Introduction
Age-related macular degeneration (AMD) is a leading
cause of blindness for those over the age of 60, affecting millions
of people. The disease is associated with impaired sight, but also
with emotional distress, depression and anxiety (1). New vessels generated by
neovascularization usually undergo repeated regeneration, which
leads to bleeding and vessel destruction due to stress as a result
of increased permeability. Certain pathways associated with
neovascularization are linked to increased expression levels of
vascular endothelial growth factor (VEGF) (2–4).
Thus, VEGF is a major component of the neovascularization
process.
VEGF-A, also known as VEGF, belongs to the
cysteine-knot superfamily of growth factors. The characteristics of
VEGF are determined by a cysteine residue (5,6). The
anti-angiogenic effects of monoclonal anti-VEGF antibodies,
including ranibizumab and bevacizumab have been confirmed by
previous studies (7–10). However, multiple injections are
required and certain patients do not respond to intravitreal
anti-VEGF antibody administration (11–14).
Recently, multiple efforts have been made to overcome these
limitations (15–18).
VEGF transcription is activated by growth factors
and hypoxia (19). The
3′-untranslated regions (3′-UTRs) of resulting transcripts contain
adenosine and uridine rich elements (AREs), which determine mRNA
stability. ARE-mediated post-transcriptional regulation is
facilitated by trans-acting ARE-binding proteins, which form stable
complexes with the 3′-UTR and regulate the decay of VEGF mRNA
(20,21). Therefore, the relative abundance of
these ARE-binding proteins determines the level of VEGF transcripts
(22,23). Tristetraprolin (TTP) is a 34 kDa
member of the CCCH class of tandem zinc finger proteins (24–26).
TTP was first demonstrated to interact with AREs in mRNAs, and its
list of known and likely targets continues to grow. However, to the
best of our knowledge, the effects of TTP in retinal pigment
epithelial (RPE) cells have not been examined.
The aims of the present study were to investigate
the effects of TTP on VEGF mRNA and protein expression levels in
ARPE-19 cells under hypoxic conditions and to consider the
possibility that TTP may be useful as a novel treatment tool for
neovascular AMD (nAMD).
Materials and methods
Cell culture and treatment
ARPE-19 cells (American Type Culture Collection,
Manassas, VA, USA) were cultured in Dulbecco's modified Eagle's
medium/Nutrient Mixture F-12 (DMEM/F12; Gibco; Thermo Fisher
Scientific, Inc., Waltham, MA, USA) supplemented with 10% fetal
bovine serum (FBS; Gibco; Thermo Fisher Scientific, Inc.), 100
µg/ml streptomycin and 100 U/ml penicillin (Gibco; Thermo Fisher
Scientific, Inc.). The cells were maintained at 37°C in an
atmosphere containing 5% CO2.
Plasmids, transfection, and hypoxic
conditions
To overexpress TTP, ARPE-19 cells were transfected
with a pcDNA6/V5-HisA vector containing the TTP coding region
(pcDNA6/V5-TTP; Invitrogen; Thermo Fisher Scientific, Inc.)
(27) using TurboFect™ in
vitro transfection reagent (Thermo Fisher Scientific, Inc.).
The ARPE-19 cells were incubated at 37°C in 1% O2 and 5% CO2 to
mimic hypoxia.
Reverse transcription-polymerase chain
reaction (RT-PCR)
Total RNA was extracted using TRIzol reagent
(Invitrogen; Thermo Fisher Scientific, Inc.) following the
manufacturer's protocols. Total RNA (1 µg) was used for cDNA
synthesis using an iCycler thermocycler (Bio-Rad Laboratories,
Inc., Hercules, CA, USA). RT-PCR was performed using rTaq
polymerase (Elpis Biotech, Daejeon, Korea). VEGF cDNA amplification
conditions were as follows: 30 cycles of 94°C for 30 sec, 55°C for
30 sec and 72°C for 1 min. hGAPDH cDNA amplification conditions
were as follows: 20 cycles of 94°C for 30 sec, 55°C for 30 sec and
72°C for 1 min. The primers were synthesized on the basis of the
human TTP, VEGF, and hGAPDH cDNA sequences in the National Center
for Biotechnology Information data bank. The sequences of the
primers used for PCR were as follows: Forward,
5′-AGGCCAATCGCCACCCCAAA-3′ and reverse, 5′-GTGCCAGGGGCAGCAGAGAA-3′
for TTP; forward, 5′-GTGGACATCTTCCAGGAGTA-3′ and reverse,
5′-GTGCTGTAGGAAGCTCATCT-3′ for VEGF; and forward,
5′-AGCTGAACGGGAAGCTCACT-3′ and reverse, 5′-TGCTGTAGCCAAATTCGTTG-3′
for hGAPDH (Bioneer Corporation, Daejeon, Korea).
