Introduction
Diabetes is one of the most common chronic diseases,
which occurs when the pancreas does not produce enough insulin, or
when the body cannot effectively use the insulin it produces. The
World Health Organization estimates the incidence of diabetes to be
9% among adults >18 years old (1). In 2012 alone, diabetes was the direct
cause of 1.5 million mortalities. Of diabetes-associated
mortalities, >80% occur in low- and middle-income countries
(2). There are two types of
diabetes: Type 1 diabetes is characterized by a lack of insulin
production, whereas Type 2 diabetes is caused by the body's
ineffective use of insulin. Both types of diabetes share a common
syndrome, which is disordered glucose homeostasis. Disorder in
glucose homeostasis is caused by defects in the
phosphatidylinositol 3-kinase (PI3K)/Akt serine/threonine kinase
(Akt) and AMP-activated protein kinase (Ampk) pathways in
insulin-sensitive tissues, which then lead to the accumulation of
glucose in the blood. The regulation of glucose uptake is critical
for the maintenance of glucose homeostasis. Glucose uptake is
dependent on the translocation of glucose transporter type 4
(Glut4) to the plasma membrane. There are two major signaling
pathways that regulate the translocation of Glut4. The first is the
insulin activated signaling pathway through insulin receptor
substrate 1 (IRS1) and PI3K. The second is the insulin-independent
signaling pathway activated by Ampk. Decreases in
insulin-stimulated glucose uptake in the skeletal muscle caused by
insulin resistance is a symptom of individuals with Type 2 diabetes
(3).
The treatment of diabetes has become a social focus.
Existing hypoglycemic drugs, including insulin and other
conventional drugs, predominantly aim to relieve the symptoms of a
particular type (Type I or II) of diabetes. These drugs may produce
certain drug dependencies and side effects. In recent years,
interest in using plant extracts as a strategy to prevent or treat
diabetes has grown, as they are natural products and are considered
to have fewer side effects than conventional treatments.
Momordica charantia, commonly termed bitter melon, is one of
the popular plants that has been used as medicinal plant for
treating diabetes and various other diseases.
M. charantia, a perennial climber, is a
tropical and subtropical vine of the family Cucurbitaceae. The
fruit, and the whole plant, has been demonstrated to possess
antidiabetic, antiviral, ant-bacterial and anticancer activities
(4). Extracts of M.
charantia have been demonstrated to increase cellular glucose
uptake by upregulating Glut4 and PI3K, which lead to enhanced
cellular insulin signaling pathways (5). Many phytochemicals with hypoglycemic
properties have been isolated from the fruit of M.
charantia, including glycosides (momordin, charantin),
alkaloids (momordicin) and polypeptides. These agents have been
demonstrated to promote the translocation of glucose transporters
and increase the activity of Ampk in insulin resistant animal
models (6,7). The protein extract from the fruit
pulp of M. charantia also stimulates the uptake of glucose
into C2C12 myocytes (8).
The compound (19R,23E)-5β, 19-epoxy-19-methoxy
cucurbita-6,23,25-trien-3 β-o-l (compound K16) was previously
evaluated for its inhibitory activities toward protein tyrosine
phosphatase 1B, a tyrosine phosphatase that has been implicated as
a key target for therapy against Type II diabetes. Additionally,
the extraction and identification of two cucurbitane-type
triterpenoids from M. charantia were previously reported,
and these compound exhibit significant cytotoxicity against cancer
cells (9). The present study
investigated the antidiabetic activity of compound K16 in
alloxan-induced diabetic mice and explored the associated
underlying mechanism.
Materials and methods
Chemical and reagents
2,4,5,6-Tetraoxypyrimidine (alloxan) was purchased
from Sigma-Aldrich (Merck Millipore, Darmstadt, Germany).
Metformine was purchased from Shanxi Baozhilin Pharmaceuticals Co.,
Ltd. (Shanxi, China). Compound K16, a cucurbitane-type triterpenoid
isolated from M. charantia was provided by Shenyang
Pharmaceutical University (Shenyang, China) (10), and its structure is presented in
Fig. 1. Antibodies against insulin
receptor (IR; cat. no. 3025s; Cell Signaling Technology, Inc.,
Danvers, MA, USA), and phosphorylated IR (cat. no. 3024s; Cell
Signaling Technology, Inc.), IRS1 (phospho-Ser636; cat. no.
D120888; Bio Basic Canada, Inc., Markham, ON, Canada), and glycogen
synthase kinase 3β (GSK3β; phospho-Tyr216; cat. no. D155143; Bio
Basic Canada, Inc.) were used. Antibody against phosphorylated Akt
(cat. no. 13038P) was obtained from Cell Signaling Tachnology, Inc.
Antibody against α-tubulin (cat. no. Ab102) was purchased from
Vazyme (Piscataway, NJ, USA).
