Introduction
Cerebral ischemia/reperfusion (I/R) injury occurs as
a complication of stroke following thrombolysis and a recurrence of
ductal patency. The mechanisms of I/R injury are not clearly
understood, and identification of effective and safe treatments for
ischemic stroke is required.
The Golgi apparatus is an organelle essential for
protein synthesis and maturation. In response to conditions of
stress, including physiological imbalances or disruption of cell
morphology, the transcription of Golgi-associated genes can be
upregulated to restore homeostasis or induce apoptosis; this
mechanism is termed the Golgi stress response (1,2).
Research suggests the extracellular signal-regulated kinase (ERK)
signaling pathway is involved in the response to oxidative stress.
A previous study demonstrated that the ERK signaling pathway
regulates phosphorylation of the Golgi reassembly and stacking
protein 65 (GRASP65), resulting in Golgi cisternal unstacking
(3).
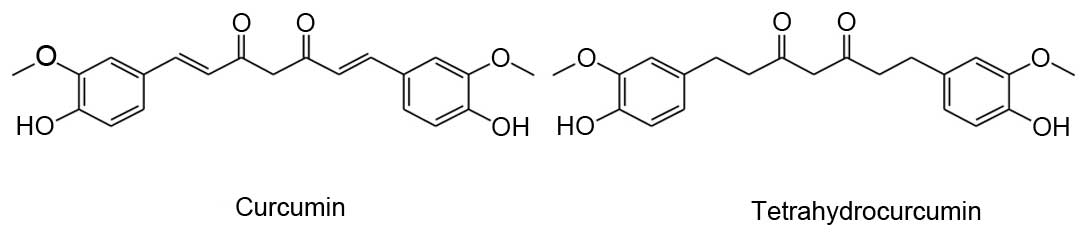
Curcumin (Cur), a yellow dye in the crude drug
‘Turmeric’ (Curcumae rhizoma) from the rhizome of Curcuma
longa L., is reported to have anti-inflammatory, anti-oxidative
and anti-tumor effects (4–6). Tetrahydrocurcumin (THC) is an active
metabolite of Cur, and has been identified in human and rat
intestinal mucosa, and in hepatic cytosol (7). THC and Cur have identical β-diketone
structures and phenolic groups, but differ in that THC lacks the
double bonds of Cur (Fig. 1).
Recent research suggests that THC exerts greater anti-oxidant
activity than Cur in certain in vitro and in vivo
systems (8–10).
The present study examined the protective effects of
THC against cerebral I/R injury in mice, and reviewed the
mechanisms of Golgi stress-induced cerebral I/R injury via the ERK
signaling pathway.
Materials and methods
Animals
Male specific pathogen-free ICR mice (n=100; 2
months old; 23–27 g) were provided by the Experimental Animal
Center of Wenzhou Medical University (Wenzhou, China). Mice had
ad libitum access to food and water and were housed in
temperature- and humidity-controlled conditions (temperature;
22±1°C; humidity 56±5%) with a 12-h light/dark cycle. All mice were
treated humanely, and the study was approved by the Experimental
Animal Ethics Committee of Wenzhou Medical University.
Chemicals
THC was purchased from Dalian Meilun Biotech Co.,
Ltd. (Dalian, China). Sodium chloride injections (0.9%) were
purchased from Hangzhou Minsheng Pharmaceutical Group Co., Ltd.
(Hangzhou, China). Dimethyl sulfoxide (DMSO), chloral hydrate (10%;
Sinopharm Chemical Reagent Co., Ltd., Shanghai, China) and
2,3,5-triphenyl-2H-tetrazolium chloride (TTC) were purchased from
Sigma-Aldrich (Merck Millipore, Darmstadt, Germany).
Paraformaldehyde (4%) was provided by the Experimental Neural
Organisms Institution of Wenzhou Medical University (Wenzhou,
China). Total superoxide dismutase (T-SOD) kits, lipid peroxidation
[malondialdehyde (MDA)] assay kits, total protein kits and
bicinchoninic acid (BCA) protein assay kits were purchased from
Nanjing Jiancheng Bioengineering Institute (Nanjing, China).
