Introduction
Hepatitis B virus (HBV) is a major cause of liver
cirrhosis and hepatocellular carcinoma (HCC), which is a severe
threat to patient health. Approximately 400 million people have
been diagnosed in worldwide, and in addition, the risk of
HBV-associated mortality is greater than 15% (1–5).
Although several therapies targeting virus invasion and replication
have exhibited some curative effects, it remains difficult to
thoroughly eradicate HBV. This is due to the fact that the cccDNA
reservoir persists in infected cells, and in addition that the
immunosuppressive environment induced by HBV infection counteracts
the antiviral response of the local innate immune system and
impairs the specific immune response, resulting in defective immune
surveillance and a chronic viral infection (6–9). The
HBV-induced systemic immune tolerance (IT) generally presents with
the characteristics of a shift of T helper cell (Th) 1/Th2 balance,
a deficient cytotoxic T-lymphocyte response to hepatitis B surface
antigen (HBsAg) or hepatitis B core antigen and an increased
proportion of regulatory T cells (Tregs) (10–16).
However, the alterations to T cell-mediated specific immunity in
HBV infection, and how HBV induces the immunosuppression remain
unclear.
Interleukin 33 (IL-33), a novel member of the IL-1
family, has been identified as the special ligand for the receptor
suppression of tumorigenicity 2 (ST2), which is selectively
expressed on Th2 cells however not on Th1 cells. IL-33 was
initially recognized as a specific Th2 effector, inducing the
production of IL-4, IL-5, IL-6, IL-10 and IL-13, and leading to the
IT of Th2-associated diseases including asthma, atopic dermatitis
and anaphylaxis (17–19). Further investigations indicated
that the IL-33/ST2 axis was not regulating the Th2 response alone,
however was additionally acting as an important component in
Th1/Th17 and innate inflammation (20,21).
In addition, IL-33 has been reported to exhibit various protective
effects in cardiovascular conditions including atherosclerosis and
cardiac remodeling, in addition to obesity and type 2 diabetes
(22–25). In cardiomyocytes and hepatocytes,
IL-33 has been reported to protect against apoptosis (26). The mechanism of IL-33 on regulating
T-cell subsets is complex, and whether IL-33 participates in the
regulation of T-cell mediated IT during HBV infection remains to be
investigated.
In the current study, the distribution of different
T-lymphocyte subsets in was investigated in patients with HBV at
different immune phases, and in addition, the effect of IL-33 on
the regulation of T subset distribution and the levels of relative
inflammatory cytokines was assessed. Furthermore, the interaction
between IL-33 and HBV × protein (HBx) was also explored. The
results of the current study suggested that clinical therapy
targeting IL-33 may be a potential method to reverse IT in patients
with HBV.
Materials and methods
Patients
A total of 50 patients with chronic HBV and 20
vaccinated healthy controls (HCs) were recruited from the
Department of Hepatology of the First Hospital of Jilin University
(Changchun, China). Patients with chronic HBV were categorized
according to the disease phase: IT phase (n=17) and immune
activation (IA) phase (n=18). The remaining 15 patients were
interferon (IFN)-α therapy-induced responders with HBsAg
seroconversion (RP). None of the patients had received antiviral
treatment within the previous 6 months. All individuals were
negative for other infectious diseases, autoimmune disorders and
malignancies. The clinical characteristics of these participants
are listed in Table I. The levels
of serum aspartate aminotransferase (AST), alanine transaminase
(ALT) and α-fetoprotein (AFP) in participants were detected using
the Biochemistry Automatic Analyzer (Roche Diagnostics, Lewes, UK).
The Ethical Committee of the First Hospital of Jilin University had
approved the experiment, and all participants had provided written
informed consent.
 | Table I.Clinical characteristics of
participants in the study. |
Table I.
Clinical characteristics of
participants in the study.
| Group | HC n=20 | IT n=17 | IA n=18 | RP n=15 |
|---|
| Sex
(male/female) | 10/10 | 12/5 | 15/3 | 11/4 |
| Age (years) | 28 (25–35) | 33 (18–49) | 36 (24–52) | 37 (20–49) |
| ALT (U/l) | 24 (13–27) | 26 (16–37) | 292
(66–985)a | 36 (16–43) |
| AST (U/l) | 22 (15–32) | 25 (19–32) | 328
(47–875)a | 19 (12–38) |
| AFP (ng/ml) | 7 (2–11) | 64
(42–87)a | 194
(12–723)a | 28 (9–47) |
| HBV DNA (100
IU/ml) | ND | 8.2 (1.8–8.9) | 6.8 (3.3–8.8) | LDL |
Cell culture and transfection
HepG2.2.1.5 (derived from HepG2 cells transfected
with a plasmid carrying two head-to-tail copies of the HBV genome
DNA serotype ayw) cell line was originally obtained from the
American Type Culture Collection (Manassas, VA, USA) and were
maintained at 37°C in 5% CO2 in Dulbecco's modified
Eagle's medium supplemented with 10% fetal bovine serum
(Invitrogen; Thermo Fisher Scientific, Inc., Waltham, MA, USA). To
silence the HBx gene, the specific short hairpin RNA (shRNA) was
inserted into the shRNA pSIREN expression vector. The shRNA
sequences targeting HBx were as follows: HBx-shRNA forward
5′-GGATCCAGGTCTTTGTACTAGGAGGCTCCACCAGCCTCCTAGTACAAAGACCTT-3′;
reverse
5′-GAATTCAAGGTCTTTGTACTAGGAGGCTGGTGGAGCCTCCTAGTACAAAGACCT-3′. Then
the recombinant vector was transfected into HBV-persistent
HepG2.2.1.5 cells. The day before plasmid transfection, HepG2.2.1.5
(2×106) were seeded onto 6-well plates (Costar; Corning
Incorporated, Corning, NY, USA) at 80% confluency. Following 24 h
incubation at 37°C, cells were transfected with pSIREN-HBx-shRNA or
empty pSIREN vectors separately according to the manufacturer's
instructions of Lipofectamine 2000 (Invitrogen; Thermo Fisher
Scientific, Inc.). Cells were collected using centrifugation at
1,100 × g for 10 min at room temperature 48 h subsequent to
transfection. A total of 50 ng/ml IL-33 (PeproTech, Inc., Rocky
Hill, NJ, USA) was used to stimulate HepG2.2.1.5 and HBx-deficient
HepG2.2.1.5 cells in vitro. In order to inhibit the IL-33
relative signaling, the ST2 blocking antibody (sc-18687P; Santa
Cruz Biotechnology, Inc., Dallas, TX, USA) was used to inhibit the
normal binding of IL-33 to its receptor ST2L.
