Introduction
Immunoglobulins (Igs) are important immunological
molecules and were considered to be specific products of B
lymphocytes. However, in 2003, Qiu et al (1) demonstrated that Ig may be derived
from and expressed by human cancer cells of epithelial origin. Ig
derived from non-B cells, including human epithelial cancer cells
and certain normal epithelial cells, is referred to as non-B Ig.
The existence of non-B Ig has now been confirmed by further
studies, which in addition have revealed that cancer-derived Ig
contributes to the malignization, migration and proliferation of
cancer cells (2,3).
Lee et al (4) generated the monoclonal antibody
(mAb), RP215 (hybridoma, HB10095; US patent no. US5650291) and
demonstrated that it bound a certain molecule in numerous cancer
cell lines, as well as serum and tissue sections from patients with
various cancers, including ovarian, cervical, endometrial, breast,
stomach and colon cancer; however, RP215 did not bind to healthy
cells (5–8). The molecule recognized by RP215 was
named cancer-associated antigen 215 (CA215). As it is markedly
homologous to normal human IgG heavy chain, CA215 was considered a
non-B Ig (4). CA215 may be an
important pan-biomarker for numerous human cancers and a potential
target for clinical monitoring. Lee et al (4,9)
further confirmed that the epitope recognized by RP215 was a
carbohydrate-associated epitope on CA215 (CA215C). Using RP215 to
target CA215 through CA215C inhibited cancer cell proliferation,
induced apoptosis in vitro and inhibited the growth of human
tumor cells in nude mice (10,11).
Therefore, RP215 may be a potential therapeutic agent for the
treatment of cancer, and has been developed as a therapeutic
humanized antibody (10). The
specific binding between RP215 and CA215C suggests that CA215C may
be a potential vaccine candidate to induce active immunity against
cancer. However, as CA215C is a carbohydrate-associated epitope(s)
with weak immunogenicity, it is not suitable as a vaccine
candidate. As thymus-independent antigens (TI-Ag),
carbohydrate-associated epitopes do not induce specific cellular
immune responses and effective secondary antibody responses.
The aim of the present study was to develop CA215C
epitopes into therapeutic tumor vaccine candidates by enabling them
to induce effective adaptive immune responses. In the present
study, peptide mimics of CA215C epitopes were screened from a
random phage display peptide library using the mAb RP215 as the
target molecule. The synthetic peptide mimics were recognized by
RP215 specifically, and effectively induced antisera/antibody
production in mice, specifically targeting CA215 on human cancer
tissues, suggesting that these peptide mimics may be potential
candidates for tumor vaccine development.
Materials and methods
Reagents
The Ph.D.™-12 phage display library was purchased
from New England Biolabs Ltd. (Hitchen, UK). The mAb RP215 and
purified CA215 were obtained from Professor GregoryLee at the
University of British Columbia (Vancouver, BC, Canada). The mAbs
4D11 (12) and 2H4 (13) were prepared in our laboratory as
previously described. Goat anti-human IgG polyclonal antibody
(catalog no. 05204005) and horseradish peroxidase (HRP)-conjugated
goat anti-human IgG (catalog no. 05204001) were purchased from
Multi Sciences (Lianke) Biotech Co., Ltd. (Hangzhou, China).
Peroxidase AffiniPure goat anti-mouse IgG (catalog no. 115-035-003)
was purchased from Jackson ImmunoResearch Laboratories Co., Inc.
(West Grove, PA, USA). HRP-labeled anti-M13 (catalog no. 27942101)
was obtained from GE Healthcare Life Sciences (Chalfont, UK).
Normal human IgG (catalog no. VGNHH-12000C) was purchased from
Multi Science (Lianke) Biotech Co., Ltd. Normal mouse IgG (catalog
no. CDF01-100UG) was purchased from EMD Millipore (Billerica, MA,
USA). Streptavidin-HRP was purchased from Beijing Dingguochangsheng
Biotechnology Co., Ltd. (Beijing, China). The synthetic peptides
were produced by Hybio Pharmaceutical Co., Ltd. (Shenzhen, China).
The enhanced HRP-diaminobenzidine (DAB) chromogenic substrate kit
was purchased from Tiangen Biotech Co., Ltd. (Beijing, China) and
the endogenous biotin-avidin blocking kit was purchased from
Shanghai YuanMu Biological Technology Co., Ltd. (Shanghai, China).
All of the chemicals used in this study were obtained from
Guangzhou Jinhuada Chemical Reagent Co., Ltd. (Guangzhou, China),
unless otherwise stated.
Mice
A total of 15 female BALB/cmice (age, 6–8 weeks)
were purchased from the Animal Center of Southern Medical
University (Guangzhou, China). Mice were housed at a temperature of
18–22°C and 50–60% humidity under a 10/14-h light/dark cycle and
with free access to food and water. All procedures were conducted
in accordance with the National Institutes of Health Guide for the
Care and Use of Laboratory Animals, and with the Guidelines for the
Care and Use of Animals established by the Animal Care and Use
Committee of the Southern Medical University. The present study was
approved by the Ethical Committee of Southern Medical
University.