Quantitative PCR (qPCR)
For RNA kinetic analysis, the quantity of VEGF mRNA
was assessed in the presence of actinomycin D (Sigma-Aldrich; Merck
Millipore, Darmstadt, Germany) using qPCR. The reactions were
performed using EvaGreen® qPCR Master mix (Applied
Biological Materials Inc., Richmond, BC, Canada) and a LightCycler
480 instrument II (Roche Applied Science, Madison, WI, USA). VEGF
and hGAPDH cDNA amplification conditions were as follows: 95°C for
30 sec, 60°C for 30 sec and 72°C for 30 sec for 45 cycles. The
results were analyzed using melting curves and agarose gel
electrophoresis. The PCR primer pairs were as follows: Forward,
5′-CCCCATCCCTGTGGGCCTTG-3′ and reverse, 5′-ACCGCCTCGGCTTGTCACAT-3′
for VEGF; and forward, 5′-GCACCCCTGGCCAAGGTCAT-3′ and reverse
5′-ACGCCACAGTTTCCCGGAGG-3′ for hGAPDH (Bioneer Corporation).
Relative quantification of gene expression was analyzed using the
2-∆∆Cq method using GAPDH as the endogenous control (28).
Western blotting
Cells were harvested, centrifuged at 890 × g
for 1 min, and lysed in lysis buffer [50 mM Tris-Cl (pH 8.0;
Amresco LLC, Solon, OH, USA), 150 mM NaCl (Amresco LLC), 0.1% SDS
(Amresco LLC), 0.02% sodium azide (Amresco LLC), 1% NP-40
(Sigma-Aldrich; Merck Millipore), 0.5% sodium deoxycholate
(Sigma-Aldrich; Merck Millipore) and proteinase inhibitor cocktail
(phenylmethylsulphonyl fluoride, 100 µg/ml; aprotinin, 1 µg/ml;
leupeptin, 0.5 µg/ml; Roche Applied Science)]. Total protein
concentration was determined using a bicinchoninic acid protein
assay system (Pierce Biotechnology, Inc., Rockford, IL, USA).
Equivalent quantities of total protein (20–30 µg) were separated by
SDS-PAGE using 10–15% polyacrylamide gel, and then transferred to a
nitrocellulose membrane (Whatman; GE Healthcare Life Sciences,
Chalfont, UK) using a semi-dry transfer apparatus (Bio-Rad
Laboratories, Inc.) submerged in transfer buffer (25 mM Tris, pH
8.3, 192 mM glycine, and 20% methanol). The membrane was blocked
with 5% skimmed milk in 0.1% Tween-20/Tris-buffered saline (TBST)
and incubated with with the following primary antibodies: Anti-TTP
(T5327; 1:1,000; Sigma-Aldrich; Merck Millipore), anti-VEGF
(ab46154; 1:1,000; Abcam, Cambridge, UK) or anti-β-actin (A5441;
1:10,000; Sigma-Aldrich; Merck Millipore) antibodies at 4°C
overnight. Subsequently, the blots were washed in TBST and
incubated with goat anti-rabbit (cat. no. 31430) and goat
anti-mouse (cat. no. 31460) immunoglobulin G secondary antibodies
(1:10,000; Thermo Fisher Scientific, Inc.) for 45 min.
Immunoreactivity was detected by enhanced chemiluminescence
(Advansta, Inc., Menlo Park, CA, USA) and images were captured
using a LAS 4000 Bioimager (Fujifilm Holdings Corporation, Tokyo,
Japan).
Enzyme-linked immunosorbent assay
(ELISA)
ARPE-19 cells were plated onto 6-well cell culture
plates at a density of 5×104 cells/well. The DMEM/F12
was supplemented with 1% FBS, 100 U/ml penicillin, and 100 µg/ml
streptomycin. VEGF secretion levels were measured using the Human
VEGF-ELISA Research-Use-Only kit (KHG0111) from Invitrogen (Thermo
Fisher Scientific, Inc.), according to the manufacturer's
protocols.
Luciferase assay
A luciferase assay was performed to determine
whether the 3′-UTR of the VEGF mRNA is required for TTP-mediated
destabilization. ARPE-19 cells were co-transfected with designated
constructs, such as psiCHECK-VEGF 3′-UTR constructs and
pcDNA6/V5-TTP using TurboFect™ in vitro transfection reagent
(Thermo Fisher Scientific, Inc.). The transfected cells were lysed
with lysis buffer (Promega Corporation, Madison, WI, USA) and mixed
with luciferase assay reagent (Promega Corporation) and the
chemiluminescent signal was measured using the Infinite 200 PRO
system (Tecan Group, Ltd., Mannedorf, Switzerland). Firefly
luciferase was normalized to Renilla luciferase in each
sample. All luciferase assays reported here represent at least
three independent experiments, each consisting of three wells per
transfection.
