Introduction
Cardiovascular diseases have the highest morbidity
rate worldwide, including angina, coronary heart disease,
hypertension and myocardial infarction (MI) (1). A previous study determined that by
the year 2020, MI may be the primary cause of mortality worldwide
(2). MI occurs when coronary blood
flow is inadequate, leading to cardiac dysfunction, arrhythmias and
sudden mortality (3). Prevention
of myocardial ischemia/reperfusion (I/R) injury is vital for
successful coronary heart disease surgery and recovery (4). It is important to develop novel
treatments to inhibit or reduce disease process and gain time for
subsequent treatment. Traditional Chinese Medicine has gained
increasing attention for the treatment of various diseases. The use
of Chinese medicine for ventricular remodeling treatments has been
investigated previously; however, it has been demonstrated that
using Traditional Chinese Medicine may affect the occurrence and
development of ventricular remodeling in a number of aspects, for
example proliferation and apoptosis (5). Additionally, previous studies have
determined that specific agents, including statins, angiotensin
converting enzyme inhibitors and angiotensin II receptor
inhibitors, may improve ventricular remodeling by increasing cell
apoptosis and reducing cell proliferation in smooth muscle cells in
hypertensive animal models (6,7).
Danshen is a Traditional Chinese Medicine and is
primarily used as treatment for cardiovascular diseases, including
angina pectoris, MI and stroke (8). Previous in vitro and in
vivo studies revealed that danshen exerts various
pharmacological effects, including relaxation of the coronary
artery, anticoagulation, reduction of myocardial I/R injury and
antiarrhythmic effects (9–11). By contrast, ligustrazine has been
previously reported to increase coronary blood flow and systemic
circulation by protecting mitochondria and improving energy
metabolism, scavenging oxygen free radicals in order to inhibit
lipid peroxidation, inhibition of apoptosis and protection of
myocardial cells, reducing the inflammatory reaction, mitigating
cell injury, and protecting myocardial cells (12–14).
Our previous study demonstrated that a compound of Salvia
miltiorrhiza and ligustrazine may act synergistically on the
cardiovascular system in rats (15). The present study determined the
effect of S. miltiorrhiza and ligustrazine injection (SLI)
(ratio of S. miltiorrhiza to ligustrazine was 1:50) on
myocardial I/R injury and investigated the underlying molecular
mechanism.
Materials and methods
Animals and treatment
SLI was obtained from Jilin Sichang Pharmaceutical
Co. Ltd. (Jilin, China). A total of 80 male Sprague-Dawley rats
(weight, 250–280 g; age, 8 weeks) were purchased from the Animal
Research Center of Sun Yat-sen University (Guangzhou, China). The
animal procedures were performed according to a protocol approved
by the Central Animal Facility of Sun Yat-sen University (no. scxk
2011-0029). All animals were housed in a room with a 12 h
light-dark cycle at a temperature 22–24°C and humidity of 50–60%,
with standard laboratory rodent chow and water ad libitum.
Rats were euthanized by overdose of anesthetic.
Myocardial I/R injury model of
rats
The cardiac I/R surgery was performed on the rats,
as previously described with certain modifications (16). The rats were anaesthetized via
intraperitoneal injection of pentobarbital sodium (60 mg/kg body
weight; Merck Millipore, Darmstadt, Germany), using a heating pad
to maintain animal body temperature at 37°C during surgery. A
tracheotomy was performed and the trachea was cannulated with a
mechanical ventilator (Alcott Biology, Shanghai, China) to
establish artificial respiration using oxygen at a frequency of
70–80 breaths/min and tidal volume of 15 ml/kg. The chest was
opened at the left fourth intercostal space to expose the heart
using a left thoracotomy. The left anterior descending (LAD) artery
was passed using a 6–0 silk suture, its ends were threaded through
a tube in order to form a snare. Successful LAD artery occlusion
was confirmed by electrocardiogram (ECG) to identify injury and
myocardial cyanosis. The heart was subjected to regional ischemia
for 35 min, followed by coronary reperfusion for 2 h following the
release of the tube.
Treatment groups
The animals were randomly divided into the following
six treatment groups (n=8/group): i) Sham, ii) I/R, iii) Low
(L-)SLI, iv) Medium (M-)SLI, v) High (H-)SLI; vi) verapamil (Ver;
Shandong Xinhua Pharmaceutical Co., Ltd., Zibo, China) groups. The
animals in the sham group underwent identical surgical procedures
without LAD ligation. In the I/R group, the coronary artery was
occluded and vehicle (veh) treatment was administered. For the SLI
treatment groups, SLI was dissolved in saline at a final
concentration of 6.8, 20.4 and 61.2 mg/kg (0.133:6.667, 0.4:20 and
1.2:60 mg/kg S. miltiorrhiza:ligustrazine) for the L-, M-
and H-SLI groups, respectively, administered by intraperitoneal
injection once a day for 3 days prior to surgery. The rats in the
Ver group were treated with 6 mg/kg Ver by intraperitoneal
injection once/day for 3 days.
Hemodynamics and ECG in rats
The right carotid artery was isolated and a catheter
was inserted. A biotic signal system (PowerLab; AD Instruments,
Sydney, Australia) was used to record various cardiac functions of
the rats in the different groups, including the left ventricular
systolic pressure (LVSP), left ventricular diastolic pressure
(LVDP), first derivative of left ventricular pressure (±dp/dt),
heart rate (HR) and ECG.