Animal care and experimental
protocol
Male C57BL/6J mice (5 weeks old; 18–20 g) obtained
from Liao Ning Chang Sheng Biotechnology Co., Ltd. (Benxi, China)
were maintained in a 12 h light/dark cycle, and provided with water
and food ad libitum. The animals were housed in cages
maintained at 21±2.0°C with 50±5% humidity. The study was approved
by the ethics committee of Liaoning Traditional Chinese Medicine
University (Shenyang, China).
Establishment of the diabetic mouse
model
After 1 week of acclimation, male mice (6 weeks old)
were randomly divided into 2 groups: Control group (n=10) and model
group (n=40). Mice in the control group mice were administered 0.9%
saline solution, whereas those in the model group received alloxan
(50 mg/kg body weight). The saline solution and alloxan were
administered via intraperitoneal injection, and a total of three
injections were given, at 48 h intervals. Subsequently, blood
samples were collected from the tails of the animals and the
glucose levels in the blood samples were measured with a glucometer
(Roche Diagnostics, Basel, Switzerland). The animals were subjected
to a 16-h fasting period prior to the blood glucose test.
Successful establishment of this model was based on mice exhibiting
a blood glucose concentration between 10 and 14 mM, and exhibiting
behavioral changes, including polydipsia, polyphagia and polyuria
(11). Only these animals were
used in subsequent experiments.
Compound K16 supplementation and
sample collection
The alloxan-induced hyperglycemic mice were randomly
sorted into 4 groups (n=10 per group), with each group having
approximately the equivalent average body weight. One group was
administered 0.9% saline solution (alloxan group). Another group
was administered metformin (10 mg/kg; metformine group) as a
positive control as metformin improves insulin resistance. The
remaining two groups were each administered compound K16 (25 or 50
mg/kg body weight). Administration of the drugs was performed by
oral gavage, and the treatment lasted for 4 weeks, with three
treatments per week. Simultaneously, mice in the control group,
(without administration of alloxan) also received 0.9% saline
solution via oral gavage for the equivalent duration and frequency.
These mice were considered as healthy control mice.
Analysis of blood glucose level and
glucose tolerance
Blood glucose levels were measured weekly. Blood
samples were collected from the tail of the animals following a
fasting period of 16 h. The glucose levels in the samples were
measured with a glucometer. Glucose tolerance was determined by the
intraperitoneal glucose tolerance test (IPGTT), which was performed
at week 4 of treatment. Again, the animals were subjected to a
fasting period of 16 h, and blood samples were then collected from
the tail before (0 time) and at 5, 15, 30, 60 and 120 min after
intraperitoneal injection of glucose (1 mg/kg body weight). The
glucose levels in the blood samples were measured by a glucometer.
Area under the curve of glucose concentration vs. time plot was
calculated using GraphPad Prism 5 software (GraphPad Software,
Inc., La Jolla, CA, USA).
Analysis of serum cholesterol and
triglyceride levels, and organ index
At the end of week 4, blood samples were collected
by eyeball extirpating and the animals were sacrificed by cervical
dislocation. Serum was isolated from the blood samples by
centrifugation at 5,000 × g min-1 for 10 min and the levels
of cholesterol and triglycerides in the serum were then measured
with a cholesterol (CHO) and triglyceride (TG) kit (Beijing BHKT
Clinical Reagent Co., Ltd., Beijing, China). The liver, kidney,
spleen and skeletal muscle were removed from the animals. They were
weighed, snap-frozen in liquid nitrogen and stored immediately at
−80°C. The organ index was calculated as: Organ index = organ
weight/body weight.
Reverse transcription-quantitative
polymerase chain reaction (RT-qPCR)
RT-qPCR was performed to measure the expression
levels of Glut4 and Ampkα1 in the liver of mice from different
treatment groups. Total RNA was extracted from the liver tissue of
three mice from each group. Liver tissue (20 mg) was ground in
liquid nitrogen in a mortar and the total RNA was extracted from
the tissue homogenate using TRIzol (Beijing Kang Century
Biotechnology Co., Ltd., Beijing, China). RT-qPCR analysis was
performed as described previously (12) using the ABI Prism 7900-HT Real-Time
PCR System (Applied Biosystem; Thermo Fisher Scientific, Inc.,
Waltham, MA, USA). Primers used in the RT-qPCR were as follow:
Glut4, 5′-CTTGGCTCCCTTCAGTTTGG-3′ (forward),
5′-CTACCCAGCCACGTTGCATT-3′ (reverse); Ampkα1,
5′-AAGCCGACCCAATGACATCA-3′ (forward) and 5′-CTTCCTTCGTACACGCAAAT-3′
(reverse); β-actin, 5′-AGGCAAACCGTGAAAAGATG-3′ (forward) and
5′-AGGCAAACCGTGAAAAGATG-3′ (reverse). β-actin was used as an
internal control and expression levels were calculated using the
ΔΔCq method (13).
