Introduction
Parkinson's disease (PD) is a common
neuro-degenerative disorder that affects more than 0.1% of the
population >40 years of age (1). The majority of PD patients present
with slowed movement, rest tremors, rigidity and an abnormal
posture. PD is associated with the progressive loss of dopaminergic
(DA) neurons in the substantia nigra (SN), however, its etiology
remains unclear. Neuro-inflammation has been implicated in the
pathogenesis of PD (1). Currently
available therapeutic agents provide symptomatic improvement;
however, no disease-modifying treatments have been developed.
Therefore, novel therapeutic targets for PD are required, and a
promising target is the Rho/Rho-associated, coiled-coil-containing
protein kinases (ROCKs) signaling pathway.
ROCKs are serine/threonine (Ser/Thr) protein kinases
of which there are two isoforms, ROCKI and ROCKII, that are encoded
by two separate genes. These two proteins share 92% homology in
their kinase domains. ROCKI is primarily expressed in non-neuronal
tissues, while ROCKII is predominantly expressed in the brain.
Previous studies suggest that each isoform performs distinct
functions. For instance, ROCKI expression is upregulated upon
macrophage adhesion, whereas phagocytic activity is downregulated
in ROCKII-depleted macrophages, but not in ROCKI-depleted cells
(2,3). In addition, ROCKs are markedly
homologous with additional cyclic adenosine monophosphate
(AMP)-dependent, cyclic guanosine monophosphate-dependent and
protein kinase C kinases. ROCKs are involved in a wide variety of
physiological and pathological processes, including
differentiation, neurite growth and plasticity of neurons (4). In addition, ROCKs influence
inflammatory responses by regulating migration and adhesion of
leukocytes, phagocytosis of macrophages and secretion of
pro-inflammatory cytokines (5–8).
ROCKs have been investigated in neuro-degenerative
disorders, such as Alzheimer's disease (AD) (9). Previous studies have reported that
inhibiting ROCK alleviated DA neuron loss induced by
1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine (MPTP) in mice
(10,11). However, due to the substantial
homology between ROCKs and additional kinases, currently used ROCKs
inhibitors do not exclusively inhibit ROCKs. For example, fasudil
has low selectivity for various other kinases, including protein
kinase N (PKN), stress-induced kinase 1 (MSK1), mitogen-activated
protein kinase 1b (MAPK1b) and protein kinase A (PKA) and moderate
selectivity for AMP-activated protein kinase (AMPK) and
phosphorylase kinase (PHK) (12).
In addition, every ROCK inhibitor indiscriminately targets ROCKI
and ROCKII. Consequently, it is considered to be impossible to
accurately evaluate the role of ROCKs in PD using ROCKs inhibitors.
The aim of the present study was to investigate the role of ROCKII
in PD by constructing a lentivirus-based small hairpin (sh)RNA
system that specifically interferes with the expression of ROCKII,
in order to examine its neuro-protective effects during
MPTP-induced neuron loss in the SN of mice.
Materials and methods
Animals
A total of 10 female C57BL/6 mice (age, 10–12 weeks;
weight 22–25 g) were purchased from Shanghai Laboratory Animal
Center (Shanghai, China). Mice were maintained at 25±2°C under 12 h
light/dark cycles, and had access to food and water ad
libitum. Animal procedures were performed in accordance with
the International Council for Laboratory Animal Science guidelines,
and the study received ethical approval from the Ethics Committee
of Fudan University (Shanghai, China).
Antibodies
The mouse anti-mouse ROCKII monoclonal antibody
(dilution, 1:1,000; cat. no. 610624; BD Biosciences, Franklin
Lakes, NJ, USA), mouse anti-mouse inducible nitric oxide synthase
(iNOS) monoclonal antibody (dilution, 1:200; cat. no. 610329; BD
Biosciences), rabbit anti-mouse tyrosine hydroxylase (TH)
polyclonal antibody (dilution, 1:1,000; cat. no. AB152; EMD
Millipore, Billerica, MA, USA), mouse anti-mouse TH monoclonal
antibody (dilution, 1:1,000; cat. no. MAB318; EMD Millipore), mouse
anti-mouse cluster of differentiation (CD) 11b monoclonal antibody
(dilution, 1:200; cat. no. 14-0112-82; eBioscience, Inc., San
Diego, CA, USA), rabbit anti-mouse Toll-like receptor (TLR) 2
monoclonal antibody (dilution, 1:500; cat. no. 3268-1; Epitomics,
Burlingame, CA, USA), rabbit anti-mouse phosphorylated TANK-binding
kinase (p-TBK) 1 (Ser172) monoclonal antibody (dilution, 1:500;
cat. no. 3300-1; Epitomics), rabbit anti-mouse p-inhibitor of κBα
(IκBα) polyclonal antibody (dilution, 1:500; cat. no. EPR6235
(2); Epitomics), rabbit anti-mouse
GAPDH monoclonal antibody (dilution, 1:10,000; cat. no. 2251-1;
Epitomics), rabbit anti-mouse p-IκB kinase (IKK) α/β (Ser176/180)
monoclonal antibody (dilution, 1:500; cat. no. 2697; Cell Signaling
Technology, Inc., Danvers, MA, USA), rabbit anti-mouse p-myosin
phosphate target subunit (MYPT) 1 (Thr696) polyclonal antibody
(dilution, 1:200; cat. no. 5163; Cell Signaling Technology, Inc.),
rabbit anti-mouse p-myosin light chain (MLC) 2 (Ser19) polyclonal
antibody (dilution, 1:200; cat. no. 3671; Cell Signaling
Technology, Inc.) and rabbit anti-mouse p-nuclear factor (NF)-κB
p65 (Ser536) monoclonal antibody (dilution, 1:1,000; cat. no. 3033;
Cell Signaling Technology, Inc.) were used for the purposes of the
current study. The secondary antibodies used in this study were all
purchased from Thermo Fisher Scientific, Inc., (Waltham, MA, USA),
and included the following: Alexa Fluor 555-conjugated goat
anti-mouse IgG (H+L; cat. no. A-21422); Alexa Fluor 555-conjugated
goat anti-rabbit IgG (H+L; cat. no. A-21428); Alexa Fluor
488-conjugated goat anti-rabbit IgG (H+L; cat. no. A-11008); Alexa
Fluor 488-conjugated goat anti-mouse IgG (H+L; cat. no A-11001);
horseradish peroxidase (HRP)-conjugated goat anti-rabbit IgG (H+L;
cat. no. 31460); and HRP-conjugated goat anti-mouse IgG (H+L; cat.
no. 31430).
BV2 microglia culture and
1-methyl-4-phenylpyridinium (MPP+) treatment
Mouse BV2 microglia cells were purchased from
Shanghai Fuxiang Biotechnology Co., Ltd. (Shanghai, China) and
cultured in Dulbecco's modified Eagle's medium (Gibco; Thermo
Fisher Scientific, Inc.) supplemented with 10% fetal bovine serum
(Gibco; Thermo Fisher Scientific, Inc.), 100 U/ml penicillin, and
100 µg/ml streptomycin at 37°C in a 95% humidified 5% CO2 cell
culture incubator. Cells were challenged with 0.5 mM MPP+
(Sigma-Aldrich; Merck Millipore, Darmstadt, Germany) once for 24 h
between 5 and 8 days following lentivirus transfection. BV2 cells
were then collected and lysed in radioimmunoprecpitation (RIPA)
lysis buffer (Beyotime Institute of Biotechnology, Shanghai, China)
to extract total protein, and the lysates were centrifuged at 4°C
for 5 min at 10,000 × g. The supernatant was stored at −20°C
until required.
