Introduction
Numerous studies have demonstrated that the majority
of retinal ganglion cells (RGCs) undergo cell death following optic
nerve (ON) injury, which leads to irreversible visual impairment
(1–4). The ON crush (ONC) model is commonly
used to analyze neurodegenerative processes in the optic nerve and
retina (2–4). It is well known that delayed RGC
apoptosis is activated following ONC. However, the detailed
mechanisms underlying RGC apoptosis following ONC remain to be
elucidated.
HS-1-associated protein X-1 (Hax-1) was identified
as a 35 kDa multi-functional protein encoded by the Hax-1 gene
(5), and was suggested to be
expressed in various rodent and human tissues (6–8).
Previous studies have demonstrated that abnormal expression of
Hax-1 may be associated with development and disease of the nervous
system, including traumatic brain injury and cerebral ischemia
(9–11). It has also been reported that Hax-1
interacts with various apoptosis-associated proteins, including
high temperature regulated A2 (HtrA2) and caspase-3 (7). Thus, it is possible that Hax-1 is
important in RGC apoptosis following ONC. However, its regulatory
mechanism following ON injury remains unknown.
The present study demonstrated temporal-spatial
patterns of Hax-1 expression in rat retina following ONC. This
research may improve understanding of the physiological functions
of Hax-1 in apoptosis of RGCs following ONC, and its association
with the cellular and molecular mechanisms.
Materials and methods
Experimental animals
A total of 96 rats (age, 6–8 weeks) were handled in
accordance with the Association for Research in Vision and
Ophthalmology Statement on Use of Animals in Ophthalmic and Vision
Research, and experimental protocols were approved by the Ethics
Committee of the People's Hospital of Guangxi Zhuang Autonomous
Region (Nanning, China). Healthy male Sprague-Dawley rats (weight,
220–275 g) supplied by the Medical Laboratory Animal Center
(Guangxi Medical University, Guangxi, China) were kept under
standardized conditions with a 12h light/dark cycle, temperature of
~23°C and 60% humidity. They had access to rodent chow and water
was available. The animals were used for western blotting analysis
(8 groups of 8, n=64), immunofluorescence studies (2 groups of 8,
n=16) and terminal deoxynucleotidyl transferase-mediated
biotinylated-dUTP nick-end labeling (TUNEL) staining (2 groups of
8, n=16).
Surgery
Briefly, rats were anesthetized with 7% chloral
hydrate solution (6 ml/kg body weight) and the surgical procedure
to produce the ONC model was performed as described previously
(3). The left ON was exposed under
a surgical microscope and crushed with forceps 2 mm behind the eye
for 10 sec. A sham operation was performed on the right ON, in
which the ON was exposed but not crushed.
Tissue lysis and western blotting
The rats were sacrificed at different time points by
administering an overdose of anesthesia (650 mg/kg chloral
hydrate). Retina tissues were harvested and stored at −80°C until
use. Cells were lysed in lysis buffer to collect total protein [50
mmol/l Tris (pH 7.5), 1% Triton X-100, 1% NP-40, 10% sodium dodecyl
sulfate, 0.5% sodium deoxycholate, 5 mmol/l Tris EDTA, 10 µg/ml
leupeptin and 10 µg/ml aprotinin) and centrifuging for 20 min at
15,000 × g in a microcentrifuge at 4°C was conducted to
collect the supernatant. Protein concentrations were determined
using the Bradford assay (Bio-Rad Laboratories, Inc., Hercules, CA,
USA). The resulting supernatants (50 µg protein) were subsequently
subjected to 10% SDS-polyacrylamide gel electrophoresis and
transferred to polyvinylidine difluoride membranes (EMD Millipore,
Billerica, MA, USA). Following a blocking step with 5% non-fat
milk, primary antibodies against Hax-1 (1:500; cat. no. sc-28268;
Santa Cruz Biotechnology, Inc., Dallas, TX, USA), active caspase-3
(1:1,000; cat. no. 9661; Cell Signaling Technology, Inc., Danvers,
MA, USA), HtrA2 (1:500; cat. no. sc-25606; Santa Cruz
Biotechnology, Inc.) and β-actin (1:1,000; cat. no. ab8227; Abcam,
Cambridge, MA, USA) were incubated with the membrane at 4°C
overnight. Subsequently, the membranes were washed in Tris/HCl
saline buffer supplemented with 0.1% Tween-20 (pH 7.4) three times
at room temperature (5 min/wash). Following incubation with the
appropriate horseradish peroxidase-conjugated goat anti-rabbit
secondary antibody (1:2,000; cat. no. 4030-05; SouthernBiotech,
Birmingham, AL, USA) for 2 h at room temperature, the blots were
developed using an enhanced chemiluminescence system (Pierce;
Thermo Fisher Scientific, Inc., Waltham, MA, USA).
