Introduction
Diabetes mellitus (DM) is a group of metabolic
diseases, which is characterized by chronic hyperglycemia and
glucose intolerance, and may result in multi-organ dysfunction
(1). It is one of the most common
chronic diseases worldwide, the incidence of which is increasing at
an alarming rate due to various factors (2,3),
including increased economic development, which often leads to
changes in diet and lifestyle habits, and increased obesity
(4,5). A previous study reported that >60%
of the worldwide population with DM resides in Asia (6). The morbidity and mortality rate of
patients with DM remains high, regardless of extensive
investigations into potential treatments, and DM is considered a
contemporary challenge for public health. The primary cause of
morbidity and mortality in patients with DM is diabetic micro- and
macroangiopathy complications, also termed diabetic angiopathy,
this may lead to accelerated and aggravated forms of
atherosclerosis, renal failure, retinopathy, neuropathy and
amputation (7,8). Therefore, it is extremely important
to determine the pathogenesis of diabetic angiopathy and develop
targeted clinical treatment strategies for the future.
American ginseng (Panax quinquefolius) and
Asian ginseng (Panax ginseng) have been used as medicinal
plants for the treatment of hyperglycemia, diabetes, and their
associated complications (9–13).
P. ginseng has been officially approved in China as an
important ingredient in herbal therapeutic agents used for treating
DM (14). The major active
components of ginseng are ginsenosides (GSS). The mechanism of
action of ginseng and GSS in terms of DM treatment is complex. A
previous study demonstrated that their positive therapeutic effects
may be associated with the modulation of insulin secretion and
glucose metabolism, or regulation of the inflammatory pathway in
insulin-dependent and -independent processes (15). GSS Re is the predominant
protopanaxatriol in ginseng. However, the efficacy of GSS Re
treatment on diabetic angiopathy remains to be elucidated.
The present study aimed to investigate the
protective and anti-angiopathy effects of GSS treatment in rats
with early stage diabetes, type 1 DM (T1DM) and type 2 DM (T2DM).
The levels of blood glucose, insulin, lipid metabolism markers, and
endothelial cell function markers were determined, and the
expression of the mitogen-activated protein kinase (MAPK) signaling
pathway proteins, including p38 MAPK, extracellular
signal-regulated kinase 1/2 (ERK1/2) and c-Jun N-terminal kinase
(JNK), were determined.
Materials and methods
Animals and drugs
A total of 72 adult male Wistar rats (age, 7–8
weeks; weight, 200–250 g), purchased from the Shanghai SLAC
Laboratory Animal Co., Ltd. (Shanghai, China), were used in the
current study. All of the rats were housed individually in
ventilated cages, with ad libitum access to a standard diet
and tap water, and were maintained at controlled temperature (25°C)
and humidity (50%) under a 12-h light/12-h dark cycle. The study
was approved by the ethics committee of The First Affiliated
Hospital of Sun Yat-sen University (Guangzhou, China) The animal
care and use was monitored by Sun Yat-sen University animal care
committee (Guangzhou, China) and was in accordance with National
Institutes of Health guidelines (16). GSS Re was obtained from Sangong
Pharmaceutical Co., Ltd. (Shanghai, China). Alloxan monohydrate and
streptozocin (STZ) were purchased from Sigma-Aldrich; Merck
Millipore (Darmstadt, Germany).
Experimental design and diabetes
induction
Diabetic angiopathy occurs during the early stage of
diabetes, T1DM and T2DM. In the present study, early diabetes, T1DM
and T2DM were induced by three different methods: The
administration of a high-sucrose-high-fat diet, alloxan monohydrate
or STZ, respectively. The rats were randomly assigned into four
groups (n=6 in each model/group): i) Control; ii) control + GSS;
iii) DM; and iv) DM + GSS. The rats in the control group were fed a
standard chow diet for 8 weeks and received intragastric
administration of normal saline (20 mg/kg) for an additional 8
weeks. In the control + GSS group, rats were fed a standard chow
diet for 8 weeks and received intragastric administration of GSS
(20 mg/kg) for an additional 8 weeks. The rats in the DM group (n=6
in each model) were fed a high-sucrose-high-fat diet for 8 weeks,
followed by induction of T1DM or T2DM, and a further 8-week
high-sucrose-high-fat diet. In the DM + GSS group (n=6 in each
model), following DM induction, rats were treated by intragastric
administration of GSS (20 mg/kg) for an additional 8 weeks.
For the induction of early stage diabetes, the rats
received a high-sucrose-high-fat diet for 8 weeks. For the
induction of T1DM and T2DM, the rats were initially fed a
high-sucrose-high-fat diet for 8 weeks, and were then fasted for 18
h. In order to induce T1DM the rats received intraperitoneal
injection with alloxan monohydrate (120 mg/kg dissolved in normal
saline) every other day for 4 days. T2DM was induced by a single
administration of STZ (50 mg/kg dissolved in 0.9% sterile sodium
chloride; i.p). Due to acute hypoglycemia, the rats were supplied
with 10% sucrose solution for 48 h in place of drinking water. To
confirm that the induction of diabetes was successful, blood
samples were collected from the tail-end part of each rat and the
blood glucose levels were determined using a glucometer. The rats
with a blood glucose level ≥300 mg/dl (16.7 mmol/l) were regarded
as diabetic.
Plasma biochemistry
Blood glucose was determined using a glucose
monitoring system (Medtronic MiniMed, Inc., Northridge, CA, USA).