Peripheral blood mononuclear cell
(PBMC) isolation and flow cytometry
PBMCs from venous blood samples were isolated by
Ficoll-Paque (GE Healthcare Life Sciences, Chalfont, UK)
density-gradient centrifugation at 1,100 × g for 30 min at
room temperature. For analyzing the distribution of T-lymphocyte
subsets, separated PBMCs were stained with different
fluorescein-labeled antibodies (Abs). Surface staining was
performed using the following monoclonal Abs: Anti-human
CD4-phycoerythrin (PE; 12-0049-42), anti-human CD8-fluorescein
isothiocyanate (9011–0087), anti-human CD45RA-allophycocyanin
(17–0458), anti-human CCR7-PE-cyanine7 (25–1979) and the
corresponding fluorescence-conjugated immunoglobulin G isotypes
(all antibodies from eBioscience, Inc., San Diego, CA, USA). A
minimum of 50,000 cells prepared for phenotypic analysis were
collected using a FACSCalibur (BD Pharmingen, San Diego, CA, USA)
analytical instrument and were analyzed by FlowJo software, version
7.6 (Tree Star, Inc., Ashland, OR, USA).
Enzyme-linked immunosorbent assay
(ELISA)
Venous blood (10 ml) from patients with HBV and HCs
were collected and centrifuged at 1,100 × g for 10 min at
room temperature. Subsequently, the blood plasma samples were used
to detect inflammatory factors using ELISA. The ELISA kit (Roche
Diagnostics) was used to detect the serum or supernatant levels of
IL-4, IL-5, IL-10, IL-12, IFN-γ and tumor necrosis factor α (TNF-α)
according to the manufacturer's instructions. The absorbance of the
plates was read at 450 nm using an Automated Microplated Reader
(BioTek Instruments, Inc., Winooski, VT, USA).
Reverse transcription-quantitative
polymerase chain reaction (RT-qPCR)
The total RNA from HepG2.2.1.5 and HBx-deficient
HepG2.2.1.5 cells were isolated with TRIzol (Invitrogen; Thermo
Fisher Scientific, Inc.). Reverse transcription was conducted using
500 ng total RNA with the RevertAid First Strand cDNA Synthesis kit
(Thermo Fisher Scientific, Inc.).
IL-33-specific RT-qPCR amplification was performed
with Power SYBR Green Master Mix (containing SYBR® Green
I Dye, AmpliTaq Gold® DNA Polymerase, dNTPs, passive
reference and optimized buffer) using ABI 7300 (Applied Biosystems;
Thermo Fisher Scientific, Inc.). The qPCR cycling conditions were
as follows: 95°C for 10 min followed by 40 cycles of 95°C for 15
sec and 60°C for 1 min, and all experiments were repeated three
times. Relative gene expression was calculated with the
2−ΔΔCq method (27)
following normalization to the expression of GAPDH. The primers
used were as follows: IL-33, sense 5′-CACCCCTCAAATGAATCAGG-3′ and
antisense 5′-GGAGCTCCACAGAGTGTTCC-3′; HBx, sense
5′-CGACCGACCTTGAGGCATACT-3′ and antisense
5′-TTAGGCAGAGGTGAAAAAGTTG-3′.
Western blotting
HepG2.2.1.5 and HBx-deficient HepG2.2.1.5 cells were
harvested and then lysed in RIPA buffer (Beyotime Institute of
Biotechnology, Haimen, China). Following centrifugation at 10,000 ×
g for 15 min at 4°C, whole cell lysates were subjected to
10% SDS-polyacrylamide gel electrophoresis, and transferred onto
polyvinylidene difluoride membranes (GE Healthcare Life Sciences).
Sequentially, the membranes were incubated with the indicated
antibodies. The primary antibodies for IL-33 (sc-130625), HBx
(sc-57760) and GAPDH (sc-32233), in addition to the goat anti-mouse
IgG horseradish peroxidase-conjugated secondary antibody (sc-2302)
were all purchased from Santa Cruz Biotechnology, Inc.
Statistical analysis
All data are representative of three independent
experiments and are expressed as the median ± range or mean ±
standard deviation as indicated. Statistical analysis was conducted
using Student's t-test. P<0.05 was considered to indicate
a statistically significant difference. All analyses were performed
using GraphPad software, version 5.0 (GraphPad, Inc., La Jolla, CA,
USA).
Results
T-lymphocyte subset distribution in
patients with HBV at different immune stages
To investigate the frequencies of different T-cell
subsets in patients with HBV at different immune stages; IT, immune
clearance (IC), IA and RP (28);
PBMCs were isolated. Subsequently, the proportional changes of
naïve T cells (TN; CD45RA+CCR7+), central memory T cells (TCM;
CD45RA-CCR7+) and effector memory T cells (TEM; CD45RA-CCR7-) were
assessed (29,30). As presented in Fig. 1, there was no significant
difference in the frequencies of CD4+ TN and CD8+ TN in patients
and HC. The proportions of CD4+ TCM and CD4+ TEM were markedly
increased in patients with HBV compared with HC, however appeared
at similar levels within the three patient groups. Similar to the
results of CD4+ TCM and CD4+ TEM, the frequencies of CD8+ TCM and
CD8+ TEM were increased in IT and IA compared with HC. However,
different to the results of CD4+, the CD8+ TCM and TEM subsets were
significantly reduced in the RP group compared with that of IT and
IA. In particular, the proportion of CD8+ TEM was lower in RP than
in HC, and levels were statistically greater in IA compared with
IT. This data indicated that levels of CD8+ TEM cells were
associated with the immune state of patients with HBV. The results
indicated that CD8+ was more sensitive to HBV activation and IFN-α
based therapy. The frequency of CD8+ TEM cells may be an effective
marker to assess the immune state of patients with HBV and the
effect of clinical therapy.