Immunofluorescence
The rats (n=3 per time point) were anesthetized at
the designated time points and perfused with saline (20 ml)
followed by 4% paraformaldehyde and 0.5% picric acid in 0.1 M
phosphate buffer (pH 7.4, 20 ml). Subsequently, the rats were fixed
in 4% paraformaldehyde overnight at 4°C. The eyes were dehydrated
and cryosections (7 µm) were produced. Briefly, the cryosections
were washed with 1X Tris-buffered saline twice, and then were
blocked with 1% bovine serum albumin (Wuhan Boster Biological
Technology Ltd., Wuhan, China) for 1 h. Subsequently, the sections
were incubated with antibodies against anti-Hax-1 (1:200; cat. no.
sc-34273; Santa Cruz Biotechnology, Inc.), and neuronal marker of
RGCs, anti-NeuN (1:500; cat. no. ABN78; EMD Millipore), anti-HtrA2
(1:200; cat. no. sc-25606; Santa Cruz Biotechnology, Inc.) and
apoptosis marker anti-active caspase-3 (1:200; cat. no. sc-22171;
Santa Cruz Biotechnology, Inc.) overnight at 4°C in a humidified
box. Following incubation with the primary antibodies, the
cryosections were washed three times in phosphate-buffered saline
(PBS), followed by an incubation with donkey anti-goat Alexa
Fluor® 488 (cat. no. ab150129) and donkey anti-rabbit
Alexa Fluor® 555 (cat. no. ab150074) secondary
antibodies (1:1,000; Abcam) for 2 h at 4°C. The stained sections
were examined at ×20 or ×40 magnification on a fluorescence
microscope (Leica Microsystems GmbH, Wetzlar, Germany).
TUNEL staining
TUNEL staining was employed using the In Situ
Cell Death Detection Kit, Fluorescence (Roche Applied Science,
Mannheim, Germany). The cryosections were rinsed with PBS and
treated with 1% Triton-100 in PBS for 2 min on ice. The Slides were
rinsed in PBS and incubated for 60 min at 37°C with 50 µl of TUNEL
reaction mixture. Following washing with PBS three times, the
slides were analyzed on a fluorescence microscope.
Quantitative analysis
A total of three adjacent sections (50 µm apart) per
animal were obtained for each rat. Sections were double labeled for
Hax-1 with phenotype-specific markers, NeuN and active caspase-3.
The number of TUNEL and Hax-1/TUNEL-positive cells in the ganglion
cell layer (GCL) was counted in a 400×400 µm measuring frame. The
cell counts in the three or four adjacent sections were then used
to determine the total number of TUNEL-positive cells or
Hax-1/TUNEL-positive cells/mm2. A minimum of 200
TUNEL-positive cells were counted in each section.
Statistical analysis
All collected data were analyzed with SPSS software
(version 13.0; SPSS, Inc., Chicago, IL, USA). Data are presented as
the mean ± standard error of the mean and each experiment was
repeated at least three times. All statistical analyses were
determined by one-way analysis of variance followed by Tukey's
post-hoc multiple comparison tests. P<0.05 was considered to
indicate a statistically significant difference.