Tissue samples
Tissue samples, including hepatic and rectal
carcinoma tissues, were collected from the Department of Pathology
of NanFang Hospital (Guangzhou, China). Written informed consent
was obtained from patients or their families. All procedures were
performed with the approval of the Institutional Review Board of
Southern Medical University. For immunohistochemistry (IHC), biopsy
tissues had been fixed immediately in 10% buffered formalin, and 24
h later were dehydrated in increasing concentrations of alcohol and
embedded in paraffin. Serial 5-µm thick sections were then cut
using a microtome.
Screening and characterization of
phage clones that mimic CA215C
Screening of the phage display peptides
Phage clones that mimicked CA215C were screened from
a 12-mer linear phage display peptide library based on an
antigen-antibody reaction described previously (14). Panning of the phage display library
was performed according to the manufacturer's protocol. Briefly,
RP215 diluted to 5 µg/ml with 0.1 M NaHCO3 (pH 8.6) was coated onto
96-well plates as a target protein. A total of 5×109
phage particles were added to the wells and incubated at room
temperature for 1 h. The non-binding phage clones were washed off
with phosphate buffered saline (PBS) containing 0.1% Tween-20
(PBST). The bound phage clones were eluted using 0.2 M glycine-HCl
buffer (pH 2.2) and were immediately neutralized with 1 M Tris-HCl
(pH 9.1). The eluted phage clones were titrated on isopropyl
β-D-1-thiogalactopyranoside (IPTG)/X-gal agar plates, amplified
with host strain ER2738, purified with polyethylene glycol (PEG)
8,000/NaCl and subsequently used for the next round of panning.
Following two further rounds of panning, single clones were
selected randomly from IPTG/X-gal agar plates and analyzed with a
phage enzyme-linked immunosorbent assay (ELISA).
Specific binding of the phage clones to RP215 mAb
by phage ELISA
ELISA plates were coated with 50 µl (2 µg/ml) RP215
as a capture antibody. 4D11, an unrelated mAb, served as an isotype
control. Phage particles (1×107 plaque-forming units/ml,
50 µl/well) from each purified phage clone were added to the
plates; an irrelevant phage clone [Advanced Oxidation Protein
Products (AOPP)-related phage clone] obtained from colleagues
(15) and dilution buffer were
used as control groups. The unbound phage particles were washed
away with PBST. HRP-labeled anti-M13 (MEK13 phage vector, diluted
1:5,000 with PBST) was added as a detection antibody. The color
reaction was developed using tetramethylbenzidine (TMB) with
hydrogen peroxide and terminated with 2 M H2SO4. Subsequently, the
absorbance values were read at 450 nm using a microplate reader
(BioTek Instruments, Inc., Winooski, VT, USA).
Sequencing of the phage clones
Positive phage clones were amplified and purified
with a PEG/NaCl solution, and the single-stranded DNA was isolated
with sodium iodide. Following examination by agarose gel
electrophoresis, the single-stranded DNA was sequenced by
Invitrogen; Thermo Fisher Scientific, Inc. (Waltham, MA, USA).
Synthesis and characterization of the
CA215C peptide mimics
Peptide synthesis
Hybio Pharmaceutical Co., Ltd. synthesized three
biotinylated single-chain peptides, R2, R13 and R42, which were
derived from phage clones 2, 13 and 42, respectively. The accuracy
and purity of the final products were confirmed by mass
spectrometry and high-performance liquid chromatography, which
demonstrated the correct sequence and 90% purity.
Antigenicity determination by ELISA
Streptavidin was diluted to 5 µg/ml with PBS and
immobilized onto microplates. The biotinylated peptides R2, R13 and
R42 and the unrelated peptide mimic P39-1 (14) were then serially diluted with PBST,
added to the microplates and incubated for 2 h at room temperature.
Subsequently, unbound peptides were washed off with PBST, 1 µg/ml
RP215 or control mouse IgG was added to the microplates and
incubated for 30 min and plates were washed with PBST. Finally,
peroxidase AffiniPure goat anti-mouse IgG diluted 1:5,000 with PBST
was added, and the color was developed using TMB plus hydrogen
peroxide.
To determine whether there were common epitopes
between the biotinylated peptides and the human IgG, 2 µg/ml
goat-anti-human IgG was immobilized on the microplates as the
capture antibody. The biotinylated peptides R2 and R42 and human
IgG as a positive control were added onto the microplates and
incubated at 37°C for 30 min. Following washing with PBST,
streptavidin-HRP or peroxidase AffiniPure goat anti-mouse IgG
diluted 1:5,000 was added to develop a color reaction with TMB.
