Introduction
Osteosarcoma (OS) is the most common primary
malignant bone tumor in children and adolescents, and demonstrates
a high tendency to metastasize (1). The estimated worldwide incidence rate
is 4,000,000 cases/year (2).
Although the 5-year survival rates of patients with primary OS has
been significantly improved from 30–50% to 60–90% by combining
surgery with multiagent chemotherapy (3–5), the
efficacy of chemotherapy is reduced by the development of drug
resistance (6). It is estimated
that <30% of patients with recurrent disease achieve 5-year
survival (7–9). Therefore, the identification of novel
molecular targets and signaling pathways is necessary to improve
the effectiveness of chemotherapy for the management of OS.
Nuclear transcription factor Y (NF-Y) is a
ubiquitous protein, composed of three subunits; NF-YA, NF-YB and
NF-YC. The genes that encode these proteins are highly conserved
from yeast to mammals, with NF-YA acting as the regulatory subunit
of the trimer (10). It has been
demonstrated that NF-Y regulates gene expression by binding to
promoter regions, and NF-Y is essential for cell proliferation
(11–13). Multiple studies have recently
demonstrated that increased expression of NF-Y in various cancers
is associated with poor prognosis (14–16).
However, whether elevated expression of NF-Y promotes a malignant
phenotype in OS cells, and the molecular mechanisms underlying
these putative effects remains unknown.
Fatty acid synthase (FASN) is an important enzyme
involved in energy metabolism. Our previous studies suggested that
FASN is overexpressed in OS cells and promoted its malignant
phenotype (17,18). However, the mechanism underlying
FASN overexpression remains unclear.
The present study aimed to determine whether NF-YA
is involved in mediating the malignant phenotype of OS via
regulation of FASN expression, by investigating the malignant
phenotype of OS cells and FASN expression following knockdown or
overexpression of NF-YA.
Materials and methods
Cell lines
The human OS cell lines, U2-OS and HOS, and the
human osteoblast cell line, HOB, were purchased from the Cell Bank
of Type Culture Collection of the Chinese Academy of Sciences
(Shanghai, China). The U2-OS and HOS cells were cultured in
Ham's/F-12 medium (Gibco; Thermo Fisher Scientific, Inc., Waltham,
MA, USA), and HOB cells were cultured in Dulbecco's modified Eagle
medium (Gibco; Thermo Fisher Scientific, Inc.), both supplemented
with 10% fetal bovine serum (FBS; Gibco; Thermo Fisher Scientific,
Inc.), 100 U/ml penicillin and 100 U/ml streptomycin. Cells were
cultured at 37°C in 5% CO2.
RNA isolation and reverse
transcription-quantitative polymerase chain reaction (RT-qPCR)
Total RNA from OS and HOB cells was extracted using
TRIzol® (Invitrogen; Thermo Fisher Scientific, Inc.)
according to the manufacturer's protocol. Total RNA concentration
was determined by measuring absorbance at a wavelength of 260 nm
and purity was determined by calculating the 260/280 nm ratio with
a BioPhotometer (Eppendorf, Hamburg, Germany). The mRNA expression
levels of NF-YA and FASN were evaluated by RT-qPCR, using β-actin
as the internal reference gene. The Two-Step RT kit (Promega
Corporation, Madison, WI, USA) was used according to the
manufacturer's protocol to synthesize cDNA, which was used as the
template for amplification. TaqMan® Real-Time PCR Master
Mixes (Thermo Fisher Scientific, Inc.) were used for amplification
under the following cycling conditions: An initial denaturation
step at 95°C for 1 min, followed by 40 cycles of denaturation at
95°C for 15 sec, annealing at 58°C for 20 sec and extension at 72°C
for 20 sec. Data was normalized using the 2−ΔΔCq method
(19). All procedures were
performed according to the manufacturer's protocols, with primer
sequences as listed in Table I. A
total of six independent experiments were performed over multiple
days.
 | Table I.Primer information. |
Table I.