Results
Temporal Hax-1 protein expression by
western blotting following ONC
To quantify the expression pattern of Hax-1 in the
retina, western blotting was conducted at the designated time
points following ONC. It was demonstrated that Hax-1 was low in
sham-operated retina tissue and maintained that level at 1 day
after ONC. Subsequently, Hax-1 protein expression gradually
increased and reached a maximal value at 3 days (P<0.01), prior
to returning to normal levels on day 7 (Fig. 1). These results suggested that
Hax-1 protein may be upregulated by ON injury.
Changes in expression and distribution
of Hax-1 indicated by immunofluorescence staining in the retina
following ONC
To investigate the distribution of Hax-1 following
ONC, double-labeling immunocytochemistry was used to determine the
cellular localization of Hax-1 and NeuN (a marker of RGCs; Fig. 2). As Hax-1 protein reached a
maximal value at 3 days after ONC, this time point was selected for
further examination. Hax-1 expression was demonstrated in RGCs.
Notably, the expression was markedly increased in RGCs at 3 days
after ONC compared with the sham retina (Figs. 2A and B). Hax-1 expression was
predominantly located in NeuN-positive cells (Fig. 2C-F). These findings indicated that
the temporal pattern of Hax-1 following ONC was consistent with the
results of the western blotting, and suggested the localization of
Hax-1 appeared to be confined to the RGCs.
Hax-1 was relevant to RGC apoptosis in
retinal tissue following ONC
In order to investigate the apoptosis of RGCs, TUNEL
staining was performed at 3 days after ONC to further determine
whether Hax-1 expression was involved in the changes in RGC
biological functions, including apoptosis (Fig. 3). Apoptosis of RGCs, as assessed by
TUNEL staining, was relatively lower in retinas from the
sham-operated group (Fig. 3A). The
number of TUNEL and Hax-1/TUNEL-positive cells was significantly
increased at 3 days after ONC (Fig.
3B-G; P<0.05).
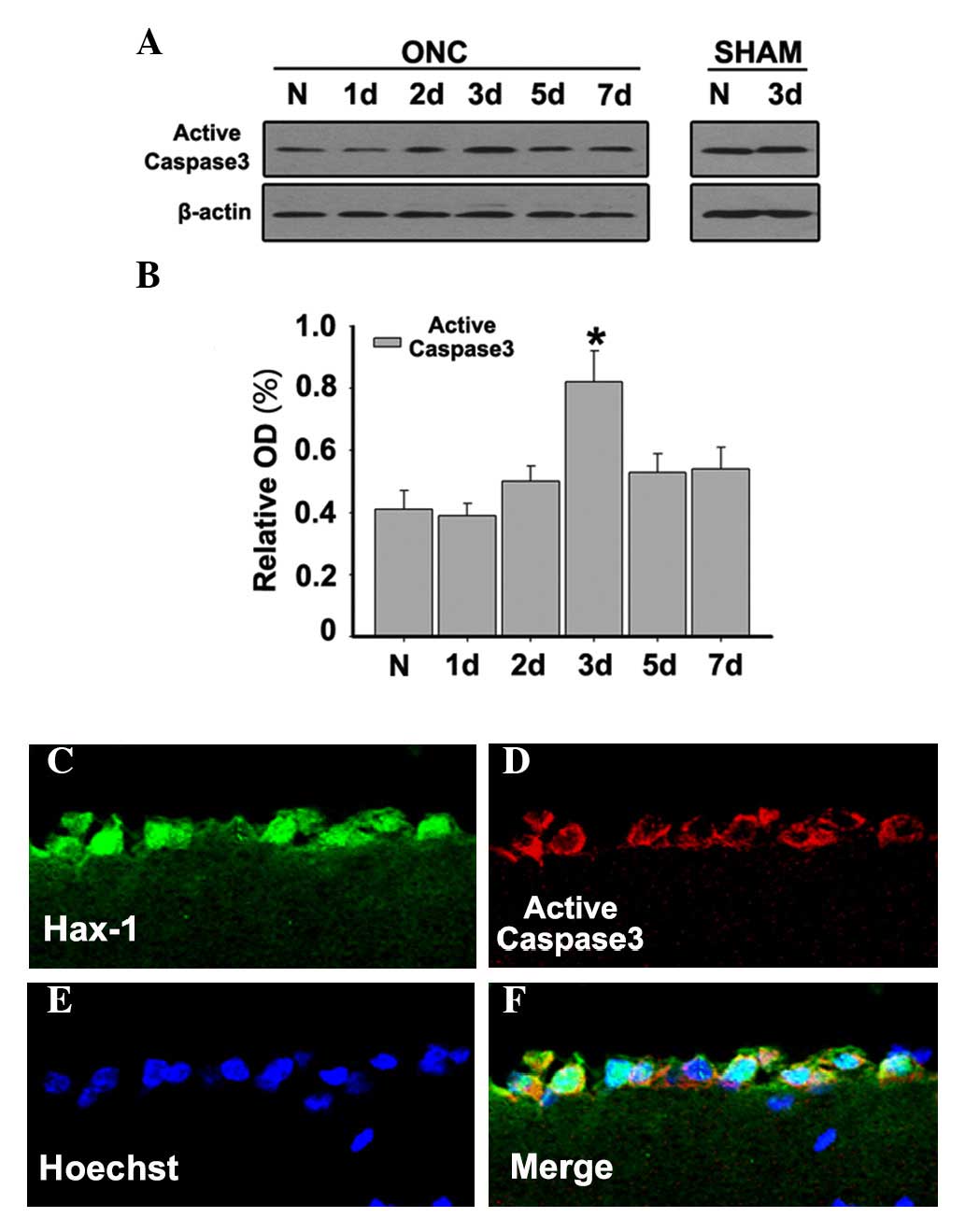