Determination of lactate dehydrogenase
(LDH), superoxide dismutase (SOD), creatine kinase (CK) and
malondialdehyde (MDA) activity in blood serum
After 3 h reperfusion, blood samples were collected
from the abdominal aortic artery and blood serum was stored at
−80°C until analysis. The levels of LDH, SOD, CK and MDA were
determined using a Hitachi 7180 automated biochemical analyzer
system (Diamond Diagnostics, Holliston, MA, USA).
Determination of the infarct size
The size of the cardiac infarct was determined using
a triphenyl tetrazolium chloride (TTC) staining method, as
previously described (17,18). Rats were sacrificed by
intraperitoneal injectino of an overdose of pentobarbital sodium
anesthesia (300 mg/kg body weight) subsequent to the collection of
the serum samples, the heart was excised and weighed immediately.
The left ventricle was sliced parallel to the atrioventricular
groove into five sections with a thickness of 2–3 mm. The
myocardial slices were incubated for 15 min at 37°C in 1% TTC
solution in phosphate-buffered saline. Next, images of the slices
were captured. The infarct area was analyzed using Image-Pro Plus
version 5.0 software (Media Cybernetics, Inc., Rockville, MD, USA).
Infarct size was expressed as a percentage, calculated by dividing
the infarct mass of the left ventricle by its total mass. This was
analyzed using Image J version 1.26 (National Institutes of Health,
Bethesda, MD, USA).
Histopathological examination of the
cardiac tissues
Cardiac tissues were obtained below ligation to the
apex area of the heart and fixed in 10% buffered formalin. Tissue
sections (~7 µm) were prepared from the paraffin-embedded tissues.
The tissues were stained with hematoxylin and eosin. The extent of
the tissue injury was determined using a microscope at a
magnification × 400.
Cell culture and hypoxia/reoxygenation
(H/R) injury
The H9C2 rat cardiomyocyte cell line was obtained
from the China Infrastructure of Cell Line Resources (Chinese
Academy of Medical Sciences, Beijing, China). H9C2 cells were
cultured in Dulbecco's modified Eagle's medium (Gibco; Thermo
Fisher Scientific, Inc., Waltham, MA, USA) with 10% fetal calf
serum (PAA; GE Healthcare Life Sciences, Pittsburgh, PA, USA) and
1% of penicillin-streptomycin, and were incubated at 37°C, with 5%
CO2. New medium was applied every 2–3 days. The cells
were subjected to experimental procedures at 80–90% confluence.
The H/R injury model was implemented, as previously
described (1–3). Briefly, H9C2 cells were exposed to
ischemia by replacing the medium with an ‘hypoxic buffer’ (0.9 mM
NaH2PO4, 6.0 mM NaHCO3, 98.5 mM
NaCl, 1.2 mM MgSO4, 10 mM KCl, 1.8 mM CaCl2,
40 mM sodium lactate, 20 mM HEPES; pH 6.8). The cells were
incubated in a hypoxic chamber at 37°C for 2 h in a humidified
atmosphere of 5% CO2 and 95% nitrogen. Following 2 h of
hypoxic conditions, the cells were exposed to reoxygenation by
replacing the medium with a ‘reoxygenation buffer’ (20.0 mM
NaHCO3, 0.9 mM NaH2PO4, 1.8 mM
CaCl2, 1.2 mM MgSO4, 20.0 mM HEPES, 5.0 mM
KCl, 129.5 mM NaCl, 5.5 mM glucose; pH 7.4) in a standard incubator
for 3 h. The cells in the control group were cultured with normal
Tyrode's solution (pH 7.4; Beijing Reagant Biological Technology
Co., Ltd., Beijing, China) in a standard incubator for 5 h
(4). A total of five treatment
groups were established: i) Control; ii) H/R; iii) L-SLI; iv)
M-SLI; and v) H-SLI. SLI was administered prior to 2-h H/R injury
within the normal culture medium at 37°C.
Cell viability assay
Cell viability was determined using a Cell Counting
Kit (CCK)-8 assay (Beyotime Institute of Biotechnology, Inc.,
Guangzhou, China), according to the manufacturer's protocol. H9C2
cells were seeded at a density of 5×104 cells/well into
96-well plates. Following the aforementioned treatments, the cells
were exposed to 10 µl CCK-8 solution for 2 h and the absorbance was
determined at 450 nm using a microplate reader (BioTek Instruments,
Inc., Winooski, VT, USA).
Western blot analysis
H9C2 cells were lysed in lysis buffer (Beyotime
Institute of Biotechnology, Inc.) containing protease inhibitor
cocktail (Merck Millipore) on ice for 30 min. Cellular proteins
were collected using a cell scraper and the lysates were
centrifuged at 4°C for 15 min at 10,000 × g. A BCA Protein
Assay kit (Beyotime Institute of Biotechnology, Inc.) was used to
determine protein concentrations. Equal quantities of protein (2
µg/µl) were separated on 8% sodium dodecyl sulphate-polyacrylamide
gels and transferred onto a polyvinylidene difluoride membrane (EMD
Millipore, Billerica, MA, USA). The membranes were blocked with 5%
non-fat dry milk for 30 min at room temperature. Following
blocking, the membranes were incubated with the primary antibodies
against rabbit IgG p-eNOS/eNOS (1:1,000; BD Biosciences, Franklin
Lakes, NJ, USA), rabbit IgG p-Akt/Akt (1:1,000; BD Biosciences),
Bcl-2 (1:1,000; Cell Signaling Technology, Inc., Danvers, MA, USA),
Bax (1:2,000; Cell Signaling Technology, Inc.) and caspase-3
(1:3,000; Cell Signaling Technology, Inc.) overnight at 4°C. The
membranes were washed with Tris-buffered saline containing Tween 20
and were subsequently incubated with secondary antibodies:
Anti-mouse (IgG; 1:5,000) or horseradish peroxidase-conjugated
anti-rabbit (IgG; 1:1,000) for 1 h (all obtained from Cell
Signaling Technology, Inc.). The membranes were washed as before.