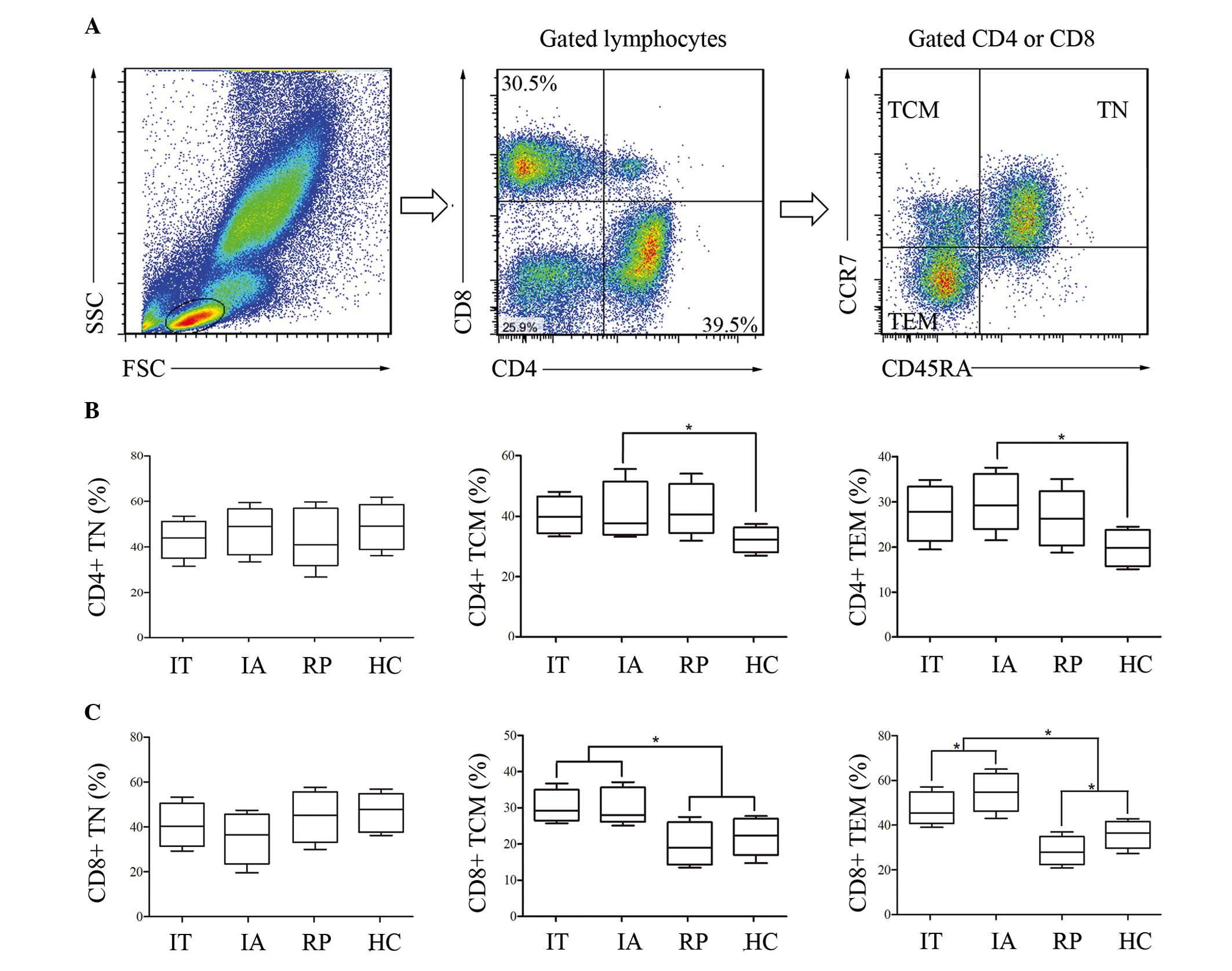 | Figure 1.Differential distribution of
circulating CD8+ and CD4+ T-lymphocyte subsets in HBV-infected
patients at various immune phases. PBMCs from patients with HBV and
HCs were isolated and categorized as TN (CD45RA+CCR7+), TCM
(CD45RA-CCR7+) and TEM (CD45RA-CCR7-). (A) The flow cytometry chart
represented the gating strategy. Detailed proportions of naïve and
memory (B) CD4+ T cells, in addition to (C) CD8+ T subsets in the
participants were presented in the form of a box plot. The boxes
represented the 5th, 25th, 75th and 95th percentiles, and the solid
line indicates the median value of each subset. *P<0.05. HBV,
hepatitis B virus; PBMCs, peripheral blood mononuclear cells; HC,
healthy controls; TN, naïve T cells; TCM, central memory T cells;
TEM, effector memory T cells; IT, immune tolerance; IA, immune
activation; RP, responders with hepatitis B surface antigen
seroconversion. |
Additionally, the serum levels of IL-4, IL-5, IL-10,
IL-12, IFN-γ and TNF-α were measured in order to evaluate the
immune status of the participants. As presented in Fig. 2, the levels of Th2-associated
factors (IL-4, IL-5 and IL-10) were all increased in IT and IA,
however a clear reduction was observed in the RP group, which may
have been induced by an IFN-α based therapy. The concentrations of
Th1-associated factors (IL-12, IFN-γ and TNF-α) appeared in general
at similar low levels in IT and IA, while the patients who had been
treated exhibited increased levels. The data indicated that there
was a clear Th2-dominant immune response in patients with HBV, and
the IFN-α based therapy predominantly improved the Th1 response
while inhibiting the Th2 reaction.