Western blot analyses
The expression levels of several key proteins
involved in the insulin signaling pathway in the liver were
analyzed by western blotting. Liver tissue was extracted in
radioimmunoprecipitation assay cell lysis buffer (Cell Signaling
Technology, Inc.) for 15 min on ice (12). Subsequently, the extract was
briefly sonicated and then centrifuged at 12,280 × g for 15
min at 4°C. The supernatant of the sample was retained and the
protein concentration in the supernatant was measured by
bicinchoninic acid assay. Aliquots of the supernatant containing
total protein (40 µg) was resolved in 10% SDS-polyacrylamide gel
and the protein bands were then transferred to a nitrocellulose
membrane and blocked with 5% bovine serum albumin for 3 h at room
temperature. Then the membranes were probed with the appropriate
primary antibody overnight at 4°C followed by horseradish
peroxidase conjugated secondary antibody (1:5,000; cat. nos. BL003A
and BL001A; Biosharp Inc., Hefei, China) at room temperature for 2
h. Primary antibodies were used at the following dilutions: IR
(1:1,000); phosphorylated IR (1:1,000); IRS1 (1:1,000); GSK3β
(1:1,000); p-GSK3β (1:500); phosphorylated Akt (1:1,000); and
α-tubulin (1:10,000). Positive signals of the blot were detected by
an enhanced chemiluminescence assay (GE Healthcare Life Sciences,
Chalfont, UK). The relative density of proteins was analyzed using
ImageJ software (version 1.48; National Institutes of Health,
Bethesda, MD, USA).
Statistical analyses
Data were analyzed by one-way analysis of variance
followed by the Student-Newman-Keuls test performed with the SPSS
software (version 16; SPSS, Inc., Chicago, IL, USA). Data are
expressed as the mean ± standard error and P<0.05 was considered
to indicate a statistically significant difference.
Results
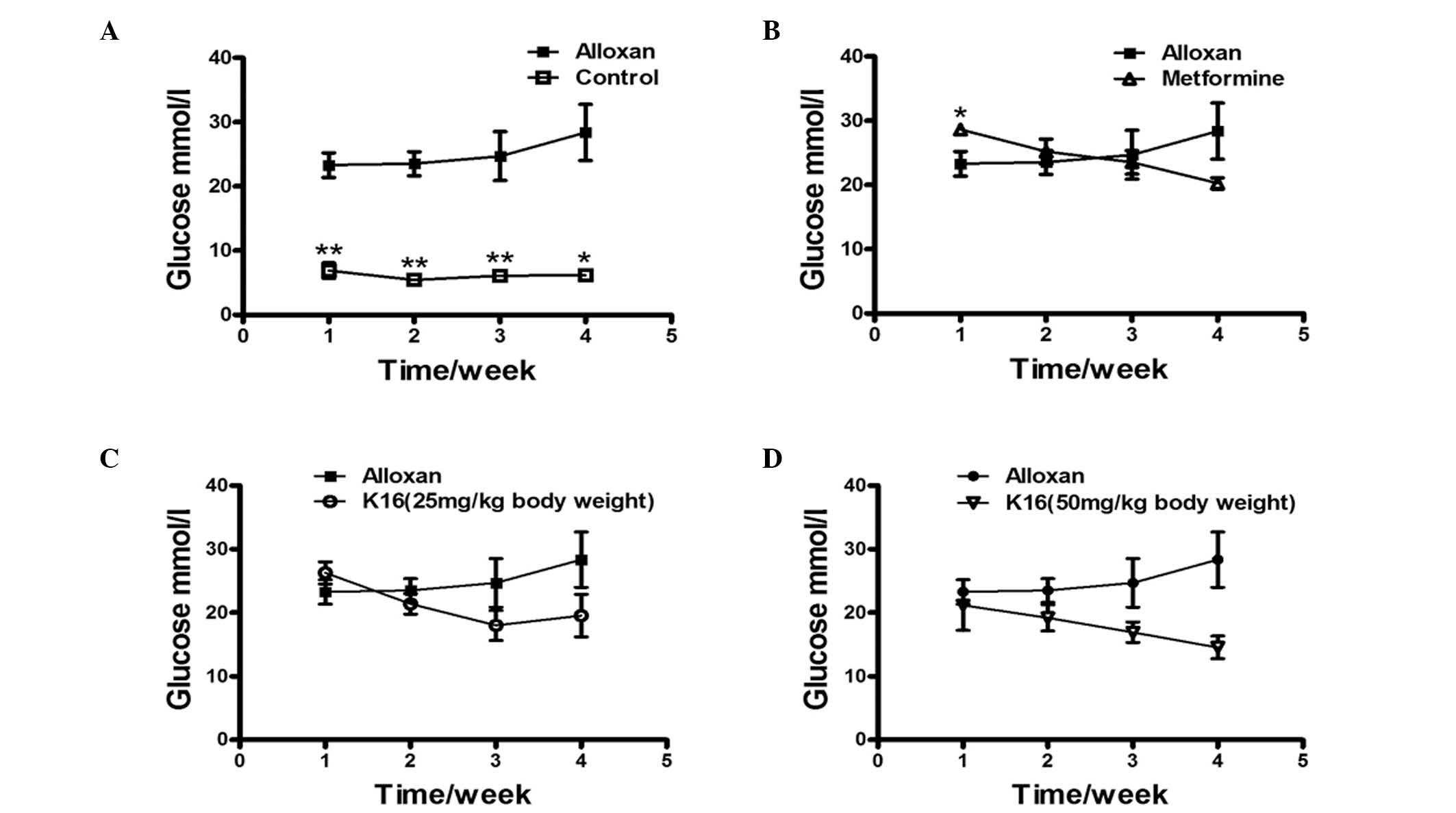
Effect of compound K16 on blood
glucose levels of alloxan-induced diabetic mice
The effect of compound K16 on the blood glucose
levels of alloxan-induced diabetic mice was investigated by
administering mice with compound K16 over a period of 4 weeks and
monitoring the changes in the blood glucose levels each week. The
blood glucose level of healthy control group was approximately
one-third of the level of the alloxan-induced diabetic mice
(alloxan group) and remained almost unchanged over the entire four
weeks (P<0.05; Fig. 2A). The
blood glucose level of the metformine group was higher than that of
alloxan group on the first week, but then decreased steadily to
~70% of the level of the alloxan group by week 4 (Fig. 2B). The blood glucose levels of the
two K16 groups were similar to that of the alloxan group week 1,
however, the levels decreased over the following 3 weeks Although
with the lower dosage of K16 the blood glucose level appeared to
increase slightly at week 4, the high dosage of K16 reduced the
blood glucose level to ~50% the level of the alloxan group
(Fig. 2C and D). These results
demonstrated the positive antidiabetic activity of K16 regarding
its ability to reduce blood glucose levels in drug-induced diabetic
mice, although the extent of reduction did not reach the blood
glucose level of healthy mice.