Electrophoretic mobility shift assay
(EMSA)
The biotinylated RNA probes for the wild-type (WT)
VEGF
(5′-GGUACUUAUUUAAUAGCCCUUUUUAAUUAGAAAUUAAAACAGUUAAUUUAAUUAA-3′) and
mutant (mut-VEGF-EMSA;
5′-GGUACUUAGGUAAUAGCCCUUUUUAAUUAGAAAUUAAAACAGUUAAGGUAAUUAA-3′) were
synthesized by Samchully Pharm Co. (Seoul, Korea) as described
previously (23). In the mutant
(MuT) RNA probes, which were used as negative controls, the AUUUA
sequences of the AREs were replaced with AGCA. Cytoplasmic extracts
were prepared from pcDNA6/V5-TTP transfected ARPE-19 cells using
NE-PER Nuclear and Cytoplasmic Extraction reagent kit (Thermo
Fisher Scientific, Inc.). RNA EMSA was performed using the
LightShift™ Chemiluminescent EMSA kit according to the
manufacturer's instructions. TTP antibody (T5327) or control
(I-5381) antibodies (Sigma-Aldrich; Merck Millipore) were added to
the reaction mixtures (1 or 5 µg/µl). Subsequent to the addition of
the antibodies, reaction mixtures were incubated overnight at 4°C.
Images were captured using a LAS 4000 Bioimager (Fujifilm).
Human umbilical vein endothelial cell
(HUVEC) tube formation assay
ARPE-19 cells were cultured in DMEM/F12 media
containing 0.1% FBS, 100 U/ml penicillin, and 100 µg/ml
streptomycin, and were maintained at 37°C and 5% CO2. The cells
were exposed to hypoxic conditions in a hypoxic chamber for 24 h.
The conditioned medium (CM) obtained from the ARPE-19 cells was
transferred to HUVECs (PromoCell GmbH, Heidelberg, Germany) that
were seeded onto 96-well plates coated with Matrigel™ at a density
of 4×103 cells/well. The CM-treated HUVECs were
incubated for 48 h, and then the branch numbers were calculated
using ImageJ software (imagej.nih.gov).
Statistical analysis
Data are expressed as the mean ± standard error of
the mean. Statistical significance was determined using Student's
t-tests (GraphPad Prism, version 5; GraphPad Software, Inc., La
Jolla, CA, USA). P<0.05 was considered to indicate a
statistically significant difference.
Results
TTP reduces the expression and
secretion levels of hypoxia-induced VEGF
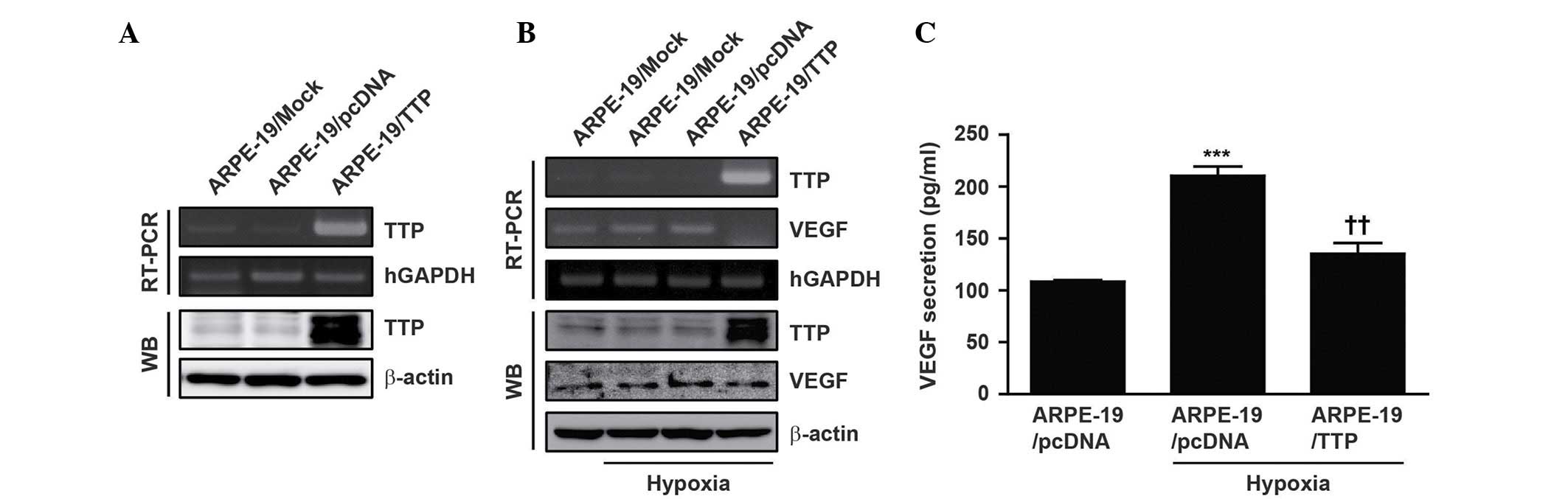
To establish transient expression of TTP, a TTP
expression vector (pcDNA6/V5-TTP) was transfected into ARPE-19
cells (ARPE-19/TTP) under normoxic conditions. As a negative
control, ARPE-19 cells were transiently transfected with empty
pcDNA6/V5 vectors. Overexpression of TTP was confirmed by RT-PCR
and western blotting (Fig. 1A).