Immunization of mice with peptide-bovine serum
albumin (BSA) conjugates
Initially, peptide mimics were cross-linked with
BSA, which acted as a carrier protein. Each of the peptide mimics
(2 mg) was diluted with 500 µl 0.1 M 2-(N-Morpholino)
ethanesulfonic acid monohydrate (MES) solution (pH 4.5); in
addition, 4 mg BSA was diluted with MES solution. Peptide (500 µl)
and 400 µl BSA were mixed together, and 100 µl of freshly prepared
1-(3-dimethylaminopropyl)-3-ethylcarbodiimide solution [10 mg/ml in
double distilled (dd)H2O] was added to the peptide-BSA mixture,
which was then incubated for 4 h at room temperature with gentle
agitation. Finally, the peptide-BSA mixture was dialyzed with ddH2O
for 48 h at 4°C. The conjugates were named R2-BSA/R42-BSA. A total
of 15 BALB/c mice were divided randomly into three groups, and each
group was immunized with BSA, R2-BSA or R42-BSA. The immunogens
were emulsified with Freund's complete adjuvant and administered
via subcutaneous injection for the first immunization. A total of 4
weeks later, five more immunization boosters were performed with
Freund's incomplete adjuvant by intraperitoneal injection, with one
injection every 2 weeks. The six total immunizations in all of the
groups were performed with 100 µg immunogen. Finally, antiserum was
collected from the tail veins of anesthetized mice and stored at
−20°C.
Mouse antiserum identification
CA215C binding to murine antisera was detected using
ELISA. CA215 (2 µg/ml, 50 µl/well) was coated onto microplates and
normal human IgG served as a control. Following blocking with
casein solution, the antisera, diluted 1:400 in PBST, were added to
the microplates (50 µl per well) and incubated for 30 min at 37°C.
Peroxidase AffiniPure goat anti-mouse IgG antibody diluted 1:5,000
in PBST was then added to the microplates as a detection antibody.
Color was developed with TMB and the reaction terminated with 2 M
H2SO4. The absorbance values were read at 450 nm on a microplate
reader.
IHC was performed using antisera on patient samples
as previously described by Lee et al (8). Antisera were purified with octylic
acid ammonium sulfate precipitation method and the concentration
was determined using a UV spectrophotometer. Normal goat serum was
used to block any non-specific binding. The following antibodies
were diluted in PBS with 2% BSA and incubated on ice for 20 min: 5
µg/ml RP215 (positive control), 5 µg/ml 2H4 (negative/isotype
control), 30 µg/ml anti-R2/R42-BSA antisera and 30 µg/ml anti-BSA
antisera (carrier protein control). These were then added to the
slides and incubated in a humidified chamber overnight at 4°C.
Peroxidase AffiniPure goat anti-mouse IgG (0.8 µg/ml in PBS with 2%
BSA) was added to each slide for 40 min at 37°C. Finally, staining
of the tissue sections was performed with an enhanced HRP-DAB
chromogenic substrate kit. The sections were counterstained with
hematoxylin and visualized under a light microscope (Olympus
Corporation, Tokyo, Japan).
Competitive inhibition of peptide-BSA
antisera
Endogenous biotin from the cancer tissue was blocked
with an endogenous biotin-avidin blocking kit. Normal goat serum
was added to the slides and incubated for 2 h at room temperature
to prevent any non-specific binding. Anti-peptide-BSA antisera (30
µg/ml) and anti-BSA antisera (30 µg/ml) in PBS with 2% BSA were
incubated on ice for 20 min and then added to the slides as the
experimental and negative control groups, respectively. Slides were
incubated in a humidified chamber overnight at 4°C. Subsequently,
the slides were brought to room temperature and washed four times
with PBS at 5-min intervals on a shaker. Biotinylated RP215 (5
µg/ml in PBS with 2% BSA) was then added to each slide except for
the blank control and incubated for 30 min at 37°C. The slides were
washed as previously, and incubated with 3% H2O2 in PBS at room
temperature for 10 min to remove any endogenous peroxidase
activity. The slides were washed three times with PBS at 5-min
intervals. Streptavidin-HRP (1 µg/ml in PBS with 2% BSA) was added
to each slide for 20 min at 37°C. Slides were washed as previously,
and staining was performed using an enhanced HRP-DAB chromogenic
substrate kit. Sections were counterstained with hematoxylin and
visualized under a light microscope.
Statistical analysis
Data are expressed as the mean ± standard deviation.
Data were analyzed using two-tailed t-tests, using GraphPad Prism
software version 6 (GraphPad Software, Inc., La Jolla, CA, USA).
P<0.05 was considered to indicate a statistically significant
difference.