Caspase-3 represents a specific marker of a
subpopulation of apoptotic cells. Western blots were used to
determine the expression levels of active caspase-3. It was
demonstrated that the expression of active caspase-3 was relatively
low in normal retinas, but it increased and reached a maximum at 3
days after ONC (Figs. 4A and
B;P<0.05). To further investigate the distribution and
co-localization of Hax-1 and active caspase-3 at 3 days after ONC,
double immunofluorescent microscopy was performed to detect active
caspase-3 and Hax-1. The results demonstrated that the
co-localization of Hax-1 and active-caspase-3 was observed at 3
days after ONC (Fig. 4C-F). These
results indicated that Hax-1 is important in RGC apoptosis in a
caspase-dependent way following ONC.
Association of Hax-1 with HtrA2 in the
retina following ONC
To investigate the exact role of Hax-1 in RGC
apoptosis following ONC, western blotting was performed to
determine the expression levels of HtrA2. HtrA2 exhibited a marked
upregulation at 2 and 3 days after ONC (Figs. 5A and B; P<0.05 and P<0.01,
respectively). Furthermore, the co-localization of Hax-1 and HtrA2
was observed by double immunofluorescent stain at 3 days after ONC
(Fig. 5C-F).
Discussion
ONC initiates a host of progressive molecular and
cellular events, which evolve over the subsequent hours and days,
resulting in neuronal survival, apoptosis, reactive gliosis and
inflammation (12–15). These secondary events lead to
additional cell apoptosis, axonal degeneration, tissue injury,
impaired regeneration, and functional disabilities (1,16,17).
RGC apoptosis is one of the most severe responses following ON
injury, it may result in loss of RGCs and poor visual function
(18–20). Thus, improved understanding of the
molecular mechanisms involved in the death of RGCs would benefit
the development of neuroprotective therapeutic strategies for the
treatment of ON injury. However, the exact mechanism underlying RGC
death following ON injury remains to be elucidated.
