Introduction
Histone methylation is conducted by the coordination
of specific histone methyltransferase and demethylase, as a
reversible dynamic process. The abnormal expression of histone
methyltransferase and demethylase are important in the generation
and development of glioma. It has been demonstrated that there were
marked changes to expression of histone methyltransferases,
including histone-lysine-N-methyltransferase SETD7, histone-lysine
N-methyltransferase 2A and myeloid/lymphoid or mixed-lineage
leukemia 4, and to demethylases, including lysine-specific
demethylase 2A and lysine-specific demethylase 2B in glioma cells
(1). G9a, as the important
methyltransferase of euchromatin, is important in the regulation of
epigenetic inheritance. Huang et al (2) demonstrated that G9a and G9a-like
protein 1 (GLP) may methylate tumor suppressor gene TP53,
resulting in methylated TP53, which has no activity
(2). H3K9 methylation induced by
G9a may hold the ability of transcriptional inhibition. However,
H3K9 methylation is important in transcriptional silencing, X
chromatin deactivation and tumor generation and development.
BIX-01294 is an artificial inhibitor of G9a with high selectivity.
Kondo et al (3)
demonstrated that following BIX-01294 treatment, the activity of
G9a and H3K9 methylation decreased, leading to a decrease in
chromosome stability, an increase in the apoptosis of tumor cells
and cycle arrest (3).
The present study investigated the differential
expression and clinical significance of histone methylase G9a,
histone H3K9me2 and histone H3K9me1 in human brain glioma and
adjacent tissues. By observing the influence of BIX-01294 on
biological changes, histone methylation and acetylation in U251
cells, a possible mechanism was also investigated.
Materials and methods
Subjects
All the patients (20 males and 21 females; mean age,
34.6) were hospitalized for neurosurgery in Zhangzhou Affiliated
Hospital of Fujian Medical University (Zhangzhou, China) between
January 2010 to June 2013. All the patients were diagnosed with
glioma and underwent complete follow-up treatment, without any
therapy received prior to surgery. The data, including pathological
specimens and clinical materials were collected. According to the
World Health Organization (WHO) classification standard of central
nervous system neoplasms (4), the
patients were graded into WHO I (8 patients), WHO II (21 patients),
WHO III (15 patients) and WHO IV (6 patients). The tumor specimens
and samples from the junctional area between the tumor and normal
brain tissue were collected to serve as the experimental group and
control group. Written informed consent was obtained from the
participants prior to the start of the study. This study was
approved by the Ethics Committee of Zhangzhou Affiliated Hospital
of Fujian Medical University.
Agents
BIX-01294 was obtained from Sigma-Aldrich (Merck
Millipore, Darmstadt, Germany). Fetal bovine serum (FBS) was
obtained from Hangzhou Sijiqing Bioengineering Material Co., Ltd.,
Hangzhou, China. Antibodies against G9a (cat. no. 09-071) H3K9me1
(cat. no. 07-450), H3K9me2 (cat. no. 16–187), H3K9me3 (cat. no.
07–442), H3K27me1 (cat. no. 07–448), H3K27me2 (cat. no. 07–452),
Acteylated (Act)-H3 (cat. no. 07–677-I), caspase-9 (cat. no.
05–672), caspase-3 (cat. no. 05–654), B-cell lymphoma 2 (Bcl-2;
cat. no. 05–826), Bcl-2-associated X protein (Bax; cat. no. AB2915)
and β-actin (cat. no. 04–1116) were obtained from Upstate
Biotechnology, Inc. (Lake Placid, NY, USA) and used at dilutions
between 1:200-1:500. Goat anti-rabbit (cat. no. sc-3837) and goat
anti-mouse (cat. no. sc-395758) antibodies were obtained from Santa
Cruz Biotechnology, Inc. and used at dilutions 1:2,000–1:5,000.
Cell culture
The U251 human glioma cell line was obtained from
Shanghai Institute for Biological Sciences, Chinese Academy of
Sciences (Shanghai, China). RPMI 1640 was obtained from Gibco
(Thermo Fisher Scientific Inc., Waltham, MA, USA), containing 10%
FBS and 2 mM L-glutamine. U251 cells were cultured at 37°C with
saturated humidity and 5% CO2. The cells were passaged every 3 to 4
days, with 0.25% trypsin digesting for 2–3 min followed by seeding
of the cell suspension to the required concentration. Prior to
seeding, the activity of U251 cells was detected by trypan blue
staining.
Detection of G9a, H3K9me2 and H3K9me1
expression in glioma and adjacent tissues
The proteins were detected using the
streptavidin-peroxidase method (5). G9a (1:200), H3K9me2 (1:300) and
H3K9me1 (1:300) antibodies were used. The results of the
immunohistochemistry were judged by the semi-quantitative integral
method, according to the sum of the color strength and the
percentage of stained cells. Color strength grade: Without
staining, 0; weak staining, 1; moderate staining, 2; and strong
staining, 3. Coloring percentage: Without staining, 0; <25%, 1;
25–50%, 2; 50–75%, 3; and >75%, 4. Sum of the two: 0, negative;
1–2, + (negative); 3–4, ++ (positive); 5–6, +++ (positive); and 7,
++++ (positive). All the data were evaluated by experienced
pathologists using double blinding.