Primer information.
| Primer (product
size) | Sequence (5′-3′) |
|---|
| FASN (262 bp) | Forward:
GTCGGAGAACTTGCAGGAGT |
|
| Reverse:
TCCTCGGAGTGAATCTGGGT |
| β-actin (295 bp) | Forward:
TCACCCACACTGTGCCATCATCGA |
|
| Reverse:
CAGCGGAACCGCTCATTGCCAATGG |
| NF-YA (238 bp) | Forward:
TTGTTGGTCAGGGTTTACAGC |
|
| Reverse:
ACGCTCCACGATGTCACTAA |
Lentivirus vector construction and
cell transfection
HEK293 cells were used to produce lentiviral
vectors, with the viral titer of 2×108. To construct
vectors for upregulating and downregulating NF-YA and FASN
expression, the wild-type sequences and reverse complementary
sequences
(5′-ACTGACTGACCAAACAGCAATAGTTCGACAGAGCACAGGACACAAGGCCTGTTAC-3′)
were inserted into lentivirus vectors (Biomiga Inc., San Diego, CA,
USA). U2-OS and HOS cells were transfected using polybrene
(Genomeditech, Shanghai, China) with lentivirus vectors
(multiplicity of infection=20) to upregulate NF-YA (Lv-up-NF-YA),
downregulate NF-YA (Lv-down-NF-YA) and downregulate FASN
(Lv-down-FASN). A non-targeting lentivirus vector (Lv-neg) was used
as a negative control. The transfection efficiency was evaluated by
fluorescence microscopy, as the lentiviral vectors expressed green
fluorescent protein.
Western blot analysis
Total protein from cells was extracted using
radioimmunoprecipitation assay lysis buffer (Invitrogen; Thermo
Fisher Scientific, Inc.) containing 60 µg/ml phenylmethylsulfonyl
fluoride, according to the manufacturer's protocol. Protein
concentration was determined using the Bradford assay. Proteins (10
µg) were separated by SDS-PAGE and transferred to polyvinylidene
difluoride membranes. The membranes were probed with the following
primary antibodies overnight at 4°C: mouse anti-NF-YA (1:500;
catalog no. sc-17753; Santa Cruz Biotechnology, Inc., Dallas, TX,
USA), mouse anti-FASN (1:500; catalog no. sc-55580; Santa Cruz
Biotechnology, Inc.) and mouse anti-β-actin antibody (1:2,000;
catalog no. sc-47778; Santa Cruz Biotechnology, Inc.). Following
incubation with a goat anti-mouse horseradish peroxidase-conjugated
secondary antibody (1:5,000; catalog no. 610-103-043; Rockland
Immunochemicals, Inc., Pottstown, PA, USA) for 1.5 h at room
temperature, immunoreactive bands were visualized using an Enhanced
Chemiluminescence reagent (Thermo Fisher Scientific, Inc.). The
intensity of western blot bands was measured using ImageJ software
version 1.48 (National Institutes of Health, Bethesda, MD, USA).
Six independent experiments were performed over multiple days.
Migration assays
Cell migration was assessed using a ‘wound-healing
assay’, which measures the ability of cells to migrate into a
two-dimensional space in vitro. In brief, cells were
cultured to 80% confluence in 6-well tissue culture dishes to a
density of ~5×106 cells/well. A line of cells was
removed by scraping a line of cells from the center of the plate
using a rubber policeman (Thermo Fisher Scientific, Inc.). Cultures
were rinsed with PBS and fresh medium alone without 10% FBS was
then added before the cells were incubated at 37°C for 24 h. Images
were captured at 1 and 24 h using an electron microscope and camera
(Canon, Inc., Tokyo, Japan), and the migrated distance was measured
using ImageJ software version 1.48 (National Institutes of Health).
For each sample, the cell migration rate was calculated by counting
the number of migrated cells in three fields of view per well. A
total of six independent experiments were performed on separate
days.