Serum levels of insulin (Rat Ins1/Insulin ELISA kit; Sigma-Aldrich;
Merck Millipore), vascular endothelial growth factor (VEGF; Rat
VEGF ELISA kit; Sigma-Aldrich; Merck Millipore), endothelin (ET;
Endothelin 1 ELISA kit; Abcam, Cambridge, MA, USA), and
interleukin-6 (IL-6; Rat IL-6 ELISA kit; Abcam) were determined by
commercial enzyme-linked immunosorbent assay kits according to the
manufacturer's protocols. Serum levels of nitric oxide (NO) were
determined by the Griess reaction, according to the manufacturer's
protocol of an NO kit (R&D Systems, Inc., Minneapolis, MN,
USA). High-density lipoprotein (HDL), triglyceride (TG) and total
cholesterol (TC) were quantified by enzymatic colorimetric analysis
(Roche Diagnostics GmbH, Mannheim, Germany). The concentration of
lipoprotein(a) (Lp-a) was determined using an immunonephelometric
method with N Latex Lp (a) reagent, according to the manufacturer's
protocol (Siemens Healthcare GmbH, Erlangen, Germany).
Metabolic analyses
The rats were fasted for 6 h prior to the oral
glucose tolerance test (OGTT). Blood samples were collected from
the lateral saphenous vein at baseline (0 min) and then at 15, 30,
60, 90 and 120 min following the oral administration of 2 g/kg
glucose. Glucose levels were assessed using a 2300 Stat Plus
glucose analyzer (YSI, Inc., Yellow Springs, OH, USA). In order to
perform the insulin tolerance test (ITT), the rats were fasted for
4 h and an intraperitoneal injection of insulin (0.5 U/kg)
(Humulin; Lilly USA, LLC, Indianapolis, IN, USA) was administered.
Blood glucose was monitored at the indicated time points (baseline
0, 15, 30, 60, 90 and 120 min).
Tissue preparation and western blot
analysis
The T2DM rats were sacrificed using
ketamine/xylazine (160/24 mg/kg). The aortic tissues were
immediately harvested and washed with cold saline. The tissues were
stored at −80°C for further protein assays. Total protein was
extracted from the aortas using NP40 protein lysis buffer (Thermo
Fisher Scientific, Inc., Waltham, MA, USA), and protein
concentration was assessed using the DC Protein Assay (Bio-Rad
Laboratories, Inc., Hercules, CA, USA). Proteins (20 µg) were
resolved on a 10% sodium dodecyl sulfate (SDS)-polyacrylamide gel
and were transferred on to a polyvinylidene difluoride membrane.
Subsequently, the membrane was blocked with 5% fresh non-fat dry
milk in Tris-buffered saline with 0.1% Tween-20 solution for 2 h at
room temperature, and was incubated with the following rabbit
monoclonal primary antibodies overnight at 4°C: Phosphorylated
(p)-p38 MAPK (p-p38; 1:1,000; cat. no. 4511), p-ERK1/2 (1:1,000;
cat. no. 4094) and p-JNK (1:1,000; cat. no. 4668; all Cell
Signaling Technology Inc., Danvers, MA, USA). Glyceraldehyde
3-phosphate dehydrogenase was used as an internal housekeeping
control with a rabbit monoclonal anti-GAPDH antibody (1:1,000; Cell
Signaling Technology, Inc.; cat. no. 5174. Following incubation for
1 h at room temperature (25°C) with the goat anti-rabbit
horseradish peroxidase-conjugated secondary antibodies (1:5,000;
Cell Signaling Technology, Inc.; cat. no. 7071), enhanced
chemiluminescence (Pierce ECL Western Blotting Substrate; Thermo
Fisher Scientific, Inc.) and densitometric analysis were performed
using ImageLab software (version 2.0.1; Bio-Rad Laboratories,
Inc.).
Statistical analysis
All data are presented as the mean ± standard
deviation. Comparisons between groups were calculated using
analysis of variance followed by a post-hoc Tukey's test if data
were parametric. If data were non-parametric a Kruskal-Wallis test
was conducted followed by a Mann-Whitney U test. Statistical
analyses were performed using statistical package for the social
sciences (SPSS) version 16.0 (SPSS, Inc., Chicago, IL, USA)
statistical software. P<0.05 was considered to indicate a
statistically significant difference.