Anti-GRASP65 (sc-398363) and anti-phospho (p)-GRASP65 (sc-389542)
antibodies were purchased from Santa Cruz Biotechnology, Inc.
(Dallas, TX, USA), and anti-ERK (4696S) and anti-pERK (9106L)
antibodies were purchased from Cell Signaling Technology, Inc.
(Danvers, MA, USA).
Animal surgery and THC
administration
The mice were randomly divided into 5 groups (n=20
per group) as follows: Sham-operated controls (group A), I/R group
(group B), low-dose THC (5 mg/kg; group C1), moderate-dose THC (10
mg/kg; group C2) and high-dose THC (25 mg/kg; group C3).
Cerebral ischemia was induced by Pulsinelli's
four-vessel occlusion method (11). All surgeries were performed by the
same person. Under anesthesia with 10% chloral hydrate (3 mg/kg), a
1.5 cm incision was made in the middle of the neck and the jugular
muscles were separated. The bilateral common vertebral arteries
were identified and occluded by electrocoagulation. The incision
was sutured and the animals were allowed to recover. The following
day, the procedure was repeated and the common carotid arteries
were occluded for 5 min using vitreous needles and small bulldog
clamps. Sham-operated animals underwent the same surgical procedure
without occlusion. Treatment injections were administered after 5
min of reperfusion. Groups A and B received intraperitoneal (i.p.)
injections of equal volumes of normal saline. A 2% THC solution was
prepared in DMSO; groups C1, C2 and C3 received i.p. injections of
THC at a low (5 mg/kg), moderate (10 mg/kg), or high (25 mg/kg)
dose, respectively.
Neuroethological assessment
After 24 h of reperfusion, mice were evaluated for
exponents of stroke (Table I) and
neurological symptoms (Table II)
(12).
 | Table I.Exponents of stroke. |
Table I.
Exponents of stroke.
| Symptom | Score |
|---|
| Messy fur,
tremor | 1 |
| Decreasing exercise
or bovine movement | 1 |
| Bovine feeling of
ears | 3 |
| Turned up head | 3 |
| Eyes opened | 3 |
| Posterior limb shaped
like Chinese character eight (八) | 3 |
| Upper eyelid
drooping | 1 |
| Circling | 3 |
| Eclampsia or
paroxysmal movement | 3 |
| Exceedingly weak | 6 |
 | Table II.Neurological symptom assessment. |
Table II.
Neurological symptom assessment.
| Symptom | Score |
|---|
| Idiopathic
excavation | 0 |
| Walking while being
stimulated | 1 |
| Cannot move | 2 |
| Normal gait | 0 |
| Ataxia | 1 |
| Crawling | 2 |
| No gait | 3 |
| Able to feed | 0 |
| Cannot feed | 1 |
| Able to drink | 0 |
| Cannot drink | 1 |
| Removable to
pains | 0 |
| Only head or trunk
can move | 1 |
| Limbs can retrace or
no reaction | 3 |
Specimen collection and preparation of
pathological sections
After 24 h of reperfusion, mice were anesthetized
with 10% chloral hydrate prior to decapitation, then were
transcardially perfused with normal saline. The brain was dissected
via craniotomy and fixed in a 4% paraformaldehyde solution for 24 h
prior to conventional paraffin embedding. The specimens were
sectioned coronally (behind the optic chiasm) at a thickness of 5
µm. Sections were stained with hematoxylin and eosin (HE), as
previously described (13).
TTC staining
Mice were decapitated after 24 h of reperfusion. The
brain was removed and cooled in normal saline at 4°C for 10 min.
The specimens were sectioned coronally into four 2-mm-thick slices
using a brain matrix. Slices were incubated with a 2% aqueous
solution of TTC in the dark for 30 min at 37°C in a water bath, and
then images using a digital camera (Canon 600D; Canon, Inc., Tokyo,
Japan). Unstained areas were defined as infarcted and measured
using image analysis software (Image-Pro Plus, version 6.0; Media
Cybernetics, Inc., Rockville, MD, USA). Total infarct volume in the
brain was calculated as the sum of the infarct volumes of the four
brain slices (14).