The ARPE-19 cells were incubated at 37°C in 1% O2 and 5% CO2 in
order to mimic hypoxia. After 24 h, VEGF expression was induced
under hypoxic conditions (Fig.
1B).
A previous study reported that TTP promotes the
decay of VEGF transcripts in human colon cancer (23). By contrast, overexpression of TTP
in ARPE-19/TTP cells reduced hypoxia-induced VEGF expression
(Fig. 1B). Next, it was examined
whether overexpression of TTP could reduce the level of VEGF
secretion. Secretion of VEGF into the extracellular space was
assessed by ELISA. Overexpression of TTP in ARPE-19/TTP cells
significantly reduced the level of secreted hypoxia-induced VEGF
(P<0.01; Fig. 1C). These
results indicate that overexpression of TTP reduces the expression
and secretion levels of hypoxia-induced VEGF.
VEGF AREs are essential for the
inhibitory effect of TTP
To determine whether the 3′-UTR of the VEGF mRNA is
required for TTP-mediated destabilization, ARPE-19 cells were
co-transfected with pcDNA6/V5-TTP and a psiCHECK2 luciferase
expression vector containing the VEGF 3′-UTR. The luciferase
activity in cells transfected with pcDNA6/V5-TTP was significantly
lower than that in cells transfected with pcDNA6/V5 empty vector
(P<0.05; Fig. 2). This result
suggests that the 3′-UTR of VEGF mRNA is involved in its
destabilization via the inhibitory activity of TTP.
TTP binds to VEGF AREs
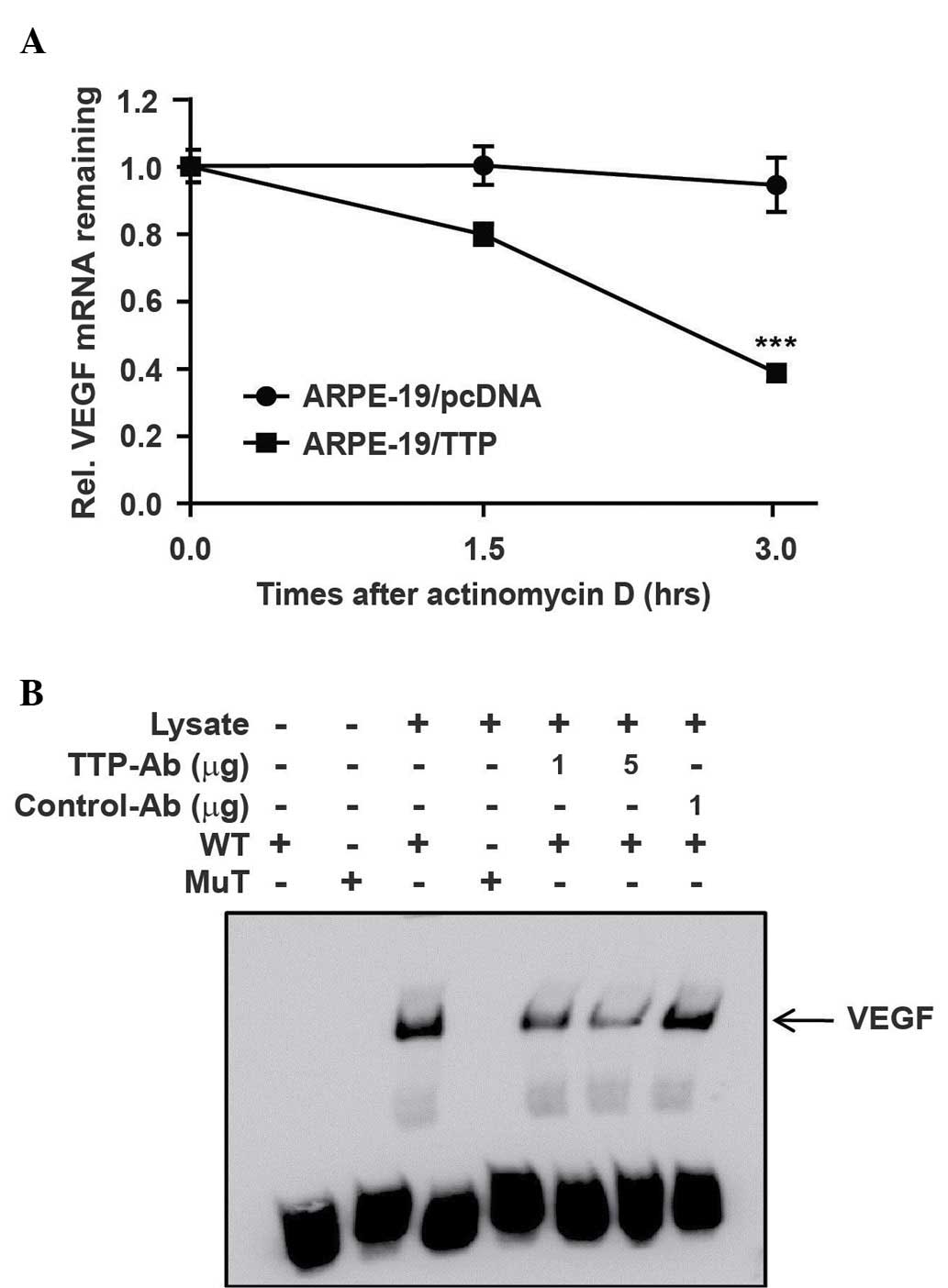
To investigate whether the reduced expression of
VEGF resulted from decay of the VEGF mRNA, the half-life of this
mRNA was measured by qPCR in ARPE-19 cells transfected with
pcDNA6/V5-TTP (ARPE-19/TTP) or pcDNA6/V5 empty vector
(ARPE-19/pcDNA6). The ARPE-19 cells were incubated for 24 h under
hypoxic conditions. Following actinomycin D treatment, the
half-life of VEGF mRNA was 1.8 h in ARPE-19/TTP cells and 6.5 h in
ARPE-19/pcDNA6 cells (Fig. 3A).