Results
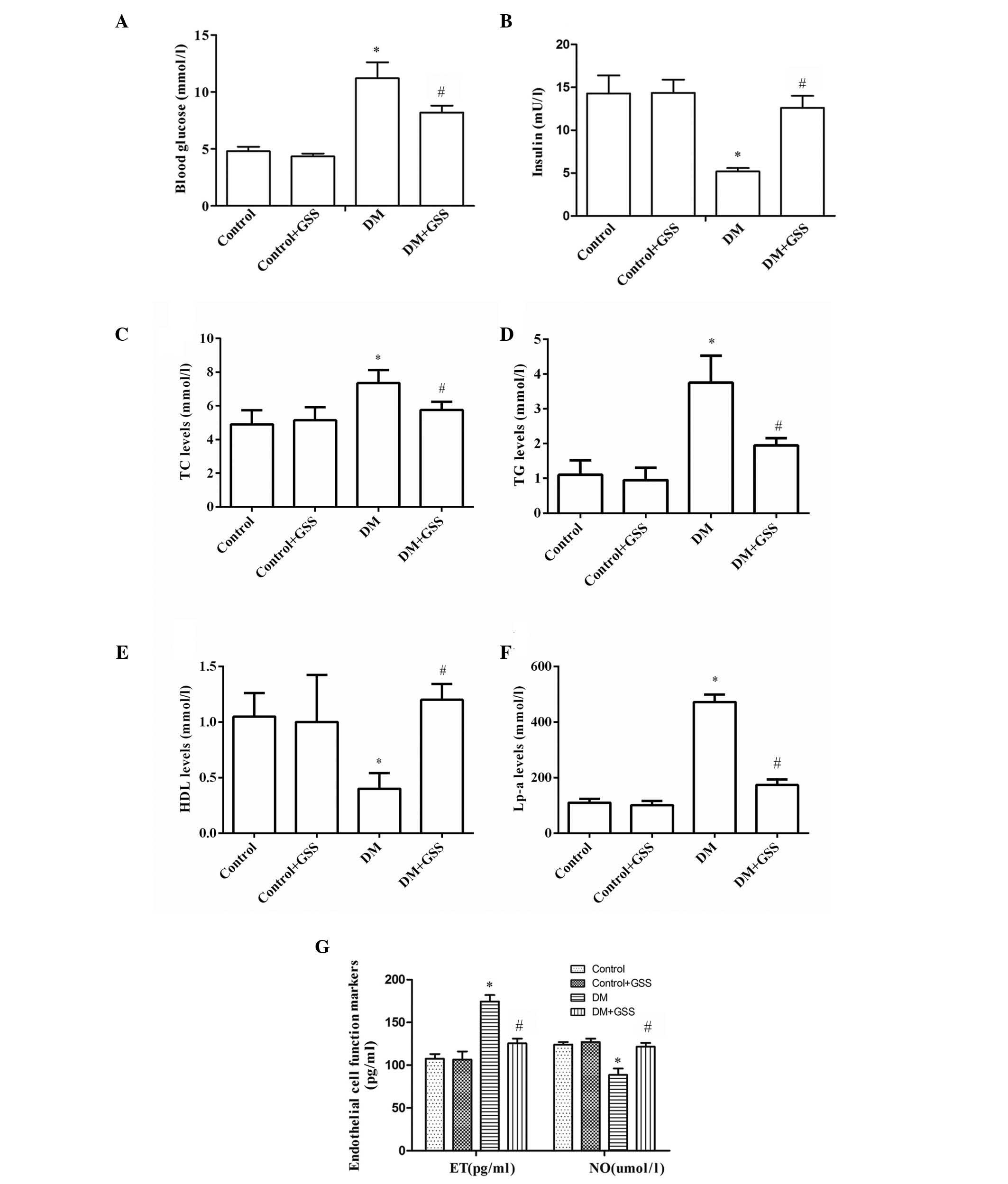
GSS exerts a positive effect on
vascular injury due to the onset of early stage diabetes
To determine the effects of GSS on vascular injury
resulting from early stage diabetes, the levels of blood glucose,
insulin, lipid metabolism markers (TC, TG, HDL, and Lp-a) and
endothelial cell function markers (ET and NO) were determined
following treatment with GSS. The levels of blood glucose were
significantly higher in the DM group compared with the control and
control + GSS groups (P<0.05; Fig.
1A). However, blood levels were significantly decreased in the
DM + GSS group compared with the DM group (P<0.05; Fig. 1A). The blood insulin levels in the
DM group were significantly lower when compared with the control
and control + GSS groups (P<0.05; Fig. 1B). However, insulin levels were
significantly increased in the DM + GSS group compared with the DM
group (P<0.05; Fig. 1B). These
results indicate that GSS may increase the levels of insulin and
decrease the levels of blood glucose in rats during the early stage
of diabetes. The levels of TC, TG and Lp-a were significantly
increased in the DM group compared with the control groups
(P<0.05; Fig. 1C-F); however,
following treatment with GSS they were significantly reduced
compared with the DM group (P<0.05; Fig. 1C-F). Notably, the levels of HDL
were significantly reduced in the DM group compared with the
control groups (P<0.05; Fig.
1C-F). However, in the GSS-treated group HDL levels were higher
compared with the DM group. Therefore, GSS treatment reduced the
levels of TC, TG and Lp-a and increased the levels of HDL, thus
indicating that GSS may effectively correct abnormal lipid
metabolism. In addition, it was determined that GSS treatment
significantly increased the levels of NO; however, the levels of ET
were reduced compared with the DM group (P<0.05; Fig. 1G), thus suggesting that GSS may
improve the endothelial cell dysfunction that frequently occurs
during the progression of diabetes.