Assessment of cerebral SOD and
MDA
Cerebral SOD and MDA levels were measured in brain
tissue homogenates prepared with a 0.9% saline solution and
centrifuged at 1,006 × g for 10 min at 4°C. Supernatants
were collected and analyzed using the SOD and MDA kits.
Concentrations of SOD and MDA were calculated based on the optical
density readings obtained at 550 and 552 nm, respectively, using
the Multiskan Spectrum microplate reader (Thermo Fisher Scientific,
Inc., Waltham, MA, USA).
Western blot analysis of ERK, pERK,
GRASP65 and pGRASP65
ERK, pERK, GRASP65 and pGRASP65 levels in brain
tissue homogenates were determined by western blotting. Protein
extraction kits from were purchased from Jiancheng Bioengineering
Institute (Nanjing, China). Total protein levels in the homogenate
samples were determined using the BCA protein assay kit. Equal
amounts of protein (20 µg sample/lane) from each sample were then
subjected to 10% SDS-PAGE. The gel was transferred to a a
polyvinylidene difluoride membrane, and proteins were detected with
antibodies against ERK (1:1,000), pERK (1:1,000), GRASP65 (1:200),
and pGRASP65 (1:500). β-actin (1:4,000; Santa Cruz Biotechnology,
Inc.) was used as a loading control. Subsequently, the membranes
were incubated with horseradish peroxidase-conjugated anti-rabbit
(KS001) or anti-goat (KS003) secondary antibodies (dilution,
1:3,000) for 2 h at 24°C, and were visualized using Western
Blotting Chemiluminescence Reagent, followed by exposure to X-ray
films. Blots were quantified using BandScan software (version 5.0;
Glyko, Inc., Novato, CA, USA).
Statistical analysis
Statistical comparisons were made using analysis of
variance (ANOVA) followed by post-hoc Bonferroni tests. Data are
presented as the mean ± standard devation. Neuroethological and
stroke assessment data were analyzed using the Friedman test.
Friedman two-way ANOVA was used for multiple comparisons.
Statistical analyses were performed using SPSS software (version
19.0; IBM SPSS, Armonk, NY, USA). P<0.05 were considered to
indicate a statistically significant difference.
Results
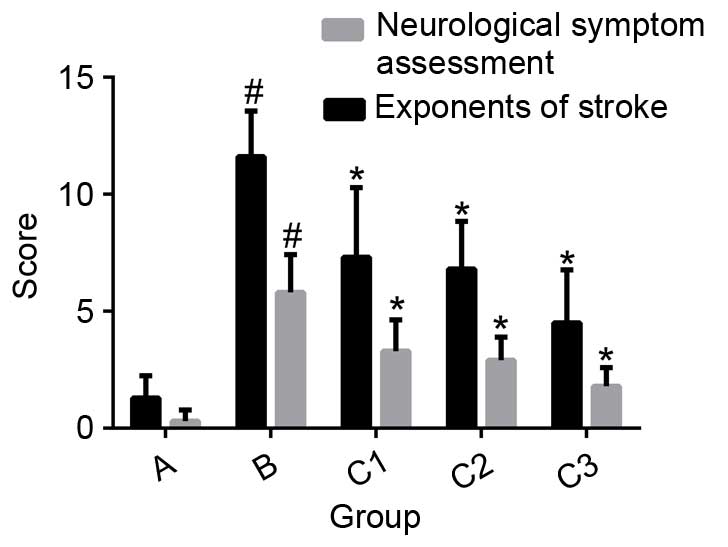
Neuroethological assessment following
cerebral I/R
After 24 h of reperfusion, various neurological
symptoms were observed: Decreased exercise, lack of feeding,
bovine-like reaction, ataxia (in some mice), circling with the tail
lifted, posterior limbs positioned as the Chinese character for
eight (八), upper eyelid drooping or inability to open the eyes, and
messy fur. Compared with group B (normal saline-injected mice), the
exponents of stroke and neurological assessment scored were
significantly decreased in the THC-treated groups (P<0.01;
Fig. 2).
