Introduction
Colorectal cancer (CRC) is the third most common
cancer in humans and has a high mortality rate, it is an important
public health problem worldwide (1). Various types of therapeutic
strategies have been used for the treatment of colon cancer,
including radiotherapy, chemotherapy, targeted therapy, and immune
therapy, however, the five-year survival rate of metastatic colon
cancer remains <10% (2).
Chemotherapy is one of the most commonly used therapeutic
strategies for metastatic colon cancer, agents used include
5-fluorouracil (5-FU), oxaliplatin and irinotecan, however,
development of resistance markedly limits their application in
clinical use (3–5). For example, 5-FU, which has been used
for many years, has a single agent effective rate of 24% (2). Although oxaliplatin improves the
response rate of patients in advanced colon cancer, >40%
patients develop serious resistance (6). Resistance to irinotecan, as a
first-line therapy for metastatic colon cancer in combination with
other antitumor agents, has also been reported in recent years
(7). The development of resistance
has been hypothesized to occur via multiple different mechanisms, a
number of which are supported with clinical data. However, the
development of resistance requires further elucidation.
Gap junctions composed of connexin directly connect
the cytoplasm of neighboring cells, thereby mediating direct
intercellular movement of cytoplasmic signaling molecules (8). Signaling molecules with weight <1
kDa can be transferred via these channels, including cyclic
adenosine monophosphate, cyclic guanosine monophosphate, calcium
and glutathione. Almost every cellular and tissue level process can
be influenced by this type of direct communication pathway, such as
differentiation, migration, and apoptosis (9). Thus, gap junctions are known to have
important roles in cancer biology and drug resistance (10,11).
Cx43 (molecular weight, 43 kDa) is regarded as one of the most
important known connexins, and it is often associated with drug
resistance. For example, alteration of Cx43 contributes to
temozolomide resistance in glioblastoma multiforme (10). Yu et al (12) demonstrated that Cx43 reversed the
resistance of A549 lung adenocarcinoma cells to cisplatin by
inhibiting epithelial-mesenchymal transition. It has not, to the
best of our knowledge, yet been reported whether resistance to
5-FU, oxaliplatin and irinotecan in colon cancer is associated with
Cx43. Thus, the current study investigated effects of the Cx43 gap
junction on these commonly used chemotherapeutic agents in colon
cancer cells to indicate a novel basis for therapeutic strategy
development for combating drug resistance.
Materials and methods
Cell line and cell culture
The RKO human colon cancer cell line was obtained
from American Type Culture Collection (Manassas, VA, USA) and
cultured in Eagle's minimum essential medium (Invitrogen; Thermo
Fisher Scientific, Inc., Waltham, MA, USA), supplemented with 10%
fetal bovine serum and 100 U/ml penicillin-streptomycin (both from
Invitrogen; Thermo Fisher Scientific, Inc.) at 37°C in a 5% CO2
incubator with 90% humidity.
Colony-forming assay
Colony-forming assays at high and low cell density
was used to detect the toxicity dependent on gap junctions. In
culture at a high cell density, cells were seeded at 30,000
cells/cm2 to ensure that cultures were 70–100% confluent at the
time of therapeutic agent exposure. At this density, each cell was
in contact with approximately four to five others cells and there
was substantial opportunity for gap junction formation. Cells were
treated with 5-FU (0–250 µM; Sigma-Aldrich; Merck Millipore,
Darmstadt, Germany), oxaliplatin (0–125 µM; Sigma-Aldrich; Merck
Millipore), or irinotecan (0–12.5 µM; Sigma-Aldrich; Merck
Millipore) for 24 h, and subsequently washed with Eagle's minimum
essential medium, harvested by trypsinization (Invitrogen; Thermo
Fisher Scientific, Inc.), counted, diluted, and seeded into
six-well dishes at 100 cells/cm2. Colony formation was assessed by
staining with crystal violet (Sigma-Aldrich; Merck Millipore) and
assessed at 7 days. Colonies containing >50 cells were scored
under an Eclipse E800 light microscope (Nikon Corporation, Tokyo,
Japan). At low cell density culture, cells were seeded into
six-well plates at 100 cells/cm2 directly and treated with the same
5-FU, oxaliplatin or irinotecan concentrations for 24 h following
attachment. Subsequently, the colony formation was assessed as
described above for the high density cell culture (13).
Gap26, retinoic acid (RA) treatment,
and survival assays
RKO cells were pretreated with connexin channel
inhibitors 300 µM Gap26 (Sigma-Aldrich; Merck Millipore), a
connexin mimetic peptide, for 1 h, and 10 µM retinoic acid
(Sigma-Aldrich; Merck Millipore), a Cx43 expression enhancer, for 1
h prior to a parachute dye-coupling assay or survival assays.
Dimethyl sulfoxide (DMSO) was used as a solvent for Gap26 and RA
(Sigma-Aldrich; Merck Millipore). Cell growth was determined in
24-well plates with the Cell Counting Kit-8 (CCK-8) assay (Dojindo
Molecular Technologies, Inc., Tokyo, Japan), which was conducted
according to the manufacturer's protocols.
Parachute dye-coupling assay
A parachute dye-coupling assay was used to detect
gap junction function. The donor and receiver cells were grown to
confluence. Donor cells were labeled with 5 µM CM-DiI (Invitrogen;
Thermo Fisher Scientific, Inc.), which did not spread to coupled
cells, and 5 µM calcein-acetoxymethyl ester (Invitrogen; Thermo
Fisher Scientific, Inc.), which was converted into the gap
junction-permeable dye calcein in an intracellular process.
Subsequently, at a 1:150 donor/receiver ratio, donor cells were
seeded onto the receiver cells. Donor cells and receiver cells
formed gap junctions. After 4 h, gap junction function was examined
with the fluorescence microscope (Eclipse E800). The mean number of
receiver cells containing dye per donor cell was counted and
normalized to that of control cultures (14).