Transwell invasion assays
Invasion of OS cells was measured using the BD
BioCoat Matrigel Invasion Chamber (BD Biosciences, Franklin Lakes,
NJ, USA) according to the manufacturer's protocol. The medium in
the lower chamber contained 5% fetal calf serum (Gibco; Thermo
Fisher Scientific, Inc.) as a source of chemoattractants. Cells
(1×103/well) were suspended in serum-free medium and
added to the upper chambers at the same time. Following 24 h of
incubation, cells that passed through the Matrigel-coated membrane
were fixed in methanol for 10 min and stained with Diff-Quik
(Sysmex Corporation, Kobe, Japan). Images (original magnification,
×400) were captured using an electron microscope and camera (Canon,
Inc.). Images of three fields of view per membrane were captured
and analyzed using ImageJ software version 1.48 (National
Institutes of Health), with six independent experiments performed
on separate days.
Statistical analysis
Data are expressed as the mean ± standard deviation.
Student's t-tests were used for two-sample comparisons and one-way
analysis of variance was used to compare >3 samples. P<0.05
was considered to indicate a statistically significant difference.
All analyses were performed using SPSS software (version, 13.0;
SPSS, Inc., Chicago, IL, USA).
Results
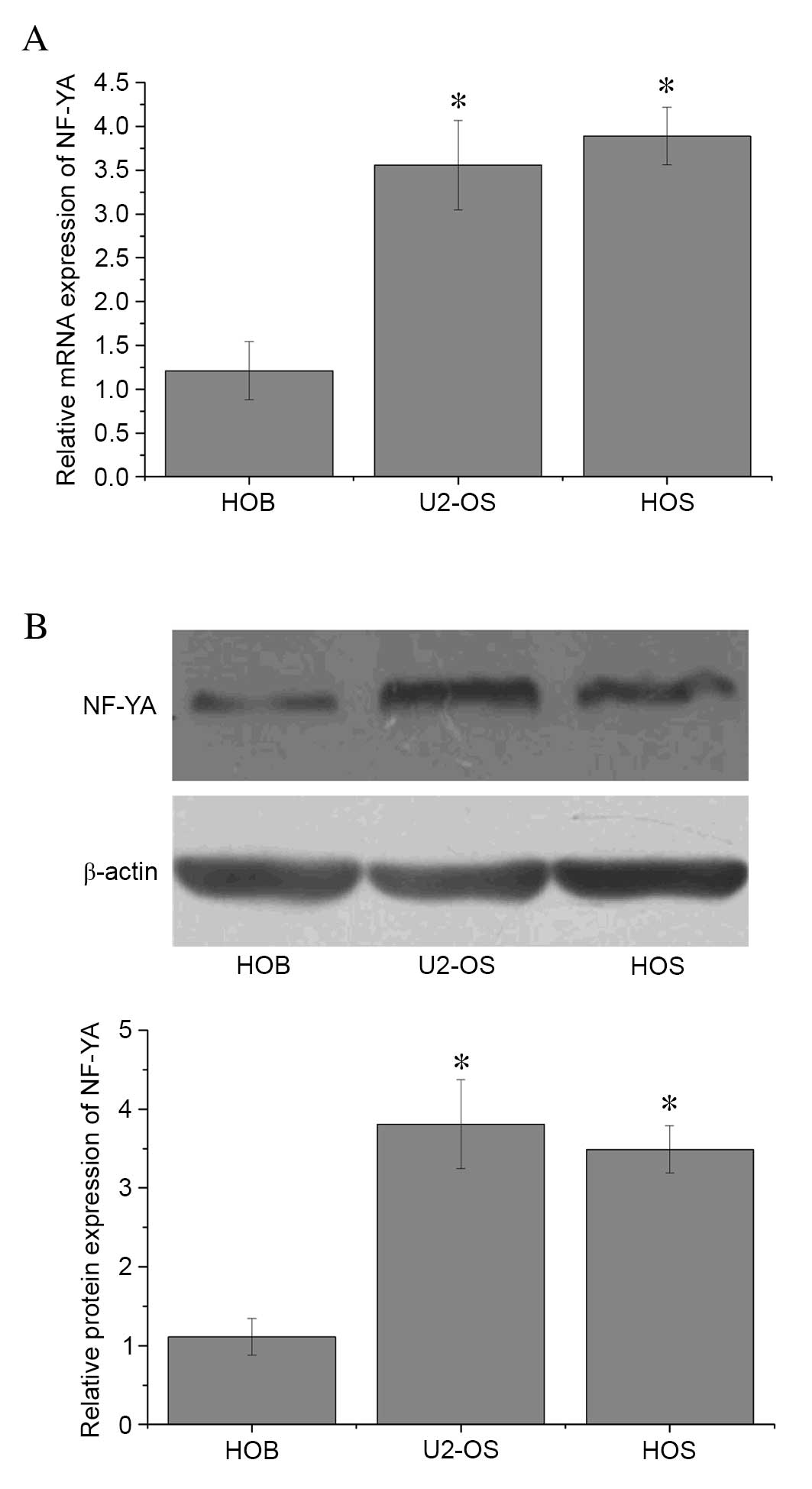
Elevated NF-YA expression in OS
cells
The mRNA and protein expression levels of NF-YA were
assessed in U2-OS, HOS and HOB cells by RT-qPCR and western blot
analyses, respectively. Expression levels of NF-YA mRNA (Fig. 1A) and protein (Fig. 1B) were significantly lower in the
HOB osteoblast cell line when compared with the U2-OS and HOS OS
cell lines, (P=0.01; Fig. 1A).
These results suggest that increased NF-YA expression may be a
feature of OS cells.
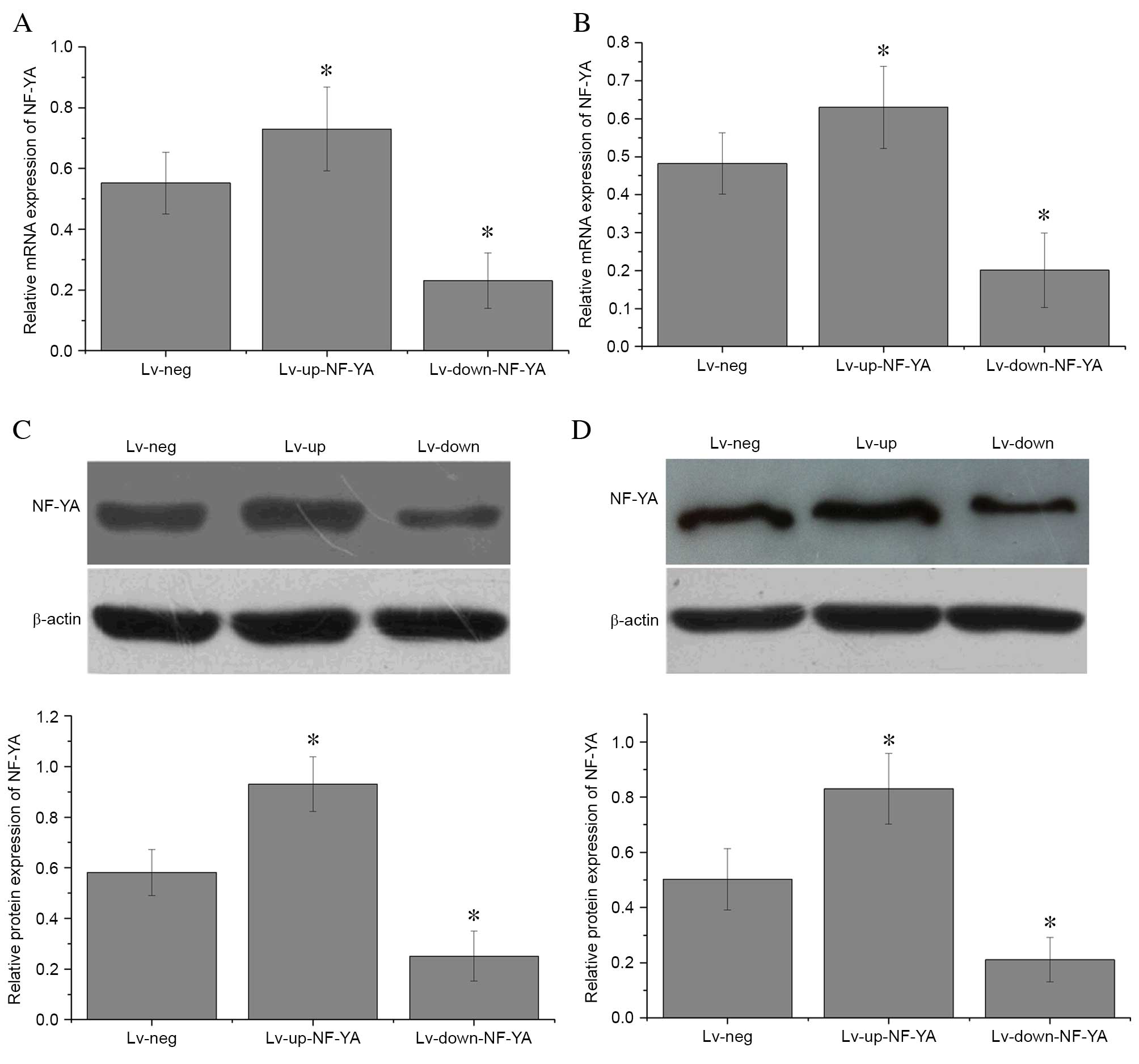
Construction of lentivirus vectors and
transfection into OS cells
U2-OS and HOS cells were transfected for 6 h with
lentivirus vectors to upregulate (Lv-up-NF-YA) or downregulate
(Lv-down-NF-YA) NF-YA expression. A non-targeting control (Lv-neg)
was used as a negative control. NF-YA mRNA (Fig. 2A and B) and protein (Fig. 2C and D) expression levels were then