Compound K16 improves the glucose
tolerance of alloxan-induced diabetic mice
The effect of compound K16 on the blood glucose
tolerance of diabetic mice was investigated by measuring the blood
glucose level of the animal prior to and following administration
of glucose at week 4, and subsequently monitoring the changes in
blood glucose levels after 5, 10, 30, 60 and 120 min. The blood
glucose level of control mice was approximately one third the
levels of alloxan-induced diabetic mice (P<0.05), and peaked at
about 13 mmol/l after 15 min, and then decreased to baseline level
after 120 min (Fig. 3A). The blood
glucose level of alloxan group followed essentially the same
pattern, peaking at 15 min, with a concentration of about 36 mmol/l
and decreased to baseline levels after 120 min. The blood glucose
levels the metformine and two K16 groups over the entire 120 min
were fairly similar; lower than the levels of the alloxan group
(Fig. 3B-D). To compare the
absolute change in the blood glucose over the entire 120 min
period, the IPGTT AUC was calculated to demonstrate the total
change in glucose. The results demonstrated that no significant
difference in glucose tolerance was observed between the metformine
treatment and the no treatment group (alloxan group; Fig. 3E). However, the AUC of the K16
groups was significantly reduced compared with the alloxan group
(P<0.05), indicating that compound K16 improved the glucose
tolerance in drug-induced diabetic mice by eliminating exogenously
administered glucose from the blood.
 | Figure 3.Effects of compound K16 on glucose
tolerance. IPGTT was performed after a fasting period of 16 h. Time
course of blood glucose concentrations during IPGTT was determined
in the (A) control, (B) positive control (metformin), (C) mice
treated with compound K16 at the concentration of 25 mg/kg body
weight and (D) mice treated with compound K16 at the concentration
of 50 mg/kg body weight. (E) AUC of IPGTT for each group. Data are
expressed as the the mean ± standard error (n≥2). *P<0.05,
**P<0.01 vs. alloxan. K16, (19R,23E)-5β,
19-epoxy-19-methoxy-cucurbita-6,23,25-trien-3 β-o-l0; IPGTT,
intraperitoneal glucose tolerance test; AUC, area under curve. |
Compound K16 decreases serum lipids in
alloxan-induced diabetic mice
Alloxan-induced diabetic mice treated with compound
K16 over a period of 4 weeks were sacrificed at the end of the week
4. The TG levels of alloxan-induced diabetic mice were ~50%
higher than the level of control mice (P<0.01; Fig. 4A). The levels of serum TG in the
two K16 groups were significantly decreased compared with the
alloxan group (P<0.01), to levels that were even lower than the
healthy control mice. As for serum CHO level, the alloxan group
exhibited levels ~30% higher than the healthy control group
(P<0.01), with the metformine group yielding even higher levels
(Fig. 4B), whereas the K16 groups
exhibited reduced serum cholesterol levels compared with the
alloxan group. However, only the high dosage (50 mg/kg) of compound
K16 resulted in a significant reduction relative to the alloxan
group (P<0.01; Fig. 4B). These
results indicated that compound K16 reduces the serum TG and CHO
levels of drug-induced diabetic mice.