Western blotting
Cells were washed three times with wash buffer [0.01
mol/l phosphate-buffered saline, 0.138 mol/l NaCl, 0.02% NaN3 (pH
7.4)] and then incubated with 0.05 ml/cm2 lysis buffer (Nanjing
Keygen Biotech Co., Ltd., Nanjing, China) for 2 h at 4°C. The
bicinchoninic acid (BCA) method using the BCA Protein Assay kit
(Nanjing Keygen Biotech Co., Ltd.) was used to measure protein
concentrations. Cell lysates (30 µg) were separated by SDS-PAGE on
10% Tris-glycine mini-gels (Invitrogen; Thermo Fisher Scientific,
Inc.) and transferred onto a polyvinylidene difluoride membrane
(Bio-Rad Laboratories, Inc., Hercules, CA, USA) and 5% nonfat dry
milk (Sigma-Aldrich; Merck Millipore) was used to block the
membranes at room temperature for 30 min. Subsequently, the
membranes were immunoblotted using mouse monoclonal anti-Cx43
antibody (1:4,000; Sigma-Aldrich; Merck Millipore; cat. no. C8093)
and mouse monoclonal anti-β-actin antibody (1:10,000;
Sigma-Aldrich; Merck Millipore; cat. no. A1978) overnight at 4°C.
Following a number of washes with Tris-buffered saline and Tween-20
(0.05%), the membranes were incubated for 1 h at room temperature
with polyclonal goat anti-mouse horseradish peroxidase
(HRP)-conjugated secondary antibodies (1:4,000; Sigma-Aldrich;
Merck Millipore; cat. no. M6898). The protein bands were detected
with an enhanced chemiluminescence system (KGP1125; Nanjing KeyGen
Biotech. Co., Ltd.). Protein band sizes were estimated using Alpha
View software (version 2.2.14407; ProteinSimple, San Jose, CA,
USA).
Cx43 knock-down with small interfering
RNA (siRNA) transfection
Two specific siRNAs were used to target the Cx43
gene and reduce expression, the sequences were as follows:
GCTGGTTACTGGTGACAGA for siRNA1-Cx43; and CCGCAATTACAACAAGCAA for
siRNA2-Cx43. A non-specific siRNA-NC was used as a negative
control. Lipofectamine 2000 (Invitrogen; Thermo Fisher Scientific,
Inc.) was used to transfect siRNA according to the manufacturer's
protocols (15).
Statistical analysis
Statistical analysis was performed using SPSS 15.0
software (SPSS, Inc., Chicago, IL, USA). Multiple comparisons among
groups were analyzed using one-way analysis of variance, followed
by Tukey's post hoc comparisons. P<0.05 was considered to
indicate a statistically significant difference.
Results
Association between toxicity and
concentration of 5-FU, oxaliplatin and irinotecan depends on cell
density
The RKO human colon cancer cell line was cultured
under two different conditions, at low density and high density
cell culture. At low density cell culture the cells were well
dispersed as single cells as the cell density was 100 cells/cm2
and, thus, no gap junctions were formed. At high density cell
culture, cells were 70–100% confluent at the time of therapeutic
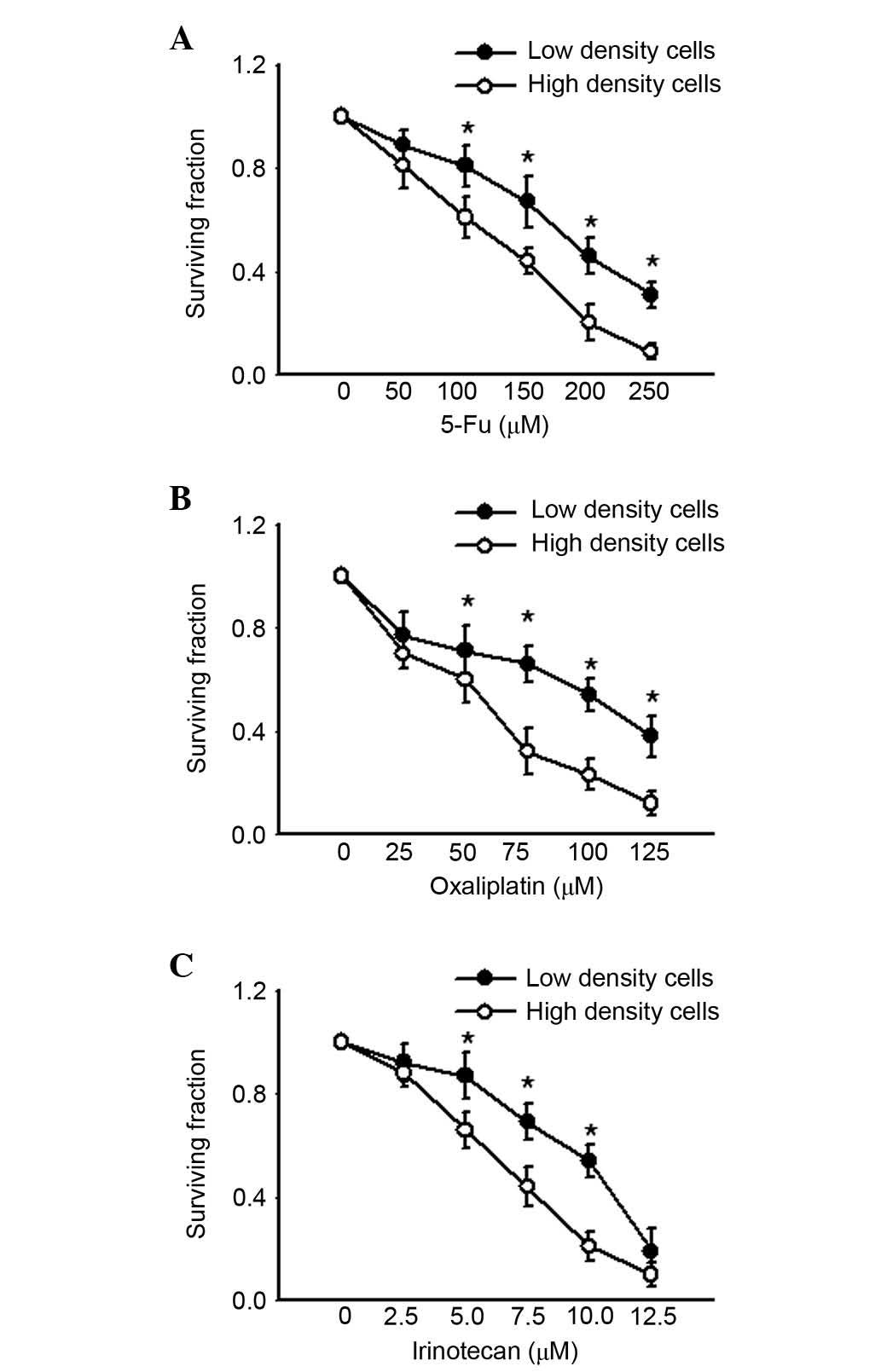
agent exposure, which allowed formation of gap junctions. Fig. 1 presents the survival of cultures
exposed to 5-FU, oxaliplatin and irinotecan for 24 h under low- or
high-density conditions. All three chemotherapeutic agents
decreased clonogenic survival of cells at low and high density in a
concentration-dependent manner. However, as the concentrations of
the agents increased, cell survival was significantly lower in high
density cell culture compared with low density (P<0.05). These
results indicated that the cell toxicity of 5-FU, oxaliplatin and
irinotecan was density-dependent, and greater in high density cell
culture where gap junctions were formed.