assessed by RT-qPCR and western blot analyses, respectively. NF-YA
mRNA expression was significantly decreased when compared with
Lv-neg in U2-OS (P=0.01; Fig. 2A)
and HOS cells (P=0.01; Fig. 2B)
transfected with Lv-down-NF-YA. By contrast, NF-YA expression was
significantly enhanced in U2-OS (P=0.03; Fig. 2A) and HOS cells (P=0.01; Fig. 2B) transfected with Lv-up-NF-YA
compared with those transfected with Lv-neg. In addition, the
protein expression levels of NF-YA in U2-OS (P=0.01; Fig. 2C) and HOS cells (P=0.01; Fig. 2D) transfected with Lv-up-NF-YA were
significantly increased compared with Lv-neg-transfected cells.
Furthermore, NF-YA protein expression was significantly lower in
U2-OS (P<0.001; Fig. 2C) and
HOS cells (P=0.01; Fig. 2D)
transfected with Lv-down-NF-YA compared with those transfected with
Lv-neg. These results indicate that the plasmids targeting NF-YA
were constructed and transfected into OS cells successfully.
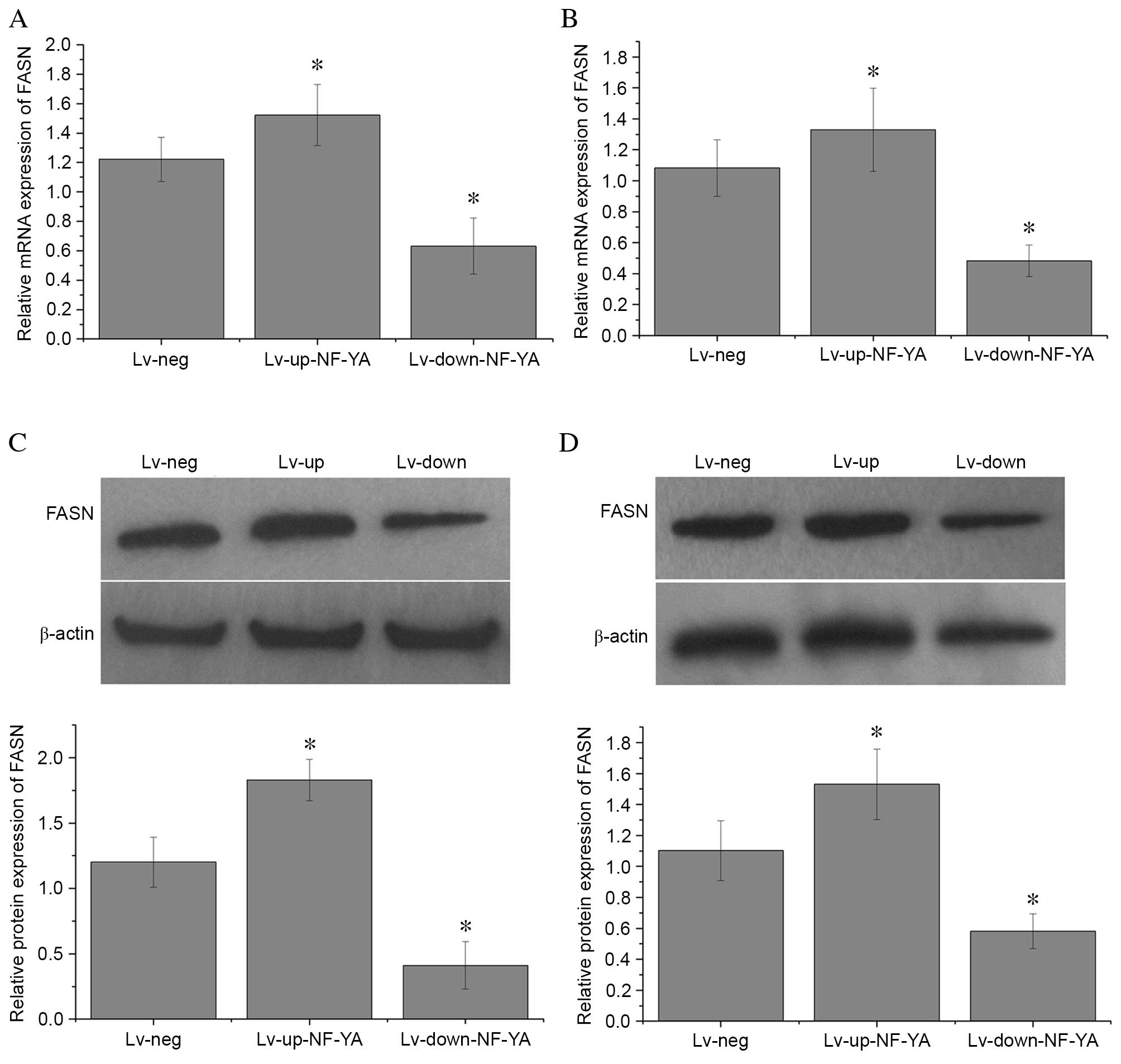
NF-YA regulates FASN expression in OS
cells