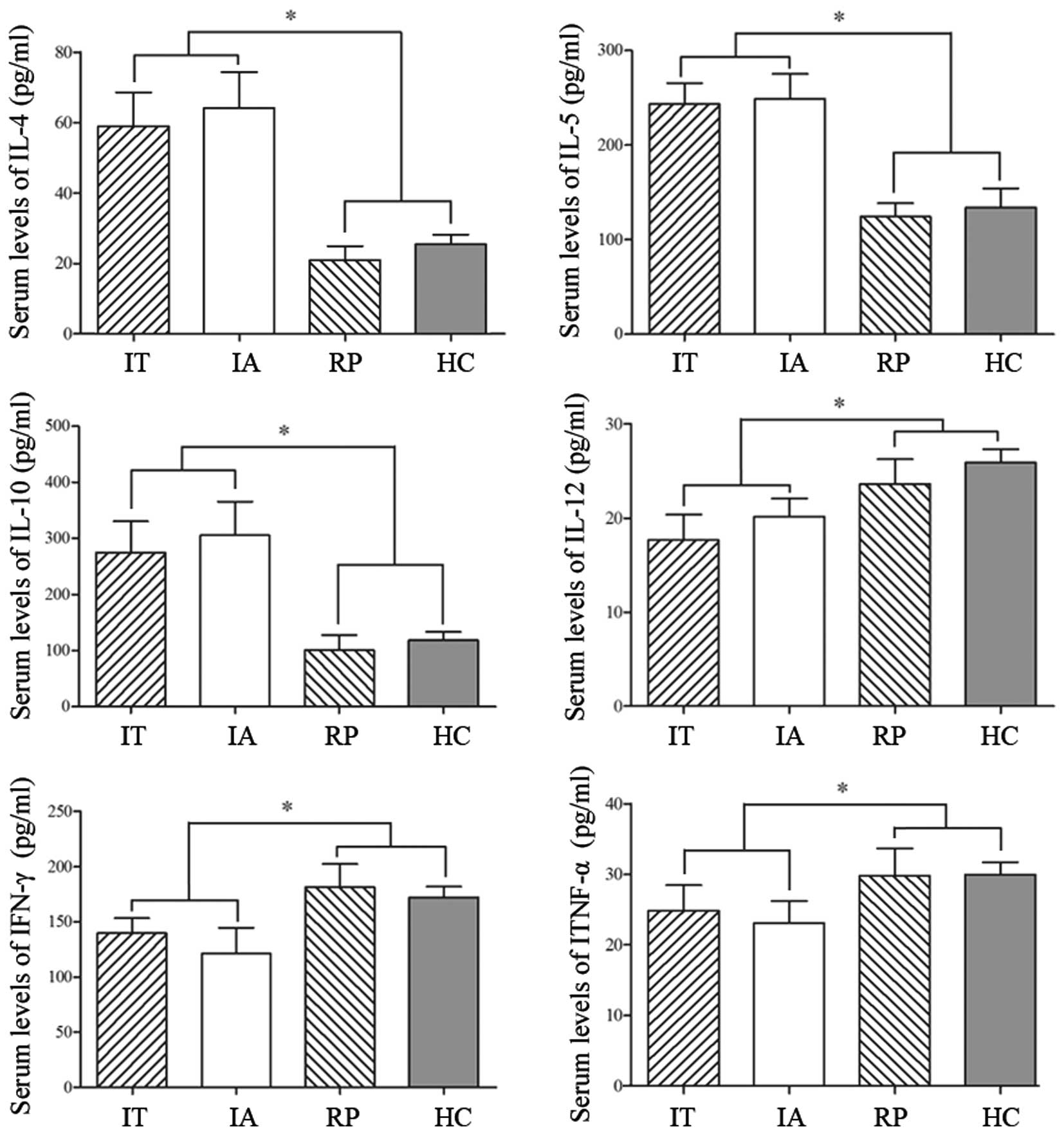 | Figure 2.The immune state in HBV patients at
different immune stages. Venous blood (10 ml) from patients with
HBV and HCs was separated to detect inflammatory factors using
ELISA. The levels of IL-12, IFN-γ and TNF-α indicated a
Th1-mediated immune response, while IL-4, IL-5 and IL-10 are
associated with the Th2-mediated immune response. The data are
expressed as the mean ± standard deviation. *P<0.05. HBV,
hepatitis B virus; IL-12, interleukin 12; IFN-γ, interferon γ;
TNF-α, tumor necrosis factor α; Th, T helper; IT, immune tolerance;
IA, immune activation; RP, responders with hepatitis B surface
antigen seroconversion; HC, healthy controls. |
The effect of IL-33 on the
distribution of T-lymphocyte subsets
As demonstrated by a previous study, serum IL-33
levels were closely associated with liver damage in patients with
chronic hepatitis B (31);
meanwhile, IL-33 could enhance humoral immunity against HBV
infection through activating T follicular helper cells (32). In the current study, in order to
investigate the effect of IL-33 on T-cell subsets, PBMCs were
isolated from patients with HBV at the IT stage (since the
frequency of CD8+ TEM was already at a very high level in the IA
phase), and were stimulated with 50 ng/ml IL-33 for 0, 2, 6 and 12
h respectively. As presented in Fig.
3A, the percentages of CD4+ TN, TCM and TEM cells were not
significantly altered obviously following stimulation with IL-33.
The frequency of CD8+ TCM also seemed not to be influenced by
IL-33, however the proportion of CD8+ TEM was reduced while CD8+ TN
increased over time of IL-33 treatment. The concentrations of
Th2-secreting cytokines were additionally upregulated along with
the duration of IL-33 treatment, which indicated a trend towards
Th2 response (Fig. 3B). These
results demonstrated that IL-33-induced IT may be mediated by the
modulation of CD8+ TN and CD8+ TEM cells.