β-actin (IgG; 1:3,000; Cell Signaling Technology, Inc.) was used as
the internal reference. The protein bands were obtained using an
enhanced chemiluminescence system (Beyotime Institute of
Biotechnology, Inc.). The band density was scanned and quantified
using Image-J software (National Institutes of Health).
Statistical analysis
The data are expressed as the mean ± standard
deviation. Statistical comparisons between groups were performed
using one-way analysis of variance. SPSS software, version 19
(SPSS, Inc., Chicago, IL, USA). P<0.05 was considered to
indicate a statistically significant difference.
Results
SLI improves cardiac function
Changes in the hemodynamic data that occurred during
the experiments are presented in Tables I–III. No difference in the baseline was
observed between the different groups. Additionally, no significant
difference was identified during the experiment in the sham group
(Table I). Pretreatment with all
doses of SLI and Ver significantly increased LVSP (P<0.05;
Table I) and the +dp/dt max
(P<0.05; Table II) compared
with the I/R group. LVDP (P<0.05; Table I) and the -dp/dt max (P<0.05;
Table II) were significantly
decreased in response to pretreatment with SLI and Ver. However, no
significant difference was observed in the heart rate between the
different groups during coronary artery occlusion and reperfusion
(Table III).
 | Table I.Effects of SLI on LVSP and LVDP of
myocardial infarction in rats. |
Table I.
Effects of SLI on LVSP and LVDP of
myocardial infarction in rats.
|
| LVSP (mmHg) | LVDP (mmHg) |
|---|
|
|
|
|
|---|
| Group | Baseline | Ischemia | Reperfusion | Baseline | Ischemia | Reperfusion |
|---|
| Sham | 149.81±4.10 | 150.26±5.50 | 147.03±4.90 | 24.49±2.10 | 23.81±2.50 | 24.52±2.90 |
| I/R | 136.32±11.70 |
96.78±13.87c |
91.73±13.79c | 23.88±5.19 |
15.92±4.10c |
17.75±4.93c |
| L-SLI | 139.22±7.22 |
99.08±8.23a |
100.84±7.79a | 22.45±6.22 | 17.34±5.23 | 16.83±6.97 |
| M-SLI | 142.48±16.72 |
106.85±22.15a |
107.05±11.86a | 25.72±3.44 | 15.05±4.97 | 17.15±4.60 |
| H-SLI | 140.30±18.23 |
103.73±26.87a |
113.90±11.12b | 24.88±3.19 |
25.59±4.96b |
26.59±4.42b |
| Ver | 152.57±5.23 |
114.30±6.15b |
111.20±4.78b | 22.07±5.32 |
20.60±4.15a |
25.60±4.78b |
 | Table III.Effects of SLI on HR of myocardial
infarction in rats. |
Table III.
Effects of SLI on HR of myocardial
infarction in rats.
|
| HR (beats/min) |
|---|
|
|
|
|---|
| Group | Baseline | Ischemia | Reperfusion |
|---|
| Sham | 376.42±32.69 | 368.18±25.75 | 372.63±22.64 |
| I/R | 399.83±52.75 | 363.33±27.46 | 374.00±32.30 |
| L-SLI | 385.50±63.78 | 371.00±64.11 | 389.25±27.97 |
| M-SLI | 387.75±23.21 | 353.25±47.32 | 395.00±76.15 |
| H-SLI | 392.00±36.06 | 341.67±20.23 | 322.67±60.80 |
| Ver | 368.50±19.09 | 300.00±75.54 | 334.00±77.78 |
 | Table II.Effects of SLI on +dp/dt (max) and
-dp/dt (max) of myocardial infarction in rats. |
Table II.
Effects of SLI on +dp/dt (max) and
-dp/dt (max) of myocardial infarction in rats.