MTS assay to detect the cell growth
curve following different concentrations of BIX-01294
Cells in the logarithmic growth phase were seeded in
a 96-well plate (Costar; Corning Incorporated, Corning, NY, USA) at
a concentration of 1.0×105/ml (100 µl each well). BIX-10294 was
added and the concentration adjusted to 0, 1, 2, 4 and 8 µmol/l
with 6 wells for each group. After 24 h, 48 h and 72 h at 37°C, 100
µl MTS (5 mg/ml; Sigma-Aldrich; Merck Millipore) was added and
incubated for 4 h at 37°C. The cells were centrifuged at 800 ×
g for 5 min and the supernatant discarded. DMSO (100 µl;
Sigma-Aldrich; Merck Millipore) was added and mixed fully. The
absorbance (value A) was detected at wavelengths of 492 and 630 nm
using a microplate reader. The cell proliferation rate was
calculated according to the 0 µM (blank). Cell proliferation rate
(%)= (Aexperiment - Ablank) / (Acontrol-Ablank) × 100%. Repeated
totally 3 times.
Apoptosis detection by TUNEL
method
U251 cells in the logarithmic growth phase were
seeded on 6-well plates at 1×106 cells per well, a cover glass was
also placed in each well. After 24 h, BIX-01294 was added, the
concentration was adjusted to 0, 2, 4 and 8 µmol/l and the cells
were incubated for 24 h at 37°C. The TUNEL assay was conducted
according to the manufacturer's protocols (DeadEnd™ Fluorometric
TUNEL System; Promega Corporation, Madison, WI, USA) and cells were
imaged using light microscopy.
Western blotting to detect the changes
of apoptosis-associated proteins following BIX-01294 treatment
The cells were centrifuged and collected following
treatment for 24 h. The cells were washed twice with PBS. Lysis
buffer (100 µl) and 1 µl enzyme inhibitor were added to 1×106 cells
on ice for 30 min. The cells were then centrifuged at 10,000 × g,
at 4°C for 10 min to extract the protein and protein quantification
was conducted using the bicinchoninic acid method. The proteins (20
µg per lane) were separated by 12% SDS-PAGE gel electrophoresis and
transferred to a polyvinylidene fluoride membrane (EMD Millipore,
Billerica, MA, USA). Following transfer, membranes were blocked
with 5% non-fat milk for 1 h at room temperature, and incubated
with primary antibodies, H3K9me (1:500), H3K9me2 (1:500), H3K9me3
(1:500), H3K27me (1:1,000), H3K27me2 (1:1,000), Act-H3 (1:2,000),
caspase 9 (1:600), caspase 3 (1:600), Bcl-2 (1:500) and Bax
(1:500), at 4°C overnight. The membrane was then washed with
Tris-buffered saline and goat anti-mouse secondary antibodies
(1:5,000) were added and incubated for 1 h at room temperature.
Following washing with TBS, the proteins were analyzed by
chemiluminescence using β-actin as a loading control. The images
were analyzed by AlphaDigiDoc imaging analysis software (version
7.1; Alpha Innotec, Kasendorf, Germany).
Statistical analysis
Data are presented as the mean ± standard deviation.
All the data were analyzed by SPSS 13.0 software (SPSS, Inc.,
Chicago, IL, USA). Homogeneity of variance and a normality test
were conducted and one-way analysis of variance was used to analyze
the results. P<0.05 was considered to indicate a statistically
significant difference.
Results
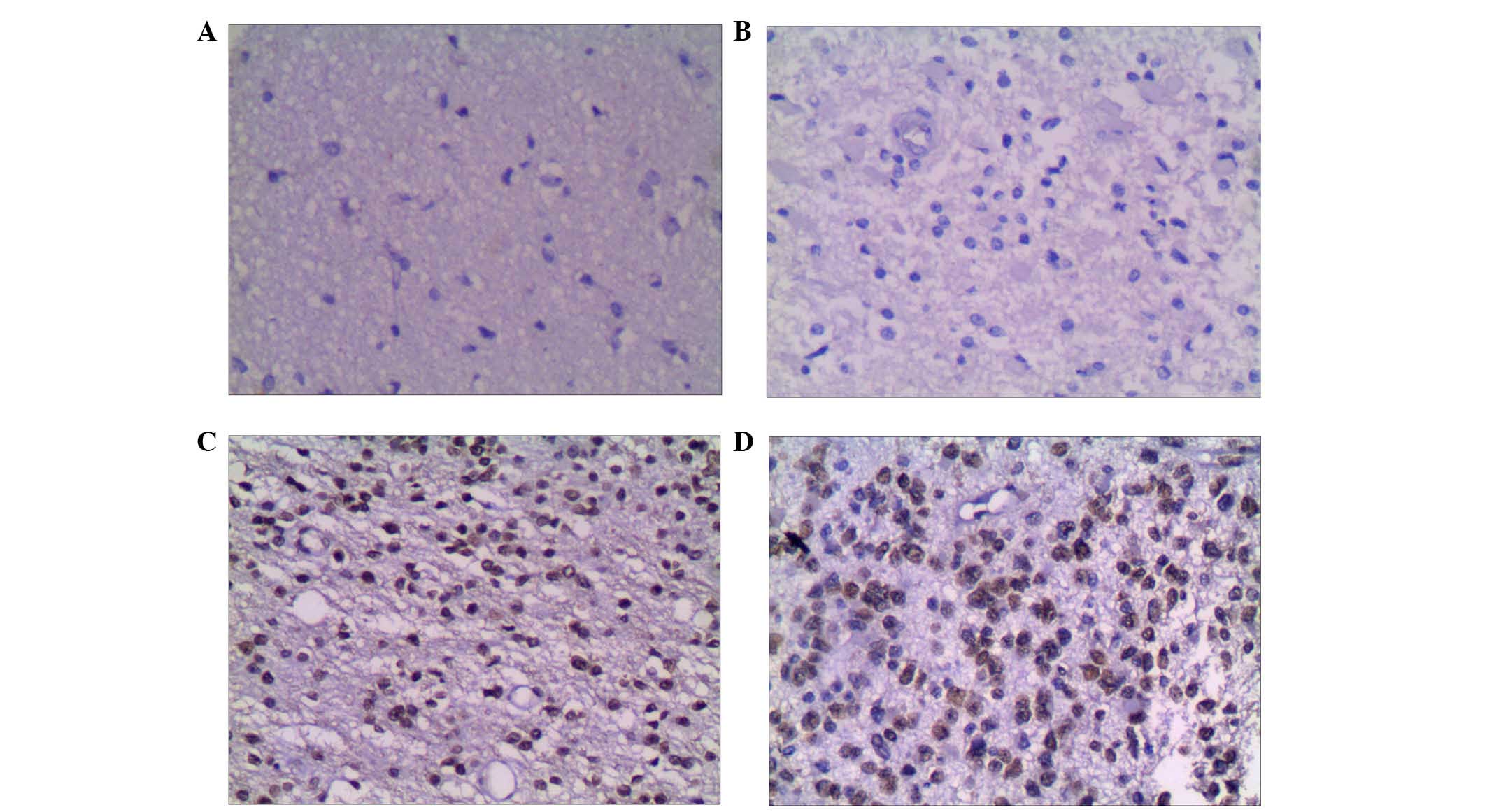
G9a is differentially expressed in
glioma and adjacent tissues
In glioma tissues, the positive rate of G9a was 86%
(43/50), which was significantly higher than that in the adjacent
tissues, 42% (21/50; χ2=19.14, P<0.01; Table I). In grade I glioma tissues, G9a
expression included 4 classified as ++, 1 classified as +++ and 0