In the present study, the expression profiles of
Hax-1 in adult rat retina were investigated following acute ON
injury. It was demonstrated that the protein expression levels of
Hax-1 were significantly upregulated following ONC. Furthermore, it
was observed that Hax-1 was significantly increased in the GCL at 3
day after ONC, as indicated by immunofluorescent staining. In
addition, based on the double immunofluorescent staining, the
co-localization of Hax-1 and NeuN was detected in the retina. This
result suggested that the localization of Hax-1 appeared to be
confined to the RGCs. The co-localization of Hax-1/active caspase-3
and Hax-1/TUNEL-positive cells was detected in RGCs, thus
suggesting that Hax-1 may participate in RGC apoptosis regulation.
Quantitative analysis indicated that the expression patterns of
active caspase-3 and TUNEL-positive cells were parallel with that
of Hax-1. These data were consistent with the hypothesis that Hax-1
was implicated in pathophysiology of ONs following ONC.
Previous studies have demonstrated that Hax-1 is
important in nervous system disorders, including traumatic brain
injury, cerebral ischemia and seizure-induced hippocampal injury
(9–11). It has been widely reported that
Hax-1 interacts with apoptosis-associated proteins, including
HtrA2, protease-activated receptor 1 (Par1), caspase-3, caspase-9
or Bcl-2-associated X protein (8,21–23).
Numerous studies have demonstrated that the activation of caspase-3
was the key effector of RGC apoptosis following ONC (24–26).
Previously, Hax-1 has been identified as a new substrate of
caspase-3, which prevents apoptosis by inhibiting the catalytic
activation of caspase-3 (27).
Caspase-3 cleaves Hax-1 at the Asp127 residue during apoptosis
(27). Conversely, overexpression
of Hax-1 had an inhibitory effect on caspase-3 activity (22,27,28).
In the present study, the temporal pattern of the expression of
Hax-1 is paralleled with the expression of active caspase-3 in the
retina following ONC. As Hax-1 has these antiapoptotic features, it
was hypothesized that upregulation of Hax-1 may inhibit the
caspase-3-mediated process of RGC apoptosis following ONC.
In addition, Hax-1 has been demonstrated to interact
with HtrA2 via Parl and to promote survival of lymphocytes and
neurons (23,29). HtrA2 has been identified as an
apoptosis-regulating protein (30–32).
During apoptosis, HtrA2 is released from the intermembrane space of
the mitochondria into the cytosol, where it likely promotes
neuronal cell death via promoting caspase activation. In the
current study, HtrA2 expression was paralleled with that of Hax-1
in the retina following ONC. These results suggested that Hax-1 may
participate in regulating RGC apoptosis via interacting with
caspase-3 and HtrA2 following ONC.
In conclusion, the present study is the first, to
the best of our knowledge, to demonstrate that Hax-1 is
significantly upregulated in RGCs after ONC. These findings
suggested that Hax-1 may participate in regulating RGC apoptosis
after ONC. Further studies are required to confirm whether Hax-1
has neuroprotective functions following ONC, which may aid
understanding of a novel molecular pathway of RGC apoptosis
following ONC, and a novel strategy for the treatment of ON
injury.
Acknowledgements
The present study was supported by the National
Natural Science Foundation of China (grant nos. 81460087, 81560166,
81660161 and 81660168), the Natural Science Foundation of Guangxi
Zhuang Autonomous Region (grant nos. 2012GXNSFAA276039 and
2011GXNSFA018228) and the Science Fund Project of People's Hospital
of Guangxi Zhuang Autonomous Region (grant nos. qn2014-1 and
qn2014-2).