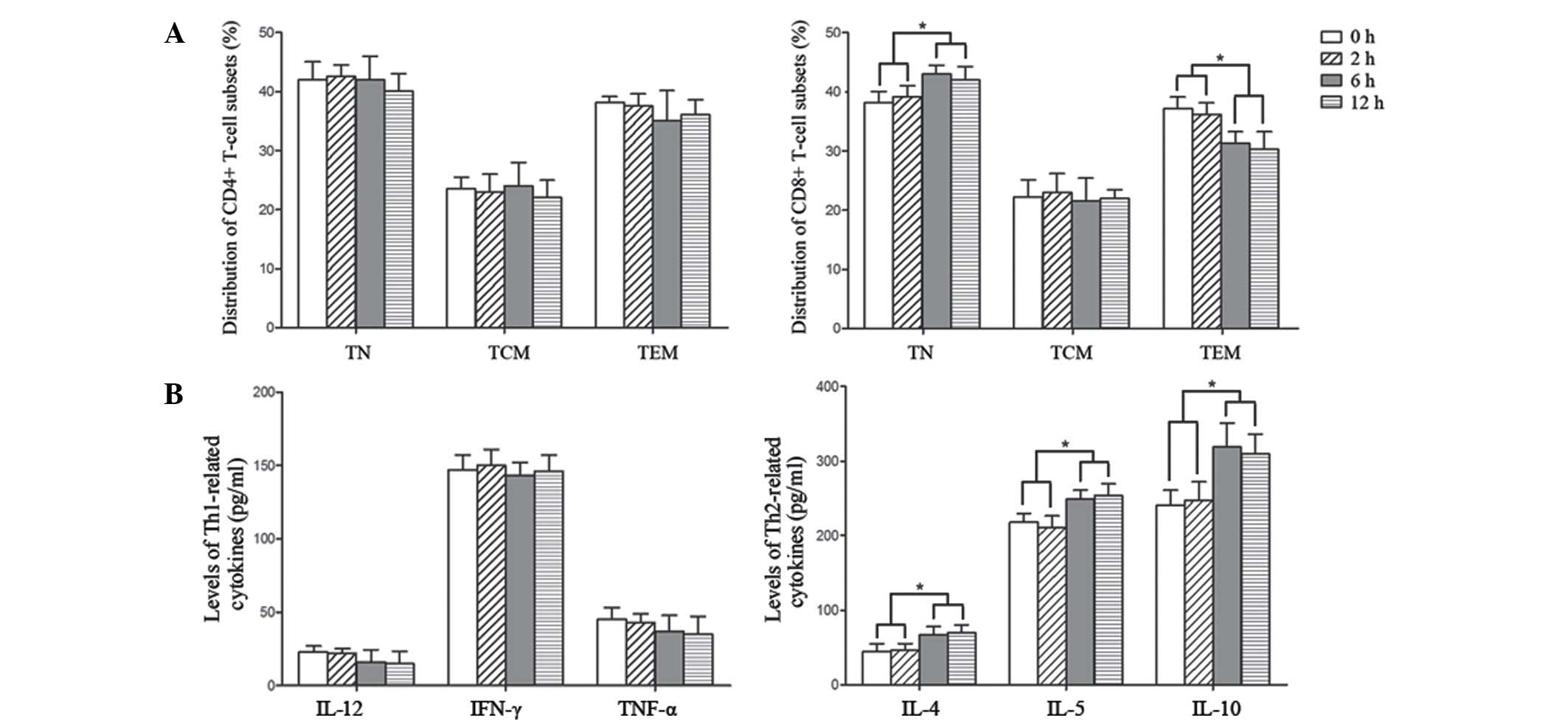 | Figure 3.The frequency changes of T-cell
subsets induced by IL-33. PBMCs from healthy controls were isolated
and stimulated with 50 ng/ml IL-33 for 0, 2, 6 and 12 h. (A)
Significant increases in CD8+ TN and reductions in CD8+ TEM were
observed following IL-33 stimulation for 6 h. (B) Expression of
Th2-associated cytokines were increased while Th1-associated
factors were reduced as a result of IL-33 treatment. The data are
presented as the mean ± standard deviation. *P<0.05. IL-33,
interleukin 33; PBMC, peripheral blood mononuclear cells; TN, naïve
T cells; TCM, central memory T cells; TEM, effector memory T cells;
Th, T helper; IFN-γ, interferon γ; TNF-α, tumor necrosis factor
α. |
The interaction between IL-33 and
HBx
As a key risk factor involved in HBV chronic
infection, the HBx protein is able to bind directly to DNA and
perform transcriptional activation. HBx has been demonstrated to
accelerate the progress of HCC in numerous aspects, including
involvement in apoptosis, proliferation, inflammation,
angiogenesis, immune responses and multi-drug resistance (33,34).
In order to investigate whether IL-33 could been regulated by HBx,
a vector containing HBx-gene-silencing shRNA was constructed, and
it was then transferred into HepG2.2.1.5 cells. Subsequently, the
expression of IL-33 was detected in HepG2.2.1.5 and HBx-deficient
HepG2.2.1.5 cells, respectively. The expression levels of IL-33
were observed to be at a higher level in HepG2.2.1.5 than in
HBx-deficient HepG2.2.1.5 cells at both transcriptional and
translational levels (Fig. 4A). To
clarify whether IL-33 could additionally influence HBx, the ST2
blocking antibody was used in order to antagonize IL-33-mediated
signaling transduction. As presented in Fig. 4B, although the levels of HBx
exhibited certain changes in the IL-33 stimulating and ST-2
blocking groups when compared with the untreated group, the
differences had no statistical significance. These data
demonstrated that HBx may directly regulate IL-33, however, IL-33
was not a key factor to affect the expression of HBx. HBx
production is clearly modulated by more complex cell signaling
networks, which remain to be fully elucidated.