Effect of THC on ischemic neuronal
necrosis
In sham-operated mice (group A), cells were closely
arranged, regularly shaped and uniformly stained by HE (Fig. 3). By contrast, cells from mice with
I/R injury (group B) were loosely arranged with various abnormal
pathological changes, including karyopyknosis, interstitial edema,
neuronal degeneration and vacuolization. Cells from mice
administered the high dose of THC (25 mg/kg; group C3) were mostly
normal, though karyopyknosis was evident in some cells (Fig. 3). Cells from mice administered the
low dose of THC (5 mg/kg; group C1) exhibited more severe
apoptosis, with higher levels of karyopyknosis, irregular
arrangement, interstitial edema, neuronal degeneration and
vacuolization compared with group C3. In mice administered the
moderate dose of THC (10 mg/kg; group C2), the cellular effects
were between those observed in groups C1 and C3 (Fig. 3).
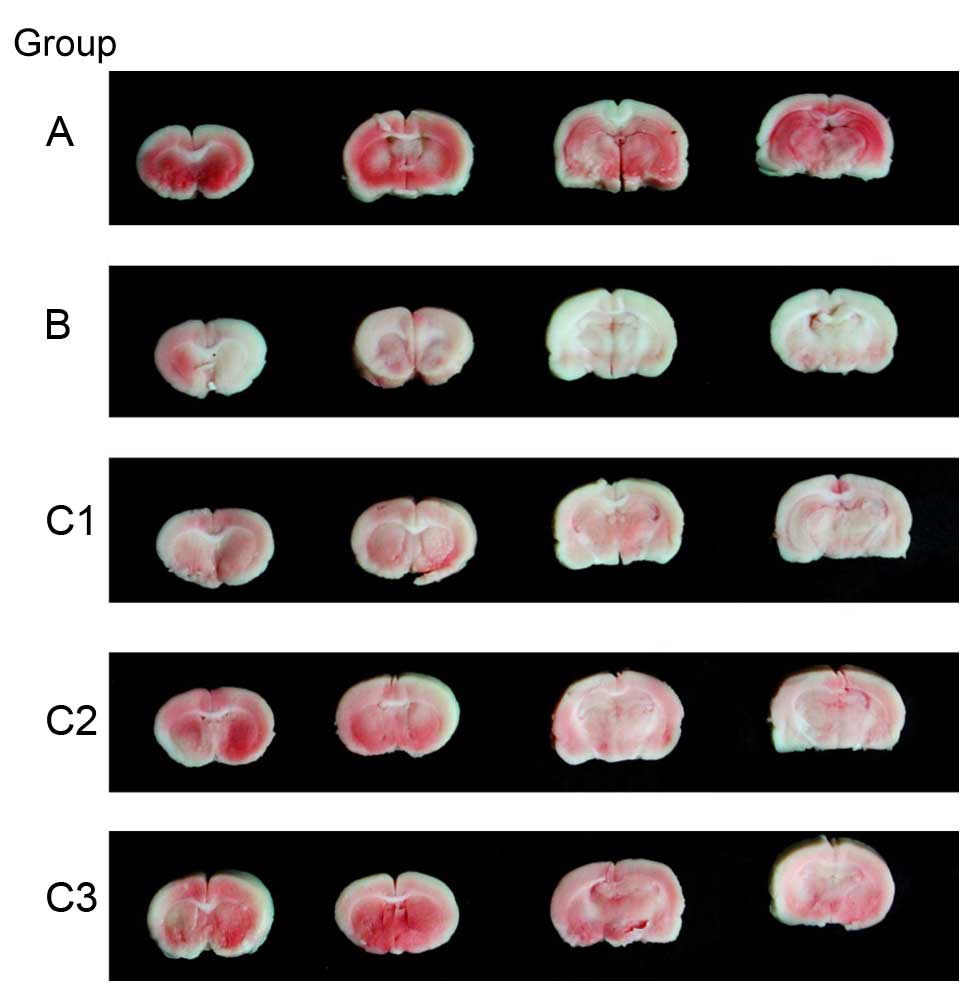
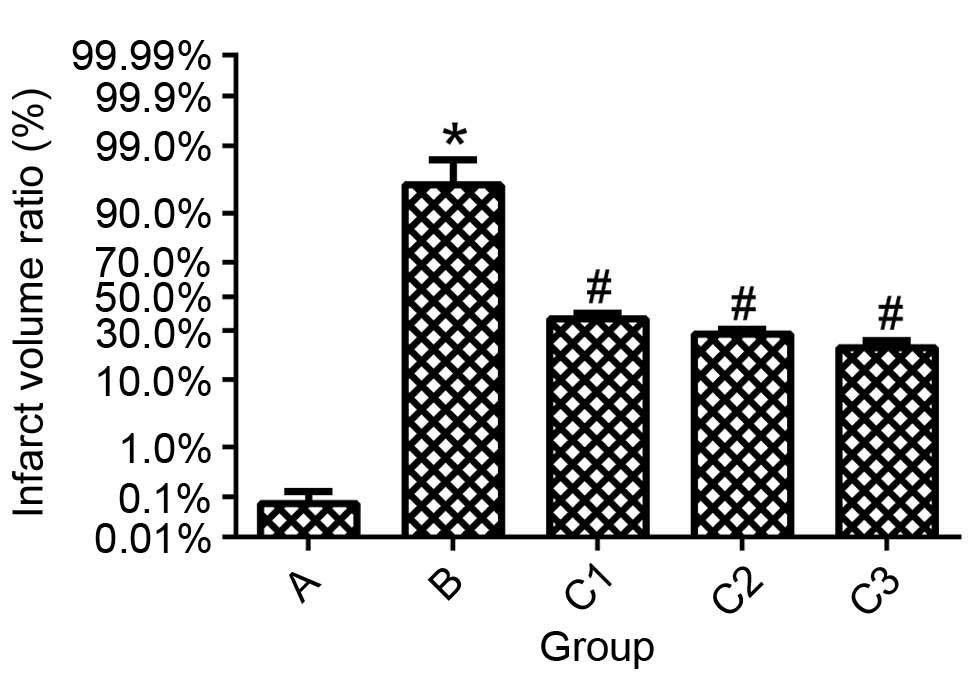
Effect of THC on infarct size
following I/R injury
After 24 h of reperfusion, extensive infarction was
evident in group B mice. The infarct size was significantly reduced
in all THC treatment groups compared with group B (P<0.01;
Figs. 4 and 5), and the effect was dose-dependent. No
infarction was observed in the sham-operated group.
Effect of THC on SOD and MDA
Compared with group B, mice administered THC
exhibited significantly increased levels of SOD (P<0.01) and
lower levels of MDA in brain tissue homogenates (P<0.01;
Fig. 6). The effects of THC on SOD
and MDA were dose-dependent.
Effect of THC on the expression of
ERK, pERK, GRASP65 and pGRASP65
Western blot analysis demonstrated the protein
expression of ERK in ischemic brain tissue did not differ the
between groups (P>0.05; Fig.
7). However, the level of pERK and the pERK/ERK ratio were
significantly increased in group B compared with group A
(P<0.01; Fig. 7). The pERK/ERK
ratio as significantly reduced in groups C1 (P<0.05), C2
(P<0.01) and C3 (P<0.01) compared with group B (Fig. 7). This suggests that the ERK
signaling pathway was suppressed by THC.
 | Figure 7.ERK, pERK, GRASP65 and pGRASP65
expression in mice from each group following reperfusion for 24 h.