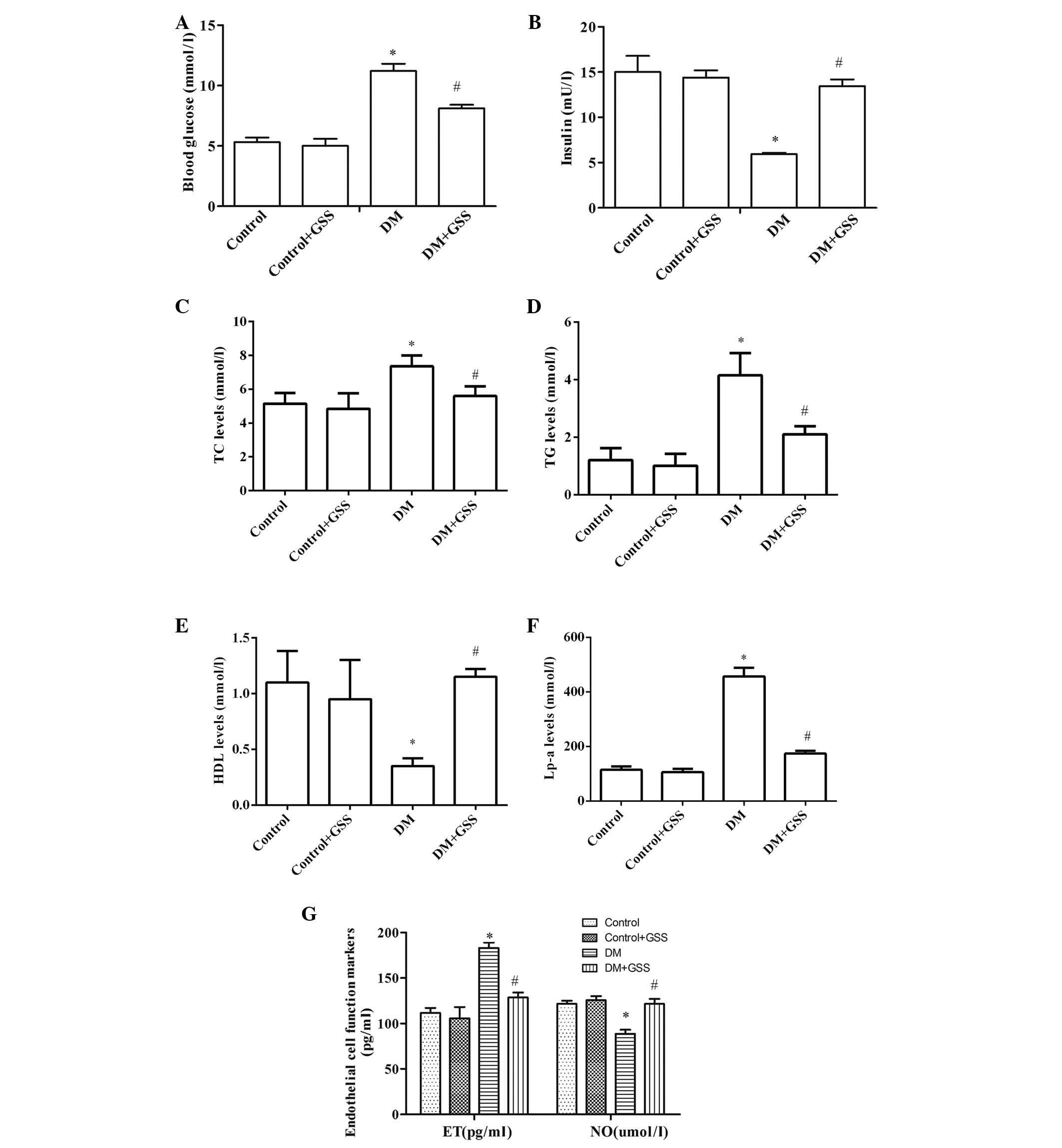
GSS reduces vascular injury resulting
from the induction of T1DM
In order to determine the effects of GSS on vascular
injury resulting from T1DM the levels of blood glucose, insulin,
TC, TG, HDL, Lp-a, and ET and NO were determined following
administration of GSS in T1DM-induced rats (Fig. 2). The levels of blood glucose
(Fig. 2A), TC, TG, Lp-a (Fig. 2C-F) and ET (Fig. 2G) in the DM group were
significantly increased when compared with the control groups
(P<0.05). Furthermore, significantly decreased levels of insulin
(Fig. 2B), HDL (Fig. 2C-F) and NO (Fig. 2G) were observed in the DM group
compared with the controls (P<0.05). The opposite was observed
for these markers in the group treated with GSS compared with the
DM group (P<0.05; Fig. 2).
These results indicate that GSS treatment may protect against
vascular injury during the pathogenesis of T1DM.
GSS reduces vascular injury resulting
from T2DM
In order to determine the effects of GSS on vascular
injury caused by T2DM OGTT and ITT were performed, and the levels
of TC, TG, HDL, Lp-a, VEGF and IL-6 were determined (Fig. 3). GSS was able to effectively
reduce the levels of blood glucose (Fig. 3A), TC, TG, Lp-a (Fig. 3C-F), VEGF and IL-6 in the DM + GSS
group compared with the DM group (Fig.
3G; P<0.05). Increased levels of insulin (Fig. 3B) and HDL (Fig. 3E) were also observed compared with
the DM group (P<0.05), indicating that GSS treatment may protect
against vascular injury due to T2DM.