Glossary
Abbreviations
Abbreviations:
|
Hax-1
|
HS-1-associated protein X-1
|
|
HtrA2
|
high-temperature-regulated A2
|
|
ONC
|
optic nerve crush
|
|
RGCs
|
retinal ganglion cells
|
|
PAGE
|
polyacrylamide gel electrophoresis
|
|
TUNEL
|
terminal deoxynucleotidyl
transferase-mediated biotinylated-dUTP nick-end labeling
|
|
SEM
|
standard error of mean
|
References
|
1
|
Li HY, Ruan YW, Ren CR, Cui Q and So KF:
Mechanisms of secondary degeneration after partial optic nerve
transection. Neural Regen Res. 9:565–574. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Schwartz M: Optic nerve crush: Protection
and regeneration. Brain Res Bull. 62:467–471. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Xu F, Huang H, Wu Y, Lu L, Jiang L, Chen
L, Zeng S, Li L and Li M: Upregulation of Gem relates to retinal
ganglion cells apoptosis after optic nerve crush in adult rats. J
Mol Histol. 45:565–571. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Wu Y, Xu F, Huang H, Chen L, Wen M, Jiang
L, Lu L, Li L, Song D, Zeng S, et al: Up-regulation of SKIP relates
to retinal ganglion cells apoptosis after optic nerve crush in
vivo. J Mol Histol. 45:715–721. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Suzuki Y, Demoliere C, Kitamura D,
Takeshita H, Deuschle U and Watanabe T: HAX-1, a novel
intracellular protein, localized on mitochondria, directly
associates with HS1, a substrate of Src family tyrosine kinases. J
Immunol. 158:2736–2744. 1997.PubMed/NCBI
|
|
6
|
Simmen T: Hax-1: A regulator of calcium
signaling and apoptosis progression with multiple roles in human
disease. Expert Opin Ther Targets. 15:741–751. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Yap SV, Koontz JM and
Kontrogianni-Konstantopoulos A: HAX-1: A family of apoptotic
regulators in health and disease. J Cell Physiol. 226:2752–2761.
2011. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Fadeel B and Grzybowska E: HAX-1: A
multifunctional protein with emerging roles in human disease.
Biochim Biophys Acta. 1790:1139–1148. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Hu J, Mu C and Hao J: Cerebral ischemia
reduces expression of Hs1-associated protein X-1 (Hax-1) in mouse
brain. Neurosci Lett. 534:338–343. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Rami A, Kim M, Niquet J and Langhagen A:
Alterations in the expression of the anti-apoptotic factor HAX-1
upon seizures-induced hippocampal injury in the neonatal rat brain.
Neurochem Res. 37:116–125. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Shi W, Zhao W, Shen A, Shao B, Wu X, Yang
J, Ni L, Wu Q and Chen J: Traumatic brain injury induces an
up-regulation of Hs1-associated protein X-1 (Hax-1) in rat brain
cortex. Neurochem Res. 36:375–382. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Suzuki H, Oku H, Horie T, Morishita S,
Tonari M, Oku K, Okubo A, Kida T, Mimura M, Fukumoto M, et al:
Changes in expression of aquaporin-4 and aquaporin-9 in optic nerve
after crushing in rats. PLoS One. 9:e1146942014. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Mac Nair CE, Fernandes KA, Schlamp CL,
Libby RT and Nickells RW: Tumor necrosis factor alpha has an early
protective effect on retinal ganglion cells after optic nerve
crush. J Neuroinflammation. 11:1942014. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Yang L, Miao L, Liang F, Huang H, Teng X,
Li S, Nuriddinov J, Selzer ME and Hu Y: The mTORC1 effectors S6K1
and 4E-BP play different roles in CNS axon regeneration. Nat
Commun. 5:54162014. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Huang SP and Tsai RK: Efficacy of
granulocyte-colony stimulating factor treatment in a rat model of
anterior ischemic optic neuropathy. Neural Regen Res. 9:1502–1505.
