption of Na+
and Cl− in the medullary and cortical portions of the
distal tubule, but also in urine concentration, and in the
establishment and maintenance of the corticomedullary osmotic
gradient (34).
Previous studies have shown that the concentration
of adenosine and mRNA expression of CLCNKB increase when
NaCl transport is upregulated, and that activation of the ClC-Kb
channel enhances NaCl transport (35,36).
A mutation in the CLCNKB gene can result in Bartter syndrome
type III, (predominantly from reduced reabsorption of
Na+ and Cl− in the TAL and distal convoluted
tubule, and chronic hypotension) and salt-sensitive hypertension
(35). These studies indicate that
changes in chloride channel activity may be one of the factors
affecting blood pressure. As already mentioned, adenosine is
important in regulating kidney function through binding to
adenosine receptors. There are four types of adenosine receptors:
A1, A2a, A2b and A3 (37). Each of
these is widely expressed in the kidney and it has been confirmed
that the adenosine receptors have different binding affinities to
adenosine (38). When the
concentration of adenosine in the extracellular fluid is low
(~50–200 nM) (23), adenosine
binds primarily to the A1 receptor. Under these conditions, kidney
function is protected by the inhibition of NaCl transport in the
TAL, and decreased consumption of energy and O2
(18). By contrast, under certain
pathophysiological conditions, including ischemia and hypoxia,
cells release an increased quantity of adenosine into the
extracellular fluid (>1 µM) (39,40).
A high concentration of adenosine primarily binds to the
A2 receptor, leading to the overtransportation of NaCl
and overconsumption of O2 (18). Therefore, these pathophysiological
conditions damage kidney function.
It has been previously established that the effects
of adenosine are mediated by several pathways, including the
cAMP-PKA and PLA2-AA pathways (37,41).
A previous study also showed that a high-salt diet can increase the
production of adenosine in mIMCD-K2 cells, and stimulate
Cl− secretion by binding to the A2 receptor and
activating the CFTR channel through the cAMP-PKA pathway (42). In addition, our previous study
demonstrated that adenosine and its analog, N6-cyclohexyladenosine,
enhance apical and basolateral potassium channel activity in the
TAL through the cAMP-PKA pathway, which promotes K+
outflow and regulates Cl− secretion (43–45).
It was found that 5 µM AA inhibits the activity of the 50 pS
potassium channel and the 10 pS chloride channel, which are located
in the basolateral mTAL. These effects were also achieved by
20-HETE, which is generated via the cytochrome P-450 monooxygenase
pathway (46).
In conclusion, the data obtained in the present
study showed the effects of adenosine on the mRNA expression of
CLCNKB in the basolateral mTAL of the rat kidney. As
adenosine assists in the regulation of salt balance in the blood,
it is a potential therapeutic target for certain diseases,
including salt-sensitive hypertension. However, the results of the
present study are limited as the mechanisms underlying the effect
of adenosine on chloride channels remain to be fully elucidated.
Therefore, additional investigations are required to assess the
effects of adenosine on chloride channels and other channels under
conditions of a high-salt diet.
Acknowledgements
This study was partially supported by a grant from
the Chinese National Nature Science Foundations (grant nos.
31171110 and 31671196).
References
|
1
|
Bell PD, Lapointe JY, Sabirov R, Hayashi
S, Peti-Peterdi J, Manabe K, Kovacs G and Okada Y: Macula densa
cell signaling involves ATP release through a maxi anion channel.
Proc Natl Acad Sci USA. 100:4322–4327. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Jackson EK, Mi Z, Gillespie DG and Dubey
RK: Metabolism of cAMP to adenosine in the renal vasculature. J
Pharmacol Exp Ther. 283:177–182. 1997.PubMed/NCBI
|
|
3
|
Ward NC, Croft KD, Blacker D, Hankey GJ,
Barden A, Mori TA, Puddey IB and Beer CD: Cytochrome P450
metabolites of arachidonic acid are elevated in stroke patients
compared with healthy controls. Clin Sci (Lond). 121:501–507. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Navar LG, Inscho EW, Majid SA, Imig JD,
Harrison-Bernard LM and Mitchell KD: Paracrine regulation of the
renal microcirculation. Physiol Rev. 76:425–536. 1996.PubMed/NCBI
|
|
5
|
Navar LG, Harrison-Bernard LM, Nishiyama A
and Kobori H: Regulation of intrarenal angiotensin II in
hypertension. Hypertension. 39:316–322. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Schnermann J and Levine DZ: Paracrine
factors in tubuloglomerular feedback: Adenosine, ATP, and nitric
oxide. Annu Rev Physiol. 65:501–529. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Wilcox CS: Reactive oxygen species: Role
in blood pressure and kidney function. Curr Hypertens Rep.