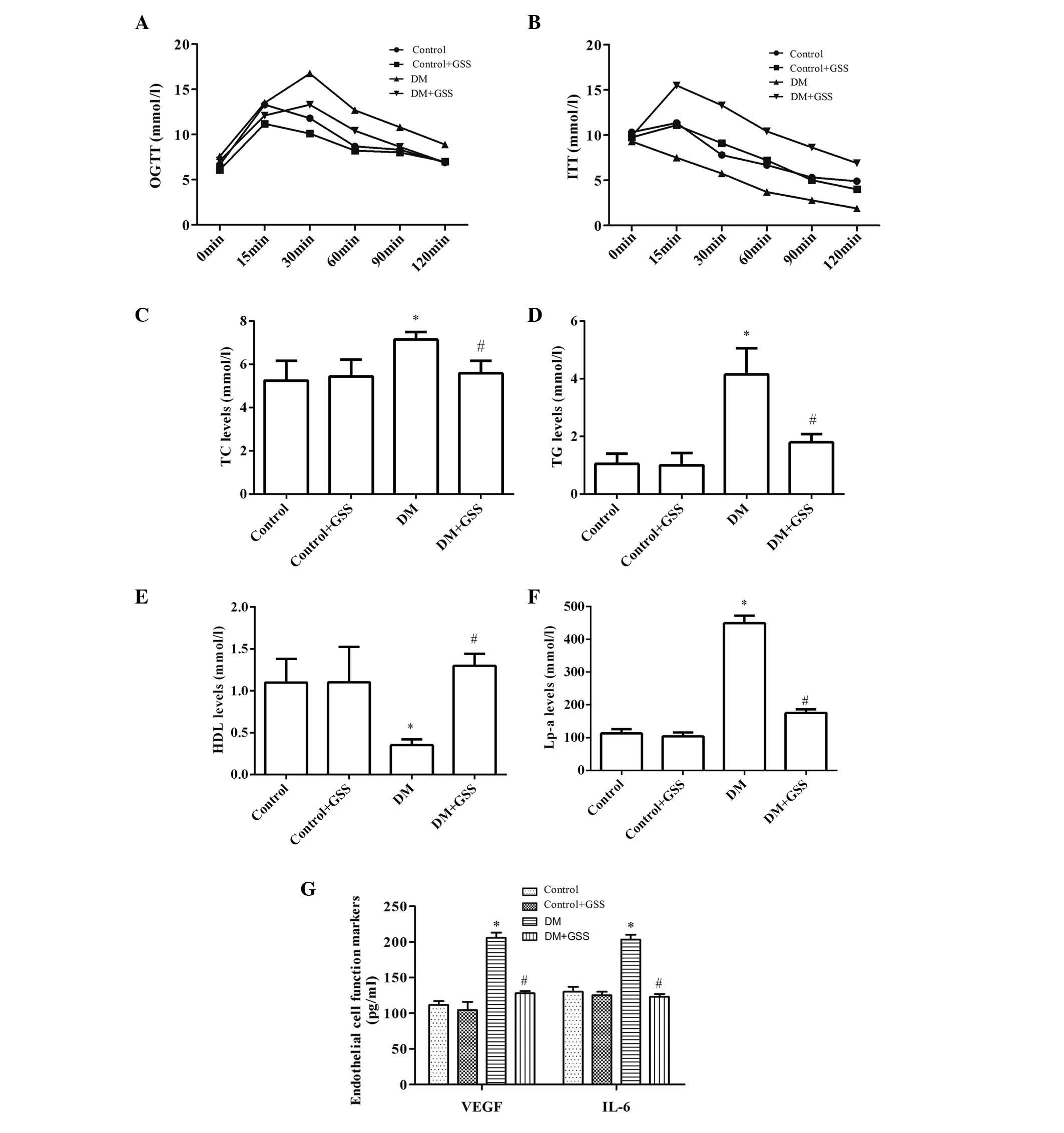 | Figure 3.Effects of GSS on vascular injury
caused by type 2 diabetes mellitus. (A) Different levels of blood
glucose revealed by OGTT. (B) Different levels of insulin
determined by ITT. Levels of (C-F) lipid metabolism markers and (G)
endothelial cell function markers. *P<0.05 vs. the control and
control +GSS groups; #P<0.05 vs. the DM group. Data
are presented as the mean ± standard deviation. A one-way analysis
of variance with a post-hoc Tukey's test was used to identify
significant differences among groups (A and B). A Kruskal-Wallis
test followed by a Mann-Whitney U test was performed to detect
significant differences among groups (C-G). GSS, ginsenoside; DM,
diabetes mellitus; OGTT, oral glucose tolerance test; ITT, insulin
tolerance test; TC, total cholesterol; TG, triglyceride; HDL,
high-density lipoprotein; Lp-a, lipoprotein(a); VEGF, vascular
endothelial growth factor; and IL-6, interleukin-6. |
Molecular mechanism underlying GSS
effects on diabetic angiopathy
In order to determine the molecular mechanism, which
allowed for GSS to improve vascular injury resulting from T2DM, the
phosphorylation levels of p38 MAPK, ERK1/2 and JNK in aortic
tissues were determined (Fig. 4).
The phosphorylation levels of p38 MAPK (Fig. 4A), ERK1/2 (Fig. 4B) and JNK (Fig. 4C) were compared with total p38,
ERK1/2 and JNK, respectively, and determined to be significantly
reduced in the DM + GSS group compared with the DM group
(P<0.05). These results suggest that during vascular injury GSS
may activate p38 MAPK, ERK1/2 and JNK signaling.
Discussion
In the absence of effective intervention strategies
for DM and diabetic angiopathy, targeted treatments may be a novel
alternative. The use of P. quinquefolius or P.
ginseng may be beneficial due to their anti-glycemic effects
and lack of systemic toxicity (17). The present study demonstrated that
GSS may exert protective and anti-angiopathy effects during DM,
including the early stage of diabetes, T1DM and T2DM, via increased
insulin levels, reduced blood glucose levels, corrected abnormal
lipid metabolism and improved endothelial cell dysfunction. This
may be achieved via activation of p38 MAPK, ERK1/2 and JNK
signaling.