Cell toxicity of 5-FU, oxaliplatin and
irinotecan was mediated by gap junctions
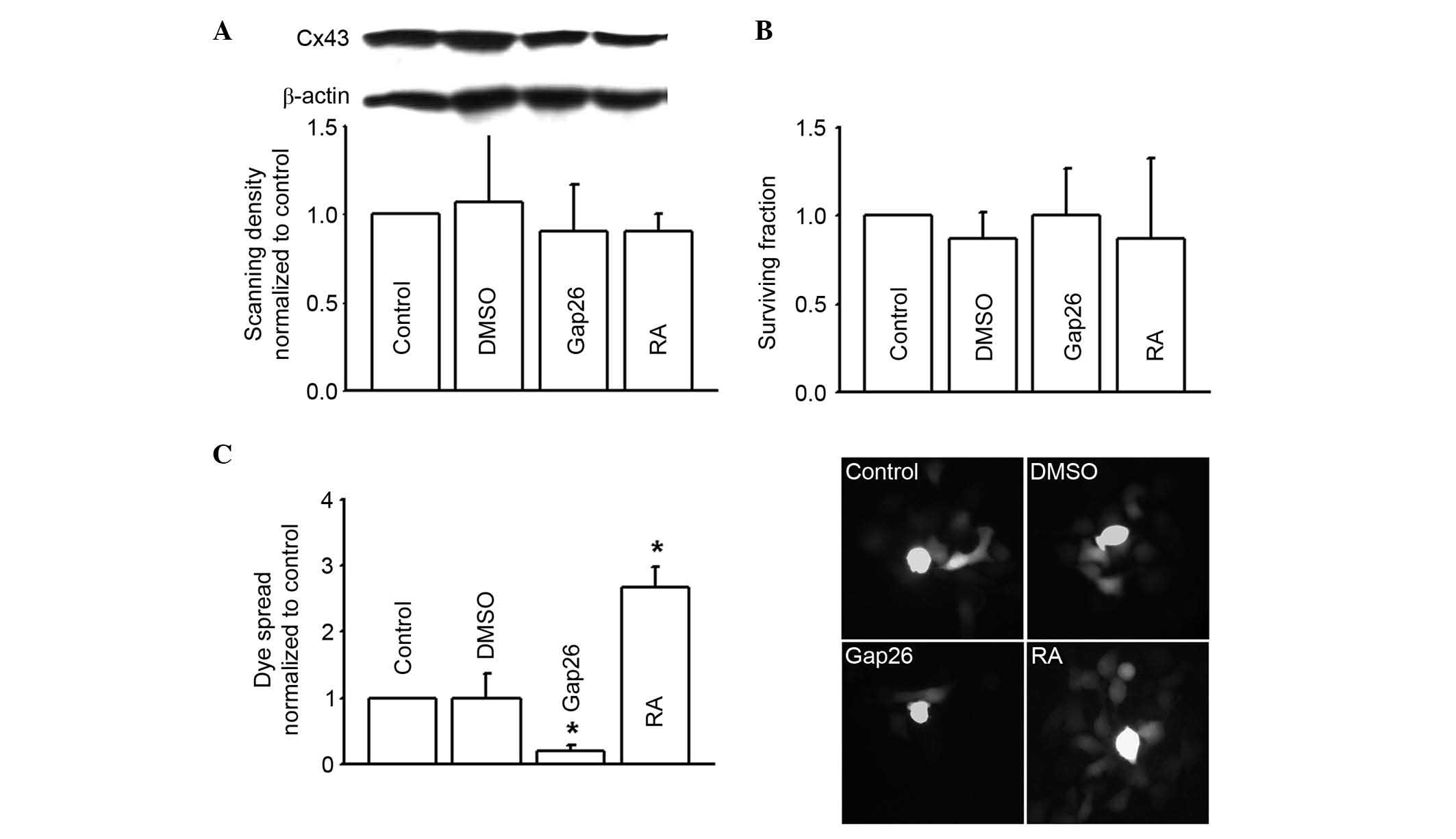
Loss of gap junctions is widely considered to be
associated with tumorigenic phenotypes, however, the RKO human
colon cancer cell line used in the present study highly expressed
Cx43 (Fig. 2A). The current study
aimed to investigate the function of the Cx43 gap junction on the
toxicity of commonly used chemotherapeutic agents targeting colon
cancer cells. Thus, roles of the gap junction composed of Cx43 on
5-FU, oxaliplatin and irinotecan toxicity in RKO cell line were
determined. Pharmacological inhibitors or enhancers were used to
alter the function of gap junctions composed of Cx43. Results
demonstrated that dye coupling was significantly reduced by the
inhibitor Gap26, but significantly increased by the enhancer RA
(P<0.05). Neither Gap26 or RA alone, or the solvent DMSO, had
any toxicity on the RKO cells or altered Cx43 expression (Fig. 2). Gap26 and RA were demonstrated to
alter the function of Cx43 gap junctions.