This result indicates that overexpression of TTP significantly
induces the decay of VEGF mRNA (P<0.001).
To examine whether TTP interacts directly with the
AREs in the 3′-UTR of VEGF mRNA, an RNA EMSA was performed using
biotinylated RNA probes containing the WT or MuT AREs of VEGF. The
RNA probes used in EMSA were the same as those used in the
luciferase assay. In the MuT RNA probe used as a negative control,
AUUUA sequence of the VEGF-ARE-WT was replaced with AGCA.
Cytoplasmic extracts prepared from ARPE-19 cells cultured under
hypoxic conditions were incubated with biotinylated RNA probes
containing the WT or MuT AREs of VEGF 3′UTR. When RNA EMSA was
performed using the VEGF-ARE-WT probe, a dominant RNA-protein
complex was formed. However, this complex was not formed with
VEGF-ARE-MuT probe. Complex formation was reduced in the presence
of an anti-TTP antibody (Fig. 3B).
Overall, these results suggest that TTP regulates the expression of
VEGF mRNA by binding to AREs in its 3′UTR.
TTP inhibits neovascularization
indirectly
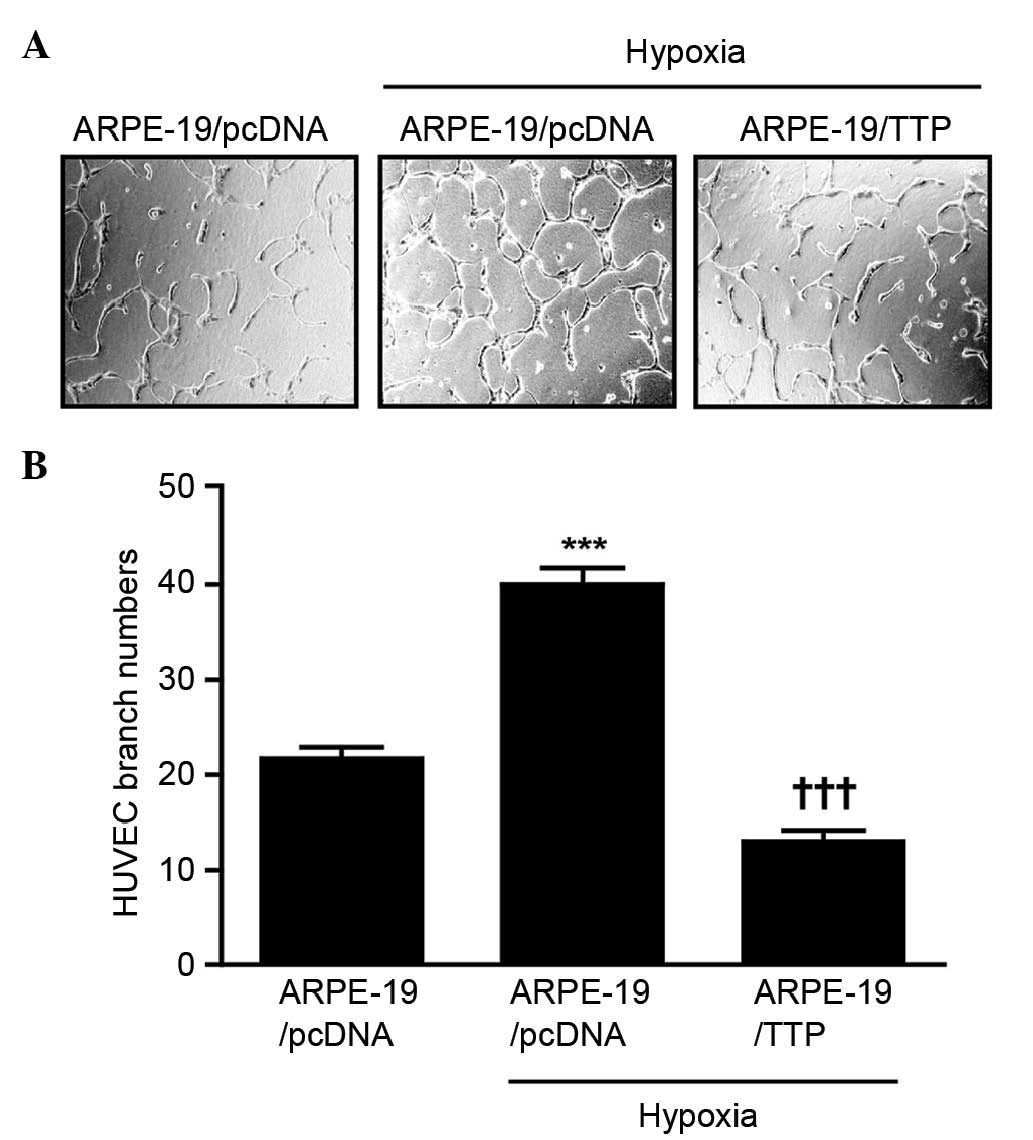
To determine the effect of TTP overexpression on
neovascularization indirectly, a HUVEC tube formation assay was
used. ARPE-19 cells transfected with pcDNA6/V5-TTP (ARPE-19/TTP) or
pcDNA6/V5 empty vector (ARPE-19/pcDNA6) were incubated at 37°C in
1% O2 and 5% CO2 to mimic hypoxia. After 24 h, CM from the cells
was transferred to HUVECs, which were incubated for 48 h. HUVECs
treated with hypoxia CM from ARPE-19/pcDNA6 cells had a larger
number of branch points than those treated with normoxia CM from
ARPE-19/pcDNA6. However, HUVECs treated with hypoxia CM from
ARPE-19/TTP cells had a significantly lower number of branch points
than those treated with hypoxia CM from ARPE-19/pcDNA cells
(P<0.001; Fig. 4A and B). These
results suggest that overexpression TTP inhibits neovascularization
indirectly.
Discussion
TTP, a tandem CCCH zinc-finger RNA-binding protein,
regulates the decay of mRNAs that contain multiple AREs (29). The number of published papers
discussing TTP has increased gradually since 1990 with >40
TTP-associated papers published in 2012 and interest in this
protein is increasing (24).
Previous studies demonstrated that TTP induces the decay of
ARE-containing mRNAs (23,30,31),