**P<0.01 vs. Group A, *#P>0.05 vs. Group A,
#P<0.05 vs. Group B, *P<0.01 vs. Group B,
##P>0.05 vs. Group B. Data are presented as the mean
± standard deviation; n=10. Group A, sham; Group B, control; Group
C1, low-dose tetrahydrocurcumin; Group C2, moderate-dose
tetrahydrocurcumin; Group C3, high-dose tetrahydrocurcumin; ERK,
extracellular-signal regulated kinase; p, phosphorylated; GRASP65,
Golgi reassembly and stacking protein 65. |
Expression of total GRASP65 did not differ
significantly between groups (P>0.05; Fig. 7). However, the level of pGRASP65
was significantly increased in group B compared with group A
(P<0.01; Fig. 7). In groups C1,
C2 and C3, pGRASP65 was significantly reduced compared with group B
(P<0.01; Fig. 7).
The results of the present study suggest that the
upregulated phosphorylation of GRASP65 following I/R injury may be
due to, at least partially, the increased activation of the ERK
signaling pathway induced by THC. Thus, THC protects against
cerebral I/R injury via suppression of GRASP65 phosphorylation, and
this effect may be mediated by the ERK signaling pathway.
Discussion
The ERK signaling pathway has an important role in
cerebral I/R injury. ERK is involved in growth and differentiation,
mediated by growth factor receptors, and is also activated by
oxidative stress, cerebral ischemia and the release of
neurotransmitters under certain pathological conditions (15,16).
In the current study, the level of pERK and the pERK/ERK ratio were
significantly increased in ischemic mice compared with
sham-operated controls, indicating that the ERK pathway was
activated in response to cerebral I/R injury. In mice administered
THC, pERK was significantly reduced compared with the control,
suggesting that THC inhibited I/R injury-induced activation of the
ERK pathway. This effect was dose-dependent, which provides further
evidence of the neuroprotective properties of THC.
GRASPs are membrane proteins involved in Golgi
stacking. They regulate Golgi assembly, and cell migration,
division and apoptosis. Specifically, GRASP65 mediates Golgi
morphological changes during pathophysiological conditions, and is
involved in cell division and apoptosis (17–21).
In the present study, expression of GRASP65 and
pGRASP65 were elevated following cerebral I/R injury compared with
sham-operated animals (P<0.01). Previous research has
demonstrated that GRASP65 is phosphorylated by ERK, cyclin
dependent kinase 1 and polo like kinase 1 during cell division,
which leads to depolymerization and division of the Golgi (17,18,20–22).
The ERK signaling pathway may be involved in
neuronal degeneration following I/R injury. In mice treated with
THC, a dose-dependent decrease in the phosphorylation of GRASP65
was observed, and this effect may be mediated by ERK. The Golgi is
essential for the endoplasmic reticulum and mitochondria during
conditions of oxidative stress as a downstream target organelle
associated with phosphorylation of GRASP65. The Golgi stress
response suppresses the synthesis of required proteins, and thereby
impacts the extent of I/R injury.
Reactive oxygen species (ROS) are produced as a
result of incomplete reduction of oxygen within the electron
transport chain. Examples of ROS include superoxide anion, singlet
oxygen, hydrogen peroxide and hydroxyl radicals. These ROS
typically function as signaling molecules, however during stress,
they can lead to cell damage and tissue necrosis. Anti-oxidant
enzymes, including glutathione reductase, catalase and SOD, are
effective at neutralizing ROS. During I/R injury, SOD is involved
in maintaining homeostasis and minimizing the damaging effects of
ROS (23–25). MDA is an end product of lipid
peroxidation in cells, and increased levels of ROS can trigger
overproduction of MDA. SOD and MDA are often used as markers of
oxidative stress and anti-oxidant status, respectively. Cur is
reported to have anti-oxidative properties and a neuroprotective
effect against cerebral I/R injury (26). For example, Wang et al
demonstrated that Cur attenuates oxidative stress injury induced by
hypoxic-ischemic brain damage in rats. In the present study, mice
treated with THC exhibited increased levels of SOD following I/R
injury compared with mice given saline, and this effect was
dose-dependent. Levels of MDA were increased following I/R injury,
and THC attenuated this effect in a dose-dependent manner. The
evaluation of neurological behavior revealed a significant
dose-dependent effect of THC.