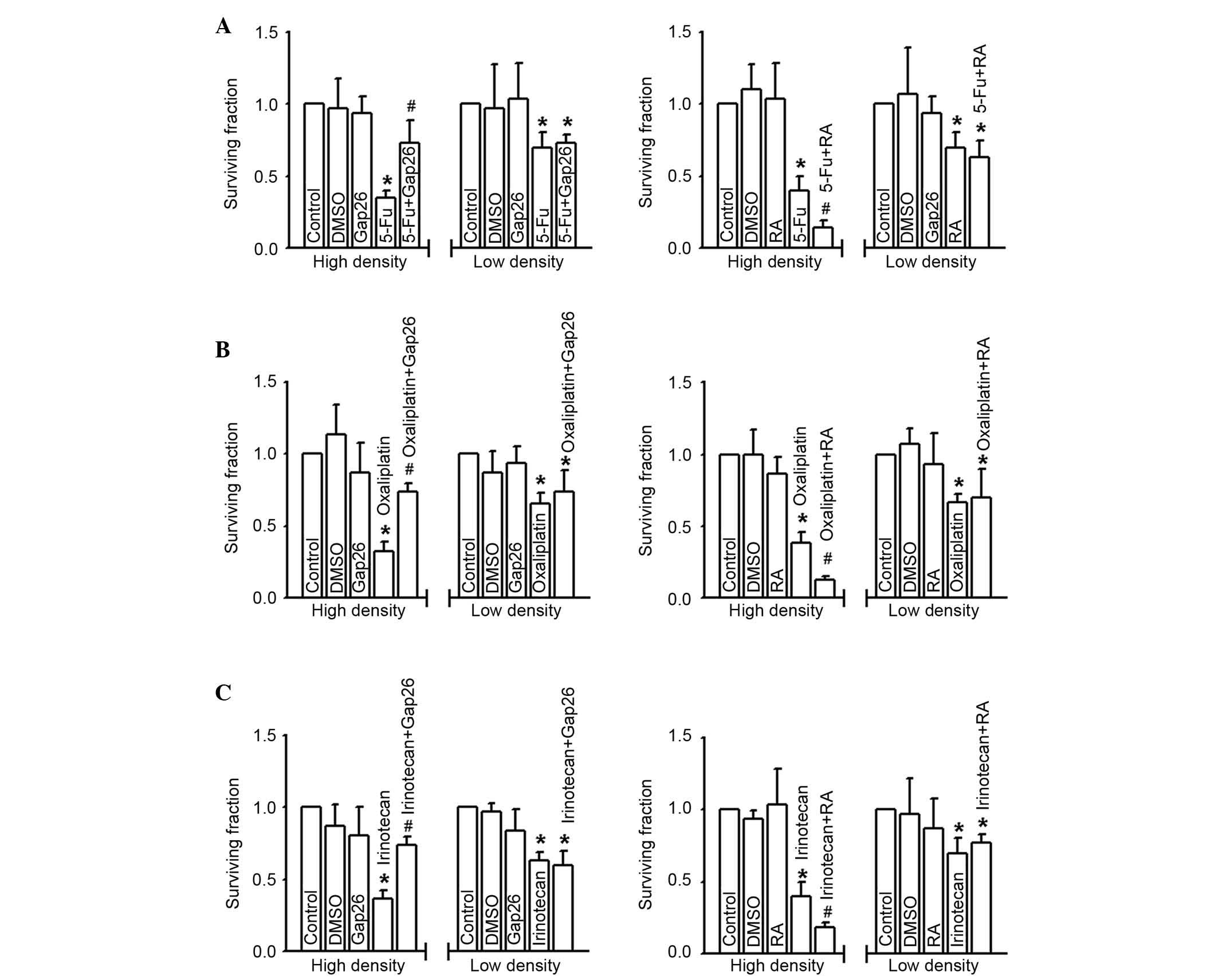
Fig. 3 presents the
effects of 5-FU, oxaliplatin and irinotecan toxicity on RKO cells
at low and high density cell culture. Survival fraction (detected
by colony-forming assay) was significantly downregulated following
treatment of RKO cells with 5-FU (200 µM), oxaliplatin (100 µM), or
irinotecan (10 µM) for 24 h at high cell density (gap junctions
formed; P<0.05), suggesting the cells were more sensitive to the
three commonly used chemotherapy agents. Notably, the cytotoxicity
of 5-FU, oxaliplatin and irinotecan was attenuated following Gap26
pretreatment at the concentration verified to inhibit gap junction
function in these cells (P<0.05), but exacerbated following RA
treatment in RKO cells cultured in high-density (P<0.05)
(Fig. 3). By contrast, at low
density cell culture (gap junction not formed), the cytotoxicity of
5-FU, oxaliplatin and irinotecan was not significantly different
irrespective of Gap26 or RA pretreatment compared with control
groups (Fig. 3). The results
demonstrate the effects of altering gap junction function on 5-FU,
oxaliplatin and irinotecan toxicity only occurs following high
density cell culture, which supports the hypothesis that gap
junctions are important in the efficacy of chemotherapeutic
agents.