4:160–166. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Inscho EW: Modulation of renal
microvascular function by adenosine. Am J Physiol Regul Integr Comp
Physiol. 285:R23–R25. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Fredholm BB: Adenosine, an endogenous
distress signal, modulates tissue damage and repair. Cell Death
Differ. 14:1315–1323. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Kinsey GR, Huang L, Jaworska K,
Khutsishvili K, Becker DA, Ye H, Lobo PI and Okusa MD: Autocrine
adenosine signaling promotes regulatory T cell-mediated renal
protection. J Am Soc Nephrol. 23:1528–1537. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Kim SM, Mizel D, Huang YG, Briggs JP and
Schnermann J: Adenosine as a mediator of macula densa-dependent
inhibition of renin secretion. Am J Physiol Renal Physiol.
290:F1016–F1023. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Schweda F, Wagner C, Krämer BK, Schnermann
J and Kurtz A: Preserved macula densa-dependent renin secretion in
A1 adenosine receptor knockout mice. Am J Physiol Renal Physiol.
284:F770–F777. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Harris RC, Zhang MZ and Cheng HF:
Cyclooxygenase-2 and the renal renin-angiotensin system. Acta
Physiol Scand. 181:543–547. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Hansen PB and Schnermann J:
Vasoconstrictor and vasodilator effects of adenosine in the kidney.
Am J Physiol Renal Physiol. 285:F590–F599. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Sun D, Samuelson LC, Yang T, Huang Y,
Paliege A, Saunders T, Briggs J and Schnermann J: Mediation of
tubuloglomerular feedback by adenosine: Evidence from mice lacking
adenosine 1 receptors. Proc Natl Acad Sci USA. 98:9983–9988. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Carlström M, Wilcox CS and Welch WJ:
Adenosine A2A receptor activation attenuates tubuloglomerular
feedback responses by stimulation of endothelial nitric oxide
synthase. Am J Physiol Renal Physiol. 300:F457–F464. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Carroll MA, Doumad AB, Li J, Cheng MK,
Falck JR and McGiff JC: Adenosine2A receptor vasodilation of rat
preglomerular microvessels is mediated by EETs that activate the
cAMP/PKA pathway. Am J Physiol Renal Physiol. 291:F155–F161. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Di Sole F: Adenosine and renal tubular
function. Curr Opin Nephrol Hypertens. 17:399–407. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Cheng MK, Doumad AB, Jiang H, Falck JR,
McGiff JC and Carroll MA: Epoxyeicosatrienoic acids mediate
adenosine-induced vasodilation in rat preglomerular microvessels
(PGMV) via A2A receptors. Br J Pharmacol. 141:441–448. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Trinh-Trang-Tan MM, Bouby N, Coutaud C and
Bankir L: Quick isolation of rat medullary thick ascending limbs.
Enzymatic and metabolic characterization. Pflugers Arch.
407:228–234. 1986. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Hao S, Zhao H, Darzynkiewicz Z, Battula S
and Ferreri NR: Expression and function of NFAT5 in medullary thick
ascending limb (mTAL) cells. Am J Physiol Renal Physiol.
296:F1494–F1503. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Hao S, Zhao H, Darzynkiewicz Z, Battula S
and Ferreri NR: Differential regulation of NFAT5 by NKCC2 isoforms
in medullary thick ascending limb (mTAL) cells. Am J Physiol Renal
Physiol. 300:F966–F975. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Vallon V, Mühlbauer B and Osswald H:
Adenosine and kidney function. Physiol Rev. 86:901–940. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Kobayashi K, Uchida S, Mizutani S, Sasaki
S and Marumo F: Intrarenal and cellular localization of CLC-K2
protein in the mouse kidney. J Am Soc Nephrol. 12:1327–1334.
2001.PubMed/NCBI
|
|
25
|
Adachi S, Uchida S, Ito H, Hata M, Hiroe
M, Marumo F and Sasaki S: Two isoforms of a chloride channel
predominantly expressed in thick ascending limb of Henle's loop and
collecting ducts of rat kidney. J Biol Chem. 269:17677–17683.
1994.PubMed/NCBI
|
|
26
|
Yoshikawa M, Uchida S, Yamauchi A, Miyai
A, Tanaka Y, Sasaki S and Marumo F: Localization of rat CLC-K2
chloride channel mRNA in the kidney. Am J Physiol. 276:F552–F558.
1999.PubMed/NCBI
|
|
27
|
Zifarelli G and Pusch M: CLC chloride
channels and transporters: A biophysical and physiological
perspective. Rev Physiol Biochem Pharmacol. 158:23–76.
2007.PubMed/NCBI
|
|
28
|
Li C and Naren AP: CFTR chloride channel
in the apical compartments: Spatiotemporal coupling to its
interacting partners. Integr Biol (Camb). 2:161–177. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Uchida S and Sasaki S: Function of
chloride channels in the kidney. Annu Rev Physiol. 67:759–778.