classified as ++++, grade II including 8 classified as ++, 7
classified as +++ and 3 classified as ++++, grade III including 5
classified as ++, 6 classified as +++ and 3 classified as ++++, and
grade IV including 2 classified as +++ and 4 classified as ++++.
There were significant differences in the differential expression
among different grades of glioma (F=37.52, P<0.01). The higher
WHO grade was associated with higher G9a expression intensity
(Table II).
 | Table I.Differential expression of histone
methylase G9a in glioma and adjacent tissues. |
Table I.
Differential expression of histone
methylase G9a in glioma and adjacent tissues.
| Group | Cases (n) | Positive (n) | Positive rate
(%) | P |
|---|
| Glioma tissue | 50 | 43 | 86.00 |
|
| Adjacent tissue | 50 | 21 | 42.00 | <0.01 |
 | Table II.Differential expression of histone
methylase G9a in different glioma tissue. |
Table II.
Differential expression of histone
methylase G9a in different glioma tissue.
|
|
|
| WHO |
|---|
|
|
|
|
|
|---|
| Positive stage | Cases (n=) | Positive (n=) | ++ | +++ | ++++ |
|---|
| I | 8 | 5 | 4 | 1 | 0 |
| II | 21 | 18 | 8 | 7 | 3 |
| III | 15 | 14 | 5 | 6 | 3 |
| IV | 6 | 6 | 0 | 2 | 4 |
H3K9me2 is differentially expressed in
glioma and adjacent tissues
The positive rate of H3K9me2 in glioma tissues was
82% (41/50), which was significantly increased from 38% (19/50) in
adjacent tissues (χ2=18.38, P<0.01; Table III). In grade I glioma tissue,
H3K9me2 expression included 3 classified as ++, 1 classified as +++
and 0 classified as ++++, grade II including 7 classified as ++, 5
classified as +++ and 6 classified as ++++, grade III including 4
classified as ++, 5 classified as +++ and 4 classified as ++++, and
grade IV including 3 classified as +++ and 3 classified as ++++.
There were significant differences in the differential expression
among different grades of glioma (F=30.28, P<0.01). Higher WHO
grade was associated with higher H3K9me2 expression intensity
(Table IV).
 | Table III.Differential expression of H3K9me2 in
glioma and adjacent tissues. |
Table III.
Differential expression of H3K9me2 in
glioma and adjacent tissues.
| Group | Cases (n) | Positive (n) | Positive rate
(%) | P |
|---|
| Glioma tissue | 50 | 41 | 82.00 |
|
| Adjacent tissue | 50 | 19 | 38.00 | <0.01 |
 | Table IV.Differential expression of H3K9me2 in
different glioma tissue samples. |
Table IV.
Differential expression of H3K9me2 in
different glioma tissue samples.
|
|
|
| Positive |
|---|
|
|
|
|
|
|---|
| WHO stage | Cases (n) | Positive (n) | ++ | +++ | ++++ |
|---|
| I | 8 | 4 | 3 | 1 | 0 |
| II | 21 | 18 | 7 | 5 | 6 |
| III | 15 | 13 | 4 | 5 | 4 |
| IV | 6 | 6 | 0 | 3 | 3 |
H3K9me1 is differentially expressed in
glioma and adjacent tissues
The positive rate of H3K9me1 in in glioma tissues
was 54% (27/50) which was not significantly different from the
adjacent tissues were the positive rate was 44% (22/50) in adjacent
tissues (χ2=1.21, P>0.05; Table V). In grade I glioma tissues,
H3K9me2 expression included 1 classified as ++, 1 classified as +++
and 2 classified as ++++, grade II including 4 classified as ++, 5
classified as +++ and 3 classified as ++++, grade III including 2
classified as ++, 3 classified as +++ and 3 classified as ++++, and
grade IV including 1 classified as +++ and 2 classified as ++++.
There were no significant differences in the differential
expression among different grades of glioma (F=1.28, P>0.05;
Tables V and VI, Figs.
1–3).
 | Table V.Differential expression of H3K9me1 in
glioma and adjacent tissues. |
Table V.