Lentivirus-based shRNA system
construction and screening
A lentivirus-based shRNA system was constructed by
NeuronBiotech Co., Ltd. (Shanghai, China). Briefly, DH5α competent
cells (Takara Bio, Inc., Otsu, Japan) were used to produce the
lentivirus vector, pLKD.CMV.GFP.U6, with U6 promoter as the
transcriptional start site for shRNA. A 21-bp sequence containing a
stem loop sequence was inserted into the vector to produce a valid
shRNA that would interfere with ROCKII at the mRNA level
(NM_009072.2). Five candidate lentiviruses targeting five unique
sequences were constructed and an empty lentivirus vector served as
a control. Lentiviruses were transfected into the mouse BV2
microglia cell line for screening. When cell density reached ~30%,
lentiviruses were added into the culture medium with a multiplicity
of infection of 20 for 24 h. Protein levels and enzyme activity of
ROCKII in the culture medium were measured to evaluate the shRNA
interference systems once at 5–8 days following transfection.
Stereotaxic microinjection and RI in
SN
Of the shRNA systems evaluated, two were confirmed
to efficiently interfere with ROCKII expression. The two shRNA
systems were combined at a ratio of 1:1 and injected into the SN of
mice in the RI group, as previously described (13). Briefly, mice were anesthetized via
intraperitoneal injection of 400 mg/kg chloral hydrate (100 mg/ml)
and placed into the stereotaxic apparatus. Unilateral injection was
performed into the right side of the brain (3.0 mm posterior to
bregma, 1.0 mm lateral to midline and 4.0 mm ventral to the dural
surface). A total of 2 µl lentivirus was injected with a
microsyringe driven by a microdialysis pump at a rate of 0.2
µl/min. Following microinjection, the syringe needle was left in
place for 5 min prior to its withdrawal to reduce efflux of
injected liquid along the injection tract. Control mice were
injected with an empty vector system according to the same
procedure.
MPTP-induced parkinsonism
One week following microinjection, mice were
challenged with intraperitoneal injections of MPTP (Sigma-Aldrich;
Merck Millipore) dissolved in saline, which was administered every
day for 7 successive days. The MPTP dose increased from 15 mg/kg on
the first day and 20 mg/kg on the second day to a maximum of 30
mg/kg on the subsequent 5 days. Mice were maintained at 25–28°C
following each injection.
Behavioral tests
The pole test (PT) and adhesive removal test (ART)
were performed with minor modifications (14,15).
In the PT, mice were placed head-downward on the top of a 12-mm
thick, 80-cm tall, vertical pole with a rough surface. The time for
descent from the top surface to the floor was recorded. If the
duration was >120 sec, it was recorded as the default value, 120
sec. The investigator was blind to the animal grouping. In the ART,
adhesive dots were placed on the plantar surface of the two
forelimbs with equal pressure; the time for spot removal on each
side was recorded. The default duration was set at 120 sec. Mice
were trained daily for 2 continuous days ahead of the formal
assessments. Tests were conducted daily and repeated for 3
continuous days prior to sacrifice.
Tissue processing
For protein extraction, mice were anesthetized via
intraperitoneal injection of 400 mg/kg chloral hydrate (100 mg/ml,
Sinopharm Chemical Reagent Co., Ltd., Shanghai, China) and perfused
by intracardiac injection of 20 ml 0.9% sodium chloride solution.
The brain was carefully removed and homogenized in RIPA lysis
buffer (Beyotime Institute of Biotechnology) supplemented with 1 mM
protease inhibitor (phenylmethanesulfonyl fluoride; Beyotime
Institute of Biotechnology). The extracts were centrifuged at
12,000 × g for 20 min at 4°C. The supernatant was recovered
and frozen at −80°C.
For histological analysis, mice were transcardially
perfused with saline, followed by 4% paraformaldehyde (PFA) in
phosphate-buffered saline (PBS). Brains were removed and dehydrated
sequentially in 10, 20 and 30% (w/v) sucrose solution overnight at
4°C. Brains were then embedded in Tissue-Tek O.C.T Compound (Sakura
Finetek USA, Inc., Torrance, CA, USA), frozen in liquid nitrogen,
sectioned at 10 µm and stored at −20°C.
Immunofluorescence staining and DA
neuron quantification
Sections were fixed with 4% PFA at room temperature
(RT) and washed thoroughly with PBS. Sections were then incubated
for 30 min at RT in 5% fetal bovine serum (Gibco; Thermo Fisher
Scientific, Inc.) and 0.1% Triton X-100 in PBS, and then incubated
overnight at 4°C with the aforementioned primary antibodies diluted
in PBS containing 1% fetal bovine serum and/or 0.1% Triton X-100.
Following thorough washing with PBS, sections were incubated with
the corresponding aforementioned secondary antibodies labeled with
various fluorochromes at RT for 2 h. Finally, sections were washed
thoroughly and observed under a fluorescence microscope. Images
were captured with DP controller software (version, 2.0; Olympus
Corporation, Tokyo, Japan) and processed with DP manager (Olympus
Corporation). Numbers of DA neurons were estimated by counting
TH-positive cells from every twelfth 10-µm section along the SN
pars compacta (SNpc) with the aid of Image-Pro Plus software
(version, 6.0; Media Cybernetics, Inc., Rockville, MD, USA) as
previously described (16).
Briefly, boundaries of the SNpc, the area of interest, were defined
according to previously defined anatomical analysis of the mouse
(17). Positive cells beyond the
SNpc were excluded. Each RBG image was processed with a constant
‘color-cube’ segmentation setting and the same threshold, resulting
in accurately defined foreground immunofluorescent staining. The
total number of TH-positive cells in five sections of the same
mouse brain was recorded and compared between shRNA and empty
vector groups. The RBG image of each striatum section was processed
with a constant ‘color-cube’ segmentation setting and at the same
threshold and level. TH-positive areas were analyzed and the mean
number of five sections for each brain was compared between shRNA
and empty vector groups.
Western blotting and protein
quantification
The protein concentration of brain sample extracts
was determined using a bicinchoninic acid kit (Beyotime Institute
of Biotechnology) and the whole protein extracts were diluted in
RIPA lysis buffer (Beyotime Institute of Biotechnology) to a final
protein concentration of 5 µg/µl prior to western blotting. Sample
proteins (10 µl) were then separated by sodium dodecyl
sulfate-polyacrylamide gel electrophoresis (8%; Bio-Rad
Laboratories, Inc., Hercules, CA, USA) at 80 V for 30 min, before
they were transferred to nitrocellulose membranes (EMD Millipore).
After blocking with 3% bovine serum albumin (w/v) for 1 h at RT,
the membranes were incubated with primary antibodies overnight at
4°C, followed by the appropriate HRP-conjugated secondary antibody
for 2 h at RT. Protein bands were developed with chemiluminescent
HRP substrate (EMD Millipore) and imaged with ChemiDOC XRS+ system
(Bio-Rad Laboratories, Inc.) combined with Image Lab version 3.0
software (Bio-Rad Laboratories, Inc.). The signal intensity was
quantified using Image Lab version 3.0 software. The density of
each band was normalized to the band density of the constitutively
expressed gene, GAPDH.