including proto-oncogenes and those encoding growth factors (such
as VEGF) and proteins involved in inflammation and invasion
(32–34). A number of studies have used
diverse cells or tissue models to demonstrate that TTP inhibits
VEGF production by destabilizing VEGF mRNA, however, to the best of
our knowledge, no studies have examined the effects of TTP on the
eye or ARPE-19 cells (25,26,35).
Thus, the aim of the present study was to investigate the effects
of TTP on VEGF mRNA and protein levels in ARPE-19 cells under
hypoxic conditions, and to examine the possibility of using TTP as
a novel treatment tool for neovascularization and nAMD.
Endogenous expression of TTP was low in ARPE-19
cells under normoxic and hypoxic conditions, whereas VEGF
expression was increased under hypoxic conditions. On the other
hand, overexpression of TTP reduced the stability of VEGF mRNA and
the secretion of VEGF into the extracellular space of ARPE-19 cells
under hypoxic conditions. These results suggest that TTP expression
is inversely correlated with that of VEGF in ARPE-19 cells, and
that TTP does not respond directly to a hypoxic stimulus. Low
expression of TTP in ARPE-19 cells under hypoxic conditions
resembles TTP in numerous cancer cells. Furthermore, these two
states increase the expression of VEGF. These points suggest the
possibility of using TTP as a treatment tool for nAMD, in addition
to cancer.
A luciferase assay was performed to determine
whether the 3′-UTR of VEGF mRNA is required for TTP-mediated
destabilization. An RNA EMSA was also conducted to examine whether
TTP binds directly to the 3′UTR of this mRNA. As occurs in other
cells, TTP in ARPE-19 cells destabilized the VEGF mRNA and bound
directly to AREs in its 3′-UTR (23,24).
This result indicates that TTP may be used to suppress VEGF in RPE
cells when nAMD occurs under hypoxic conditions.
Anti-VEGF antibodies bind directly to VEGFs and
render them inactive in the extracellular space (9,10).
By contrast, the expression of TTP and destabilization of VEGF
mRNAs occurs in the intracellular space. For this reason, gene
transfection with a TTP-expressing pcDNA6/V5-HisA vector or
adenoviral vectors could be performed in the intracellular space.
The effective period of anti-VEGF antibody treatment is ~4 weeks
and aflibercept has a longer duration of effect (13,15).