Differential expression of H3K9me1 in
glioma and adjacent tissues.
| Group | Cases (n) | Positive (n) | Positive rate
(%) | P |
|---|
| Glioma tissue | 50 | 27 | 54.00 |
|
| Adjacent
tissue | 50 | 22 | 44.00 | <0.01 |
 | Table VI.Differential expression of H3K9me1 in
different glioma tissue samples. |
Table VI.
Differential expression of H3K9me1 in
different glioma tissue samples.
|
|
|
| Positive |
|---|
|
|
|
|
|
|---|
| WHO | Cases (n) | Positive (n) | ++ | +++ | ++++ |
|---|
| I | 8 | 4 | 1 | 1 | 2 |
| II | 21 | 12 | 4 | 5 | 3 |
| III | 15 | 8 | 2 | 3 | 3 |
| IV | 6 | 3 | 1 | 0 | 2 |
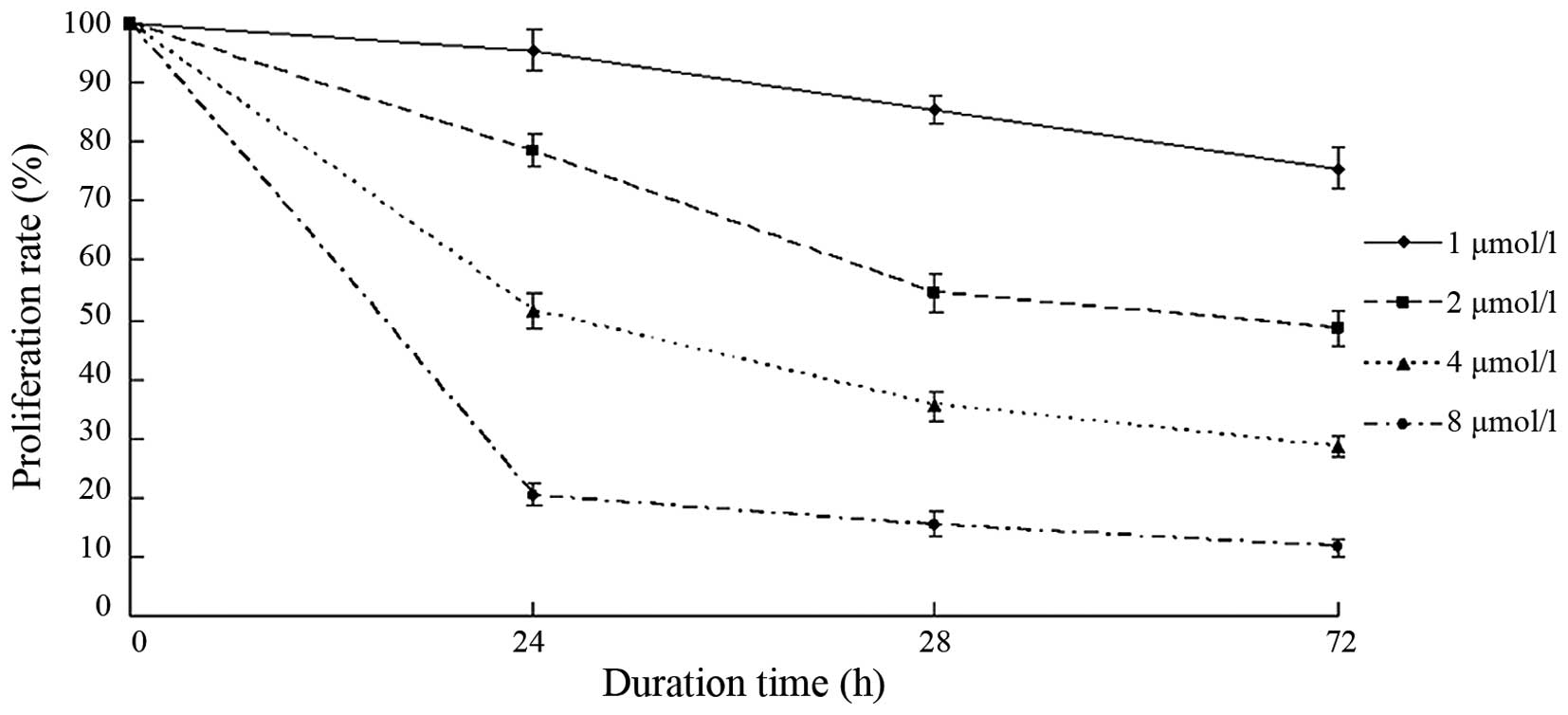
BIX-01294 inhibited the proliferation
of U251 cells
Following treatment with different concentrations of
BIX-01294 (1, 2, 4 and 8 µmol/l) for 24 h, the proliferation rates
of U251 cells were 94.12±3.41, 78.83±2.25, 53.68±2.54 and
21.04±2.07%, respectively. As the concentration increased, the
proliferation inhibition rate increased and the proliferation rate
decreased (Fig. 4). After 24 h, 48
h and 72 h of BIX-01294 treatment, the proliferation of U251 cells
decreased. The proliferation rate also decreased in a
time-dependent manner.
BIX-01294 induced apoptosis of U251
cells
Following treatment with different concentrations of
BIX-01294 (1, 2, 4 and 8 µmol/l) for 24 h, the apoptosis rates of
U251 cells were 3.67±1.42, 16.42±5.18, 35.18±3.26 and 57.52±4.37%,
respectively, which indicates apoptosis rates significantly
increased in a concentration-dependent manner (F=32.52, P<0.01;
Fig. 5).