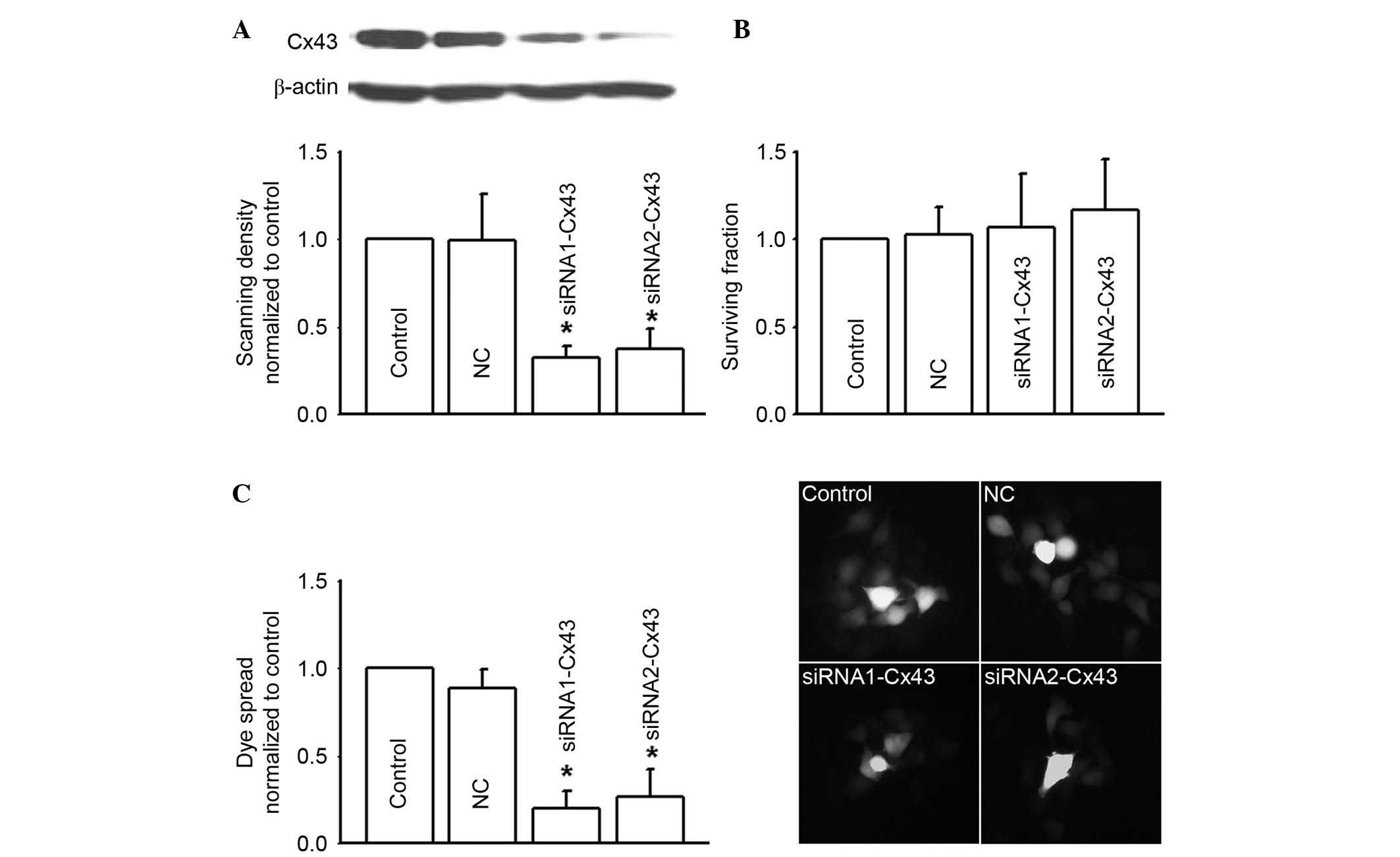
Cx43 gene knock-down inhibited Cx43
gap junction function and attenuated RKO cell toxicity of 5-FU,
oxaliplatin and irinotecan
In order to confirm the effects of Cx43 gap junction
function on the cytotoxicity of 5-FU, oxaliplatin and irinotecan,
two different Cx43 siRNAs (siRNA1-Cx43 and siRNA2-Cx43) were
synthesized to specifically knockdown Cx43 expression (Fig. 4A). Cx43 knockdown alone did not
influence RKO survival fraction (Fig.
4B). However, as Cx43 expression was depressed, dye coupling
(gap junction function) was also significantly decreased
(P<0.05; Fig. 4C).
In Fig. 5, the
CCK-8 assay was used to investigate the cytotoxicity of 5-FU,
oxaliplatin and irinotecan on RKO following depression of Cx43
expression levels with Cx43 siRNAs. Results indicated that Cx43
knock-down attenuated the cytotoxicity induced by 5-FU, oxaliplatin
and irinotecan in RKO cells, significantly increasing the survival
fraction to different degrees (P<0.05; Fig. 5A to C). These results indicate that
gap junctions composed of Cx43 are important in 5-FU, oxaliplatin
and irinotecan-induced cytotoxicity of RKO cells.
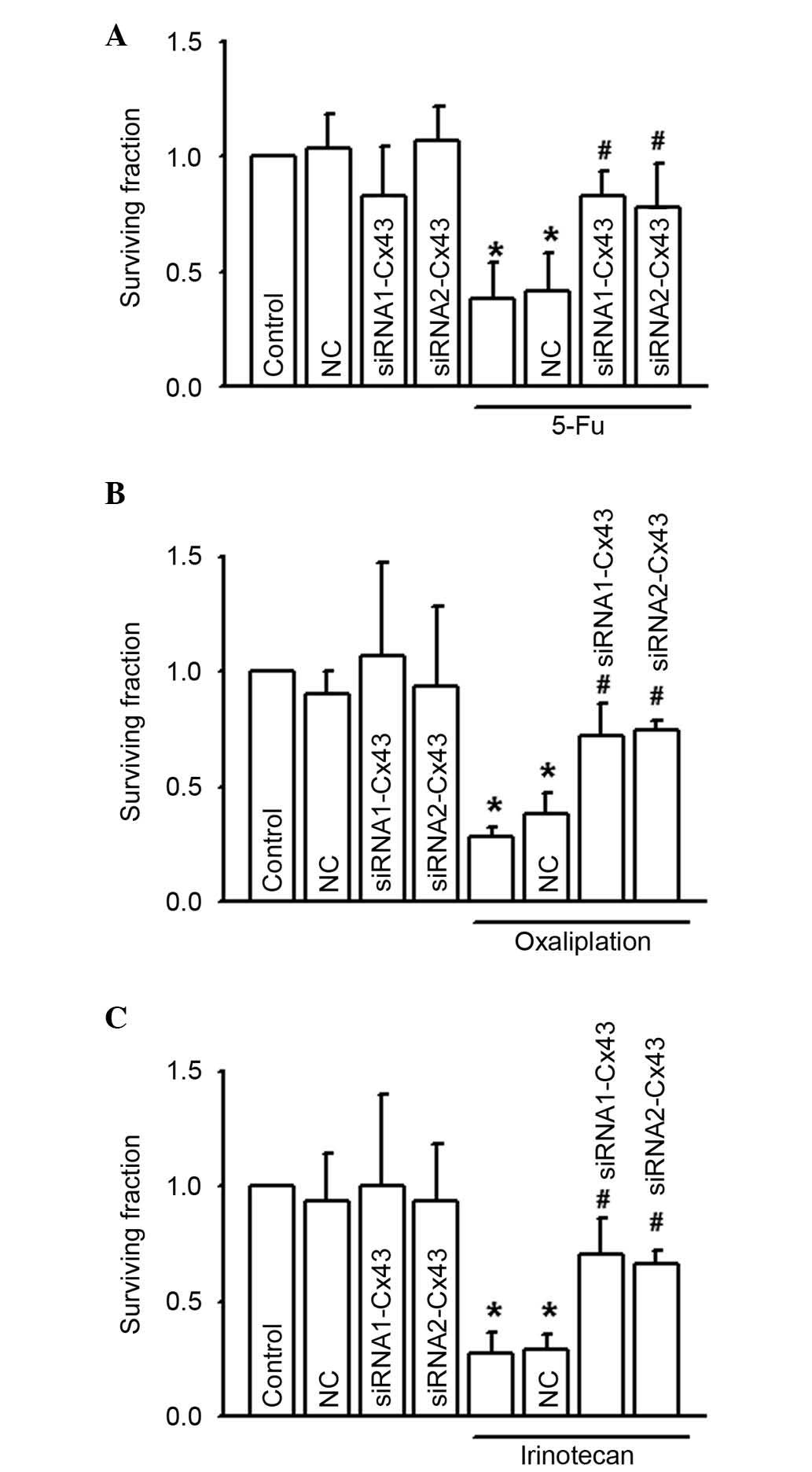 | Figure 5.RKO cell growth was increased by
specific siRNA when exposed to (A) 5-FU (200 µM, 24 h), (B)
oxaliplatin (100 µM, 24 h), or (C) irinotecan (10 µM, 24 h), n=5–7.