Results
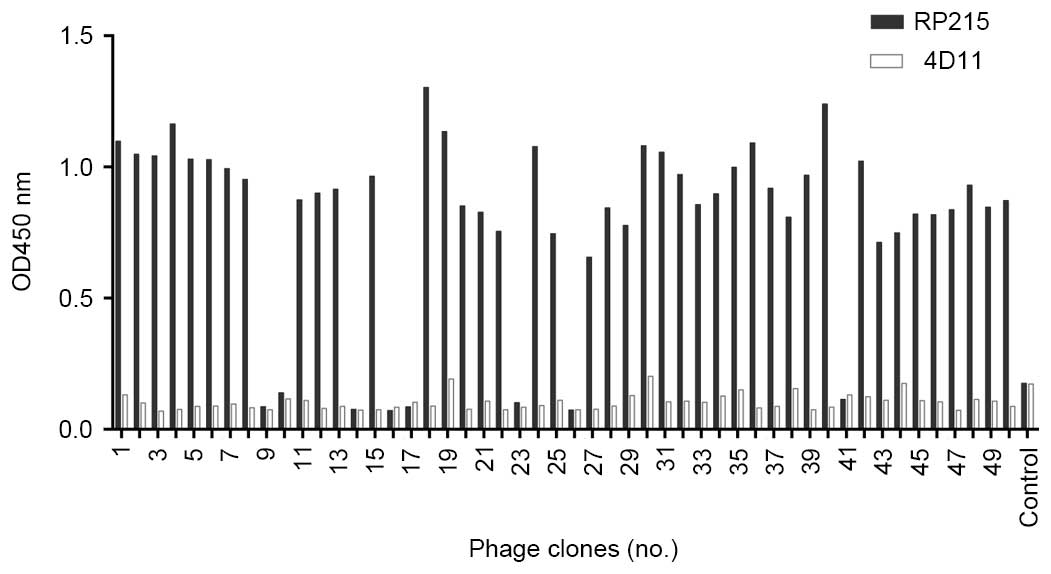
Phage clones bound to RP215
Following the third round of screening with the
anti-CA215 mAb, RP215, 50 blue phage clones were picked from the
IPTG/X-gal agar plates. To identify the specificity of the phage
clones, RP215 was used as a capture antibody, and 4D11, an
unrelated mAb, served as a control antibody. In total, 33 phage
clones demonstrated strong binding to RP215 without binding to 4D11
(Fig. 1), whereas irrelevant
control, AOPP-related phage and blank control did not bind to
RP215, suggesting that the displayed peptides on these 33 positive
phage clones may be complementary epitopes to RP215. This
experiment was performed three times, and the 30 positive phage
clones with the greatest strength and stability of binding were
selected for sequencing.
Sequence analysis of phage clones
In total, 30 positive phage clones were amplified to
obtain purified DNA for sequencing. A total of 23 sequences were
analyzed, as certain phage clones demonstrated identical sequences.
Phage clones 1, 2, 5, 6, 11 and 31 shared the conserved E-LWR
sequence (sequence I); clones 4, 12, 25 and 36 shared the E-HWR
sequence (sequence II); clones 18, 21 and 39 phage contained the
E-WR sequence (sequence III); phage clones 3 and 40 shared the EDLW
conserved sequence (sequence IV); and phage clones 7 and 42
possessed the conserved E-LWR(K) sequence (sequence V). There were
no conserved sequences in the remaining the phage clones (Table I). The sequence E-(−)WR was present
in four conserved sequence groups, although the amino acids between
amino E and WR were not identical. Furthermore, phage clones 2
(LSTKEVESLWRR), 42 (HKASWERDLWRE) and 13 (ISEKQVESLRRR) had
high-affinity binding to low RP215 concentrations (Fig. 2). Consequently, the peptide
synthesis reactions were performed based on the sequences of clones
2, 42 and 13.
 | Figure 2.Binding of phage clones with various
conserved sequences to low concentration RP215. Each phage clone
was adjusted to the same titer and binding to RP215 was assessed by
ELISA. Phage clones 1, 2, 5, 6, 11 and 31 had the E-LWR conserved
sequence; phage clones 4, 12, 25, and 36 possessed the E-HWR
sequence, phage clones 18, 21, and 39 possessed the E-WR sequence;
and phage clones 7 and 42 had the E-LWR(K) sequence. The sequences
in phage clones 13, 15, 19, 32, 33 and 35 were irregular. Phage
clones 2, 42 and 13 had high-affinity binding to low RP215
concentrations. |
 | Table I.Amino acid sequences of phage clones
that bound to RP215. |
Table I.
Amino acid sequences of phage clones
that bound to RP215.