However, the present study did not determine the effective period
of TTP-expressing vectors, despite the finding that CM from of
TTP-overexpressing ARPE-19 cells suppressed HUVEC tube formation
compared with hypoxic CM. Thus, further investigations will be
undertaken in the future.
In conclusion, TTP was demonstrated to destabilize
the VEGF mRNA in ARPE-19 cells under hypoxic conditions.
Furthermore, CM from TTP-overexpressing ARPE-19 cells suppressed
tube formation in HUVECs. These findings indicate that regulation
of TTP expression may be a promising therapeutic tool for nAMD,
although further research is required.
Acknowledgements
The present study was supported by the Biomedical
Research Institute Fund (grant no. GNUHBRIF-2013-0002) of
Gyeongsang National University Hospital (Jinju, Korea).
References
|
1
|
Berman K and Brodaty H: Psychosocial
effects of age-related macular degeneration. Int Psychogeriatr.
18:415–428. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Chan EC, van Wijngaarden P, Liu GS, Jiang
F, Peshavariya H and Dusting GJ: Involvement of Nox2 NADPH oxidase
in retinal neovascularization. Invest Ophthalmol Vis Sci.
54:7061–7067. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Engelmann D, Mayoli-Nüssle D, Mayrhofer C,
Fürst K, Alla V, Stoll A, Spitschak A, Abshagen K, Vollmar B, Ran S
and Pützer BM: E2F1 promotes angiogenesis through the
VEGF-C/VEGFR-3 axis in a feedback loop for cooperative induction of
PDGF-B. J Mol Cell Biol. 5:391–403. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Ma JF, Von Kalle M, Plautz QM, Xu F, Singh
L and Wang L: Relaxin promotes in vitro tumour growth, invasion and
angiogenesis of human Saos-2 osteosarcoma cells by AKT/VEGF
pathway. Eur Rev Med Pharmacol Sci. 17:1345–1350. 2013.PubMed/NCBI
|
|
5
|
Muller YA, Christinger HW, Keyt BA and de
Vos AM: The crystal structure of vascular endothelial growth factor
(VEGF) refined to 1.93 A resolution: Multiple copy flexibility and
receptor binding. Structure. 5:1325–1338. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Ciulla TA, Danis RP, Criswell M and Pratt
LM: Changing therapeutic paradigms for exudative age-related
macular degeneration: Antiangiogenic agents and photodynamic
therapy. Expert Opin Investig Drugs. 8:2173–2182. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Ogino K, Tsujikawa A, Yamashiro K, Ooto S,
Oishi A, Nakata I, Miyake M and Yoshimura N: Intravitreal injection
of ranibizumab for recovery of macular function in eyes with
subfoveal polypoidal choroidal vasculopathy. Invest Ophthalmol Vis
Sci. 54:3771–3779. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Frampton JE: Ranibizumab: A review of its
use in the treatment of neovascular age-related macular
degeneration. Drugs Aging. 30:331–358. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Scott AW and Bressler SB: Long-term
follow-up of vascular endothelial growth factor inhibitor therapy
for neovascular age-related macular degeneration. Curr Opin
Ophthalmol. 24:190–196. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Jiang S, Park C and Barner JC: Ranibizumab
for age-related macular degeneration: A meta-analysis of dose
effects and comparison with no anti-VEGF treatment and bevacizumab.
J Clin Pharm Ther. 39:234–239. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Binder S: Loss of reactivity in
intravitreal anti-VEGF therapy: Tachyphylaxis or tolerance? Br J
Ophthalmol. 96:1–2. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Ehlken C, Jungmann S, Bohringer D,
Agostini HT, Junker B and Pielen A: Switch of anti-VEGF agents is
an option for nonresponders in the treatment of AMD. Eye (Lond).
28:538–545. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Fassnacht-Riederle H, Becker M, Graf N and
Michels S: Effect of aflibercept in insufficient responders to
prior anti-VEGF therapy in neovascular AMD. Graefes Arch Clin Exp
Ophthalmol. 252:1705–1709. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Bakall B, Folk JC, Boldt HC, Sohn EH,
Stone EM, Russell SR and Mahajan VB: Aflibercept therapy for
exudative age-related macular degeneration resistant to bevacizumab
and ranibizumab. Am J Ophthalmol. 156:15–22. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Schmidt-Erfurth U, Kaiser PK, Korobelnik
JF, Brown DM, Chong V, Nguyen QD, Ho AC, Ogura Y, Simader C, Jaffe
GJ, et al: Intravitreal aflibercept injection for neovascular
age-related macular degeneration: Ninety-six-week results of the
VIEW studies. Ophthalmology. 121:193–201. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Fritsche LG, Fariss RN, Stambolian D,
Abecasis GR, Curcio CA and Swaroop A: Age-related macular
degeneration: Genetics and biology coming together. Annu Rev
Genomics Hum Genet. 15:151–171. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Xu XD, Li KR, Li XM, Yao J, Qin J and Yan
B: Long non-coding RNAs: New players in ocular neovascularization.