Enzyme-linked immunosorbent assays
(ELISAs)
The levels of interleukin (IL)-1β, IL-6, and tumor
necrosis factor (TNF)-α in the mouse brain were measured with the
corresponding ELISA kits (cat. nos. 900-M47, 900-K50 and 900-M54,
respectively; PeproTech, Inc., Rocky Hill, NJ, USA) according to
the manufacturer's instructions, as described previously (18).
Statistical analysis
Data were analyzed with Student's t test or
Mann-Whitney U test and P<0.05 was considered to indicate a
statistically significant difference. All statistical analyses were
performed using SPSS software version 12.0 (SPSS Inc., Chicago, IL,
USA) and data are presented as the mean ± standard error of the
mean.
Results
Expression level and activity of
ROCKII increased in the SNpc area of mice with MPTP-induced
parkinsonism
The level of ROCKII expression in the brains of the
MPTP-induced parkinsonism mice was ~1.2 times the level in the
brains of the control mice (P<0.01; Fig. 1A). The level of MYPT1 [a substrate
of ROCKII (19)] phosphorylated at
Thr696 (p-MYPT1) in the brains of the MPTP-induced parkinsonism
mice was ~1.5 times the level in the brains of the control mice
(P<0.05; Fig. 1A). The majority
of p-MYPT1-positive cells exhibited a glia-like shape (Fig. 1B and C). These results demonstrated
that ROCKII activation was involved in the MPTP-induced
parkinsonism.
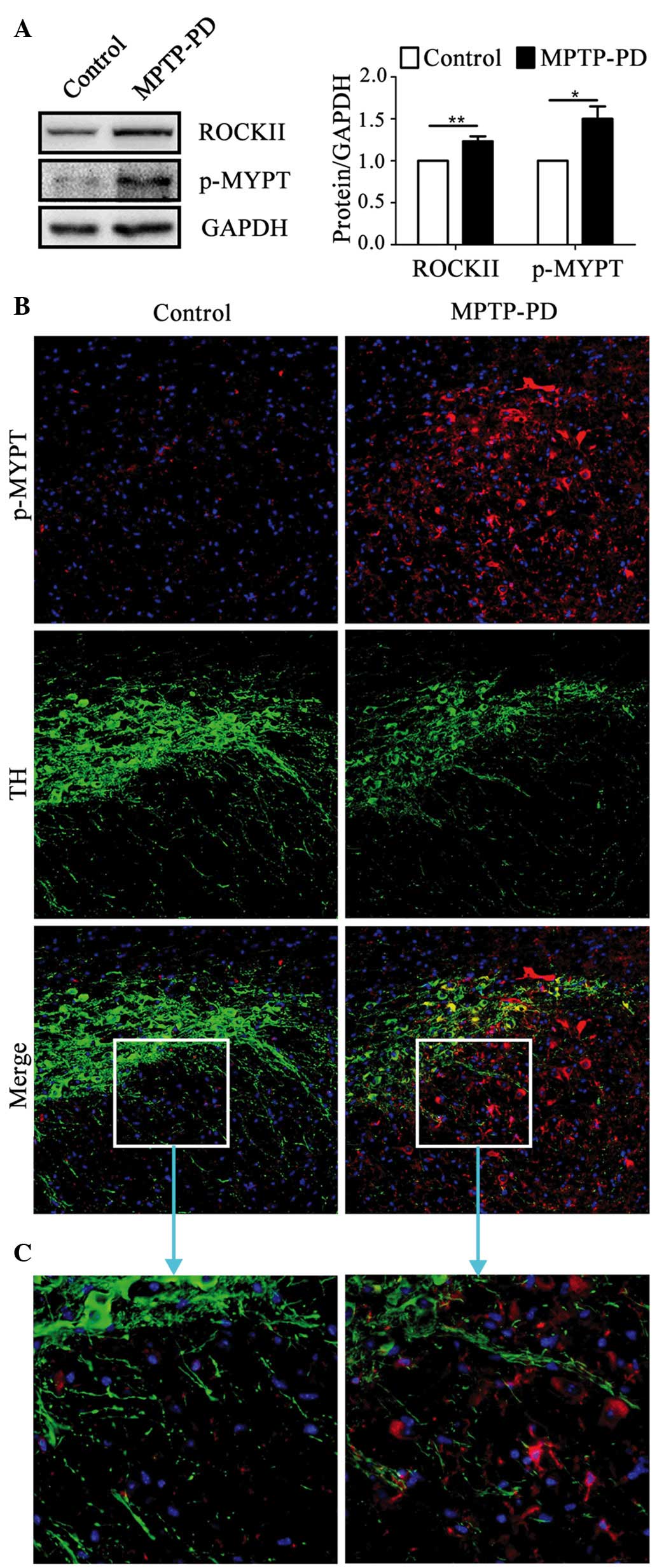 | Figure 1.ROCKII protein expression level and
enzyme activity were increased in the brains of mice with
MPTP-induced parkinsonism. (A) The protein expression levels of
ROCKII and p-MYPT increased in the brains of the MPTP-induced PD
mouse model as determined by western blotting (n=4; ROCKII,
P=0.015; p-MYPT, P=0.001). (B) p-MYPT staining of the SN of
MPTP-treated and control mice (magnification, ×20). p-MYPT-positive
cells were abundant in MPTP-treated mice compared with the control
mice. A proportion of p-MYPT-positive cells expressed TH. (C)
Magnification of the boxed areas in (B) (magnification, ×40).
ROCKII, Rho/Rho-associated, coiled-coil-containing protein kinase
II; MPTP, 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine; PD,
Parkinson's disease; p-MYPT, phosphorylated myosin phosphate target
subunit; SN, substantia nigra; TH, tyrosine hydroxylase. |
RI decreases the expression level and
activity of ROCKII in vitro and in vivo
The schematic of lentivirus construction is
presented in Fig. 2A and target
sequences of five shRNAs are listed in Table I. ROCKII expression was inhibited
to the greatest extent in shR340- and shR337-transfected cells,
with decreases of ~93% (P<0.001) and ~51% (P<0.01),
respectively, when compared with empty vector-transfected cells
(Fig. 2B). The level of MLC
phosphorylated at Ser19 (p-MLC), another substrate of ROCKII
(12), was examined. shR337 and
shR340 significantly decreased the level of p-MLC by ~80% when
compared with the empty vector (P<0.01; Fig. 2B). In addition, shR337 and shR340
markedly inhibited the expression and activity of ROCKII in the SN
of MPTP-induced parkinsonism mouse brains following injection of
combined lentiviruses into the SN region (Fig. 2C). These data demonstrate that
shR337 and shR340 lentiviruses effectively inhibited the expression
and activity of ROCKII in vitro and in the MPTP-induced
parkinsonism.