| Phage
clonesa | Amino acid
sequencesb |
|---|
| 1, 2, 5, 6, 11,
31 | E-LWRc |
| 4, 12, 25, 36 | E-HWRc |
| 18, 21, 39 | E-WRc |
| 3, 40 | EDLW |
| 7, 42 | E-LWR(K)c |
| 13, 15, 19, 32, 33,
35 | Irregular |
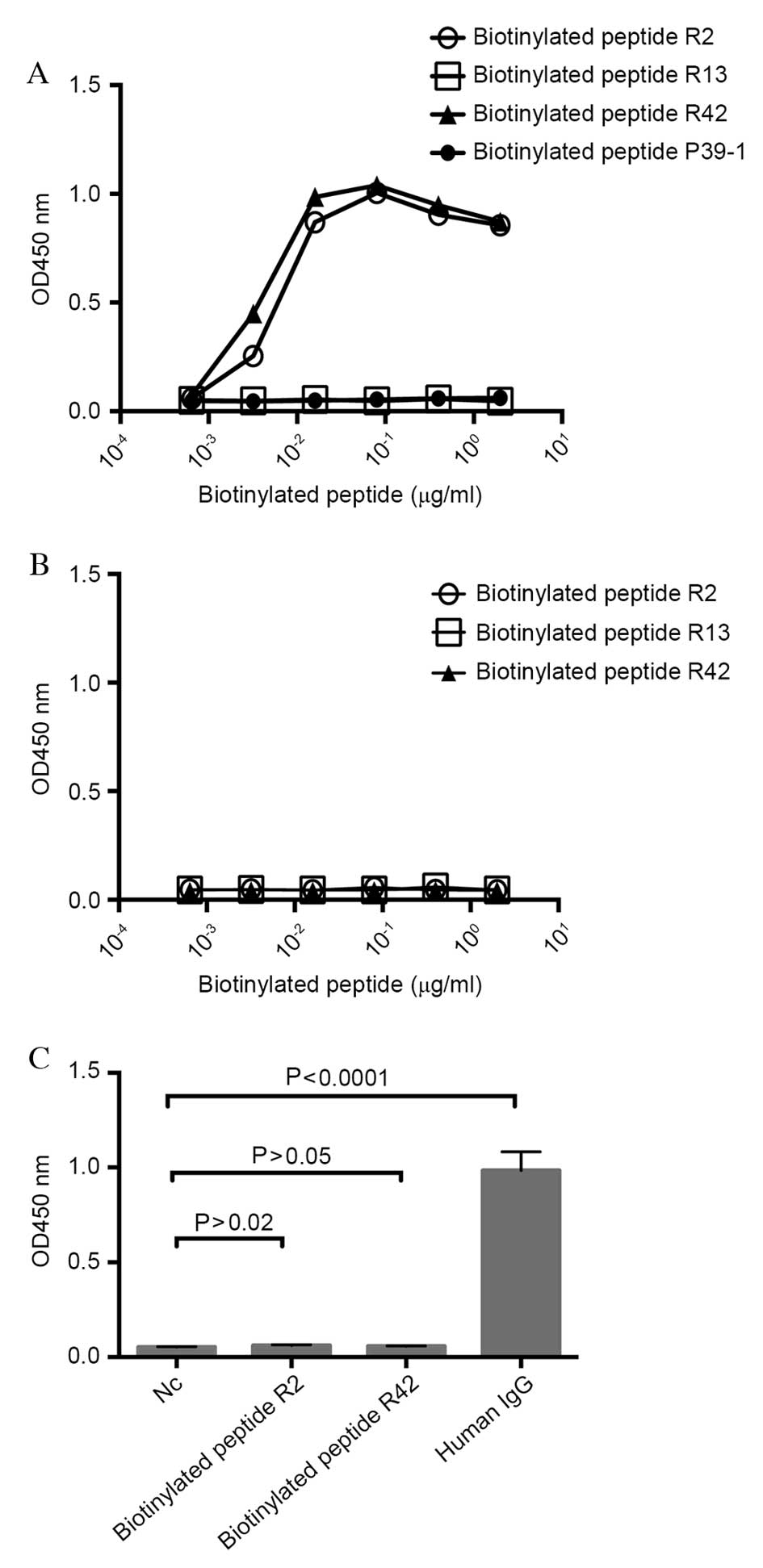
Antigenicity of the biotinylated
CA215C peptide mimics
To identify the antigenicity of peptide mimics,
short peptides were synthesized in the form of biotinylated
peptides, and binding to RP215 was assessed by ELISA. The
biotinylated peptides R2 and R42 bound to RP215 in a dose-dependent
manner, whereas the R13 biotinylated peptide and an unrelated
peptide, P39-1 (14), did not
(Fig. 3A). Additionally, no
cross-reaction of the biotinylated peptides R2, R13 and R42 with
normal mouse IgG was observed (Fig.
3B). Furthermore, there was no cross-reaction between the
biotinylated peptides R2 and R42 and goat-anti-human IgG (Fig. 3C). These results indicated that
CA215C peptide mimics with the conserved sequences from R2 and R42
demonstrated antigenicity with RP215, which suggested that the
biotinylated peptides R2 and R42 may mimic the CA215C binding
epitope of RP215.
Immunogenicity of R2 and R42
As the molecular weights of the short peptides were
too low to directly use in immunization without a carrier,
biotinylated peptides R2 and R42 were conjugated to BSA, which
served as a carrier protein. The immunogenicity of R2 and R42 short
peptides was confirmed by immunizing BALB/c mice with R2-BSA or
R42-BSA conjugates, while immunization with BSA alone served as a
control. The antisera of R2-BSA (P<0.0001) and R42-BSA
(P=0.0005) -immunized mice reacted with CA215C, whereas there was
no significant binding to normal human IgG (R2-BSA antisera,
P=0.3660; R42-BSA antisera, P=0.1601; Fig. 4). The murine antisera were then
reacted with the native CA215C epitope expressed in human tumor
tissues, by IHC. As for the RP215 positive control (Fig. 5A-a), antisera from mice immunized
with R2-BSA (Fig. 5A-b) or R42-BSA
(Fig. 5A-c) bound to human hepatic
carcinoma tissue, whereas no binding was observed using antisera
from mice immunized with BSA only (Fig. 5A-d), using the irrelevant mAb, 2H4
(Fig. 5A-e) or in the blank
control (Fig. 5A-f). Similar
results were observed in rectal carcinoma tissue (Fig. 5B). Furthermore, murine antisera
inhibited RP215 binding to tumor tissue, including hepatic
(Fig. 6A) and rectal (Fig. 6B) carcinoma tissue, compared with
controls. These results indicated that R2 and R42 peptides mimicked
the CA215C epitope characteristics and induced serum antibodies
with activities similar to RP215 in mice.