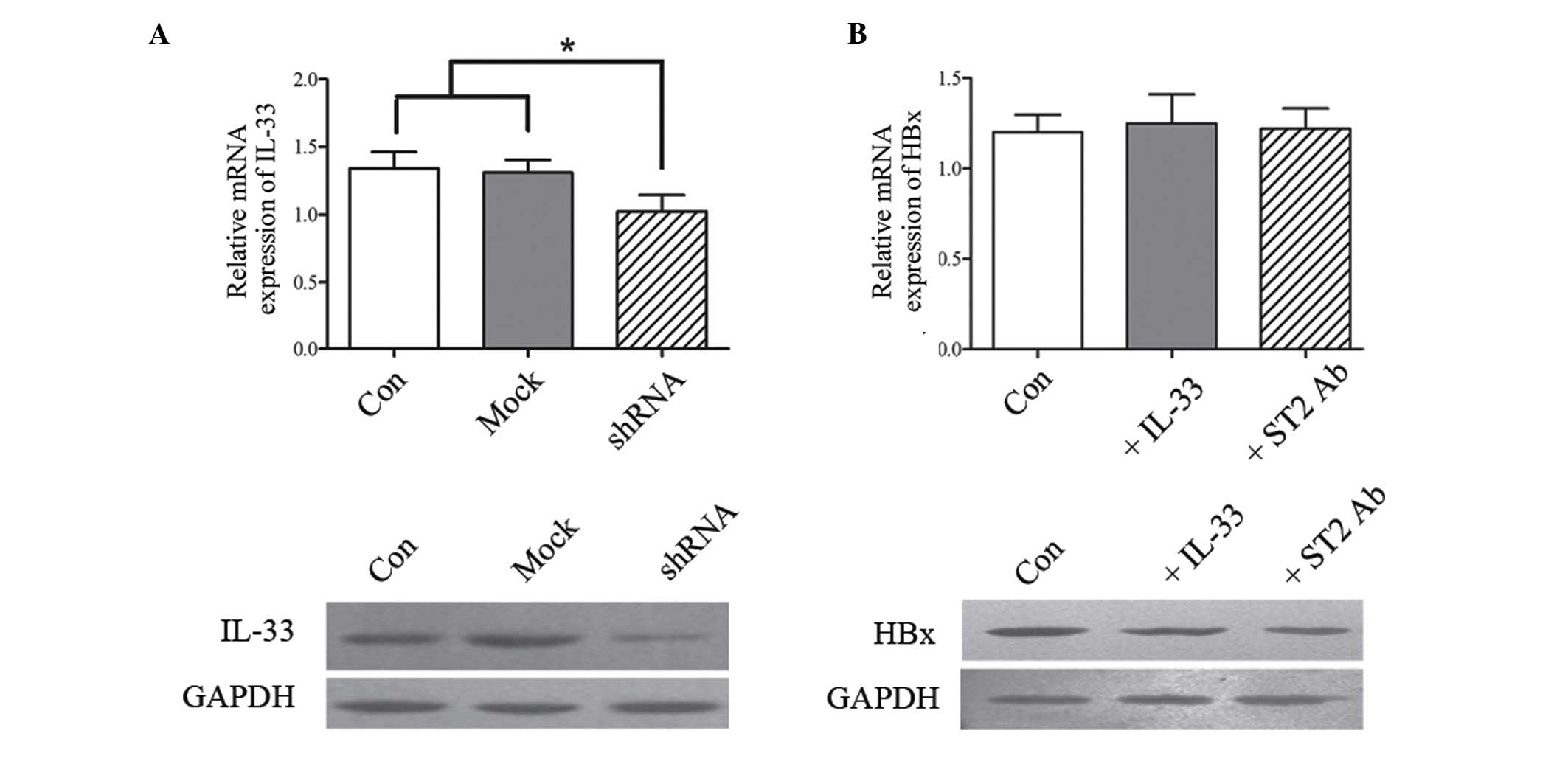 | Figure 4.The interaction between IL-33 and HBx
protein. (A) Total RNA from HepG2.2.1.5 and HBx-deficient
HepG2.2.1.5 were isolated to detect the level of intracellular
IL-33 at a transcriptional level by quantitative polymerase chain
reaction, while these cells cultured in vitro were harvested
to analyze expression of IL-33 at a translational level by western
blot analysis. (B) To clarify whether HBx was influenced by IL-33,
the expression of HBx was tested in IL-33-stimulated and ST2
antibody-blocked HepG2.2.1.5 cells at transcriptional and
translational levels using the above-mentioned methods. Con,
HepG2.2.1.5; Mock, HepG2.2.1.5 transfected with empty plasmid;
shRNA, HepG2.2.1.5 transfected with shRNA targeted HBx. *P<0.05.
IL-33, interleukin 33; HBx, hepatitis B virus × protein; ST2,
suppression of tumorigenicity 2; shRNA, short hairpin RNA; Con,
control. |
Discussion
HBV-induced systemic IT generally inhibits the
innate or adaptive immune response, resulting in a life-long
chronic viral infection (6–9). In
the current study, the distributions of TN, TCM and TEM cells were
initially investigated in patients with HBV at the IA, IT and RP
immune stages, respectively. Neither CD4+ TN nor CD8+ TN were
identified to be associated with the immune phase in participants.
Meanwhile, although the proportions of CD4+ TCM and CD4+ TEM were
greater in IT, IA and RP than in HC, there were no significant
alterations within the three patient groups. The phenomenon that
there were no clear changes of CD4+TN/TCM/TEM cells may be
associated with the various compositions of Th cells, including
immune-promoted CD4+ T cells and immune-suppressive types such as
Tregs. The integrated effects of these Th subsets may lead to the
frequency of stable CD4+ T cells in patients with HBV at different
immune stages. By contrast, although the frequencies of CD8+ TCM
and CD8+ TEM cells were identified to be increased in the IA and IT
groups, these high levels could be restored in patients at the RP
stage who had received clinical treatment. The percentage of CD8+
TEM cells was greater in IA when compared with IT, and it was
reduced to a lower level in the RP group compared with the HC
group. This indicated that CD8+ TEM, rather than other naïve and
memory CD4+ or CD8+ T subsets, may be a more sensitive marker to
evaluate the HBV activation and the effect of clinical therapy.
Furthermore, the levels of IL-4, IL-5 and IL-10 were detected,
which represented the Th2 immune response, in addition to IL-12,
IFN-γ and TNF-α, which are highly expressed in the Th1 response.