2005. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Tang CH, Hwang LY and Lee TH: Chloride
channel ClC-3 in gills of the euryhaline teleost, Tetraodon
nigroviridis: Expression, localization and the possible role of
chloride absorption. J Exp Biol. 213:683–693. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Kieferle S, Fong P, Bens M, Vandewalle A
and Jentsch TJ: Two highly homologous members of the ClC chloride
channel family in both rat and human kidney. Proc Natl Acad Sci
USA. 91:6943–6947. 1994. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Sile S, Gillani NB, Velez DR, Vanoye CG,
Yu C, Byrne LM, Gainer JV, Brown NJ, Williams SM and George AL Jr:
Functional BSND variants in essential hypertension. Am J Hypertens.
20:1176–1182. 2007.PubMed/NCBI
|
|
33
|
Favero M, Calò LA, Schiavon F and Punzi L:
Miscellaneous non-inflammatory musculoskeletal conditions.
Bartter's and Gitelman's diseases. Best Pract Res Clin Rheumatol.
25:637–648. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Sile S, Velez DR, Gillani NB, Alexander
CA, George AL Jr and Williams SM: Haplotype diversity in four genes
(CLCNKA, CLCNKB, BSND, NEDD4 L) involved in renal salt
reabsorption. Hum Hered. 65:33–46. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Krämer BK, Bergler T, Stoelcker B and
Waldegger S: Mechanisms of disease: The kidney-specific chloride
channels ClCKA and ClCKB, the Barttin subunit, and their clinical
relevance. Nat Clin Pract Nephrol. 4:38–46. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Capasso G, Rizzo M, Garavaglia ML,
Trepiccione F, Zacchia M, Mugione A, Ferrari P, Paulmichl M, Lang
F, Loffing J, et al: Upregulation of apical sodium-chloride
cotransporter and basolateral chloride channels is responsible for
the maintenance of salt-sensitive hypertension. Am J Physiol Renal
Physiol. 295:F556–F567. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Schulte G and Fredholm BB: Human adenosine
A(1), A(2A), A(2B), and A(3) receptors expressed in Chinese hamster
ovary cells all mediate the phosphorylation of
extracellular-regulated kinase 1/2. Mol Pharmacol. 58:477–482.
2000.PubMed/NCBI
|
|
38
|
Yaar R, Jones MR, Chen JF and Ravid K:
Animal models for the study of adenosine receptor function. J Cell
Physiol. 202:9–20. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Nishiyama A, Kimura S, He H, Miura K,
Rahman M, Fujisawa Y, Fukui T and Abe Y: Renal interstitial
adenosine metabolism during ischemia in dogs. Am J Physiol Renal
Physiol. 280:F231–F238. 2001.PubMed/NCBI
|
|
40
|
Latini S, Bordoni F, Pedata F and
Corradetti R: Extracellular adenosine concentrations during in
vitro ischaemia in rat hippocampal slices. Br J Pharmacol.
127:729–739. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Sexl V, Mancusi G, Höller C,
Gloria-Maercker E, Schütz W and Freissmuth M: Stimulation of the
mitogen-activated protein kinase via the A2A-adenosine receptor in
primary human endothelial cells. J Biol Chem. 272:5792–5799. 1997.
View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Rajagopal M and Pao AC: Adenosine
activates a2b receptors and enhances chloride secretion in kidney
inner medullary collecting duct cells. Hypertension. 55:1123–1128.
2010. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Gu R, Wang J, Zhang Y, Li W, Xu Y, Shan H,
Wang WH and Yang B: Adenosine stimulates the basolateral 50 pS K
channels in the thick ascending limb of the rat kidney. Am J
Physiol Renal Physiol. 293:F299–F305. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Li D, Wei Y and Wang WH: Dietary K intake
regulates the response of apical K channels to adenosine in the
thick ascending limb. Am J Physiol Renal Physiol. 287:F954–F959.
2004. View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Wang M, Sui H, Li W, Wang J, Liu Y, Gu L,
Wang WH and Gu R: Stimulation of A (2a) adenosine
receptor abolishes the inhibitory effect of arachidonic acid on the
basolateral 50-pS K channel in the thick ascending limb. Am J
Physiol Renal Physiol. 300:F906–F913. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Gu RM, Yang L, Zhang Y, Wang L, Kong S,
Zhang C, Zhai Y, Wang M, Wu P, Liu L, et al:
CYP-omega-hydroxylation-dependent metabolites of arachidonic acid
inhibit the basolateral 10 pS chloride channel in the rat thick
ascending limb. Kidney Int. 76:849–856. 2009. View Article : Google Scholar : PubMed/NCBI
|