|
| +dp/dt (max)
(mmHg/ms) | -dp/dt (max)
(mmHg/ms) |
|---|
|
|
|
|
|---|
| Group | Baseline | Ischemia | Reperfusion | Baseline | Ischemia | Reperfusion |
|---|
| Sham | 1.31±0.23 | 1.29±0.18 | 1.33±0.22 | 1.28±0.12 | 1.33±0.28 | 1.36±0.27 |
| I/R | 1.26±0.28 |
0.80±0.21c |
0.85±0.29c | 1.13±0.17 |
0.65±0.30c |
0.73±0.24c |
| L-SLI | 1.20±0.26 |
0.92±0.21a |
1.02±0.27a | 1.25±0.26 |
1.27±0.31b |
1.19±0.22b |
| M-SLI | 1.12±0.24 |
1.09±0.25a |
1.06±0.20a | 1.02±0.11 |
1.02±0.15b |
1.08±0.22a |
| H-SLI | 1.27±0.24 |
1.45±0.20b |
1.34±0.22b | 1.23±0.11 |
1.11±0.06b |
1.38±0.28b |
| Ver | 1.20±0.25 |
1.08±0.18a |
1.17±0.18a | 1.25±0.28 | 0.96±0.32 |
1.20±0.29b |
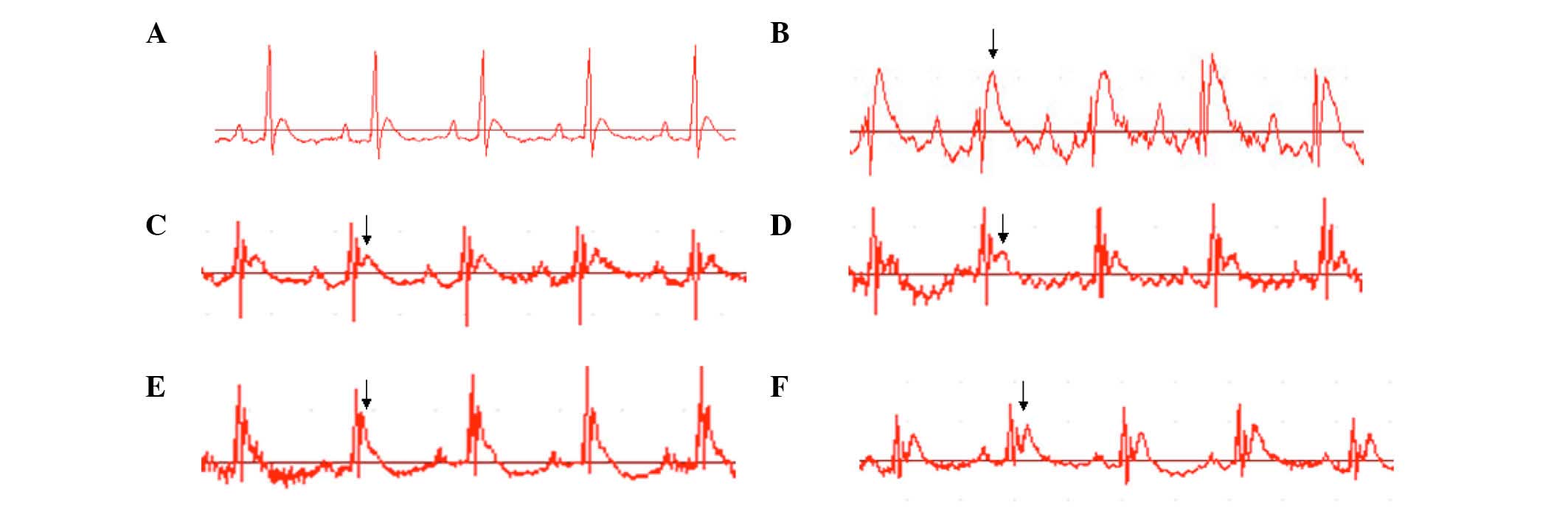
Effect of SLI on the ST segment of the
ECG in I/R rats
Changes in ECG, including the degrees of T wave and
ST segment, may be used to evaluate MI in the animals. The present
study determined that T waves were consistent in the sham group
rats. The ST-segments were elevated in the I/R group following the
ischemia surgery. The ST-segments in the remaining groups were
reduced compared with the I/R group following reperfusion (data not
shown; Fig. 1).
Effect of SLI on LDH, CK, SOD and MDA
levels in blood serum
In order to determine whether S. miltiorrhiza
and ligustrazine may reduce the damage of cardiomyocytes following
I/R, characterized by cell membrane disruption and cell content
release, the activities of CK, LDH, SOD and MDA in blood serum were
determined at the end of reperfusion. As shown in Fig. 2, the levels of CK, LDH and MDA were
increased in the I/R group and SOD levels were significantly
decreased in the I/R group compared with the sham group (P<0.05;
Fig. 2B). Pretreatment with SLI
and Ver significantly decreased the levels of CK, LDH and MDA in
blood serum (P<0.05 and P<0.01; Fig. 2A, C and D) and significantly
increased the levels of SOD compared with rats in the I/R group
(P<0.05; P<0.01; Fig. 2B).
These findings suggested that SLI may protect the heart against
I/R-induced myocardial injury in vivo.
 | Figure 2.Effects of SLI on the activity of
various molecules. The (A) LDH, (B) SOD, (C) CK and (D) MDA
activity in blood serum of rats was measured using an automated
biochemical analyzer system following myocardial I/R injury. LDH,
lactate dehydrogenase; SOD, superoxide dismutase; CK, creatine
kinase; MDA, malondialdehyde; SLI, Salvia miltiorrhiza and
ligustrazine injection; I/R, ischemia/reperfusion; L-, low-; M-,
medium-; H-, high-; Ver, verapamil. Data are presented as the mean
± standard deviation (n=8;. *P<0.05, **P<0.01 vs. I/R group;
##P<0.01 vs. sham group). |
Effect of SLI on myocardial infarct
size in I/R rats
The ratios of infarct areas are shown in Fig. 3. MI was not observed in the hearts
of the sham group, as expected. The ratio of infarct size was
50.24±9.51, 31.87±3.75, 42.63±8.56 and 28.67±7.55% in the I/R,
L-SLI, M-SLI and H-SLI groups, respectively. The M-SLI and H-SLI
pretreatment groups exhibited significantly reduced infarct size
compared with the I/R group (P<0.05 and P<0.01, respectively;
Fig. 3B). No significant
difference was observed between the L-SLI and I/R groups (Fig. 3B).