Effects of compound K16 on organ
index
The effect of compound K16 on the organs of the mice
was evaluated by determining the organ indexes (organ weight/body
weight) of the spleen, liver and kidney following sacrifice of the
animals at the end of the 4-week treatment. Overall, there was no
significant difference in the organ indexes among all the different
groups (Fig. 5). This suggested
that compound K16 exhibited no toxic effect on the animals.
Compound K16 upregulates the
expression of glycometabolism-associated genes
The aforementioned experimental results indicated
that compound K16 indeed exerted antidiabetic activity, as
demonstrated by its ability to lower blood glucose level and to
increase glucose tolerance in diabetic mice. Thus, to investigate
the mechanisms associated with the antidiabetic activity of
compound K16, the effect of the compound on the expression of genes
or the activation of proteins that are involved in glycometabolism
was analyzed. The expression/phosphorylation of IR, IRS1, GSK-3β,
Akt, Glut4 and Ampkα1 were determined in the current study
(14,15). Compared with control mice, the
phosphorylated protein levels of IR and IRS1 were significantly
reduced in the alloxan group, however this reduction was
significantly abolished in the K16 groups, at the low and high
dosages (Fig. 6A). At the high
dosage, compound K16 also significantly increased the levels of
phosphorylated GSK-3β and Akt, compared with the levels in the
alloxan group. Furthermore, RT-qPCR revealed that the mRNA levels
of Glut4 and Ampkα1 were significantly increased when the mice were
treated with metformine or compound K16 compared with the alloxan
group (Fig. 6B). These results
provided evidence that compound K16 mediated upregulation of
certain genes involved in glycometabolism, and suggested that this
may be part of the mechanism by which compound K16 exerts its
antidiabetic activity.
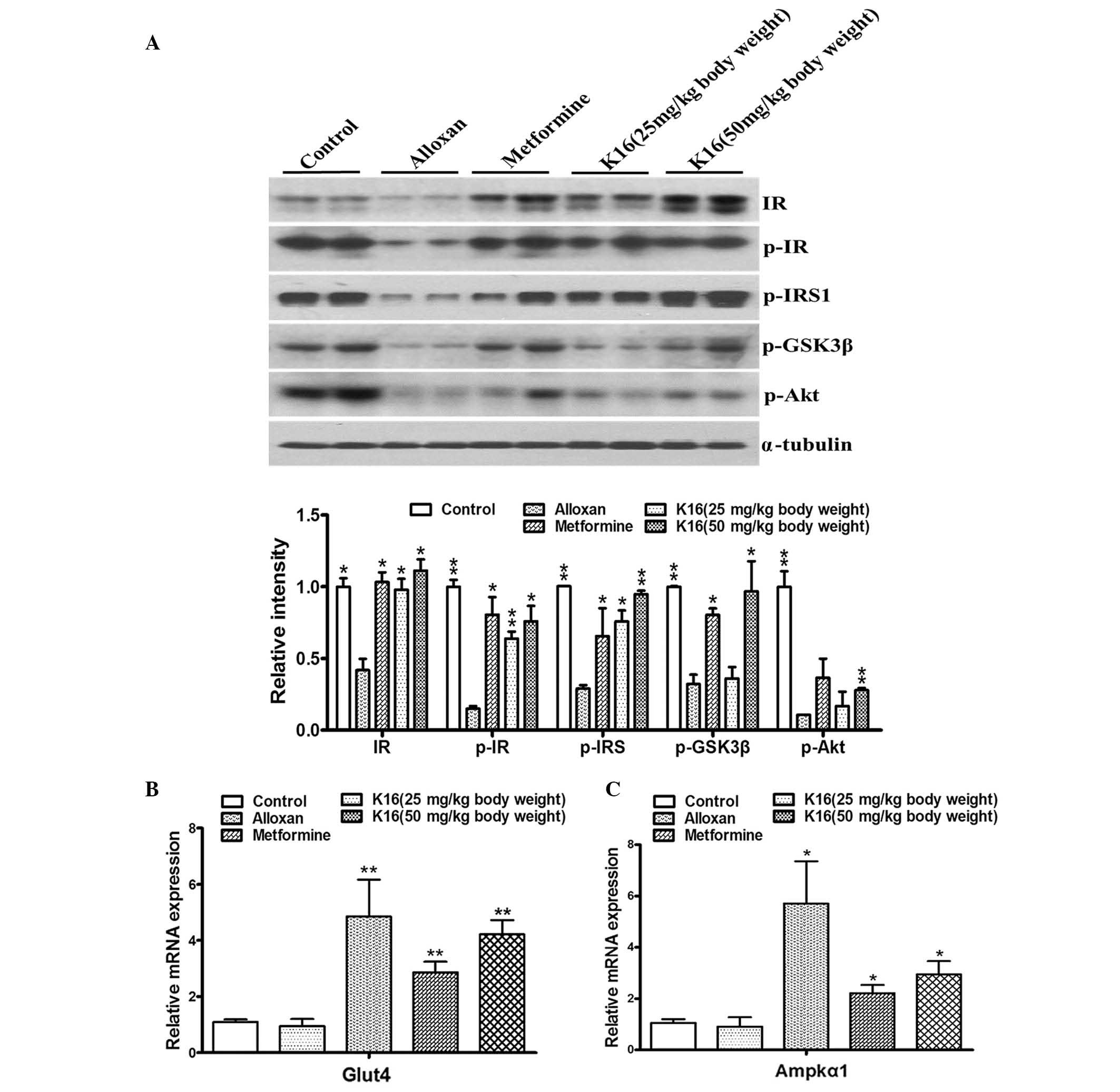 | Figure 6.Changes in insulin
signaling-associated proteins and genes in experimental mice.