The data demonstrated that the IFN-α based therapy resulted in a
marked reversal of the dominant Th2 response, and an increase in
the Th1-associated factors, resulting in a Th2 to Th1 shift.
Although there previous studies have focused upon
the role of IL-33 in mediating the Th2-associated immune response,
its effect on different T-lymphocyte subsets remains unclear. Thus,
in the current study, PBMCs were stimulated with IL-33. The main
effect of IL-33 identified was the regulation of CD8+ TEM and CD8+
TN cells, with little influence on CD4+ T subsets and CD8+ TCM
cells. Considering the Th1 to Th2 shift induced by IL-33, it was
concluded that IL-33-mediated IT partially resulted from the
imbalance of CD8+ TN and CD8+ TEM, particularly the inhibition of
the main cytotoxic lymphocytes, the CD8+ TEM cells.
Considering HBx was a key transcriptional activator
in HBV, the association between IL-33 and HBx was further
investigated. The level of IL-33 was demonstrated to be directly
diminished subsequent to silencing of the HBx gene. However, the
expression of HBx was not significantly altered following IL-33
treatment or the blocking of IL-33/ST2 signaling. Thus the IT
induced by HBV may be associated with the effect of HBx promoting
IL-33, which has been recognized as an inducer of Th2. Furthermore,
the expression of HBx appears to act independently of IL-33,
however requires additional cytokines.
In conclusion, the current study identified that
HBV-induced IT may be mediated via the regulation of IL-33 through
HBx, leading to the imbalance of CD8+ TEM and CD8+ TCM cells, in
addition to a Th2-dominant response. Thus, it is suggested that
clinical therapy targeting IL-33 may be a potential method to
enhance the immune response of patients with HBV. Further
longitudinal studies focussing upon the immunosuppression caused by
HBV and the associated inflammatory cytokines should be conducted
in order to further understand these processes.
Acknowledgements
The current study was supported by grants from the
National Natural Science Foundation of China (grant nos. 30972610
and 81273240), Jilin Province Science and Technology Agency (grant
no. 20110716), the Health Department Research Projects in Jilin
Province (grant no. 2009Z054), Norman Bethune Program of Jilin
University (grant no. 2012206) and the Special Research Foundation
of Jilin University (grant no. B03).
Glossary
Abbreviations
Abbreviations:
|
HCC
|
hepatocellular carcinoma
|
|
HBV
|
hepatitis B virus
|
|
HBx
|
HBV × protein
|
|
Th
|
T helper cell
|
|
Treg
|
regulatory T cells
|
|
ST2
|
suppression of tumorigenicity 2
|
|
IFN-γ
|
interferon γ
|
|
TNF-α
|
tumor necrosis factor α
|
|
IL-33
|
interleukin 33
|
|
HC
|
healthy controls
|
|
PBMCs
|
peripheral blood mononuclear cells
|
|
IT
|
immune tolerance
|
|
IA
|
immune activation
|
|
RP
|
responders with hepatitis B surface
antigen seroconversion
|
|
AST
|
aspartate aminotransferase
|
|
ALT
|
alanine transaminase
|
|
AFP
|
α-fetoprotein
|
|
PBS
|
phosphate-buffered saline
|
|
ELISA
|
enzyme-linked immune-sorbent assay
|
|
qRT-PCR
|
quantitative reverse
transcription-polymerase chain reaction
|
|
Abs
|
antibodies
|
References
|
1
|
Jemal A, Bray F, Center MM, Ferlay J, Ward
E and Forman D: Global cancer statistics. CA Cancer J Clin.
61:69–90. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Zender L, Villanueva A, Tovar V, Sia D,
Chiang DY and Llovet JM: Cancer gene discovery in hepatocellular
carcinoma. J Hepatol. 52:921–929. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Takayama T, Sekine T, Makuuchi M, Yamasaki
S, Kosuge T, Yamamoto J, Shimada K, Sakamoto M, Hirohashi S, Ohashi
Y and Kakizoe T: Adoptive immunotherapy to lower postsurgical
recurrence rates of hepatocellular carcinoma: A randomised trial.
Lancet. 356:802–807. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Singal AG, Nehra M, Adams-Huet B, Yopp AC,
Tiro JA, Marrero JA, Lok AS and Lee WM: Detection of hepatocellular
carcinoma at advanced stages among patients in the HALT-C trial:
Where did surveillance fail? Am J Gastroenterol. 108:425–432. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Bréchot C: Pathogenesis of hepatitis B
virus related hepatocellular carcinoma: Old and new paradigms.
Gastroenterology. 127(5): Suppl 1. S56–S61. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Tanaka T, Bai Z, Srinoulprasert Y, Yang
BG, Hayasaka H and Miyasaka M: Chemokines in tumor progression and
metastasis. Cancer Sci. 96:317–322. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Jain RK: Normalizing tumor
microenvironment to treat cancer: Bench to bedside to biomarkers. J
Clin Oncol. 31:2205–2218. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Moeini A, Cornellà H and Villanueva A:
Emerging signaling pathways in hepatocellular carcinoma. Liver
Cancer. 1:83–93. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Zheng S, Tansey WP, Hiebert SW and Zhao Z:
Integrative network analysis identifies key genes and pathways in
the progression of hepatitis C virus induced hepatocellular
carcinoma. BMC Med Genomics. 4:622011. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Yin Y, Wu C, Song J, Wang J, Zhang E, Liu
H, Yang D, Chen X, Lu M and Xu Y: DNA immunization with fusion of
CTLA-4 to hepatitis B virus (HBV) core protein enhanced Th2 type
responses and cleared HBV with an accelerated kinetic. PLoS One.