Histological effect of SLI on the
myocardium in I/R rats
The structure of the myocardium in the sham group
was normal, with uniform cytoplasmic staining, consistent round or
oval shape of cells and nuclear chromatin was uniformly distributed
(Fig. 4A). The I/R group exhibited
significant cardiomyocyte damage with extensive edema, myonecrosis
and inflammatory cell infiltration. The nuclei were also chipped
and dissolved (Fig. 4B).
Pretreatment with H-SLI and Ver protected the cardiomyocytes from
damage compared with the I/R group, as myofibril loss and
inflammatory cell infiltration were decreased (Fig. 4E and F).
 | Figure 4.Microscopy images of rat myocardial
tissue in response to various treatments. The myocardial tissue was
stained with hematoxylin-eosin in the (A) sham group, where rats
exhibited normal myocardial histology, clear transverse striations
and no inflammatory cell infiltration, (B) the I/R group that
exhibited evident swelling of myocardial cells, myonecrosis and
inflammatory cell infiltration, (C) the L-SLI group that exhibited
myocardial cell swelling degeneration, unclear arrangement, clear
transverse striations and large numbers of inflammatory cells, (D)
the M-SLI group that exhibited myocardial cell swelling, slight
degeneration, unclear horizontal striations and reduced
infiltration of inflammatory cells, (E) the H-SLI group had normal
structure, clear transverse striations and few invasive
inflammatory cells, and (F) the verapamil group with normal
arrangement of cells, clear transverse striations and few invasive
inflammatory cells. Magnification, ×400. SLI, Salvia
miltiorrhiza and ligustrazine injection; I/R,
ischemia/reperfusion; L-, low-; M-, medium-; H-, high-. |
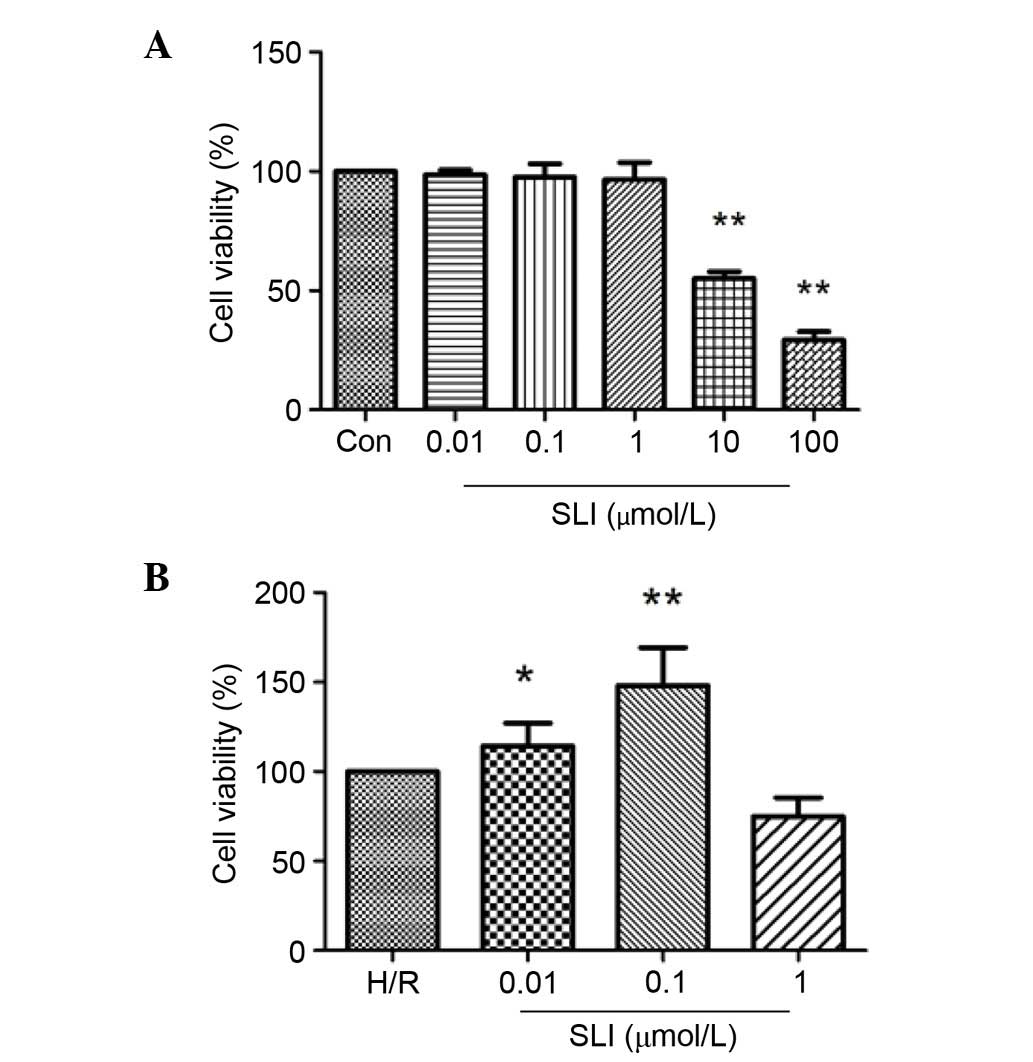
Effects of SLI on cell viability
Cell viability was assessed using a CCK-8 assay. The
viability of H9C2 cells incubated with SLI at various
concentrations for 2 h was determined (Fig. 5). SLI concentrations between 0.01
and 1 µM did not significantly affect cell viability (Fig. 5A). Conversely, 10 and 100 µM SLI
significantly reduced cell viability compared with the control
group (P<0.01; Fig. 5A)
Pretreatment with SLI reduced H/R-induced damage in H9C2 cells, and
significantly increased cell viability (P<0.05 and P<0.01;
Fig. 5B). Therefore, 0.1 µM SLI
was used in subsequent in vitro experiments.