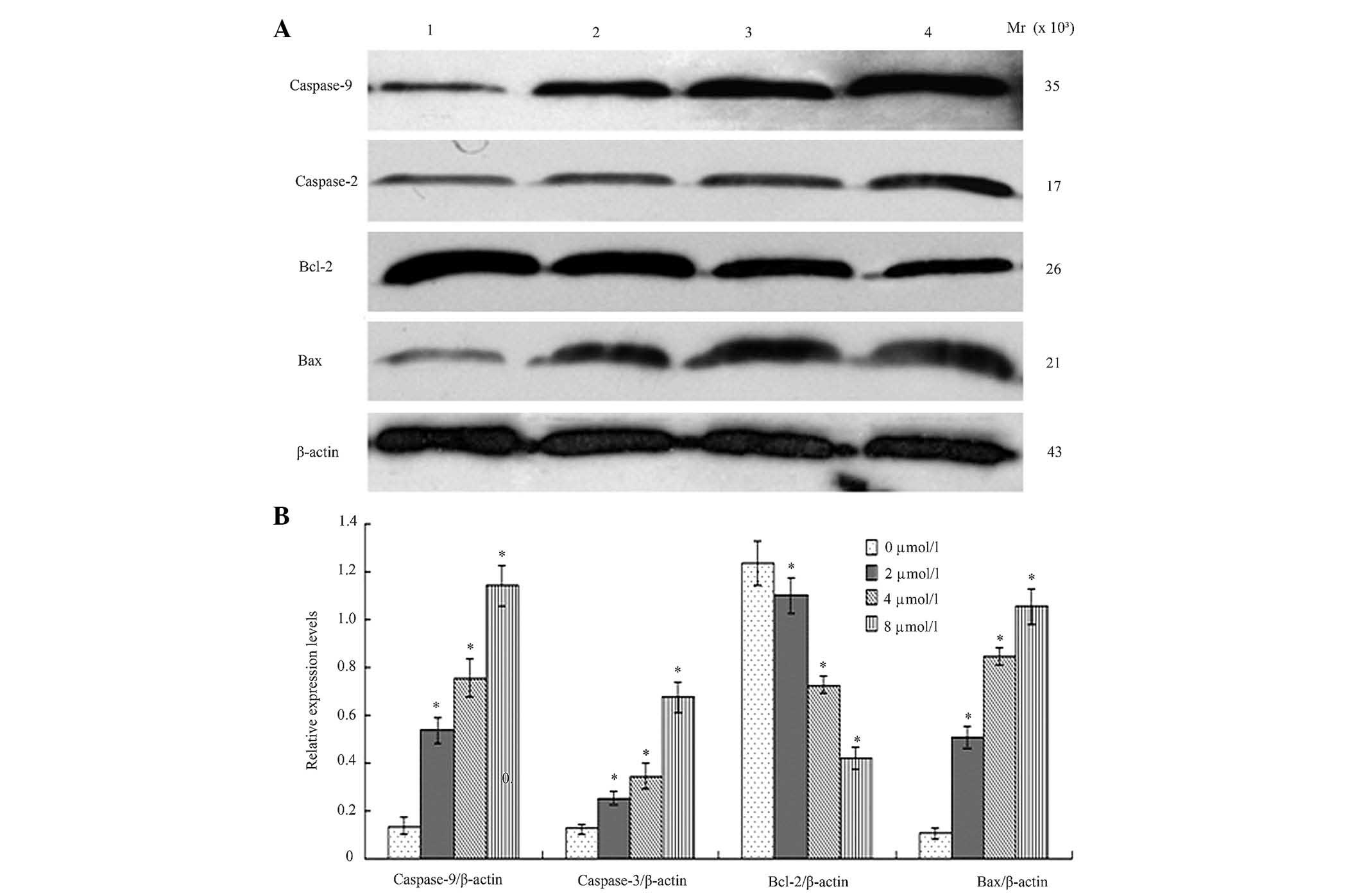
BIX-01294 altered expression levels of
apoptosis-associated proteins in U251 cells
Following treatment different concentration of
BIX-01294 (1, 2, 4 and 8 µmol/l) for 24 h, the proteins were
analyzed by western blotting and AlphaDigiDoc image analysis. Bcl-2
expression was demonstrated to be downregulated, while Bax,
caspase-9 and caspase-3 were upregulated. All the gray values of
the protein bands were compared with β-actin and statistically
compared to the 0 µmol/l (blank group), indicating significant
differences (P<0.05, Fig.
6).