Compound K16 and metformine (positive control) increased (A) the
levels of glycometabolism signaling-associated proteins and the
mRNA levels of (B) Glut4 and (C) Ampkα1 at different concentrations
in mice, compared with alloxan treatment. α-tubulin was used as a
loading control for western blotting. All experiments were
performed three times. Data are expressed as the mean ± standard
error (n≥2). *P<0.05, **P<0.01 vs. alloxan. K16,
(19R,23E)-5β, 19-epoxy-19-methoxy-cucurbita-6,23,25-trien-3 β-o-l0;
IR, insulin receptor; p-, phosphorlyated; IRS, insulin receptor
substrate; GSK3β, gylcogen syntase kinase 3β; Akt, Akt
serine/threonine kinase; Glut4, glucose transporter type 4; Ampkα1,
AMP-activated protein kinase α1. |
Discussion
The present study examined the potential
antidiabetic effect of compound K16, which was isolated from M.
charantia, a plant that is well known for its medicinal
properties against a variety of diseases, including diabetes. The
data of the present study clearly demonstrated that compound K16
reduced blood glucose levels, improve glucose tolerance and reduced
the levels of lipids (TG and CHO) in the serum of alloxan-induced
diabetic mice. Furthermore, compound K16 appeared to perform better
than the antidiabetic drug, metformine, with the high dosage (50
mg/kg compound K16) yielding marginally improved results compared
with metformin, which were not statistically significant.
Previous studies have demonstrated that the
application of M. charantia fruit extract for the treatment
of diabetes resulted in a dose-dependent hypoglycemic effect
(4,16,17).
A potential underlying mechanism of this anti-diabetic activity is
thought to be the activation of Ampk, resulting in the upregulation
of the Glut4 gene (18).
At the cellular level, a variety of natural
polyphenols, including resveratrol, green tea polyphenols and
polyphenols from Callistephus chinensis flower, have been
demonstrated to activate Glut4 by activating the Ampk branch of the
insulin-signaling pathway to stimulate glucose metabolism in fat
and muscle tissues (19–22). However, high doses of resveratrol
are less effective than low doses in the activation of Ampk and
improvement of glucose utilization. Green tea polyphenols can
relieve the condition of diabetes through modulating the expression
of key proteins involved in the insulin signaling pathway,
including IR, IRS1, Akt and GSK-3β (22,23).
IR, IRS-1, Akt are predominantly activated by phosphorylation, and
the data of the current study clearly demonstrated significant
increases in the phosphorylated forms of these proteins in the
alloxan-induced diabetic mice following treatment with compound
K16, compared with no treatment (Fig.
6A). GSK-3β is a serine/threonine protein kinase, which was
originally discovered for its involvement in regulating glycogen
synthase (23,24). Phosphorylation of GSK-3β is induced
by activated Akt, which inhibits glycogen synthesis (25). A recent study by Yang et al
(16) demonstrated that M.
momordica exerts antidiabetic activities by decreasing the
levels of the proinflammatory cytokines tumor necrosis factor-α,
interleukin 6 and C-C motif chemokine ligand 2, and inhibiting the
nuclear factor-κB (NF-κB) and c-Jun N-terminal kinase (JNK)
pathways. These authors observed significant increases in the
levels of phosphorylated IRS1 and Akt, and reduced a level of
phosphorylated JNK in mice fed with a high fat diet and 3% M.
momordica compared to those fed with a high fat diet only. The
data were consistent with the inhibitory effect on the
phosphorylation of JNK and NF-κB.