In summary, THC had a dose-dependent protective
effect against cerebral I/R injury, mediated by suppression of the
ERK signaling pathway and a subsequent reduction of GRASP65
phosphorylation. The current study investigated the cerebral
ischemia-reperfusion injury mechanism, and suggested that THC may
be a potential therapeutic agent for the prevention of ischemic
brain injury.
Acknowledgements
This project was supported by funding from the
National Students' Innovation and Entrepreneurship Training Program
(grant no. 201410343003).
Glossary
Abbreviations
Abbreviations:
|
THC
|
tetrahydrocurcumin
|
|
ERK
|
extracellular signal-regulated
kinase
|
|
GRASP
|
golgi reassembly and stacking
proteins
|
|
Cur
|
curcumin
|
|
SOD
|
superoxide dismutase
|
|
MDA
|
malondialdehyde
|
|
ROS
|
reactive oxygen species
|
|
TTC
|
tetrzolium chloride
|
References
|
1
|
Li T, You H, Zhang J, Mo X, He W, Chen Y,
Tang X, Jiang Z, Tu R, Zeng L, et al: Study of GOLPH3: A potential
stress-inducible protein from Golgi apparatus. Mol Neurobiol.
49:1449–1459. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Li T, You H, Mo X, He W, Tang X, Jiang Z,
Chen S, Chen Y, Zhang J and Hu Z: GOLPH3 mediated golgi stress
response in modulating N2A cell death upon oxygen-glucose
deprivation and reoxygenation injury. Mol Neurobiol. 53:1377–1385.
2016. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Yao Z and Seger R: The ERK signaling
cascade - views from different subcellular compartments.
Biofactors. 35:407–416. 2009. View
Article : Google Scholar : PubMed/NCBI
|
|
4
|
Chong L, Zhang W, Nie Y, Yu G, Liu L, Lin
L, Wen S, Zhu L and Li C: Protective effect of curcumin on acute
airway inflammation of allergic asthma in mice through Notch1-GATA3
signaling pathway. Inflammation. 37:1476–1485. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
García-Niño WR, Zatarain-Barrón ZL,
Hernández-Pando R, Vega-García CC, Tapia E and Pedraza-Chaverri J:
Oxidative stress markers and histological analysis in diverse
organs from rats treated with a hepatotoxic dose of Cr (VI): Effect
of curcumin. Biol Trace Elem Res. 167:130–145. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Ji JL, Huang XF and Zhu HL: Curcumin and
its formulations: Potential anti-cancer agents. Anticancer Agents
Med Chem. 12:210–218. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Naito M, Wu X, Nomura H, Kodama M, Kato Y,
Kato Y and Osawa T: The protective effects of tetrahydrocurcumin on
oxidative stress in cholesterol-fed rabbits. J Atheroscler Thromb.
9:243–250. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Song KI, Park JY, Lee S, Lee D, Jang HJ,
Kim SN, Ko H, Kim HY, Lee JW, Hwang GS, et al: Protective effect of
tetrahydrocurcumin against cisplatin-induced renal damage: In vitro
and in vivo studies. Planta Med. 81:286–291. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Muthumani M and Miltonprabu S:
Ameliorative efficacy of tetrahydrocurcumin against arsenic induced
oxidative damage, dyslipidemia and hepatic mitochondrial toxicity
in rats. Chem Biol Interact. 235:95–105. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Sangartit W, Kukongviriyapan U, Donpunha
W, Pakdeechote P, Kukongviriyapan V, Surawattanawan P and Greenwald
SE: Tetrahydrocurcumin protects against cadmium-induced
hypertension, raised arterial stiffness and vascular remodeling in
mice. PloS One. 9:e1149082014. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Pulsinelli WA and Brierley JB: A new model
of bilateral hemispheric ischemia in the unanesthetized rat.