Mol Biol Rep. 41:4493–4505. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
McLaughlin MM, Paglione MG, Slakter J,
Tolentino M, Ye L, Xu CF, Suttle AB and Kim RY: Initial exploration
of oral pazopanib in healthy participants and patients with
age-related macular degeneration. JAMA Ophthalmol. 131:1595–1601.
2013. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Arcondéguy T, Lacazette E, Millevoi S,
Prats H and Touriol C: VEGF-A mRNA processing, stability and
translation: A paradigm for intricate regulation of gene expression
at the post-transcriptional level. Nucleic Acids Res. 41:7997–8010.
2013. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Claffey KP, Shih SC, Mullen A, Dziennis S,
Cusick JL, Abrams KR, Lee SW and Detmar M: Identification of a
human VPF/VEGF 3′ untranslated region mediating hypoxia-induced
mRNA stability. Mol Biol Cell. 9:469–481. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Levy NS, Chung S, Furneaux H and Levy AP:
Hypoxic stabilization of vascular endothelial growth factor mRNA by
the RNA-binding protein HuR. J Biol Chem. 273:6417–6423. 1998.
View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Cherradi N, Lejczak C, Desroches-Castan A
and Feige JJ: Antagonistic functions of tetradecanoyl phorbol
acetate-inducible-sequence 11b and HuR in the hormonal regulation
of vascular endothelial growth factor messenger ribonucleic acid
stability by adrenocorticotropin. Mol Endocrinol. 20:916–930. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Lee HH, Son YJ, Lee WH, Park YW, Chae SW,
Cho WJ, Kim YM, Choi HJ, Choi DH, Jung SW, et al: Tristetraprolin
regulates expression of VEGF and tumorigenesis in human colon
cancer. Int J Cancer. 126:1817–1827. 2010.PubMed/NCBI
|
|
24
|
Brooks SA and Blackshear PJ:
Tristetraprolin (TTP): Interactions with mRNA and proteins, and
current thoughts on mechanisms of action. Biochim Biophys Acta.
1829:666–679. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Cha HJ, Lee HH, Chae SW, Cho WJ, Kim YM,
Choi HJ, Choi DH, Jung SW, Min YJ, Lee BJ, et al: Tristetraprolin
downregulates the expression of both VEGF and COX-2 in human colon
cancer. Hepatogastroenterology. 58:790–795. 2011.PubMed/NCBI
|
|
26
|
Brennan SE, Kuwano Y, Alkharouf N,
Blackshear PJ, Gorospe M and Wilson GM: The mRNA-destabilizing
protein tristetraprolin is suppressed in many cancers, altering
tumorigenic phenotypes and patient prognosis. Cancer Res.
69:5168–5176. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Lee HH, Vo MT, Kim HJ, Lee UH, Kim CW, Kim
HK, Ko MS, Lee WH, Cha SJ, Min YJ, et al: Stability of the LATS2
tumor suppressor gene is regulated by tristetraprolin. J Biol Chem.
285:17329–17337. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Schmittgen TD and Livak KJ: Analyzing
real-time PCR data by the comparative C(T) method. Nat Protoc.
3:1101–1108. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Baou M, Jewell A and Murphy JJ: TIS11
family proteins and their roles in posttranscriptional gene
regulation. J Biomed Biotechnol. 2009:6345202009. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Carballo E, Lai WS and Blackshear PJ:
Feedback inhibition of macrophage tumor necrosis factor-alpha
production by tristetraprolin. Science. 281:1001–1005. 1998.
View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Hau HH, Walsh RJ, Ogilvie RL, Williams DA,
Reilly CS and Bohjanen PR: Tristetraprolin recruits functional mRNA
decay complexes to ARE sequences. J Cell Biochem. 100:1477–1492.
2007. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Nanbu R, Menoud PA and Nagamine Y:
Multiple instability-regulating sites in the 3′ untranslated region
of the urokinase-type plasminogen activator mRNA. Mol Cell Biol.
14:4920–4928. 1994. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Roldan AL, Cubellis MV, Masucci MT,
Behrendt N, Lund LR, Danø K, Appella E and Blasi F: Cloning and
expression of the receptor for human urokinase plasminogen
activator, a central molecule in cell surface, plasmin dependent
proteolysis. EMBO J. 9:467–474. 1990.PubMed/NCBI
|
|
34
|
Fini ME, Plucinska IM, Mayer AS, Gross RH
and Brinckerhoff CE: A gene for rabbit synovial cell collagenase:
Member of a family of metalloproteinases that degrade the
connective tissue matrix. Biochemistry. 26:6156–6165. 1987.
View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Hacker C, Valchanova R, Adams S and Munz
B: ZFP36L1 is regulated by growth factors and cytokines in
keratinocytes and influences their VEGF production. Growth Factors.
28:178–190. 2010. View Article : Google Scholar : PubMed/NCBI
|