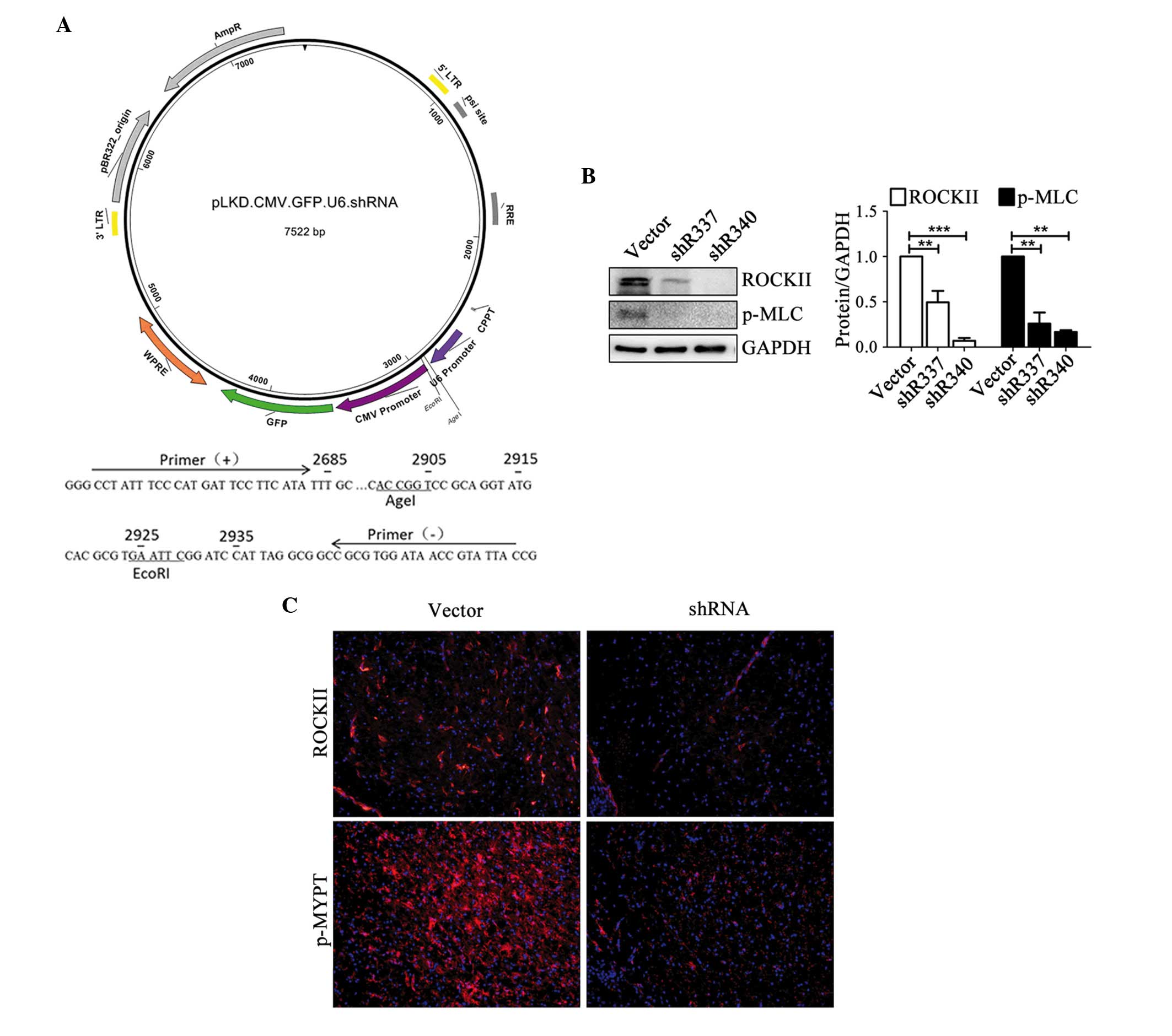 | Figure 2.Construction of a lentivirus-based
shRNA system and interference efficiency of anti-ROCKII shRNA in
vitro and in vivo. (A) Construction schematic of the
lentivirus vehicle, driven by the U6 promoter. The GFP reporter
gene is driven by the CMV promoter. (B) Interference efficiencies
of anti-ROCKII shRNAs in mouse BV2 microglia cells as determined by
western blotting. ROCKII and p-MLC protein levels were decreased
significantly following transfection with shR337 (ROCKII, P=0.007;
p-MLC, P=0.006) and shR340 (ROCKII, P<0.001; p-MLC, P=0.002),
compared with the empty vector (n=3). **P<0.01 and
***P<0.001. (C) Interference efficiencies of anti-ROCKII shRNAs
in the brains of mice determined by fluorescence (magnification,
×20). Stereotaxic injection of the combination of shR337 and shR340
at a ratio of 1:1 into the SN area markedly reduced ROCKII and
p-MYPT staining compared with the empty vector. shRNA, small
hairpin RNA; ROCKII, Rho/Rho-associated, coiled-coil-containing
protein kinase II; GFP, green fluorescent protein; CMV,
cytomegalovirus; p-MLC, phosphorylated myosin light chain; SN,
substantia nigra; p-MYPT, phosphorylated myosin phosphate target
subunit. |
 | Table I.Targeting sequences of five
anti-ROCKII shRNAs. |
Table I.
Targeting sequences of five
anti-ROCKII shRNAs.
| shRNA | Target
sequence |
|---|
| shR336 |
GCTGGAAATTACCCTTACCAA |
| shR337 |
CCCTGCAAAGTTTATTATGAT |
| shR338 |
GCATCTCTTGAAGAAACAAAT |
| shR339 |
GCTTGCACTGGATGCAATACA |
| shR340 |
GCAGAGCAGTATTTCTCAACC |
RI attenuated movement disorder and DA
neuron loss induced by MPTP
RI mice outperformed the control mice in the PT and
ART tests. In the PT (Fig. 3A), RI
mice moved from the top surface to the ground more rapidly than the
control mice (6.9±0.6 vs. 9.8±1.1 sec; P<0.05). In the ART
(Fig. 3B), RI mice removed
adhesive spots on their right forepaws more rapidly than the
control mice (7.6±0.9 vs. 13.07±2.1 sec; P<0.05). In addition,
RI mice removed adhesive spots on their right forepaws quicker than
those on their left (7.6±0.9 vs. 12.2±2.1 sec), although the
difference was not statistically significant (P=0.052; Fig. 3B).