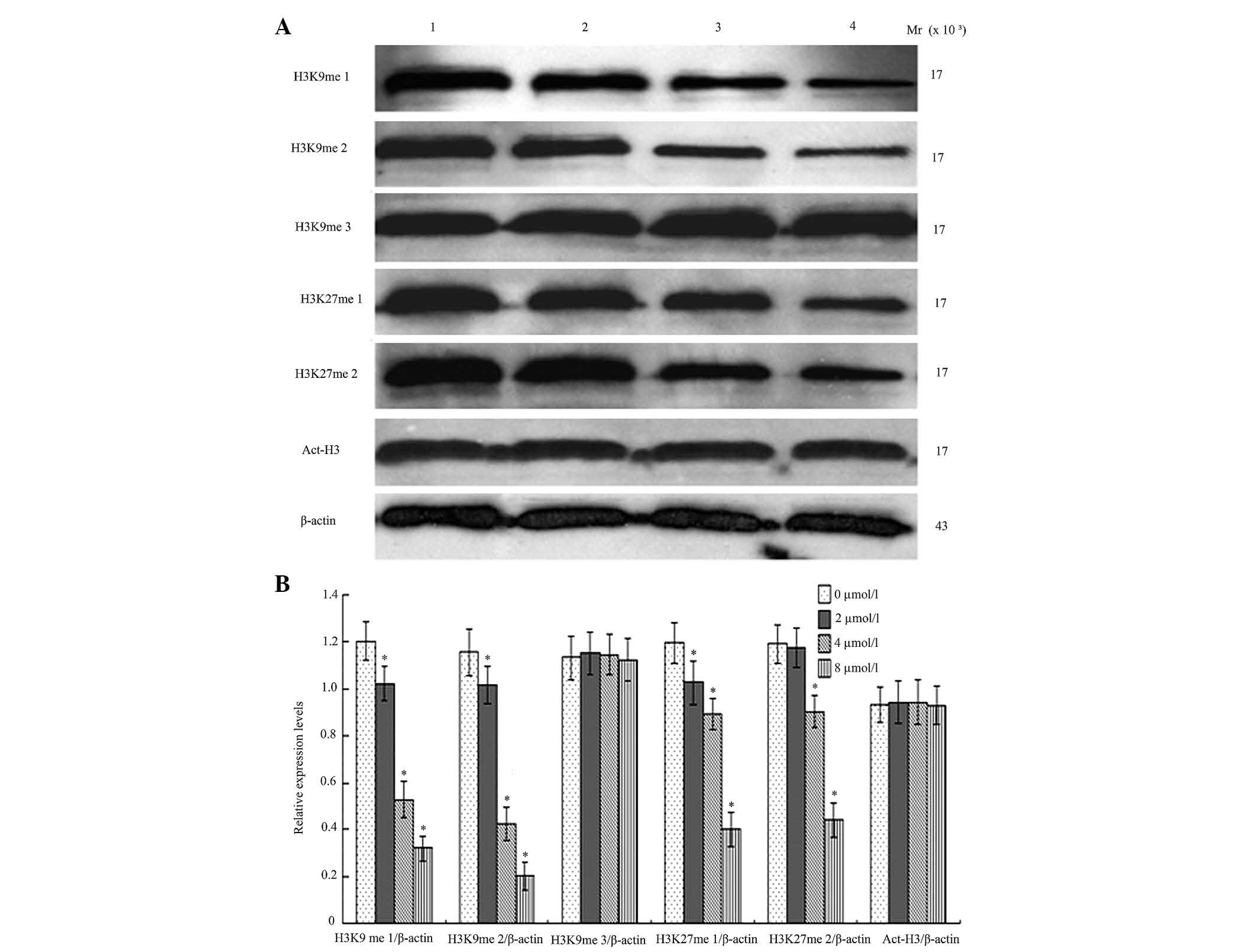
BIX-01294 affects the methylation of
H3K9 and H3K27, and the acetylation of H3
Following treatment with different concentrations of
BIX-01294 (1, 2, 4 and 8 µmol/l) for 24 h, the monomethylation and
dimethylation of H3K9 decreased, while the trimethylation was not
markedly influenced, which indicates there was activity for
monomethylation and dimethylation but reduced activity for
trimethylation. G9a was also demonstrated to exert an effect on the
first and second methylation of H3K27. Following treatment with
different concentrations of BIX-01294 (1, 2, 4 and 8 µmol/l) for 24
h, there were no marked changes to H3 acetylation (Fig. 7).
Discussion
Histone methylation is an important regulatory
mechanism in changing chromatin structure and transcription, which
is associated with the generation and development of different
types of tumor. H3K9 monomethylation, dimethylation and
trimethylation indicate transcriptional inhibition. The
trimethylation of H3K9 was associated with the closed structure of
heterochromatin, while H3K9 dimethylation was associated with
autosomal silencing (6). Stewart
et al (7) demonstrated that
H3K9 downregulated acetylation of H3 and H4, and deacetylated H3
and H4, which inhibited transcription and induced gene silencing.
However, in endotoxin-tolerant THP-1 cells, G9a bound to the
promoter region of TNFα, to promote dimethylation of H3K9
and enriched DNMTa/3b, which promoted DNA methylation and inhibited
TNFα transcription (8).
H3K9 hypermethylation in the promoter region of the suppressor gene
led to suppressor gene silencing, which is associated with the
generation of tumors. Following treatment with 5-Aza-Dc in
different types of gastric cancer cells, the dimethylation of H3K9
decreased, DNA was demethylated and P16 expression was
induced (9).
A previous study demonstrated that in gastric
carcinoma, histone methyltransferase Suv39H1 and H3K9
trimethylation were highly expressed (10). Further studies demonstrated that
Suv39H1 and H3K9 methylation were associated with tumor
differentiation, invasive depth and lymph node metastasis, which
indicated that the two may possibly promote the generation,
development, invasion and metastasis of gastric carcinoma (11,12).
In the present study, the positive rate of H3K9me2 in glioma tissue
was 82% (41/50), which was significantly different to the 38%
(19/50) in adjacent tissue (χ2=18.38, P<0.01). The
positive rate of H3K9me2 was associated with higher WHO glioma
grade. However, the positive rate of H3K9me1 in glioma tissue was
54% (27/50), and 44% (22/50) in adjacent tissue
(χ2=1.21, P>0.05). These results indicated that
H3K9me2 was associated with the generation and development of
glioma, while H3K9me1 was not important in the generation and
development of glioma. H3K9me2 may be an indicator of glioma
severity and prognosis.