2014. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Morgan-Warren PJ, Berry M, Ahmed Z, Scott
RA and Logan A: Exploiting mTOR signaling: A novel translatable
treatment strategy for traumatic optic neuropathy? Invest
Ophthalmol Vis Sci. 54:6903–6916. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Watanabe M: Regeneration of optic nerve
fibers of adult mammals. Dev Growth Differ. 52:567–576. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Schmitt HM, Pelzel HR, Schlamp CL and
Nickells RW: Histone deacetylase 3 (HDAC3) plays an important role
in retinal ganglion cell death after acute optic nerve injury. Mol
Neurodegener. 9:392014. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Shibeeb O, Wood JP, Casson RJ and Chidlow
G: Effects of a conventional photocoagulator and a 3-ns pulse laser
on preconditioning responses and retinal ganglion cell survival
after optic nerve crush. Exp Eye Res. 127:77–90. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Chen M, Xiang Z and Cai J: The
anti-apoptotic and neuro-protective effects of human umbilical cord
blood mesenchymal stem cells (hUCB-MSCs) on acute optic nerve
injury is transient. Brain Res. 1532:63–75. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Chao JR, Parganas E, Boyd K, Hong CY,
Opferman JT and Ihle JN: Hax1-mediated processing of HtrA2 by Parl
allows survival of lymphocytes and neurons. Nature. 452:98–102.
2008. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Han Y, Chen YS, Liu Z, Bodyak N, Rigor D,
Bisping E, Pu WT and Kang PM: Overexpression of HAX-1 protects
cardiac myocytes from apoptosis through caspase-9 inhibition. Circ
Res. 99:415–423. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Cilenti L, Soundarapandian MM, Kyriazis
GA, Stratico V, Singh S, Gupta S, Bonventre JV, Alnemri ES and
Zervos AS: Regulation of HAX-1 anti-apoptotic protein by Omi/HtrA2
protease during cell death. J Biol Chem. 279:50295–50301. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Wang Y, Zhang H, Liu Y, Li P, Cao Z and
Cao Y: Erythropoietin (EPO) protects against high glucose-induced
apoptosis in retinal ganglional cells. Cell Biochem Biophys.
71:749–755. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Cao Y, Li X, Shi P, Wang LX and Sui ZG:
Effects of L-carnitine on high glucose-induced oxidative stress in
retinal ganglion cells. Pharmacology. 94:123–130. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Rathnasamy G, Sivakumar V, Rangarajan P,
Foulds WS, Ling EA and Kaur C: NF-kB-mediated nitric oxide
production and activation of caspase-3 cause retinal ganglion cell
death in the hypoxic neonatal retina. Invest Ophthalmol Vis Sci.
55:5878–5889. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Lee AY, Lee Y, Park YK, Bae KH, Cho S, Lee
DH, Park BC, Kang S and Park SG: HS 1-associated protein X-1 is
cleaved by caspase-3 during apoptosis. Mol Cells. 25:86–90.
2008.PubMed/NCBI
|
|
28
|
Yedavalli VS, Shih HM, Chiang YP, Lu CY,
Chang LY, Chen MY, Chuang CY, Dayton AI, Jeang KT and Huang LM:
Human immunodeficiency virus type 1 Vpr interacts with
antiapoptotic mitochondrial protein HAX-1. J Virol. 79:13735–13746.
2005. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Li B, Hu Q, Wang H, Man N, Ren H, Wen L,
Nukina N, Fei E and Wang G: Omi/HtrA2 is a positive regulator of
autophagy that facilitates the degradation of mutant proteins
involved in neurodegenerative diseases. Cell Death Differ.
17:1773–1784. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Zurawa-Janicka D, Skorko-Glonek J and
Lipinska B: HtrA proteins as targets in therapy of cancer and other
diseases. Expert Opin Ther Targets. 14:665–679. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Bhuiyan MS and Fukunaga K: Mitochondrial
serine protease HtrA2/Omi as a potential therapeutic target. Curr
Drug Targets. 10:372–383. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Sun H, Li L, Zhou F, Zhu L, Ke K, Tan X,
Xu W, Rui Y, Zheng H, Zhou Z and Yang H: The member of high
temperature requirement family HtrA2 participates in neuronal
apoptosis after intracerebral hemorrhage in adult rats. J Mol
Histol. 44:369–379. 2013. View Article : Google Scholar : PubMed/NCBI
|