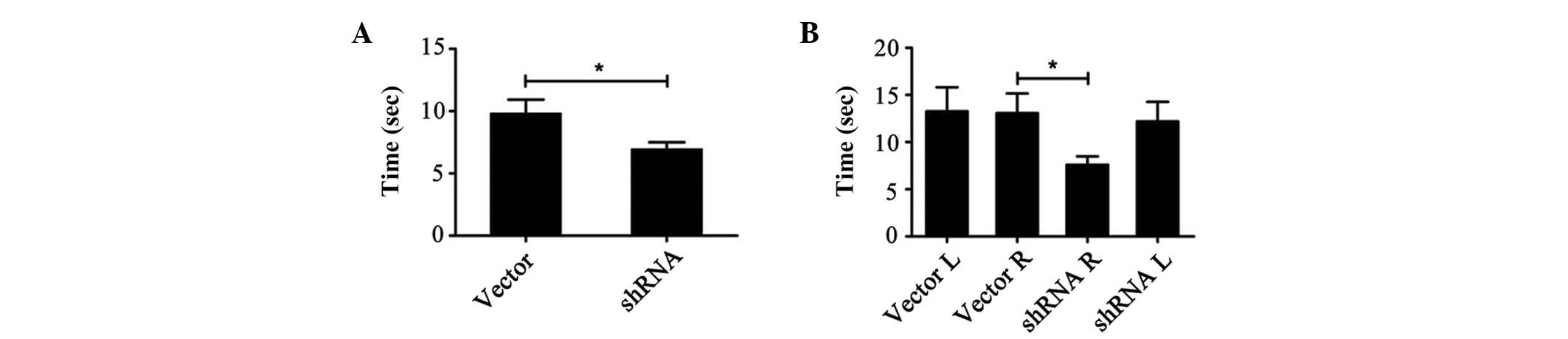 | Figure 3.RI attenuated movement disturbance
induced in mice by MPTP. (A) Pole test. RI mice move more rapidly
than control mice (n=8; P=0.036). (B) Adhesive removal test. RI
mice removed adhesive spots on their right forepaws more quickly
than the control mice (n=8; P=0.028). In addition, RI mice removed
adhesive spots on their right forepaws more rapidly than those on
their left (P=0.052). The time taken for left forepaw adhesive spot
removal did not differ between groups. *P<0.05. RI,
Rho/Rho-associated, coiled-coil-containing protein kinase II
interference; MPTP, 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine;
L, left forepaw; R, right forepaw; shRNA, small hairpin RNA. |
A notable reduction of ROCKII-positive staining was
observed in the SN region of RI mice (Fig. 4A and B; P<0.01). TH-positive
cell numbers in the SN region were markedly increased in RI mice
when compared with the control mice (Fig. 4A; P<0.01). The protein
expression levels of ROCKII and p-MYPT1 in the right hemisphere of
RI mice was reduced by 21 and 37% compared with controls,
respectively, as measured by western blotting (P<0.01; Fig. 4C). These results indicated
successful interference of ROCKII. The expression level of TH
protein in the right hemispheres of RI mice was ~1.6 times that of
the control mice (P<0.01; Fig.
4C). In addition, the TH-positive area in the striatum was
augmented in RI mice (P<0.01; Fig.
4D). These results revealed that inhibition of ROCKII
effectively improved the behavioral performance and inhibited DA
neuron loss in the MPTP-induced PD mouse model.
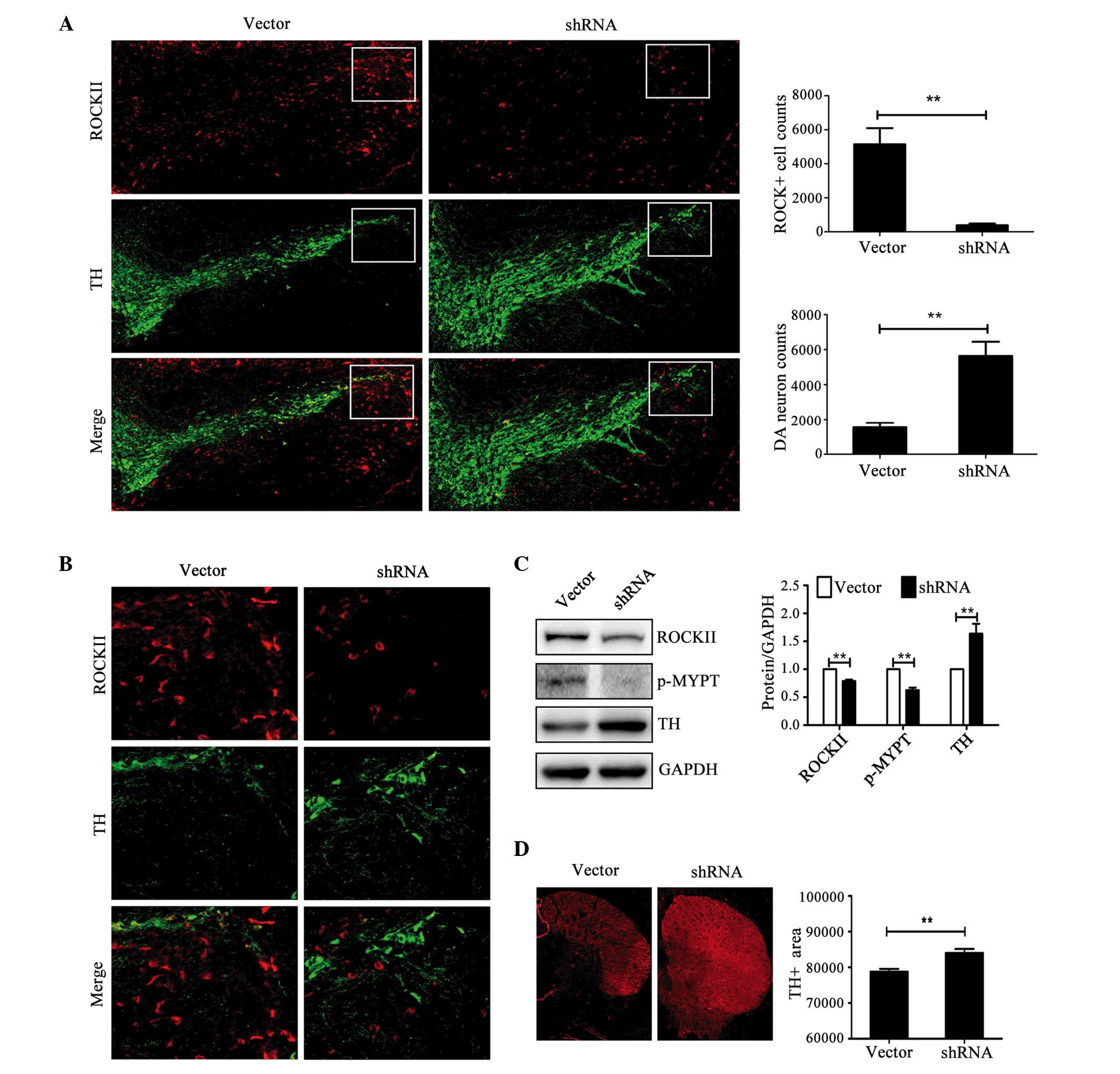 | Figure 4.RI significantly inhibited
MPTP-induced TH-positive neuron loss. (A) Local injection of shRNA
efficiently reduced the expression level of ROCKII (P=0.007) and
the TH-positive area at substantia nigra (n=5; P=0.005) compared
with the empty vector (magnification, ×20). (B) Magnification (×40)
of the boxed areas in (A). (C) The protein levels of ROCKII
(P=0.004) and p-MYPT (P=0.001) were significantly decreased and the
protein level of TH (P=0.001) significantly increased in the RI
group compared with empty vector controls (n=4). (D) TH-positive
area in the striatum was increased in the RI group compared with
the empty vector controls (magnification, ×20; n=5; P=0.005).