6:e225242011. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Barnaba V, Franco A, Paroli M, Benvenuto
R, De Petrillo G, Burgio VL, Santilio I, Balsano C, Bonavita MS,
Cappelli G, et al: Selective expansion of cytotoxic T lymphocytes
with a CD4+ CD56+ surface phenotype and a T helper type 1 profile
of cytokine secretion in the liver of patients chronically infected
with hepatitis B virus. J Immunol. 152:3074–3087. 1994.PubMed/NCBI
|
|
12
|
Kang EH, Kown TY, Oh GT, Park WF, Park SI,
Park SK and Lee YI: The flavonoid ellagic acid from a medicinal
herb inhibits host immune tolerance induced by the hepatitis B
virus-e antigen. Antiviral Res. 72:100–106. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Morrey JD, Motter NE, Chang S and Fairman
J: Breaking B and T cell tolerance using cationic lipid-DNA
complexes (CLDC) as a vaccine adjuvant with hepatitis B virus (HBV)
surface antigen in transgenic mice expressing HBV. Antiviral Res.
90:227–230. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Ye B, Liu X, Li X, Kong H, Tian L and Chen
Y: T-cell exhaustion in chronic hepatitis B infection: Current
knowledge and clinical significance. Cell Death Dis. 6:e16942015.
View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Busca A and Kumar A: Innate immune
responses in hepatitis B virus (HBV) infection. Virol J. 11:222014.
View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Kastenmuller W, Gasteiger G, Subramanian
N, Sparwasser T, Busch DH, Belkaid Y, Drexler I and Germain RN:
Regulatory T cells selectively control CD8+ T cell effector pool
size via IL-2 restriction. J Immunol. 187:3186–3197. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Schmitz J, Owyang A, Oldham E, Song Y,
Murphy E, McClanahan TK, Zurawski G, Moshrefi M, Qin J, Li X, et
al: IL-33, an interleukin-1-like cytokine that signals via the IL-1
receptor-related protein ST2 and induces T helper type 2-associated
cytokines. Immunity. 23:479–490. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Carriere V, Roussel L, Ortega N, Lacorre
DA, Americh L, Aguilar L, Bouche G and Girard JP: IL-33, the
IL-1-like cytokine ligand for ST2 receptor, is a
chromatin-associated nuclear factor in vivo. Proc Natl Acad Sci
USA. 104:282–287. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Roussel L, Erard M, Cayrol C and Girard
JP: Molecular mimicry between IL-33 and KSHV for attachment to
chromatin through the H2A-H2B acidic pocket. EMBO Rep. 9:1006–1012.
2008. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Vocca L, Di Sano C, Uasuf CG, Sala A,
Riccobono L, Gangemi S, Albano GD, Bonanno A, Gagliardo R and
Profita M: IL-33/ST2 axis controls Th2/IL-31 and Th17 immune
response in allergic airway diseases. Immunobiology. 220:954–963.
2015. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Blom L and Poulsen LK: IL-1 family members
IL-18 and IL-33 upregulate the inflammatory potential of
differentiated human Th1 and Th2 cultures. J Immunol.
189:4331–4337. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Seki K, Sanada S, Kudinova AY, Steinhauser
ML, Handa V, Gannon J and Lee RT: Interleukin-33 prevents apoptosis
and improves survival after experimental myocardial infarction
through ST2 signaling. Circ Heart Fail. 2:684–691. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
McLaren JE, Michael DR, Salter RC, Ashlin
TG, Calder CJ, Miller AM, Liew FY and Ramji DP: IL-33 reduces
macrophage foam cell formation. J Immunol. 185:1222–1229. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Wood IS, Wang B and Trayhurn P: IL-33, a
recently identified interleukin-1 gene family member, is expressed
in human adipocytes. Biochem Biophys Res Commun. 384:105–109. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Miller AM, Asquith DL, Hueber AJ, Anderson
LA, Holmes WM, McKenzie AN, Xu D, Sattar N, McInnes IB and Liew FY:
Interleukin-33 induces protective effects in adipose tissue
inflammation during obesity in mice. Circ Res. 107:650–658. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Arshad MI, Piquet-Pellorce C,
L'Helgoualc'h A, Rauch M, Patrat-Delon S, Ezan F, Lucas-Clerc C,
Nabti S, Lehuen A, Cubero FJ, et al: TRAIL but not FasL and TNFα,
regulates IL-33 expression in murine hepatocytes during acute
hepatitis. Hepatology. 56:2353–2362. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(−Delta Delta C(T)) method. Methods. 25:402–408. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Xu X, Shang Q, Chen X, Nie W, Zou Z, Huang
A, Meng M, Jin L, Xu R, Zhang JY, et al: Reversal of B-cell
hyperactivation and functional impairment is associated with HBsAg
seroconversion in chronic hepatitis B patients. Cell Mol Immunol.
12:309–316. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Hojo-Souza NS, Pereira DB, Passos LS,
Gazzinelli-Guimarães PH, Cardoso MS, Tada MS, Zanini GM,
Bartholomeu DC, Fujiwara RT and Bueno LL: Phenotypic profiling of
CD8(+) T cells during Plasmodium vivax blood-stage infection. BMC
Infect Dis. 15:352015. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Kaech SM, Wherry EJ and Ahmed R: Effector
and memory T-cell differentiation: Implications for vaccine
development. Nat Rev Immunol. 2:251–262. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Wang J, Cai Y, Ji H, Feng J, Ayana DA, Niu
J and Jiang Y: Serum IL-33 levels are associated with liver damage
in patients with chronic hepatitis B. J Interferon Cytokine Res.
32:248–253. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Zhao PW, Shi X, Li C, Ayana DA, Niu JQ,
Feng JY, Wang J and Jiang YF: IL-33 enhances humoral immunity
against chronic HBV infection through activating CD4(+)CXCR5(+) TFH
cells. J Interferon Cytokine Res. 35:454–463. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Neuveut C, Wei Y and Buendia MA:
Mechanisms of HBV-related hepatocarcinogenesis. J Hepatol.
52:594–604. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Ng SA and Lee C: Hepatitis B virus X gene
and hepatocarcinogenesis. J Gastroenterol. 46:974–990. 2011.
View Article : Google Scholar : PubMed/NCBI
|


