*P<0.05 vs. the control group; #P<0.05 vs. the
5-FU, oxaliplatin and irinotecan groups. Data are presented as the
mean ± standard error. NC and these two siRNAs alone had no
significant effect on the abovementioned parameters. 5-FU,
5-fluorouracil; NC, negative control; siRNA, small interfering
RNA. |
Discussion
The present study investigated the effect of Cx43
gap junctions on the toxicity of 5-FU, oxaliplatin and irinotecan.
Cx43 is involved in resistance to these commonly used
chemotherapeutic agents targeting colon cancer cells. The present
study demonstrated that these antitumor therapeutic agents worked
in a cell density-dependent manner. At high-density cell culture
(gap junctions formed), alteration of gap junction function using
different methods, such as the inhibitor Gap26 or the enhancer RA,
or Cx43 knock-down, affected 5-FU, oxaliplatin and
irinotecan-induced cytotoxicity. The cytotoxicity was attenuated
subsequent to depression of Cx43 gap junction functioning, but
exacerbated as Cx43 gap junction function increased. However, this
result was absent in low-density cell culture, which lacked gap
junction formation. This conclusion was consistent with previous
studies (12,13), and provided more information
regarding the importance of gap junctions composed of Cx43 in 5-FU,
oxaliplatin and irinotecan-induced cytotoxicity in RKO cells.
Gap junctions enable the direct transfer of small
molecules or electrical charge between neighboring cells, which
contributes to different physiological and pathological effects,
including cell growth, differentiation, damage, and response to
trauma (16). Molecular signals
resulting in the amplification of cytotoxicity or apoptosis are
termed ‘death signals,’ which are predominantly regulated by gap
junctions between neighboring cells, particularly in cancer cells
(17). Toxic products generated in
one cell can enter another via gap junctions and subsequently
enhance the likelihood of cell death, which in turn generates its
own toxic products as part of a positive feedback mechanism
(9). Toxic products, or ‘death
signals’ not only damage the neighboring cells directly, but also
trigger activation of different signal pathways, resulting in
cytotoxicity or apoptosis indirectly (13). This type of ‘death signal’ transfer
between the neighboring cells via gap junctions amplifies the
cytotoxicity induced by antitumor therapeutic agents, which is
termed the ‘bystander effect’ (18–20).
The identity of the ‘death signals’ has not yet been identified.
Although the possibility of calcium, reactive oxygen species or
cell metabolites have been recently discussed, more proof is
required (8).
Gap junction deficiency is widely considered to be
associated with tumorigenic phenotypes (21–23),
however, exceptions remain, including the RKO human colon cancer
cell line used in present study. Cx43 is highly expressed in RKO
cells. As previously reported, gap junctions are important in
different stages of cancer progression, including invasion,
extravasation, and metastasis in various types of cancer cell, such
as HeLa cells, breast carcinoma, melanoma, lung carcinoma and
glioma (24–27). The results of the present study
demonstrated the effects of altering gap junction function on colon
cancer chemotherapy that had not, to the best of our knowledge,
been previously reported, that enhancing the function of the Cx43
gap junction increased 5-FU, oxaliplatin and irinotecan-induced
cytotoxicity. By contrast, the cytotoxicity could be reduced by
inhibition of gap junction function.
CRC has a high mortality rate and is considered to
be the third most common cancer in humans (28). In metastatic colon cancer, the
five-year survival rate is <10% (2). 5-FU, oxaliplatin and irinotecan are
the most commonly used chemotherapeutic agents in CRC. However,
their response rates are low, even in combination with other
chemotherapeutic agents. As previously reported, 5-FU as a single
agent has an effective rate of 24%, and ~31% at higher doses,
however, dosage increase results in serious side-effects and the
development of drug resistance limits its dosage and therapeutic
effect (2). Oxaliplatin is a third
generation platinum-based antineoplastic agent, used as a
chemotherapeutic agent in advanced colon cancer treatment. Although
oxaliplatin application has notably improved response rates and
progression-free survival in advanced colon cancer, ~40% of
patients develop resistance (29).
Irinotecan is also used as a first-line therapy for metastatic
colon cancer in combination with other antitumor agents.
Unfortunately, resistance to irinotecan has been observed in the
clinic (30). Thus, it is clear
resistance to chemotherapeutic agents for colon cancers is a great
challenge in clinical use.
Toxic effects of gap junction-mediated intercellular
transfer had been investigated in a number of different systems
(31–33). Notably, it has been observed that
gap junctions composed of Cx43 act synergistically with 5-FU,
oxaliplatin and irinotecan to result in cytotoxicity. It has also
been reported that resistance to chemotherapeutic agents is
associated with loss of gap junctions in specific stages of cancer
progression. He et al (9)
and Wang et al (13)
demonstrated that gap junction recovery or enhancement improved
resistance of cisplatin. Toxic metabolites, such as 5-fluorouracil
converted from 5-FU by cytosine deaminase, can transfer between
neighboring cells via gap junctions to amplify the cytotoxicity
(34). This is consistent with the
results of the present study, which indicate that enhancing the
function of Cx43 gap junctions increased 5-FU, oxaliplatin and
irinotecan-induced cytotoxicity, which may be reduced by inhibition
of gap junction functioning, suggesting that gap junction reduction
may be an important mechanism underlying the development of
resistance. Thus, recovery of connexin expression or enhancing gap
junction functioning may be useful strategies to attenuate
resistance or increase the efficacy of anticancer chemotherapeutic
agents.