Activation of Ampk requires the presence of an
additional subunit encoded by the gene Ampkα1. Alloxan-induced
diabetic mice treated with compound K16 demonstrated a significant
increase in the level of Ampkα1 transcripts compared with untreated
mice (Fig. 6C), and this provided
evidence that K16 exerts its antidiabetic activity through
activation of Ampkα1. Furthermore, the transcript level of Glut4
was also significantly upregulated by compound K16. Glut4 is an
insulin-regulated glucose transporter, which promotes glucose
uptake into muscle tissue (7,26).
Similarly, M. momordica-derived triterpenoids have been
demonstrated to activate Ampkα1 and increase Glut4 translocation to
the plasma membrane, a mechanism that may be responsible for the
enhanced elimination of glucose from insulin resistant models in
vivo (6). The level of Glut4
in muscle tissues in Type 1 diabetes is substantially reduced. The
expression of Glut4 is regulated by the insulin signaling pathway
through IRS1 and Ampk (27–30).
An increased level of Glut4 allows more glucose to be transported
into the cells, thus, the upregulation of Glut4 was consistent with
the antidiabetic activity exhibited by compound K16.
In conclusion, the antidiabetic activity of K16 was
demonstrated through its ability to reduce blood glucose level,
increase glucose tolerance and reduce serum lipids in
alloxan-induced diabetic mice model. In addition, insight into the
underlying molecular mechanism by which compound K16 exerts its
antidiabetic effects was revealed. However, as diabetes is a
disease that requires long term and continuous medication in its
treatment, further investigation involving longer animal trials is
required to ascertain the potential of compound K16 as a
therapeutic and to further understand the mechanism of its
antidiabetic effect. However, the current findings represent a step
forward in the search for an antidiabetic compound from natural
sources that would be effective for treating diabetes, and
illustrated the importance of M. momordica as an effective
source for the search of natural antidiabetic drugs.
Acknowledgements
The work was financially supported by the program of
Liaoning Excellent Talents in University (grant no.
LETU#LR2014001), the Projects of Liaoning Province Science and
Technology Department (grant no. 2012226006) and the National
Science Foundation of China to (grant nos. 81272333, 81001003 and
81273389). The authors thank Dr Alan K Chang (Liaoning University,
Shenyang, China) for his contribution to the writing of the
manuscript and Liaoning University of Traditional Chinese Medicine
(Shenyang, China) for providing the experimental space for animal
research.
References
|
1
|
World Health Organization, . Diabetes Fact
Sheet. World Health Organization; Geneva: 2015
|
|
2
|
World Health Organization, . Global health
estimates: Deaths by cause, age, sex and country, 2000–2012. World
Health Organization; Geneva: 2014
|
|
3
|
Sharma BR, Kim HJ and Rhyu DY: Caulerpa
lentillifera extract ameliorates insulin resistance and regulates
glucose metabolism in C57BL/KsJ-db/db mice via PI3K/AKT signaling
pathway in myocytes. J Transl Med. 13:622015. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Grover JK and Yadav SP: Pharmacological
actions and potential uses of Momordica charantia: A review. J
Ethnopharmacol. 93:123–132. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Zhu Y, Dong Y, Qian X, Cui F, Guo Q, Zhou
X, Wang Y, Zhang Y and Xiong Z: Effect of superfine grinding on
antidiabetic activity of bitter melon powder. Int J Mol Sci.
13:14203–14218. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Iseli TJ, Turner N, Zeng XY, Cooney GJ,
Kraegen EW, Yao S, Ye Y, James DE and Ye JM: Activation of AMPK by
bitter melon triterpenoids involves CaMKKβ. PLoS One. 8:e623092013.
View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Tan MJ, Ye JM, Turner N, Hohnen-Behrens C,
Ke CQ, Tang CP, Chen T, Weiss HC, Gesing ER, Rowland A, et al:
Antidiabetic activities of triterpenoids isolated from bitter melon
associated with activation of the AMPK pathway. Chem Biol.
15:263–273. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Chaturvedi P: Antidiabetic potentials of
Momordica charantia: Multiple mechanisms behind the effects. J Med
Food. 15:101–107. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Wang X, Sun W, Cao J, Qu H, Bi X and Zhao
Y: Structures of new triterpenoids and cytotoxicity activities of
the isolated major compounds from the fruit of Momordica charantia
L. J Agric Food Chem. 60:3927–3933. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Zeng K, He YN, Yang D, Cao JQ, Xia XC,
Zhang SJ, Bi XL and Zhao YQ: New compounds from acid hydrolyzed
products of the fruits of Momordica charantia L. and their
inhibitory activity against protein tyrosine phosphatas 1B. Eur J
Med Chem. 81:176–180. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Li Y, Hamasaki T, Nakamichi N, Kashiwagi
T, Komatsu T, Ye J, Teruya K, Abe M, Yan H, Kinjo T, et al:
Suppressive effects of electrolyzed reduced water on
alloxan-induced apoptosis and type 1 diabetes mellitus.