SLI increases Bcl-2 and decreases Bax
and caspase-3 expression levels
Bcl-2 and Bax are important for mediating cell
survival or death following apoptotic stimuli. As presented in
Fig. 6A, pretreatment with 0.1 µM
SLI increased the expression of Bcl-2 and inhibited the expression
of Bax, resulting in a significantly increased Bcl-2/Bax ratio
compared with the H/R group (P<0.01). The caspase family of
proteins are apoptosis regulators. Caspase-3 is an important
mediator for apoptosis and may be considered as a marker of H/R
apoptosis. As presented in Fig.
6B, H/R significantly increased the activity of caspase-3
compared with the control group (P<0.05), whereas 0.1 µM SLI
significantly reduced the levels of caspase-3 compared with the H/R
group (P<0.01).
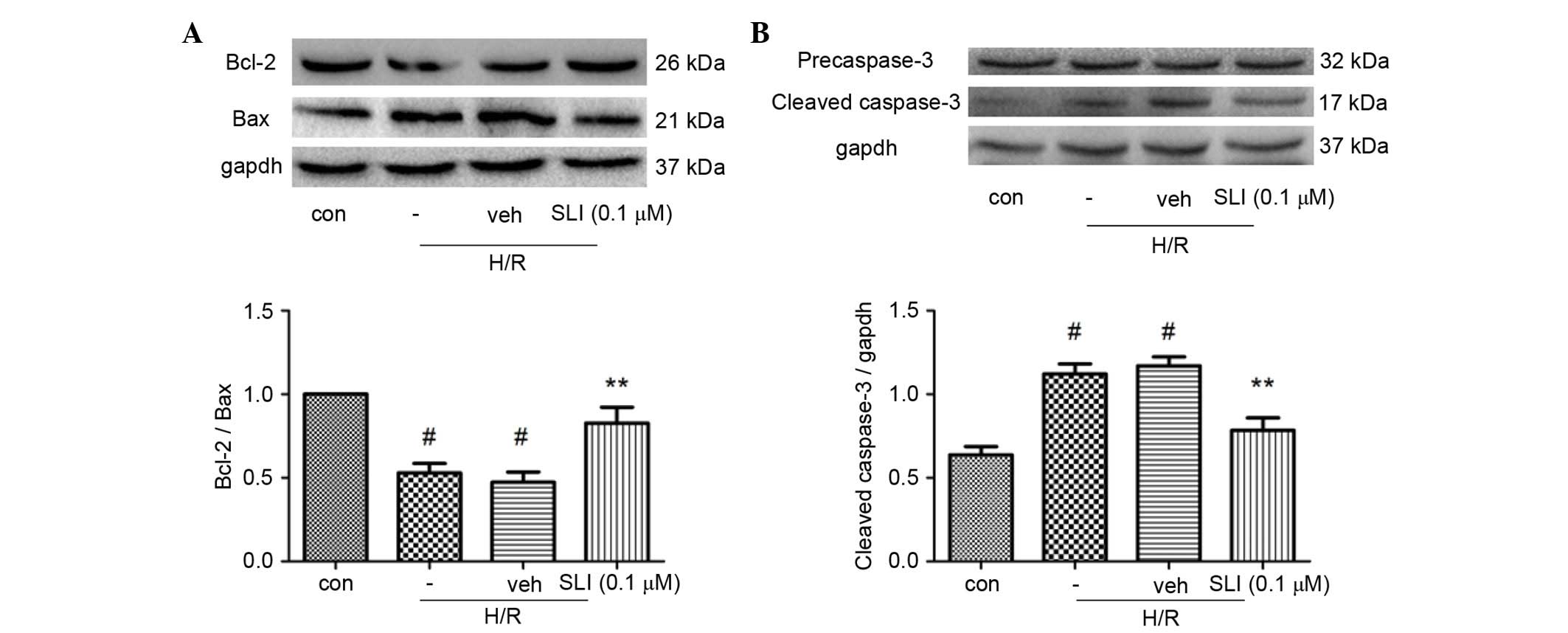 | Figure 6.Effect of SLI on Bcl-2, Bax, caspase-3
levels following H/R injury. The protein expression levels of
Bcl-2, Bax, caspase-3 subsequent to H/R injury were assessed by
western blotting. (A) Pretreatment with 0.1 µM SLI increased the
expression of Bcl-2 and inhibited the expression of Bax; therefore,
increased the Bcl-2/Bax ratio compared with the H/R group. (B)
Treatment with 0.1 µM SLI attenuated the levels of activated
caspase-3 compared with the H/R group. Data are presented as the
mean ± standard deviation (#P<0.05 vs. control group;
**P<0.01 vs. H/R group). H/R, hypoxia/reoxygenation; SLI,
Salvia miltiorrhiza and ligustrazine injection; Bcl-2,
B-cell lymphoma-2; Bax, Bcl-2-associated X protein; veh, vehicle;
con, control. |
SLI increases the phosphorylation of
Akt and eNOS
The molecular mechanism underlying SLI-mediated
cardioprotection was investigated by western blot analysis. It has
been previously reported that eNOS is a substrate for Akt and NO
production following Akt-induced eNOS phosphorylation. The protein
expression levels of p-Akt/Akt and p-eNOS in H9C2 cells were
determined following induction of H/R. SLI pretreatment
significantly increased the expression levels of p-Akt and p-eNOS
compared with the H/R group (P<0.01; Fig. 7). Simultaneously, the Akt
inhibitor, LY294002 significantly reduced the ratio of p-Akt/Akt
compared with the SLI group (P<0.05; Fig. 7A). No significant difference was
identified in the total protein expression levels of Akt and eNOS.