The early stage of diabetes is characterized by
normal levels of fasting blood-glucose; however, over time insulin
secretion decreases and fasting hyperglycemia develops. Progressive
autoimmune destruction of pancreatic islet beta cells results in
permanent insulin deficiency, which leads to T1DM (18). Environmental triggers in
genetically susceptible individuals also contribute to T1DM
progression (19). T2DM accounts
for ~90% of all cases of diabetes and is prevalent in the general
adult population; T2DM is also associated with genetic factors
(20). The mechanism of T1DM and
T2DM pathogenesis differs; however their symptoms and outcomes are
similar, including hyperglycemia, insulin deficiency, lipid
metabolic disorder and endothelial cell dysfunction. In addition,
they are associated with a high risk of developing chronic diabetic
angiopathy in various organs, including nephropathy, neuropathy,
retinopathy and atherosclerosis, which often result in an
unfavorable prognosis and may lead to a marked decline of life
expectancy for patients (21).
However, the pathogenesis of diabetic angiopathy is
complex and remains to be elucidated. Epidemiological studies and
clinical trials have confirmed that alongside various factors,
hyperglycemia and dyslipidemia initiate the pathology of the vessel
wall (22–24). Hyperglycemia contributes to the
microvascular pathology. Therefore, strict control and monitoring
of blood glucose levels is critical in order to prevent or reverse
diabetic complications, which may improve the quality of life and
possibly prolong survival in patients with DM (25,26).
In addition to hyperglycemia, impaired lipid metabolism also
contributes to the pathology of T2DM and macroangiopathy (27,28).
A previous study determined that individuals with DM frequently
have dysfunctional lipid metabolism (29). This is often reflected by increased
levels of TC, TG and very low-density lipoproteins, alongside
reduced levels of circulating HDL (30,31).
However, endothelial cells remain the most important active
participant in DM pathogenesis. Dysfunction of endothelial cells
has been considered a key factor in the pathogenesis and
development of vascular disease in patients with DM (28,32,33).
VEGF has been identified as an important survival factor for
endothelial cells and may inhibit the apoptosis of endothelial
cells (34). ET-1 is an effective
vasoconstrictor, proinflammatory and proliferative endothelial
cell-derived peptide, which is important for the modulation of
vascular function. In conjunction with NO, ET is responsible for
the progressive development of endothelial dysfunction.
Overexpression of ET-1 and its receptors has been determined to
contribute to the development of atherosclerosis and diabetic
angiopathy (35). Furthermore, a
previous study indicated that inflammatory mediators, including
tumor necrosis factor α, IL-1β and IL-6 may be associated with
diabetic angiopathy (36).
Therefore, for effective treatment of DM and diabetic angiopathy
the following measures should be taken: Control of blood glucose,
regulation of insulin levels, correction of abnormal lipid
metabolism and improvement of endothelial cell dysfunction.
Previous studies have confirmed that GSS, an active
compound of ginseng, is important for the prevention and treatment
of various diseases, including cancer, cardiovascular disease and
diabetes (37–39). In addition, previous studies have
suggested that GSS has a substantial anti-hyperglycemic effect
(40,41), anti-inflammatory activity, and is
able to reduce serum insulin and lipid levels (42–44).
The effects of GSS on reduced insulin resistance have also been
reported to be associated with JNK, nuclear factor-κB and
peroxisome proliferator activated receptors γ (45,46).
However, few studies have investigated the function of GSS in
diabetic angiopathy. The present study used GSS Re to investigate
its effect on diabetic angiopathy and the possible molecular
mechanism. In conjunction with previous studies (47,48),
the current study confirmed that GSS may reduce the levels of blood
glucose, TC, TG, Lp-a and reverse the decreased levels of insulin
and HDL in various types of diabetes, including early stage
diabetes, T1DM and T2DM. In addition, it was determined that GSS
may protect against diabetic angiopathy by decreasing levels of ET,
VEGF, and IL-6, and increasing the levels of NO. In addition to JNK
signaling, p38 MAPK and ERK1/2 signaling may also be involved in
these effects.
In conclusion, the present study provided
experimental evidence that GSS may exert protective and
anti-angiopathy effects against vascular damage induced by early
stage diabetes, T1DM and T2DM. These effects were the result of a
reduction in blood glucose levels, increased insulin levels,
improved lipid metabolism and reduced endothelial cell dysfunction.
The underlying mechanism of these effects may be the activation of
p38 MAPK, ERK1/2 and JNK signaling.
References
|
1
|
Mokini Z and Chiarelli F: The molecular
basis of diabetic microangiopathy. Pediatr Endocrinol Rev.
4:138–152. 2006.PubMed/NCBI
|
|
2
|
Whiting DR, Guariguata L, Weil C and Shaw
J: IDF diabetes atlas: Global estimates of the prevalence of
diabetes for 2011 and 2030. Diabetes Res Clin Pract. 94:311–321.
2011. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Schulze MB and Hu FB: Epidemiology of
diabetesHandbook of Epidemiology. 2nd. Ahrens W and Pigeot I:
Springer; New York: pp. 2429–2467. 2014, View Article : Google Scholar
|
|
4
|
Zhang P, Zhang X, Brown J, Vistisen D,
Sicree R, Shaw J and Nichols G: Global healthcare expenditure on
diabetes for 2010 and 2030. Diabetes Res Clin Pract. 87:293–301.