Cytotechnology. 63:119–131. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Bi X, Fang W, Wang LS, Stoner GD and Yang
W: Black raspberries inhibit intestinal tumorigenesis in apc1638+/−
and Muc2−/− mouse models of colorectal cancer. Cancer Prev Res
(Phila). 3:1443–1450. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(−Delta Delta C(T)) Method. Methods. 25:402–408. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Prabhakar V, Gupta D, Kanade P and
Radhakrishnan M: Diabetes-associated depression: The serotonergic
system as a novel multifunctional target. Indian J Pharmacol.
47:4–10. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Alvim RO, Cheuhen MR, Machado SR, Sousa AG
and Santos PC: General aspects of muscle glucose uptake. An Acad
Bras Cienc. 87:351–368. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Yang SJ, Choi JM, Park SE, Rhee EJ, Lee
WY, Oh KW, Park SW and Park CY: Preventive effects of bitter melon
(Momordica charantia) against insulin resistance and diabetes are
associated with the inhibition of NF-kappaB and JNK pathways in
high-fat-fed OLETF rats. J Nutr Biochem. 26:234–240. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Lo HY, Ho TY, Li CC, Chen JC, Liu JJ and
Hsiang CY: A novel insulin receptor-binding protein from Momordica
charantia enhances glucose uptake and glucose clearance in vitro
and in vivo through triggering insulin receptor signaling pathway.
J Agric Food Chem. 62:8952–8961. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Zheng D, MacLean PS, Pohnert SC, Knight
JB, Olson AL, Winder WW and Dohm GL: Regulation of muscle GLUT-4
transcription by AMP-activated protein kinase. J Appl Physiol
(1985). 91:1073–1083. 2001.PubMed/NCBI
|
|
19
|
Penumathsa SV, Thirunavukkarasu M, Zhan L,
Maulik G, Menon VP, Bagchi D and Maulik N: Resveratrol enhances
GLUT-4 translocation to the caveolar lipid raft fractions through
AMPK/Akt/eNOS signalling pathway in diabetic myocardium. J Cell Mol
Med. 12:2350–2361. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Turan B, Tuncay E and Vassort G:
Resveratrol and diabetic cardiac function: Focus on recent in vitro
and in vivo studies. J Bioenerg Biomembr. 44:281–296. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Dasgupta B and Milbrandt J: Resveratrol
stimulates AMP kinase activity in neurons. Proc Natl Acad Sci USA.
104:7217–7222. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Wang X, Tian J, Jiang J, Li L, Ying X,
Tian H and Nie M: Effects of green tea or green tea extract on
insulin sensitivity and glycaemic control in populations at risk of
type 2 diabetes mellitus: A systematic review and meta-analysis of
randomised controlled trials. J Hum Nutr Diet. 27:501–512. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Ozcan U, Cao Q, Yilmaz E, Lee AH, Iwakoshi
NN, Ozdelen E, Tuncman G, Görgün C, Glimcher LH and Hotamisligil
GS: Endoplasmic reticulum stress links obesity, insulin action, and
type 2 diabetes. Science. 306:457–461. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Embi N, Rylatt DB and Cohen P: Glycogen
synthase kinase-3 from rabbit skeletal muscle. Separation from
cyclic-AMP-dependent protein kinase and phosphorylase kinase. Eur J
Biochem. 107:519–527. 1980. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
de Figueiredo Souto Padron A, Salmon AB,
Bruno F, Jimenez F, Martinez HG, Halade GV, Ahuja SS, Clark RA,
DeFronzo RA, Abboud HE and El Jamali A: Nox2 mediates skeletal
muscle insulin resistance induced by a high-fat diet. J Biol Chem.
290:13427–13439. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Shih CC, Lin CH, Lin WL and Wu JB:
Momordica charantia extract on insulin resistance and the skeletal
muscle GLUT4 protein in fructose-fed rats. J Ethnopharmacol.
123:82–90. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Huang S and Czech MP: The GLUT4 glucose
transporter. Cell Metab. 5:237–252. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Cheng HL, Huang HK, Chang CI, Tsai CP and
Chou CH: A cell-based screening identifies compounds from the stem
of Momordica charantia that overcome insulin resistance and
activate AMP-activated protein kinase. J Agric Food Chem.
56:6835–6843. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
McCarty MF: Does bitter melon contain an
activator of AMP-activated kinase? Med Hypotheses. 63:340–343.
2004. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Jiang Q, Takemori AE, Sultana M,
Portoghese PS, Bowen WD, Mosberg HI and Porreca F: Differential
antagonism of opioid delta antinociception by [D-Ala2, Leu5,
Cys6]enkephalin and naltrindole 5′-isothiocyanate: Evidence for
delta receptor subtypes. J Pharmacol Exp Ther. 257:1069–1075.
1991.PubMed/NCBI
|