These findings demonstrated that SLI may activate Akt and induce
p-eNOS expression in cells where H/R is induced (Fig. 7).
Discussion
The present study evaluated the effects of SLI on
myocardial I/R injury in a rat model and H/R injury in a rat
myocardium cell line. The present study determined that SLI
attenuated the effects of I/R injury in vivo and H/R injury
in vitro.
To evaluate the effect of SLI on cardiac dysfunction
following myocardial ischemia, hemodynamic parameters such as LVSP,
LVDP and ±dp/dt max were recorded. In the present study, the I/R
injury group exhibited significant cardiac dysfunction, with
reduced LVSP, LVDP and ±dp/dt max. Pretreatment with SLI
significantly reduced the changes in LVSP, LVDP and ±dp/dt max
following the induction of I/R. Therefore, SLI may ameliorate heart
function impairment due to I/R injury. Cytosolic enzymes, including
LDH and CK, may be used as diagnostic markers of myocardial
ischemia injury, as they are released from the damaged myocardial
tissues into the blood serum when cell membrane was induced to
permeate or rupture. Therefore, LDH and CK levels in blood serum
reflected changes in membrane integrity and the extent of
myocardial injury. The present study determined that pretreatment
with SLI reduced the elevated blood serum levels of LDH and CK
induced by I/R injury, suggesting that SLI may reduce cell membrane
damage in myocardial ischemic injury.
A previous study reported that oxidative stress is
the primary contribution to myocardial I/R injury (19). Overproduction of reactive oxygen
species may also result in lipid peroxidation process. SOD is often
the primary mediator of oxygen free radicals due to its ability to
reduce the production of free radicals and alleviate H/R injury in
myocardial cells. MDA is a terminal product of lipid peroxidation
and its concentration in blood serum may reflect the extent of
myocardial injury (1). In the
present study, pretreatment with SLI significantly reduced MDA
levels and increased SOD activity. The current findings indicated
that SLI may enhance the elimination of oxygen free radicals and
reduce myocardial injury.
Calculation of the MI area and the myocardial
pathomorphism are the most intuitive methods of assessing
myocardial damage. Myocardial injury may lead to membrane damage of
cardiomyocytes with extensive edema, myonecrosis, infiltration of
inflammatory cells, disrupted cristae, chromatin condensation,
cytoplasmic vacuoles and loss of myofibrils. SLI pretreatment may
reduce cardiomyocyte membrane damage induced during MI with smaller
areas of myofibril loss and reduced inflammatory cell infiltration,
swelling and vacuolation of mitochondrial cristae and decreased the
infarct size. In order to determine the mechanism behind the
positive SLI effects in H9C2 cells. Akt was identified as the
primary target enzyme; therefore, phosphorylation and expression of
Akt may influence protein synthesis of apoptotic factors, including
inhibiting the activation of caspase-3 and increasing the Bcl-2/Bax
ratio. Conversely, previous studies have determined that
phosphorylation of Akt may activate eNOS, which is the downstream
target of RISK pathways, and may induce p-eNOS function as a
cardiovascular protective molecule (20–22).
Further investigation is required in order to fully elucidate the
molecular mechanisms behind the protective effect of SLI on I/R and
H/R -damaged myocardial tissues.
To the best of our knowledge, the present study is
the first to demonstrate that SLI exerted cardioprotective effects
in rats with myocardial I/R injury via the regulation of myocardial
enzymes and increasing the levels of enzymes responsible for
eliminating oxygen free radicals. Additionally, SLI pretreatment
may inhibit cell apoptosis via activation of the Akt-eNOS signaling
pathway.
References
|
1
|
Ren-an Q, Juan L, Chuyuan L, Wenjuan F,
Chunyan H, Xuemei Y, Lin H and Hong N: Study of the protective
mechanisms of Compound Danshen Tablet (Fufang Danshen Pian) against
myocardial ischemia/reperfusion injury via the Akt-eNOS signaling
pathway in rats. J Ethnopharmacol. 156:190–198. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Lopez AD and Murray CC: The global burden
of disease, 1990–2020. Nat Med. 4:1241–1243. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Shimokawa H and Yasuda S: Myocardial
ischemia: Current concepts and future perspectives. J Cardiol.