Discussion
In the present study, short peptide mimics of a
carbohydrate-associated epitope of a tumor antigen were
successfully obtained by screening a random phage display library
with the specific mAb, RP215. In addition, these peptide mimics
induced the production of antisera that could bind to CA215C,
suggesting that these short peptides mimicked the CA215C epitope.
Furthermore, the results indicated that the carbohydrate epitope, a
TI-Ag, was altered to a peptide epitope that may become a
thymus-dependent antigen (TD-Ag) by carrier protein conjugation or
formation of multiple antigen peptides. In the past two decades,
the importance of carbohydrate-associated epitopes of pathogen and
tumor antigens has been increasingly investigated, as numerous
studies have demonstrated that carbohydrate or glycolipid
structures or epitopes on pathogens and tumor cells are important
as potential biomarkers or vaccine candidates, including LPS
(16) and Thomsen-Friedenreich
antigen (Galβ1-3GalNAcα-O-Ser/Thr) (17). However, there are two challenges
for the application or development of vaccine candidates for
carbohydrate or glycolipid epitopes: i) These types of epitope are
difficult to obtain by biological purification or recombinant
expression and ii) the characteristics of a TI-Ag render them
incapable of inducing effective secondary antibody responses and
cellular immunity. As an alternative strategy, peptide mimics
structurally simulate non-protein epitopes and may alter them from
TI-Ag to TD-Ag. In addition, peptide mimics may be readily
synthesized and conjugated.
Prior to the present study regarding CA215C peptide
mimics, numerous studies have successfully generated peptide
mimics. For example, Monzavi-Karbassi et al (18) reported that a peptide surrogate of
GlcNAc induced an in vivo tumor-specific cellular response
to established Meth A tumors that expressed native O-GlcNAc
glycoproteins on the tumor cell surface. In addition, peptide
mimics of pathogens have been generated, particularly for
non-protein elements, including LPS (16) and peptidoglycan (14). Furthermore, peptide-mimic
strategies using random peptide libraries have been used for
nonlinear or structural epitope analyses (19,20).
However, not all synthesized peptides based on screened sequences
from peptide libraries exhibit the expected antigenicity, even
though positive phage clones or bead-conjugated peptides from
combinatory peptide libraries bind well to specific antibodies. For
example, it is very difficult to obtain short linear peptides that
mimic structural epitopes on protein molecules. In addition,
numerous short linear peptides have been synthesized based on
screened sequences from phage display peptide libraries using
antibodies to protein epitopes, that could not bind to antibodies
for screening during subsequent determination experiments; however,
longer linear or cyclical peptide mimics to non-protein epitopes
appear to be antigenic, including peptide mimics to Escherichia
coli LPS (14,16,21).
In the present study, two specific and antigenic
peptide mimics were obtained of a carbohydrate epitope recognized
by the mAb, RP215, which binds CA215C on numerous tumor cell types
and inhibits tumor cell growth (7,10).
Following three rounds of screening and positive clone
identification with sandwich ELISAs that utilized RP215 at a very
low concentration, DNA sequencing from 30 positive phage clones was
performed. From these results, five conserved sequence groups were
obtained: E-LWR, E-HWR, E-WR, EDLW and E-LWR(K); E-WR may serve as
a more conserved sequence for four of the groups. According to the
affinity of phage clones with RP215, clones 2 (LSTKEVESLWRR), 42
(HKASWERDLWRE) and 13 (ISEKQVESLRRR) were selected for peptide
synthesis. The R2 (sequence from clone 2) and R42 (sequence from
clone 42)-synthesized peptides bound RP215 at a concentration of
10−2 µg/ml; however, they did not bind with anti-human
IgG, suggesting a high degree of sensitivity and specificity.
CA215 is homologous with normal human IgG, and at
least 32 tryptic peptide fragments exhibited various degrees of
homology with human Igs (60–100%). Although N-linked and O-linked
carbohydrate composition analyses demonstrate similar compositions
of monosaccharides in normal human IgG and RP215 (mouse IgG), CA215
exhibits a distinct sugar content compared with normal human IgG
and mouse IgG. In particular, CA215 contains a reduced percentage
of N-acetylglucosamine and significantly greater quantities of
mannose, N-acetylneuraminic acid and N-glycoylneuraminic acid
(4,22). Therefore, the ideal short peptide
should mimic the CA215C epitope, a specific carbohydrate-associated
epitope on the IgG heavy chain V region from cancer cells, which is
not expressed on normal IgG; in this way the epitope mimicked by
the short peptide would not be a common epitope between normal
human IgG and CA215. Goat anti-human IgG antibody, a polyclonal
antibody that recognizes all epitopes of normal human IgG, was
almost incapable of binding to the R2 and R42 synthetic peptide
mimics. This result suggested that the R2 and R42 peptides have no
shared epitope with normal human IgG and that these synthetic R2
and R42 peptides may mimic a CA215C epitope.