**P<0.01. ROCKII, Rho/Rho-associated, coiled-coil-containing
protein kinase II; RI, ROCKII interference; MPTP,
1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine; TH, tyrosine
hydroxylase; shRNA, small hairpin RNA; p-MYPT, phosphorylated
myosin phosphate target subunit; DA, dopaminergic. |
RI inhibited microglia activation and
inflammatory responses in mice with MPTP-induced parkinsonism
The probable mechanisms underlying the DA neuron
protection of RI against MPTP were investigated. In the SN area of
MPTP-induced parkinsonism mice, the majority of ROCKII-positive
cells were co-localized with CD11b-positive microglia (Fig. 5A). As ROCKII was inhibited, the
activation of CD11b-positive microglia was markedly inhibited in
the SN area of RI mice (Fig.
5B).
 | Figure 5.RI inhibited microglia activation in
mice with MPTP-induced parkinsonism. (A) ROCKII-positive and
CD11b-positive staining is increased in the SN in mice with
MPTP-induced PD compared with the control mice (magnification ×20).
The majority of ROCKII-positive cells were CD11b-positive. (B)
CD11b-positive staining declined markedly in the RI mice as
ROCKII-positive staining declined (magnification, ×20). ROCKII,
Rho/Rho-associated, coiled-coil-containing protein kinase II; RI,
ROCKII interference; MPTP,
1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine; PD, Parkinson's
disease; CD11b, cluster of differentiation 11b; SN, substantia
nigra. |
It is widely accepted that activated microglia exert
dual functions, and are defined as pro-inflammatory (M1) and
anti-inflammatory (M2) microglia (18). Microglia in the SN region of
MPTP-induced PD mice polarized toward the M1 subtype, expressing
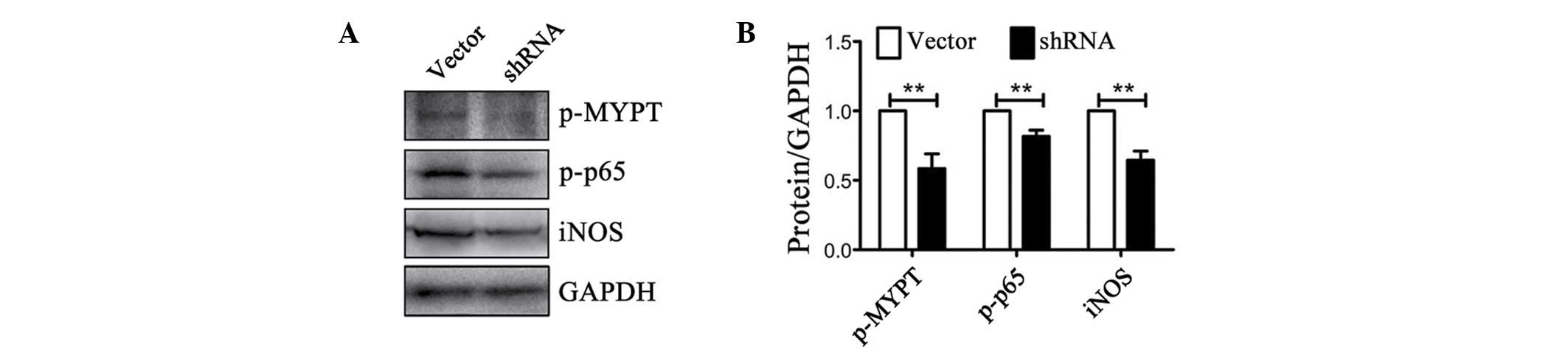
high levels of iNOS, TLR2 and NF-κB (Fig. 6A). RI attenuated iNOS and TLR2
expression levels, and inhibited IκB/IKK/TBK1/NF-κB signaling
pathway activation (P<0.05; Fig. 6B
and C).
 | Figure 6.RI inhibited microglia activation in
mice with MPTP-induced parkinsonism by inhibiting NF-κB activation.
(A) p-p65, the activated form of NF-κB, increased significantly in
the SN of MPTP-treated mice and was present in CD11b-positive cells
(magnification, ×20). In addition, iNOS and TLR2 were upregulated
in MPTP-treated mice and expressed by activated microglia. (B)
p-p65, iNOS and TLR2 all decreased in the RI group as microglia
activation was inhibited (magnification, ×20). (C) p-p65 (P=0.015),
iNOS (P=0.049) and TLR2 (P<0.001) were downregulated in RI mice.
In addition, p-IKK (P=0.028), p-IκB (P=0.002) and p-TBK1 (P=0.035)
expression levels were inhibited in RI mice (n=4). (D) The
expression levels of IL-1β (P=0.028) and IL-6 (P=0.002) were
elevated significantly in RI mice compared with the control mice.
No difference in TNF-α expression was detected. *P<0.05,
**P<0.01, ***P<0.001. RI, Rho/Rho-associated,
coiled-coil-containing protein kinase II interference; MPTP,
1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine; PD, Parkinson's
disease; CD11b, cluster of differentiation 11b; NF-κB, nuclear
factor-κB; p-p65, phosphorylated p65; SN, substantia nigra; iNOS,
inducible nitric oxide synthase; TLR2, Toll-like receptor 2; p-IKK,
phosphorylated inhibitor of κB kinase; p-IκB, phosphorylated
inhibitor of κB; p-TBK1, phosphorylated TANK-binding kinase 1; IL,
interleukin; TNF-α, tumor necrosis factor α; shRNA, small hairpin
RNA. |
The production of IL-1β and IL-6 decreased
significantly (P<0.05 and P<0.01, respectively) in the brain
of RI mice (Fig. 6D). However, the
production of TNF-α did not differ significantly (Fig. 6D). These results demonstrated that
the inhibition of ROCKII may contribute to the neuro-protection of
DA neurons against MPTP by inhibiting the activation of M1
microglia in the SN region and the release of inflammatory
cytokines.
ROCK/NF-κB axis-dependent
anti-inflammation in BV2 microglia
Whether RI influenced the activity of the NF-κB
signaling pathway and inflammatory responses was investigated in
mouse BV2 microglia cell lines. As presented in Fig. 7, RI resulted in the downregulation
of p-MYPT expression (P<0.01; Fig.
7A and B), which was accompanied by a decrease in NF-κB
activity (P<0.01; Fig. 7A and
B). In addition, the inhibition of iNOS indicated that
inflammatory M1 microglia were suppressed (P<0.01; Fig. 7A and B). In a previous study,
microglia polarization from M1 toward M2 was associated with
reduced NF-κB activity (18).
These results, combined with the in vivo data, suggest the
existence of a ROCK/NF-κB axis.
Discussion
The expression and activity of ROCKs are elevated in
neuro-degenerative diseases, such as PD (11) and AD (20). ROCK inhibitors, including Y27632 or
fasudil, have been shown to protect neurons from degeneration in
animal models of these diseases, although the underlying mechanism
remains unclear (10,20). It has been reported that ROCK
activation increases angiotensin type-1 receptor expression in
neurons, which promotes the neuro-degenerative process (21).
Previous studies have demonstrated that ROCKs are
involved in inflammation. ROCK activation increased the
permeability of endothelial cells and promoted lymphocyte
infiltration (22). In addition,
ROCK activation enhanced the phagocytic activity of macrophages
(23), and the release of reactive
oxygen species and pro-inflammatory cytokines by inflammatory cells
(24). The neuro-protective
effects of fasudil, a ROCK inhibitor (4,25)
may be partly mediated through inhibiting neuro-inflammation. In a
previous study, fasudil exhibited therapeutic potential in
experimental autoimmune encephalomyelitis (26). Fasudil rebalanced T helper 17 and
regulatory T cells, induced microglial polarization towards M2, and
inhibited inflammatory responses (26).