Lysine methylation of histone was completed by the
catalysis of histone lysine methyltransferases, while G9a is the
predominant methyltransferase of H3K9. In euchromatin, G9a
catalyzed monomethylation and dimethylation of H3K9, while
methylated H3K27 in vitro (13). G9a gene deletion led to a
decrease in H3K9me2 in the nuclear peripheral region, while some
control of gene silencing was lost (14). Previous studies have demonstrated
that the abnormal expression of histone methyltransferase and
histone demethylase were associated with the generation and
development of tumors (15). Chen
et al (16) observed that
the mRNA and protein expression levels of histone methyltransferase
G9 gene in extrahepatic bile duct carcinoma were increased
compared with the control group, which were positively associated
with lymph node metastasis and TNM staging (16). The present study determined that
the positive rate of G9a in glioma tissues was 86% (43/50), which
was significantly different from 42% (21/50) in adjacent tissues
(P<0.01). Increased H3K9me2 expression was associated with
higher WHO grade. These results indicated that G9a was expressed at
higher levels in tumor tissue samples than in normal tissue
samples, which may be targeted in therapeutic strategies. Wu et
al (9) used depsipeptide to
downregulate G9a and SUV39H1 expression, leading to a decrease in
H3K9me2/3 in the promoter region of P16. The enrichment of
DNMT1 decreased, DNA was demethylated, P16 was expressed and the
proliferation of tumor cells was inhibited.
BIX-01294 is a selective artificial inhibitor of
G9a. Kondo et al (3)
demonstrated that following BIX-01294 treatment, the activity of
G9a and H3K9 methylation decreased, leading to P16 and RASSF1A
expression and marked inhibition of PC3 prostate cancer cell growth
(3). In the present study,
following treatment with different concentrations of BIX-01294 (1,
2, 4 and, 8 µmol/l) for 24 h, the proliferation rates of U251 were
94.12±3.41, 78.83±2.25, 53.68±2.54 and 21.04±2.07%, respectively,
which indicated there was a significant concentration-dependent
increase. As the treatment time increased, the proliferation rate
decreased. Following treatment with different concentrations of
BIX-01294 (1, 2, 4 and 8 µmol/l) for 24 h, the apoptosis rates of
U251 were 3.67±1.42, 16.42±5.18, 35.18±3.26 and 57.52±4.37%, which
demonstrated the significant increase in apoptosis in a
concentration-dependent manner.
Apoptosis is the spontaneous process of programmed
cell death, whose generation is under rigorous control in the body.
The expression and regulation of the Bcl-2 family is important in
signal transduction pathways and apoptosis. The Bcl-2 family
includes two types of protein, those that are anti-apoptotic,
including Bcl-2, B-cell lymphoma-extra large, Bcl-2-like protein 2,
myeloid cell leukemia-1 and Bcl-2-related protein A1 and
pro-apoptotic proteins, including Bax, Bcl-2 homologous
antagonist/killer, BH3 interacting-domain death agonist and
Bcl-2-associated death promoter (17). Bcl-2 and Bax are the
representative anti-apoptotic and pro-apoptotic gene in Bcl-2
family, respectively. Bcl-2 inhibits apoptosis of tumor cells
induced by different factors. Bax forms an apoptosis complex and
activates caspase-9, which subsequently activates the downstream
effector proteins, including caspase-3, caspase-6 and caspase-7
(18). It was observed that
following BIX-01294 treatment, Bcl-2 protein expression was
downregulated, while Bax expression was upregulated.
Apoptosis-associated proteins caspase-9 and caspase-3 were
upregulated, which resulted in cell apoptosis.
G9a is able to monomethylate and dimethylate H3K9,
while trimethylation is reduced when incubated in vitro for
an extended amount of time. G9a and GLP in mouse
euchromatin is dependent on the heterodimer formed by the SET
domain, which may catalyze the production of H3K9me2 and H3K9me1,
enrich heterochromatin protein 1 in euchromatin and mediate
transcriptional repression (19).
Knock-out of G9a in mice reduced H3K9me2 which led to the
silencing of 167 genes that have enrichment of H3K9me2 on the
promoter (14). The present study
observed that following BIX-01294-induced inhibition of G9a
activity, methylation of H3K9me1 and H3K9me2 was downregulated,
however, the expression of H3K9me3 and Act-H3 is less altered,
which is consistent with monomethylation and dimethylation and
lacking trimethylation.
H3K27me3 was considered to be associated with
transcriptional inhibition. Following trimethylation of H3K27, the
protein regulator of cytokinesis 1 complex combined with the
specific gene locus, then inhibited the enrichment of transcription
activators, silenced tumor suppressor genes and led to carcinoma.
Enhancer of zeste homolog 2, the histone methyltransferase
catalyzing H3K27 trimethylation, is expressed in numerous tumors,
including glioma, which was associated with development and poor
prognosis (20,21). However, less research has been
conducted on H3K27me1 and H3K27me2. Previous studies demonstrated
that in the embryonic stem cell line with deletion of G9a,
the methylation of the 27th lysine in H3 decreased and
polycomb repressive complex 2 has an important role in this process
(22,23). It was observed that following
BIX-01294 treatment to inhibit G9a activity, the methylation of
H3K27me1 and H3K27me2 decreased significantly, which suggested that
G9a had the ability to methylate H3K27, however, this remains to be
investigated in a clinical setting.
In conclusion, the present study observed that
BIX-01294 inhibits the proliferation of glioma cells and induces
apoptosis. Further study demonstrated that the alteration of
methylation of H3K9 and H3K27 resulted in changes in chromosomal
conformation, which may be an underlying mechanism of inhibition of
tumor cells proliferation. BIX-01294 may be a potential novel
therapeutic agent in the treatment of glioma.
Acknowledgements
This work was supported by the Natural Science
Foundation of Fujian Province (grant no. 2016J01484) and the
Introductive Major Project of Science Research Foundation of Fujian
Province (grant no. 201212004).