2010. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Chan JC, Malik V, Jia W, Kadowaki T,
Yajnik CS, Yoon KH and Hu FB: Diabetes in Asia: Epidemiology, risk
factors and pathophysiology. JAMA. 301:2129–2140. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Ramachandran A, Snehalatha C, Shetty AS
and Nanditha A: Trends in prevalence of diabetes in Asian
countries. World J Diabetes. 3:110–117. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Head J and Fuller JH: International
variations in mortality among diabetic patients: The WHO
multinational study of vascular disease in diabetics. Diabetologia.
33:477–481. 1990. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Fioretto P, Dodson PM, Ziegler D and
Rosenson RS: Residual microvascular risk in diabetes: Unmet needs
and future directions. Nat Rev Endocrinol. 6:19–25. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Shishtar E, Jovanovski E, Jenkins A and
Vuksan V: Effects of Korean white ginseng (Panax Ginseng C.A.
Meyer) on vascular and glycemic health in Type 2 diabetes: Results
of a randomized, double blind, placebo-controlled,
multiple-crossover, acute dose escalation trial. Clin Nutr Res.
3:89–97. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Hong YJ, Kim N, Lee K, Hee Sonn C, Eun Lee
J, Tae Kim S, Ho Baeg I and Lee KM: Korean red ginseng (Panax
ginseng) ameliorates type 1 diabetes and restores immune cell
compartments. J Ethnopharmacol. 144:225–233. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Liu Z, Li W, Li X, Zhang M, Chen L, Zheng
YN, Sun GZ and Ruan CC: Antidiabetic effects of malonyl
ginsenosides from Panax ginseng on type 2 diabetic rats induced by
high-fat diet and streptozotocin. J Ethnopharmacol. 145:233–240.
2013. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Sen S, Chen S, Wu Y, Feng B, Lui EK and
Chakrabarti S: Preventive effects of North American ginseng (Panax
quinquefolius) on diabetic retinopathy and cardiomyopathy.
Phytother Res. 27:290–298. 2013. View
Article : Google Scholar : PubMed/NCBI
|
|
13
|
Mucalo I, Jovanovski E, Vuksan V, Božikov
V, Romić Z and Rahelić D: American ginseng extract (Panax
quinquefolius L.) is safe in long-term use in type 2 diabetic
patients. Evid Based Complement Alternat Med. 2014:9691682014.
View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Jia W, Gao W and Tang L: Antidiabetic
herbal drugs officially approved in China. Phytother Res.
17:1127–1134. 2003. View
Article : Google Scholar : PubMed/NCBI
|
|
15
|
Yuan HD, Kim JT, Kim SH and Chung SH:
Ginseng and diabetes: The evidences from in vitro, animal and human
studies. J Ginseng Res. 36:27–39. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Committee for the Update of the Guide for
the Care and Use of Laboratory Animals, . Guide for the Care and
Use of Laboratory Animals. 6th. National Institutes of Health;
1985
|
|
17
|
Vuksan V, Sung MK, Sievenpiper JL, Stavro
PM, Jenkins AL, Di Buono M, Lee KS, Leiter LA, Nam KY, Arnason JT,
et al: Korean red ginseng (Panax ginseng) improves glucose and
insulin regulation in well-controlled, type 2 diabetes: Results of
a randomized, double-blind, placebo-controlled study of efficacy
and safety. Nutr Metab Cardiovasc Dis. 18:46–56. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Baecher-Allan C and Hafler DA: Human
regulatory T cells and their role in autoimmune disease. Immunol
Rev. 212:203–216. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Bingley PJ, Bonifacio E and Gale EA: Can
we really predict IDDM? Diabetes. 42:213–220. 1993. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
van den Oever IA, Raterman HG, Nurmohamed
MT and Simsek S: Endothelial dysfunction, inflammation, and
apoptosis in diabetes mellitus. Mediators Inflamm. 2010:7923932010.
View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Li Y, Chen M, Xuan H and Hu F: Effects of
encapsulated propolis on blood glycemic control, lipid metabolism,
and insulin resistance in type 2 diabetes mellitus rats. Evid Based
Complement Alternat Med. 2012:9818962012. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Haller H, Drab M and Luft FC: The role of
hyperglycemia and hyperinsulinemia in the pathogenesis of diabetic
angiopathy. Clin Nephrol. 46:246–255. 1996.PubMed/NCBI
|
|
23
|
Hammes HP: Pathophysiological mechanisms
of diabetic angiopathy. J Diabetes Complications. 17:16–9. 2003.
View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Kreisberg RA: Diabetic dyslipidemia. Am J
Cardiol. 82:67U–73U; discussion 85U-86U. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Warren RE: The stepwise approach to the
management of type 2 diabetes. Diabetes Res Clin Pract. 65:(Suppl
1). S3–S8. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
The Diabetes Control and Complications
Trial Research Group, . The effect of intensive treatment of
diabetes on the development and progression of long-term
complications in insulin-dependent diabetes mellitus. The diabetes
control and complications trial research group. N Engl J Med.