Stroke. 10:267–272. 1979. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Wei W, Wu XM and Li YJ: Experimental
Methodology of Pharmacology. 4th. People's Medical Publishing
House; Beijing: pp. 1000–1003. 2010
|
|
13
|
Chang YH, Fan XN and Shi XM: HE staining
and light microscope observation method used in researches of brain
tissue. Liaoning J Trad Chin Med. 5:777–779. 2010.
|
|
14
|
Bederson JB, Pitts LH, Germano SM,
Nishimura MC, Davis RL and Bartkowski HM: Evaluation of
2,3,5-triphenyltetrazolium chloride as a stain for detection and
quantification of experimental cerebral infarction in rats. Stroke.
17:1304–1308. 1986. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Zhan L, Li D, Liang D, Wu B, Zhu P, Wang
Y, Sun W and Xu E: Activation of Akt/FoxO and inactivation of
MEK/ERK pathways contribute to induction of neuroprotection against
transient global cerebral ischemia by delayed hypoxic
postconditioning in adult rats. Neuropharmacology. 63:873–882.
2012. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Wang S, Wei H, Cai M, Lu Y, Hou W, Yang Q,
Dong H and Xiong L: Genistein attenuates brain damage induced by
transient cerebral ischemia through up-regulation of ERK activity
in ovariectomized mice. Int J Biol Sci. 10:457–465. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Veenendaal T, Jarvela T, Grieve AG, van Es
JH, Linstedt AD and Rabouille C: GRASP65 controls the cis Golgi
integrity in vivo. Biol Open. 3:431–443. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Ji G, Ji H, Mo X, Li T, Yu Y and Hu Z: The
role of GRASPs in morphological alterations of Golgi apparatus:
Mechanisms and effects. Rev Neurosci. 24:485–497. 2013.PubMed/NCBI
|
|
19
|
Lane JD, Lucocq J, Pryde J, Barr FA,
Woodman PG, Allan VJ and Lowe M: Caspase-mediated cleavage of the
stacking protein GRASP65 is required for Golgi fragmentation during
apoptosis. J Cell Biol. 156:495–509. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Wang Y, Seemann J, Pypaert M, Shorter J
and Warren G: A direct role for GRASP65 as a mitotically regulated
Golgi stacking factor. EMBO J. 22:3279–3290. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Wang Y, Satoh A and Warren G: Mapping the
functional domains of the Golgi stacking factor GRASP65. J Biol
Chem. 280:4921–4928. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Yoshimura S, Yoshioka K, Barr FA, Lowe M,
Nakayama K, Ohkuma S and Nakamura N: Convergence of cell cycle
regulation and growth factor signals on GRASP65. J Biol Chem.
280:23048–23056. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Niizuma K, Yoshioka H, Chen H, Kim GS,
Jung JE, Katsu M, Okami N and Chan PH: Mitochondrial and apoptotic
neuronal death signaling pathways in cerebral ischemia. Biochim
Biophys Acta. 1802:92–99. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Liu P, Zhao H, Wang R, Wang P, Tao Z, Gao
L, Yan F, Liu X, Yu S, Ji X and Luo Y: MicroRNA-424 protects
against focal cerebral ischemia and reperfusion injury in mice by
suppressing oxidative stress. Stroke. 46:513–519. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Xin X, Li L, Miao L, Zhang QY and Zhu YZ:
P105 S-propargyl-cysteine (ZYZ-802), a novel water-soluble donor of
endogenous hydrogen sulfide, attenuates impairment against cerebral
ischemia-reperfusion injury in rats. Nitric Oxide. 39:(Suppl).
S47–S48. 2014. View Article : Google Scholar
|
|
26
|
Wang JX and Zhu XB: Effects of curcumin on
contents of SOD and MDA in brain of neonatal rats with
hypoxic-ischemic encephalopathy. Zhong Guo Yao Li Xue Tong Bao Bian
Ji Bu. 9:1327–1328. 2013.
|