Neuro-inflammation has been observed in autopsies of
PD patients (27). Lymphocyte
infiltration, inappropriate microglia activation (27,28),
upregulation of pro-inflammatory cytokines, including IL-1β, IL-6
and TNF-α (29–31), and upregulation of
inflammation-associated signaling molecules, including iNOS and
NF-κB (31–33) have all been observed in the SN of
the brain. In addition, imaging has provided evidence of microglial
activation in early PD patients (34,35).
Furthermore, corresponding inflammatory conditions have been
described in toxin-based parkinsonism models, with high levels of
microglia in the SN (36–40). DA neurons are particularly
vulnerable to the inflammatory microenvironment, and these
responses may be crucial in aggravating DA degeneration in the SN
region (41). Thus, it is proposed
that activation of microglia is a risk factor triggering the onset
of a cascade of events leading to progressive degeneration of DA
neurons.
In our previous study, fasudil skewed LPS-stimulated
M1 microglia towards the M2 subtype, expressing reduced NF-κB
activity and inflammatory cytokine levels, including IL-1β, IL-6
and TNF-α (18). In the present
study, RI also inhibited M1 microglia activation, and the
expression of iNOS and additional pro-inflammatory molecules in the
SN region of MPTP-treated mice, as well as in mouse BV2 microglia
cells. This internal environmental change may have a
neuro-protective effect on DA neurons. In addition, a recent study
demonstrated that medium from microglia stimulated with LPS and
fasudil exerted a neuro-protective effect on rat PC12 neurons,
suggesting that inhibition of ROCK contributes to the survival of
DA neurons (18). As microglial
activation is aggressively initiated in early PD (34) and remains stable for years
(35), it may be essential to
consider ROCK inhibition in the early stages of PD to prevent
glial-mediated inflammation and neuronal elimination. Given that
MPTP/MPP+ directly damages DA neurons in vivo, whether the
shRNA directly inhibited ROCKII activity in DA neurons and thus
protected them from apoptosis, or whether this effect was an
indirect result of the inhibited pro-inflammatory effect of
microglia, could not be established in the current study. However,
experiments are planned to separately analyze neuron-targeted and
microglia-targeted shRNA systems to further investigate the
underlying mechanisms.
The greatest problem with ROCK inhibitors is that
neither Y27632 nor fasudil exclusively inhibit ROCKs. In addition,
they slightly influence PKN, MSK1, MAPK1b and PKA, and moderately
regulate AMPK and PHK (12). It
has thus been difficult to accurately evaluate the contribution of
ROCKs to the process of neuro-degeneration and investigate the
neuro-protective effect of ROCK inhibitors in neuro-degenerative
diseases. The present study used RNA interference to specifically
inhibit ROCKII expression and verify its role in an MPTP-induced PD
mouse model.
In conclusion, the results of the present study
indicate that ROCKII may serve a crucial role in MPTP-induced
parkinsonism in mice. In addition, the results demonstrate that RI
is a promising therapeutic target that inhibits the activation of
inflammatory microglia in the SN region.
Acknowledgements
The present study was supported by grants from the
National Foundation of Natural Science of China (grant nos.
81070956 and 81371414).
References
|
1
|
Dawson TM and Dawson VL: Molecular
pathways of neurodegeneration in Parkinson's disease. Science.
302:819–822. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Fox R, Nhan TQ, Law GL, Morris DR, Liles
WC and Schwartz SM: PSGL-1 and mTOR regulate translation of ROCK-1
and physiological functions of macrophages. EMBO J. 26:505–515.
2007. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Yoneda A, Multhaupt HA and Couchman JR:
The Rho kinases I and II regulate different aspects of myosin II
activity. J Cell Biol. 170:443–453. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Watanabe K, Ueno M, Kamiya D, Nishiyama A,
Matsumura M, Wataya T, Takahashi JB, Nishikawa S, Nishikawa S,
Muguruma K and Sasai Y: A ROCK inhibitor permits survival of
dissociated human embryonic stem cells. Nat Biotechnol. 25:681–686.
2007. View
Article : Google Scholar : PubMed/NCBI
|
|
5
|
Mong PY and Wang Q: Activation of Rho
kinase isoforms in lung endothelial cells during inflammation. J
Immunol. 182:2385–2394. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Aihara M, Dobashi K, Iizuka K, Nakazawa T
and Mori M: Comparison of effects of Y-27632 and Isoproterenol on
release of cytokines from human peripheral T cells. Int
Immunopharmacol. 3:1619–1625. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Aihara M, Dobashi K, Iizuka K, Nakazawa T
and Mori M: Effect of Y-27632 on release of cytokines from
peripheral T cells in asthmatic patients and normal subjects. Int
Immunopharmacol. 4:557–561. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Henry PJ, Mann TS and Goldie RG: A rho
kinase inhibitor, Y-27632 inhibits pulmonary eosinophilia,
bronchoconstriction and airways hyperresponsiveness in allergic
mice. Pulm Pharmacol Ther. 18:67–74. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Song Y, Chen X, Wang LY, Gao W and Zhu MJ:
Rho kinase inhibitor fasudil protects against β-amyloid-induced
hippocampal neurodegeneration in rats. CNS Neurosci Ther.
19:603–610. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Villar-Cheda B, Dominguez-Meijide A,
Joglar B, Rodriguez-Perez AI, Guerra MJ and Labandeira-Garcia JL:
Involvement of microglial RhoA/Rho-kinase pathway activation in the
dopaminergic neuron death. Role of angiotensin via angiotensin type
1 receptors. Neurobiol Dis. 47:268–279. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Tönges L, Frank T, Tatenhorst L, Saal KA,
Koch JC, Szego ÉM, Bähr M, Weishaupt JH and Lingor P: Inhibition of
rho kinase enhances survival of dopaminergic neurons and attenuates
axonal loss in a mouse model of Parkinson's disease. Brain.
135:3355–3370. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Mueller BK, Mack H and Teusch N: Rho
kinase, a promising drug target for neurological disorders. Nat Rev
Drug Discov. 4:387–398. 2005. View
Article : Google Scholar : PubMed/NCBI
|
|
13
|
Tokuoka H, Muramatsu S, Sumi-Ichinose C,
Sakane H, Kojima M, Aso Y, Nomura T, Metzger D and Ichinose H:
Compensatory regulation of dopamine after ablation of the tyrosine
hydroxylase gene in the nigrostriatal projection. J Biol Chem.
286:43549–43558. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Matsuura K, Kabuto H, Makino H and Ogawa
N: Pole test is a useful method for evaluating the mouse movement
disorder caused by striatal dopamine depletion. J Neurosci Methods.
73:45–48. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Bouet V, Boulouard M, Toutain J, Divoux D,
Bernaudin M, Schumann-Bard P and Freret T: The adhesive removal
test: A sensitive method to assess sensorimotor deficits in mice.