Gap junction loss was widely accepted to be
associated with tumorigenic phenotypes. As tumors progress, gap
junction functioning and connexin expression levels decrease, as
observed in HeLa cells, bladder cancer cells, lung carcinoma,
breast carcinoma, glioma or other colon cancer cell lines. All of
these tumors were not sensitive to chemotherapy agents, which
always results in a high rate of mortality (35–39).
If connexin expression could be recovered and gap junction function
enhanced, the responses of these tumors to chemotherapeutic agents
may be improved significantly.
In conclusion, the current study indicated that the
cytotoxicity of chemotherapeutic agents was attenuated with gap
junction inhibition, however was strengthened with gap junction
enhancement. This conclusion provided a novel basis for therapeutic
strategy development to combat drug resistance in numerous cell
types, not only for colon cancer cells, however additionally for
other types of cancer.
Acknowledgements
The present study was supported by the Natural
Science Foundation of Guangdong (grant no. S2011040003563) and the
National University Student Innovation Program (grant no.
201312121009).
References
|
1
|
Ikehata M, Ogawa M, Yamada Y, Tanaka S,
Ueda K and Iwakawa S: Different effects of epigenetic modifiers on
the cytotoxicity induced by 5-fluorouracil, irinotecan or
oxaliplatin in colon cancer cells. Biol Pharm Bull. 37:67–73. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Wang H, Zhang L, Yang X, Jin Y, Pei S,
Zhang D, Zhang H, Zhou B, Zhang Y and Lin D: PUMA mediates the
combinational therapy of 5-FU and NVP-BEZ235 in colon cancer.
Oncotarget. 6:14385–14398. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Chen J, Lu H, Yan D, Cui F, Wang X, Yu F,
Xue Y, Feng X, Wang J, Wang X, et al: PAK6 increase chemoresistance
and is a prognostic marker for stage II and III colon cancer
patients undergoing 5-FU based chemotherapy. Oncotarget. 6:355–367.
2015.PubMed/NCBI
|
|
4
|
Montazami N, Andish M Kheir, Majidi J,
Yousefi M, Yousefi B, Mohamadnejad L, Shanebandi D, Estiar MA,
Khaze V, Mansoori B, et al: siRNA-mediated silencing of MDR1
reverses the resistance to oxaliplatin in SW480/OxR colon cancer
cells. Cell Mol Biol (Noisy-le-grand). 61:98–103. 2015.PubMed/NCBI
|
|
5
|
Chen MC, Lee NH, Ho TJ, Hsu HH, Kuo CH,
Kuo WW, Lin YM, Tsai FJ, Tsai CH and Huang CY: Resistance to
irinotecan (CPT-11) activates epidermal growth factor
receptor/nuclear factor kappa B and increases cellular metastasis
and autophagy in LoVo colon cancer cells. Cancer Lett. 349:51–60.
2014. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Tan S, Peng X, Peng W, Zhao Y and Wei Y:
Enhancement of oxaliplatin-induced cell apoptosis and tumor
suppression by 3-methyladenine in colon cancer. Oncol Lett.
9:2056–2062. 2015.PubMed/NCBI
|
|
7
|
Nemunaitis J, Cox J, Meyer W, Courtney A
and Mues G: Irinotecan hydrochloride (CPT-11) resistance identified
by K-ras mutation in patients with progressive colon cancer after
treatment with 5-fluorouracil (5-FU). Am J Clin Oncol. 20:527–529.
1997. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Luo C, Yuan D, Li X, Yao W, Luo G, Chi X,
Li H, Irwin MG, Xia Z and Hei Z: Propofol attenuated acute kidney
injury after orthotopic liver transplantation via inhibiting gap
junction composed of connexin 32. Anesthesiology. 122:72–86. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
9
|
He B, Tong X, Wang L, Wang Q, Ye H, Liu B,
Hong X, Tao L and Harris AL: Tramadol and flurbiprofen depress the
cytotoxicity of cisplatin via their effects on gap junctions. Clin
Cancer Res. 15:5803–5810. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Gielen PR, Aftab Q, Ma N, Chen VC, Hong X,
Lozinsky S, Naus CC and Sin WC: Connexin43 confers Temozolomide
resistance in human glioma cells by modulating the mitochondrial
apoptosis pathway. Neuropharmacology. 75:539–548. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Le HT, Sin WC, Lozinsky S, Bechberger J,
Vega JL, Guo XQ, Sáez JC and Naus CC: Gap junction intercellular
communication mediated by connexin43 in astrocytes is essential for
their resistance to oxidative stress. J Biol Chem. 289:1345–1354.
2014. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Yu M, Zhang C, Li L, Dong S, Zhang N and
Tong X: Cx43 reverses the resistance of A549 lung adenocarcinoma
cells to cisplatin by inhibiting EMT. Oncol Rep. 31:2751–2758.
2014.PubMed/NCBI
|
|
13
|
Wang Q, You T, Yuan D, Han X, Hong X, He
B, Wang L, Tong X, Tao L and Harris AL: Cisplatin and oxaliplatin
inhibit gap junctional communication by direct action and by
reduction of connexin expression, thereby counteracting cytotoxic
efficacy. J Pharmacol Exp Ther. 333:903–911. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Zhao Y, Liu B, Wang Q, Yuan D, Yang Y,
Hong X, Wang X and Tao L: Propofol depresses the cytotoxicity of
X-ray irradiation through inhibition of gap junctions. Anesth
Analg. 112:1088–1095. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Yuan D, Wang Q, Wu D, Yu M, Zhang S, Li L,
Tao L and Harris AL: Monocyte-endothelial adhesion is modulated by
Cx43-stimulated ATP release from monocytes. Biochem Biophys Res
Commun. 420:536–541. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Luo C, Yuan D, Yao W, Cai J, Zhou S, Zhang
Y and Hei Z: Dexmedetomidine protects against apoptosis induced by
hypoxia/reoxygenation through the inhibition of gap junctions in
NRK-52E cells. Life Sci. 122:72–77. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Hong X, Wang Q, Yang Y, Zheng S, Tong X,
Zhang S, Tao L and Harris AL: Gap junctions propagate opposite
effects in normal and tumor testicular cells in response to
cisplatin. Cancer Lett. 317:165–171. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Garcia-Rodríguez L, Pérez-Torras S, Carrió
M, Cascante A, García-Ribas I, Mazo A and Fillat C: Connexin-26 is
a key factor mediating gemcitabine bystander effect. Mol Cancer
Ther. 10:505–517. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Sanson M, Marcaud V, Robin E, Valéry C,
Sturtz F and Zalc B: Connexin 43-mediated bystander effect in two
rat glioma cell models. Cancer Gene Ther. 9:149–155. 2002.