329:977–986. 1993. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
McGarry JD: Banting lecture 2001:
Dysregulation of fatty acid metabolism in the etiology of type 2
diabetes. Diabetes. 51:7–18. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Skrha J: Pathogenesis of angiopathy in
diabetes. Acta Diabetol. 40:(Suppl 2). S324–S329. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Bardini G, Rotella CM and Giannini S:
Dyslipidemia and diabetes: Reciprocal impact of impaired lipid
metabolism and Beta-cell dysfunction on micro- and macrovascular
complications. Rev Diabet Stud. 9:82–93. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Tilly-Kiesi M, Syvänne M, Kuusi T,
Lahdenperä S and Taskinen MR: Abnormalities of low density
lipoproteins in normolipidemic type II diabetic and nondiabetic
patients with coronary artery disease. J Lipid Res. 33:333–342.
1992.PubMed/NCBI
|
|
31
|
Stewart MW, Laker MF, Dyer RG, Game F,
Mitcheson J, Winocour PH and Alberti KG: Lipoprotein compositional
abnormalities and insulin resistance in type II diabetic patients
with mild hyperlipidemia. Arterioscler Thromb. 13:1046–1052. 1993.
View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Schalkwijk CG and Stehouwer CD: Vascular
complications in diabetes mellitus: The role of endothelial
dysfunction. Clin Sci (Lond). 109:143–159. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
De Caterina R: Endothelial dysfunctions:
Common denominators in vascular disease. Curr Opin Clin Nutr Metab
Care. 3:453–467. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Gupta K, Kshirsagar S, Li W, Gui L,
Ramakrishnan S, Gupta P, Law PY and Hebbel RP: VEGF prevents
apoptosis of human microvascular endothelial cells via opposing
effects on MAPK/ERK and SAPK/JNK signaling. Exp Cell Res.
247:495–504. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Pernow J, Shemyakin A and Böhm F: New
perspectives on endothelin-1 in atherosclerosis and diabetes
mellitus. Life Sci. 91:507–516. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Vlassara H, Cai W, Crandall J, Goldberg T,
Oberstein R, Dardaine V, Peppa M and Rayfield EJ: Inflammatory
mediators are induced by dietary glycotoxins, a major risk factor
for diabetic angiopathy. Proc Natl Acad Sci USA. 99:15596–15601.
2002. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Nakata H, Kikuchi Y, Tode T, Hirata J,
Kita T, Ishii K, Kudoh K, Nagata I and Shinomiya N: Inhibitory
effects of ginsenoside Rh2 on tumor growth in nude mice bearing
human ovarian cancer cells. Jpn J Cancer Res. 89:733–740. 1998.
View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Zhou W, Chai H, Lin PH, Lumsden AB, Yao Q
and Chen CJ: Molecular mechanisms and clinical applications of
ginseng root for cardiovascular disease. Med Sci Monit.
10:RA187–RA192. 2004.PubMed/NCBI
|
|
39
|
Xie JT, Mehendale SR, Li X, Quigg R, Wang
X, Wang CZ, Wu JA, Aung HH, A Rue P, et al: Anti-diabetic effect of
ginsenoside Re in ob/ob mice. Biochim Biophys Acta. 1740:319–325.
2004. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Han KL, Jung MH, Sohn JH and Hwang JK:
Ginsenoside 20S-protopanaxatriol (PPT) activates peroxisome
proliferator-activated receptor gamma (PPARgamma) in 3T3-L1
adipocytes. Biol Pharm Bull. 29:110–113. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Xie JT, Wang CZ, Wang AB, Wu J, Basila D
and Yuan CS: Antihyperglycemic effects of total ginsenosides from
leaves and stem of Panax ginseng. Acta Pharmacol Sin. 26:1104–1110.
2005. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Attele AS, Zhou YP, Xie JT, Wu JA, Zhang
L, Dey L, Pugh W, Rue PA, Polonsky KS and Yuan CS: Antidiabetic
effects of Panax ginseng berry extract and the identification of an
effective component. Diabetes. 51:1851–1858. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Cho WC, Chung WS, Lee SK, Leung AW, Cheng
CH and Yue KK: Ginsenoside Re of Panax ginseng possesses
significant antioxidant and antihyperlipidemic efficacies in
streptozotocin-induced diabetic rats. Eur J Pharmacol. 550:173–179.
2006. View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Park EK, Choo MK, Han MJ and Kim DH:
Ginsenoside Rh1 possesses antiallergic and anti-inflammatory
activities. Int Arch Allergy Immunol. 133:113–120. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Zhang Z, Li X, Lv W, Yang Y, Gao H, Yang
J, Shen Y and Ning G: Ginsenoside Re reduces insulin resistance
through inhibition of c-Jun NH2-terminal kinase and nuclear
factor-kappaB. Mol Endocrinol. 22:186–195. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Gao Y, Yang MF, Su YP, Jiang HM, You XJ,
Yang YJ and Zhang HL: Ginsenoside Re reduces insulin resistance
through activation of PPAR-γ pathway and inhibition of TNF-α
production. J Ethnopharmacol. 147:509–516. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
47
|
Uzayisenga R, Ayeka PA and Wang Y:
Anti-diabetic potential of Panax notoginseng saponins (PNS): a
review. Phytother Res. 28:510–516. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
48
|
Quan HY, Yuan HD, Jung MS, Ko SK, Park YG
and Chung SH: Ginsenoside Re lowers blood glucose and lipid levels
via activation of AMP-activated protein kinase in HepG2 cells and
high-fat diet fed mice. Int J Mol Med. 29:73–80. 2012.PubMed/NCBI
|