In addition, the present study confirmed that
peptide mimics of CA215C may be potential vaccine candidate
epitopes by preparing antisera following immunization with peptide
mimic conjugates and BSA as a carrier protein. The R2-BSA and
R42-BSA peptide mimics reacted with RP215 in a dose-dependent
manner; however, they did not react with goat anti-human IgG
polyclonal antibody in an ELISA experiment (data not shown). This
result demonstrated that the mimic epitopes for CA215C,
specifically recognized by RP215, remained and that the R2-BSA and
R42-BSA cross-linked peptides may be used to evaluate the efficacy
of these synthetic peptides for activating the immune system.
BALB/c mice were separately immunized with the
R2-BSA and R42-BSA conjugates and the resultant antisera were
analyzed by ELISA and IHC. Murine antisera reacted with CA215
specifically; no significant reaction with normal human or mouse
IgG occurred. Notably, murine antisera bound to human hepatic and
rectal carcinoma tissue, and this inhibited RP215 binding.
Furthermore, the binding of RP215 to cancer tissues, including
human hepatic and rectal carcinoma tissue, was inhibited by
antisera from mice immunized with R2-BSA or R42-BSA conjugates.
These results indicated that the R2 and R42 peptides mimicked a
CA215C epitope. Therefore, these synthetic peptides may be utilized
as vaccine candidate epitopes to induce specific antibodies against
cancer cells through CA215C in humans.
Further investigation is required to develop a
viable tumor vaccine from a vaccine candidate or candidate epitope.
In future studies, the immunogenicity may be enhanced by exchanging
certain amino acids, synthesizing a four-branch multiple antigenic
peptide that may not require a carrier protein (23,24)
and selecting a suitable adjuvant (25).
In conclusion, the results of the present study
demonstrated that R2-BSA and R42-BSA antisera had similar
characteristics to RP215 and that the synthetic peptides R2 and R42
may mimic the CA215C epitope. R2 and R42 peptides may therefore
have potential for development into a tumor vaccine.
Acknowledgements
The authors thank Professor Gregory Lee for donating
CA215 and the mAb, RP215. The present study was supported in part
by the Special Project on the Integration of Industry, Education
and Research of Guangdong Province (grant no. #2012B091000148), and
the Nature Science Foundation of Guangdong Province (grant no.
2014A030313281).
Glossary
Abbreviations
Abbreviations:
|
Ig
|
immunoglobulin
|
|
TI-Ag
|
thymus-independent antigen
|
|
TD-Ag
|
thymus-dependent antigen
|
|
mAb
|
monoclonal antibody
|
|
IHC
|
immunohistochemistry
|
|
HRP
|
horseradish peroxidase
|
|
TMB
|
tetramethylbenzidine
|
|
V region
|
variable region
|
|
ELISA
|
enzyme-linked immunosorbent assay
|
|
PBS
|
phosphate buffered saline
|
|
PBST
|
PBS containing 0.1% Tween-20
|
|
BSA
|
bovine serum albumin
|
|
MES
|
2-(N-Morpholino) ethansulfonic acid
monohydrate
|
|
H&E
|
hematoxylin-eosin
|
|
NC
|
negative control
|
References
|
1
|
Qiu X, Zhu X, Zhang L, Mao Y, Zhang J, Hao
P, Li G, Lv P, Li Z, Sun X, et al: Human epithelial cancers secrete
immunoglobulin g with unidentified specificity to promote growth
and survival of tumor cells. Cancer Res. 63:6488–6495.
2003.PubMed/NCBI
|
|
2
|
Li M, Feng DY, Ren W, Zheng L, Zheng H,
Tang M and Cao Y: Expression of immunoglobulin kappa light chain
constant region in abnormal human cervical epithelial cells. Int J
Biochem Cell Biol. 36:2250–2257. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Chen Z, Huang X, Ye J, Pan P, Cao Q, Yang
B, Li Z, Su M, Huang C and Gu J: Immunoglobulin G is present in a
wide variety of soft tissue tumors and correlates well with
proliferation markers and tumor grades. Cancer. 116:1953–1963.
2010. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Lee G, Laflamme E, Chien CH and Ting HH:
Molecular identity of a pan cancer marker, CA215. Cancer Biol Ther.