View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Vrionis FD, Wu JK, Qi P, Waltzman M,
Cherington V and Spray DC: The bystander effect exerted by tumor
cells expressing the herpes simplex virus thymidine kinase (HSVtk)
gene is dependent on connexin expression and cell communication via
gap junctions. Gene Ther. 4:577–585. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Gonzalez-Nieto D, Li L, Kohler A, Ghiaur
G, Ishikawa E, Sengupta A, Madhu M, Arnett JL, Santho RA, Dunn SK,
et al: Connexin-43 in the osteogenic BM niche regulates its
cellular composition and the bidirectional traffic of hematopoietic
stem cells and progenitors. Blood. 119:5144–5154. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Autsavapromporn N, de Toledo SM, Little
JB, Jay-Gerin JP, Harris AL and Azzam EI: The role of gap junction
communication and oxidative stress in the propagation of toxic
effects among high-dose α-particle-irradiated human cells. Radiat
Res. 175:347–357. 2011. View
Article : Google Scholar : PubMed/NCBI
|
|
23
|
Segretain D, Decrouy X, Dompierre J,
Escalier D, Rahman N, Fiorini C, Mograbi B, Siffroi JP, Huhtaniemi
I, Fenichel P and Pointis G: Sequestration of connexin43 in the
early endosomes: An early event of Leydig cell tumor progression.
Mol Carcinog. 38:179–187. 2003. View
Article : Google Scholar : PubMed/NCBI
|
|
24
|
Li J, Cheng L, Wang LJ, Liu HC, Li L, Wang
XL and Geng MY: Cell surface sialic acid inhibits Cx43 gap junction
functions in constructed Hela cancer cells involving in sialylated
N-cadherin. Mol Cell Biochem. 344:241–251. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Lin Q, Balasubramanian K, Fan D, Kim SJ,
Guo L, Wang H, Bar-Eli M, Aldape KD and Fidler IJ: Reactive
astrocytes protect melanoma cells from chemotherapy by sequestering
intracellular calcium through gap junction communication channels.
Neoplasia. 12:748–754. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Matesic DF, Sidorova TS, Burns TJ, Bell
AM, Tran PL, Ruch RJ and May SW: p38 MAPK activation, JNK
inhibition, neoplastic growth inhibition, and increased gap
junction communication in human lung carcinoma and Ras-transformed
cells by 4-phenyl-3-butenoic acid. J Cell Biochem. 113:269–281.
2012. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Aftab Q, Sin WC and Naus CC: Reduction in
gap junction intercellular communication promotes glioma migration.
Oncotarget. 6:11447–11464. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Grossi V, Peserico A, Tezil T and Simone
C: p38alpha MAPK pathway: A key factor in colorectal cancer therapy
and chemoresistance. World J Gastroenterol. 20:9744–9758. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Martinez-Balibrea E, Martínez-Cardús A,
Ginés A, de Porras V Ruiz, Moutinho C, Layos L, Manzano JL, Bugés
C, Bystrup S, Esteller M and Abad A: Tumor-related molecular
mechanisms of oxaliplatin resistance. Mol Cancer Ther.
14:1767–1776. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Westover D, Ling X, Lam H, Welch J, Jin C,
Gongora C, Del Rio M, Wani M and Li F: FL118, a novel camptothecin
derivative, is insensitive to ABCG2 expression and shows improved
efficacy in comparison with irinotecan in colon and lung cancer
models with ABCG2-induced resistance. Mol Cancer. 14:922015.
View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Sovadinova I, Babica P, Böke H, Kumar E,
Wilke A, Park JS, Trosko JE and Upham BL: Phosphatidylcholine
specific PLC-induced dysregulation of gap junctions, a robust
cellular response to environmental toxicants and prevention by
resveratrol in a rat liver cell model. PloS One. 10:e01244542015.
View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Dong L, Yang X, Gu W, Zhao K, Ge H, Zhou J
and Bai X: Connexin 43 mediates PFOS-induced apoptosis in
astrocytes. Chemosphere. 132:8–16. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Igarashi I, Maejima T, Kai K, Arakawa S,
Teranishi M and Sanbuissho A: Role of connexin 32 in acetaminophen
toxicity in a knockout mice model. Exp Toxicol Pathol. 66:103–110.
2014. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Lawrence TS, Rehemtulla A, Ng EY, Wilson
M, Trosko JE and Stetson PL: Preferential cytotoxicity of cells
transduced with cytosine deaminase compared to bystander cells
after treatment with 5-flucytosine. Cancer Res. 58:2588–2593.
1998.PubMed/NCBI
|
|
35
|
Sirnes S, Bruun J, Kolberg M, Kjenseth A,
Lind GE, Svindland A, Brech A, Nesbakken A, Lothe RA, Leithe E and
Rivedal E: Connexin43 acts as a colorectal cancer tumor suppressor
and predicts disease outcome. Int J Cancer. 131:570–581. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Aasen T, Hodgins MB, Edward M and Graham
SV: The relationship between connexins, gap junctions, tissue
architecture and tumour invasion, as studied in a novel in vitro
model of HPV-16-associated cervical cancer progression. Oncogene.
22:7969–7980. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Bernzweig J, Heiniger B, Prasain K, Lu J,
Hua DH and Nguyen TA: Anti-breast cancer agents, quinolines,
targeting gap junction. Med Chem. 7:448–453. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Gee J, Tanaka M and Grossman HB: Connexin
26 is abnormally expressed in bladder cancer. J Urol.
169:1135–1137. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Leithe E, Sirnes S, Omori Y and Rivedal E:
Downregulation of gap junctions in cancer cells. Crit Rev Oncog.
12:225–256. 2006. View Article : Google Scholar : PubMed/NCBI
|