Nat Protoc. 4:1560–1564. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Ojo B, Rezaie P, Gabbott PL, Cowely TR,
Medvedev NI, Lynch MA and Stewart MG: A neural cell adhesion
molecule-derived peptide, FGL, attenuates glial cell activation in
the aged hippocampus. Exp Neurol. 232:318–328. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Zaborszky L and Vadasz C: The midbrain
dopaminergic system: Anatomy and genetic variation in dopamine
neuron number of inbred mouse strains. Behav Genet. 31:47–59. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Zhang H, Li Y, Yu J, Guo M, Meng J, Liu C,
Xie Y, Feng L, Xiao B and Ma C: Rho kinase inhibitor fasudil
regulates microglia polarization and function.
Neuroimmunomodulation. 20:313–322. 2013.PubMed/NCBI
|
|
19
|
Grassie ME, Moffat LD, Walsh MP and
MacDonald JA: The myosin phosphatase targeting protein (MYPT)
family: A regulated mechanism for achieving substrate specificity
of the catalytic subunit of protein phosphatase type 1δ. Arch
Biochem Biophys. 510:147–159. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Herskowitz JH, Feng Y, Mattheyses AL,
Hales CM, Higginbotham LA, Duong DM, Montine TJ, Troncoso JC,
Thambisetty M, Seyfried NT, et al: Pharmacologic inhibition of
ROCK2 suppresses amyloid-β production in an Alzheimer's disease
mouse model. J Neurosci. 33:19086–19098. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Rodriguez-Perez AI, Dominguez-Meijide A,
Lanciego JL, Guerra MJ and Labandeira-Garcia JL: Inhibition of Rho
kinase mediates the neuroprotective effects of estrogen in the MPTP
model of Parkinson's disease. Neurobiol Dis. 58:209–219. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Noma K, Kihara Y and Higashi Y: Striking
crosstalk of ROCK signaling with endothelial function. J Cardiol.
60:1–6. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Barcia C, Ros CM, Annese V, Carrillo-de
Sauvage MA, Ros-Bernal F, Gómez A, Yuste JE, Campuzano CM, de
Pablos V, Fernandez-Villalba E and Herrero MT: ROCK/Cdc42-mediated
microglial motility and gliapse formation lead to phagocytosis of
degenerating dopaminergic neurons in vivo. Sci Rep. 2:8092012.
View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Satoh K, Fukumoto Y and Shimokawa H:
Rho-kinase: Important new therapeutic target in cardiovascular
diseases. Am J Physiol Heart Circ Physiol. 301:H287–H296. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Wu KY, Hengst U, Cox LJ, Macosko EZ,
Jeromin A, Urquhart ER and Jaffrey SR: Local translation of RhoA
regulates growth cone collapse. Nature. 436:1020–1024. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Liu CY, Guo SD, Yu JZ, Li YH, Zhang H,
Feng L, Chai Z, Yuan HJ, Yang WF, Feng QJ, et al: Fasudil mediates
cell therapy of EAE by immunomodulating encephalomyelitic T cells
and macrophages. Eur J Immunol. 45:142–152. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
McGeer PL, Itagaki S, Boyes BE and McGeer
EG: Reactive microglia are positive for HLA-DR in the substantia
nigra of Parkinson's and Alzheimer's disease brains. Neurology.
38:1285–1291. 1988. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Brochard V, Combadière B, Prigent A,
Laouar Y, Perrin A, Beray-Berthat V, Bonduelle O, Alvarez-Fischer
D, Callebert J, Launay JM, et al: Infiltration of CD4+ lymphocytes
into the brain contributes to neurodegeneration in a mouse model of
Parkinson disease. J Clin Invest. 119:182–192. 2009.PubMed/NCBI
|
|
29
|
Boka G, Anglade P, Wallach D, Javoy-Agid
F, Agid Y and Hirsch EC: Immunocytochemical analysis of tumor
necrosis factor and its receptors in Parkinson's disease. Neurosci
Lett. 172:151–154. 1994. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Mogi M, Harada M, Riederer P, Narabayashi
H, Fujita K and Nagatsu T: Tumor necrosis factor-alpha (TNF-alpha)
increases both in the brain and in the cerebrospinal fluid from
parkinsonian patients. Neurosci Lett. 165:208–210. 1994. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Mogi M, Harada M, Kondo T, Riederer P,
Inagaki H, Minami M and Nagatsu T: Interleukin-1 beta,
interleukin-6, epidermal growth factor and transforming growth
factor-alpha are elevated in the brain from parkinsonian patients.
Neurosci Lett. 180:147–150. 1994. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Mogi M, Kondo T, Mizuno Y and Nagatsu T:
p53 protein, interferon-gamma, and NF-kappaB levels are elevated in
the parkinsonian brain. Neurosci Lett. 414:94–97. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Knott C, Stern G and Wilkin GP:
Inflammatory regulators in Parkinson's disease: iNOS, lipocortin-1,
and cyclooxygenases-1 and −2. Mol Cell Neurosci. 16:724–739. 2000.
View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Ouchi Y, Yoshikawa E, Sekine Y,
Futatsubashi M, Kanno T, Ogusu T and Torizuka T: Microglial
activation and dopamine terminal loss in early Parkinson's disease.
Ann Neurol. 57:168–175. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Gerhard A, Pavese N, Hotton G, Turkheimer
F, Es M, Hammers A, Eggert K, Oertel W, Banati RB and Brooks DJ: In
vivo imaging of microglial activation with [11C](R)-PK11195 PET in
idiopathic Parkinson's disease. Neurobiol Dis. 21:404–412. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Liberatore GT, Jackson-Lewis V, Vukosavic
S, Mandir AS, Vila M, McAuliffe WG, Dawson VL, Dawson TM and
Przedborski S: Inducible nitric oxide synthase stimulates
dopaminergic neurodegeneration in the MPTP model of Parkinson
disease. Nat Med. 5:1403–1409. 1999. View
Article : Google Scholar : PubMed/NCBI
|
|
37
|
Teismann P, Tieu K, Choi DK, Wu DC, Naini
A, Hunot S, Vila M, Jackson-Lewis V and Przedborski S:
Cyclooxygenase-2 is instrumental in Parkinson's disease
neurodegeneration. Proc Natl Acad Sci USA. 100:5473–5478. 2003.
View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Cicchetti F, Lapointe N, Roberge-Tremblay
A, Saint-Pierre M, Jimenez L, Ficke BW and Gross RE: Systemic
exposure to paraquat and maneb models early Parkinson's disease in
young adult rats. Neurobiol Dis. 20:360–371. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Noelker C, Morel L, Lescot T, Osterloh A,
Alvarez-Fischer D, Breloer M, Henze C, Depboylu C, Skrzydelski D,
Michel PP, et al: Toll like receptor 4 mediates cell death in a
mouse MPTP model of Parkinson disease. Sci Rep. 3:13932013.
View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Kim WG, Mohney RP, Wilson B, Jeohn GH, Liu
B and Hong JS: Regional difference in susceptibility to
lipopolysaccharide-induced neurotoxicity in the rat brain: Role of
microglia. J Neurosci. 20:6309–6316. 2000.PubMed/NCBI
|
|
41
|
Wang C, Wang H, Luo J, Hu Y, Wei L, Duan M
and He H: Selenium deficiency impairs host innate immune response
and induces susceptibility to Listeria monocytogenes infection. BMC
Immunol. 10:552009. View Article : Google Scholar : PubMed/NCBI
|