7:2007–2014. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Lee G: Cancer cell-expressed
immunoglobulins: CA215 as a pan cancer marker and its diagnostic
applications. Cancer Biomark. 5:137–142. 2009.PubMed/NCBI
|
|
6
|
Lee G and Ge BX: Cancer cell expressions
of immunoglobulin heavy chains with unique carbohydrate-associated
biomarker. Cancer Biomark. 5:177–188. 2009.PubMed/NCBI
|
|
7
|
Lee G, Ge B, Huang TK, Zheng G, Duan J and
Wang IH: Positive identification of CA215 pan cancer biomarker from
serum specimens of cancer patients. Cancer Biomark. 6:111–117.
2010.PubMed/NCBI
|
|
8
|
Lee G, Zhu M, Ge B and Potzold S:
Widespread expressions of immunoglobulin superfamily proteins in
cancer cells. Cancer Immunol Immunother. 61:89–99. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Lee G, Cheung AP, Li B, Ge B and Chow PM:
Molecular and immuno-characteristics of immunoglobulin-like
glycoproteins in cancer cell-expressed biomarker, CA215. Immunol
Invest. 41:429–446. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Lee G and Ge B: Inhibition of in vitro
tumor cell growth by RP215 monoclonal antibody and antibodies
raised against its anti-idiotype antibodies. Cancer Immunol
Immunother. 59:1347–1356. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Lee G, Chu RA and Ting HH: Preclinical
assessment of anti-cancer drugs by using RP215 monoclonal antibody.
Cancer Biol Ther. 8:161–166. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Tang L, Zhu P, Luo C, Xu XM and Fu N:
Preparation and identification of monoclonal antibodies against
different epitopes on human zeta globin chain. Di Yi Jun Yi Da Xue
Xue Bao. 25:1394–1397. 2005.(In Chinese). PubMed/NCBI
|
|
13
|
Xiao H, Zhu P, Liu B, Pan Q, Jiang X, Xu X
and Fu N: Generation and characterization of human
delta-globin-specific monoclonal antibodies. Blood Cells Mol Dis.
44:127–132. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Chen Y, Liu B, Yang D, Li X, Wen L, Zhu P
and Fu N: Peptide mimics of peptidoglycan are vaccine candidates
and protect mice from infection with Staphylococcus aureus. J Med
Microbiol. 60:995–1002. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Hou XR, Liu BY, Tian JW, Chen YG and Fu N:
Screening and Characterization of the Mimotopes of Adveanced
Oxidation Protein Products from Phage Peptide Library. Chinese
Journal of Laboratory Diagnosis. 15:765–768. 2011.
|
|
16
|
Nagy G and Pál T: Lipopolysaccharide: A
tool and target in enterobacterial vaccine development. Biol Chem.
389:513–520. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Samuel J, Noujaim AA, MacLean GD, Suresh
MR and Longenecker BM: Analysis of human tumor associated
Thomsen-Friedenreich antigen. Cancer Res. 50:4801–4808.
1990.PubMed/NCBI
|
|
18
|
Monzavi-Karbassi B, Luo P, Jousheghany F,
Torres-Quiñones M, Cunto-Amesty G, Artaud C and Kieber-Emmons T: A
mimic of tumor rejection antigen-associated carbohydrates mediates
an antitumor cellular response. Cancer Res. 64:2162–2166. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Tiwari R, Negi SS, Braun B, Braun W, Pomés
A, Chapman MD, Goldblum RM and Midoro-Horiuti T: Validation of a
phage display and computational algorithm by mapping a
conformational epitope of Bla g 2. Int Arch Allergy Immunol.
157:323–330. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Gazarian KG, Palacios-Rodriguez Y,
Gazarian TG and Huerta L: HIV-1 V3 loop crown epitope-focused
mimotope selection by patient serum from random phage display
libraries: Implications for the epitope structural features. Mol
Immunol. 54:148–156. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Hossany RB, Johnson MA, Eniade AA and
Pinto BM: Synthesis and immunochemical characterization of protein
conjugates of carbohydrate and carbohydrate-mimetic peptides as
experimental vaccines. Bioorgan Med Chem. 12:3743–3754. 2004.
View Article : Google Scholar
|
|
22
|
Lee G and Azadi P: Peptide mapping and
glycoanalysis of cancer cell-expressed glycoproteins CA215
recognized by RP215 monoclonal antibody. J Carbohyd Chem. 31:10–30.
2012. View Article : Google Scholar
|
|
23
|
Wu YZ, Zhang JB, Chen SY, Chen A, Wang L,
Li J, Zhao T, Zou L, Tang Y, Tingrong L and Wang F: Frequencies of
epitope-specific cytotoxic T lymphocytes in active chronic viral
hepatitis B infection by using MHC class I peptide tetramers.
Immunol Lett. 92:253–258. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Yamada A, Sasada T, Noguchi M and Itoh K:
Next-generation peptide vaccines for advanced cancer. Cancer Sci.
104:15–21. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Cheng WK, Wee K, Kollmann TR and Dutz JP:
Topical CpG adjuvantation of a protein-based vaccine induces
protective immunity to Listeria monocytogenes. Clin Vaccine
Immunol. 21:329–339. 2014. View Article : Google Scholar : PubMed/NCBI
|