References
|
1
|
Parsons DW, Jones S, Zhang X, Lin JC,
Leary RJ, Angenendt P, Mankoo P, Carter H, Siu IM, Gallia GL, et
al: An integrated genomic analysis of human glioblastoma
multiforme. Science. 321:1807–1812. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Huang J, Dorsey J, Chuikov S, Pérez-Burgos
L, Zhang X, Jenuwein T, Reinberg D and Berger SL: G9a and Glp
methylate lysine 373 in the tumor suppressor p53. J Biol Chem.
285:9636–9641. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Kondo Y, Shen L, Ahmed S, Boumber Y,
Sekido Y, Haddad BR and Issa JP: Downregulation of histone H3
lysine 9 methyltransferase G9a induces centrosome disruption and
chromosome instability in cancer cells. PLoS One. 3:e20372008.
View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Fuller GN and Scheithauer BW: The 2007
Revised World Health Organization (WHO) Classification of Tumours
of the Central Nervous System: newly codified entities. Brain
Pathol. 17:304–307. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Lin X, Huang Y, Zou Y, Chen X and Ma X:
Depletion of G9a gene induces cell apoptosis in human gastric
carcinoma. Oncology Reports. 35:3041–3049. 2016.PubMed/NCBI
|
|
6
|
McGarvey KM, Fahrner JA, Greene E, Martens
J, Jenuwein T and Baylin SB: Silenced toumor stressor genes
reactivated by DNA demethylation do not return to a fully
euchromatic chromati state. Cancer Res. 66:3541–3549. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Stewart MD, Li J and Wong J: Elationship
between histone H3 lysine 9 methylation, transcription repression,
and heterochromatin protein 1 recruitment. Mol Cell Biol.
25:2525–2538. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
El Gazzar M, Yoza BK, Chen X, Hu J,
Hawkins GA and McCall CE: G9a and HP1 couple histone and DNA
methylation to TNFalpha transcription silencing during endotoxin
tolerance. J Biol Chem. 283:32198–32208. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Wu LP, Wang X, Li L, Zhao Y, Lu S, Yu Y,
Zhou W, Liu X, Yang J, Zheng Z, et al: Histone deacetylase
inhibitor depsipeptide activates silenced genes through decreasing
both CpG and H3K9 methylation on the promoter. Mol Cell Biol.
28:3219–3235. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Cai L, Ma X, Huang Y, Zou Y and Chen X:
Aberrant histone methylation and the effect of Suv39H1 siRNA on
gastric carcinoma. Oncol Rep. 31:2593–2600. 2014.PubMed/NCBI
|
|
11
|
Cattaneo F and Nucifora G: EVI1 recruits
the histone methyltransferase SUV39H1 for transcription repression.
J Cell Biochem. 105:344–352. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Goyama S, Nitta E, Yoshino T, Kako S,
Watanabe-Okochi N, Shimabe M, Imai Y, Takahashi K and Kurokawa M:
EVI-1 interacts with histone methyl transferases SUV39H1 and G9a
for transcriptional repression and bone marrow immortalization.
Leukemia. 24:81–88. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Wu H, Chen X, Xiong J, Li Y, Li H, Ding X,
Liu S, Chen S, Gao S and Zhu B: Histone methyltransferase G9a
contributes to H3K27 methylation in vivo. Cell Res. 21:365–367.
2011. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Yokochi T, Poduch K, Ryba T, Lu J,
Hiratani I, Tachibana M, Shinkai Y and Gilbert DM: G9a selectively
represses a class of late-replicating genes at the nuclear
periphery. Proc Natl Acad Sci USA. 106:19363–19368. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Zou Y, Ma X, Huang Y, Hong L and Chiao JW:
Effect of phenylhexyl isothiocyanate on aberrant histone H3
methylation in primary human acute leukaemia. J Hematol Oncol.
5:362012. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Chen Y, Luo J, Chen B, Wang J and Zou S:
Expression of histone methyltransferase G9a and clinical
significance in extrahepatic cholangiocarcinoma. Chin-Ger J Clin
Oncol. 7:10–13. 2008. View Article : Google Scholar
|
|
17
|
Sitailo LA, Jerome-Morais A and Denning
MF: Mcl-1 functions as major epidermal survival protein required
for proper keratinocyte differentiation. J Invest Dermatol.
129:1351–1360. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Theofilas P, Bedner P, Hüttmann K, Theis
M, Steinhäuser C and Frank S: The proapoptotic BCL-2 homology
domain 3-only protein Bim is not critical for acute excitotoxic
cell death. J Neuropathol Exp Neurol. 68:102–110. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Tachibana M, Matsumura Y, Fukuda M, Kimura
H and Shinkai Y: G9a/GLP complexes independently mediate H3K9 and
DNA methylation to silence transcription. EMBO J. 27:2681–2690.
2008. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Zhang YB, Niu HT, Chang JW, Dong GL and Ma
XB: EZH2 silencing by RNA interference inhibits proliferation in
bladder cancer cell lines. Eur J Cancer Care (Engl). 20:106–112.
2011. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Bryant RJ, Winder SJ, Cross SS, Hamdy FC
and Cunliffe VT: The Polycomb group protein EZH2 regulates actin
polymerization in human prostate cancer cells. Prostate.
68:255–263. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Ma DK, Chiang CH, Ponnusamy K, Ming GL and
Song H: G9a and Jhdm2a regulate embryonic stem cell fusion-induced
reprogramming of adult neural stem cells. Stem Cells. 26:2131–2141.
2008. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Ikegami K, Iwatani M, Suzuki M, Tachibana
M, Shinkai Y, Tanaka S, Greally JM, Yagi S, Hattori N and Shiota K:
Genome-wide and locus-specific DNA hypomethylation in G9a deficient
mouse embryonic stem cells. Genes Cells. 12:1–11. 2007. View Article : Google Scholar : PubMed/NCBI
|