52:67–78. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Morgan EN, Boyle EM Jr, Yun W, Kovacich
JC, Canty TG Jr, Chi E, Pohlman TH and Verrier ED:
Platelet-activating factor acetylhydrolase prevents myocardial
ischemia-reperfusion injury. Circulation. 100(19): Suppl.
II365–II368. 1999.PubMed/NCBI
|
|
5
|
Li SY, Wang XG, Ma MM, Liu Y, Du YH, Lv
XF, Zhou JG, Tang YB and Guan YY: Ginsenoside-Rd potentiates
apoptosis induced by hydrogen peroxide in basilar artery smooth
muscle cells through the mitochondrial pathway. Apoptosis.
17:113–120. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Wang Y, Li X, Wang X, Lau W, Wang Y, Xing
Y, Zhang X, Ma X and Gao F: Ginsenoside Rd attenuates myocardial
ischemia/reperfusion injury via Akt/GSK-3β signaling and inhibition
of the mitochondria-dependent apoptotic pathway. PLoS One.
8:e709562013. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Zhu J, Qiu Y, Wang Q, Zhu Y, Hu S, Zheng
L, Wang L and Zhang Y: Low dose cyclophosphamide rescues myocardial
function from ischemia-reperfusion in rats. Eur J Cardiothorac
Surg. 34:661–666. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Ji XY, Tan BK and Zhu YZ: Salvia
miltiorrhiza and ischemic diseases. Acta Pharmacol Sin.
21:1089–1094. 2000.PubMed/NCBI
|
|
9
|
Dong ZT and Jiang WD: Effect of danshensu
on isolated swine coronary artery perfusion preparation (author's
transl). Yao Xue Xue Bao. 17:226–228. 1982.(In Chinese). PubMed/NCBI
|
|
10
|
Lam FF, Yeung JH, Chan KM and Or PM:
Relaxant effects of danshen aqueous extract and its constituent
danshensu on rat coronary artery are mediated by inhibition of
calcium channels. Vascul Pharmacol. 46:271–277. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Wu L, Qiao H, Li Y and Li L: Protective
roles of puerarin and Danshensu on acute ischemic myocardial injury
in rats. Phytomedicine. 14:652–658. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Wong KL, Wu KC, Wu RS, Chou YH, Cheng TH
and Hong HJ: Tetramethylpyrazine inhibits angiotensin II-increased
NAD(P)H oxidase activity and subsequent proliferation in rat aortic
smooth muscle cells. Am J Chin Med. 35:1021–1035. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Feng L, Ke N, Cheng F, Guo Y, Li S, Li Q
and Li Y: The protective mechanism of ligustrazine against renal
ischemia/reperfusion injury. J Surg Res. 166:298–305. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Zhang XP, Wang C, Wu DJ, Ma ML and Ou JM:
Protective effects of ligustrazine, kakonein and Panax
notoginsenosides on multiple organs in rats with severe acute
pancreatitis. Methods Find Exp Clin Pharmacol. 32:631–644. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Huang WD, Yang YF, Chen JW and Zhu BH:
Synergism of salviamiltiorriza bunge and tetramethylpyrazine on
cardiovascular system in rat. Chinese Pharmacological Bulletin.
432–436. 2013.(In Chinese).
|
|
16
|
Buerke M, Murohara T, Skurk C, Nuss C,
Tomaselli K and Lefer AM: Cardioprotective effect of insulin-like
growth factor I in myocardial ischemia followed by reperfusion.
Proc Natl Acad Sci USA. 92:8031–8035. 1995. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Miki T, Miura T, Tsuchida A, Nakano A,
Hasegawa T, Fukuma T and Shimamoto K: Cardioprotective mechanism of
ischemic preconditioning is impaired by postinfarct ventricular
remodeling through angiotensin II type 1 receptor activation. J
Cardiol. 37:112–113. 2001.(In Japanese). PubMed/NCBI
|
|
18
|
Oron U, Yaakobi T, Oron A, Mordechovitz D,
Shofti R, Hayam G, Dror U, Gepstein L, Wolf T, Haudenschild C and
Haim SB: Low-energy laser irradiation reduces formation of scar
tissue after myocardial infarction in rats and dogs. Circulation.
103:296–301. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Kaminski KA, Bonda TA, Korecki J and
Musial WJ: Oxidative stress and neutrophil activation-the two
keystones of ischemia/reperfusion injury. Int J Cardiol. 86:41–59.
2002. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Schulz R, Kelm M and Heusch G: Nitric
oxide in myocardial ischemia/reperfusion injury. Cardiovasc Res.
61:402–413. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Thirunavukkarasu M, Penumathsa SV, Koneru
S, Juhasz B, Zhan L, Otani H, Bagchi D, Das DK and Maulik N:
Resveratrol alleviates cardiac dysfunction in
streptozotocin-induced diabetes: Role of nitric oxide, thioredoxin,
and heme oxygenase. Free Radic Biol Med. 43:720–729. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Vinten-Johansen J, Zhao ZQ, Jiang R, Zatta
AJ and Dobson GP: Preconditioning and postconditioning: Innate
cardioprotection from ischemia-reperfusion injury. J Appl Physiol
(1985). 103:1441–1448. 2007. View Article : Google Scholar : PubMed/NCBI
|