In order to investigate whether NF-YA levels affect
FASN expression in OS cells, the mRNA and protein expression levels
of FASN were detected in U2-OS and HOS cells transfected with
Lv-up-NF-YA, Lv-down-NF-YA and Lv-neg by RT-qPCR and western blot
analyses, respectively. FASN mRNA expression was significantly
decreased when compared with Lv-neg in U2-OS (P=0.02; Fig. 3A) and HOS cells (P=0.01; Fig. 3B) transfected with Lv-down-NF-YA.
By contrast, FASN mRNA expression was increased in U2-OS (P=0.01;
Fig. 3A) and HOS cells
(P<0.001; Fig. 3B) transfected
with Lv-up-NF-YA when compared with those transfected with Lv-neg.
In addition, FASN protein expression levels in U2-OS (P=0.01;
Fig. 3C) and HOS cells (P=0.02;
Fig. 3D) transfected with
Lv-up-NF-YA was significantly higher than those transfected with
Lv-neg. FASN protein levels were significantly decreased in U2-OS
(P=0.01; Fig. 3C) and HOS cells
(P=0.01; Fig. 3D) transfected with
Lv-down-NF-YA compared with cells treated with Lv-neg. These
findings indicate that alterations in NF-YA expression may affect
FASN expression in OS cells.
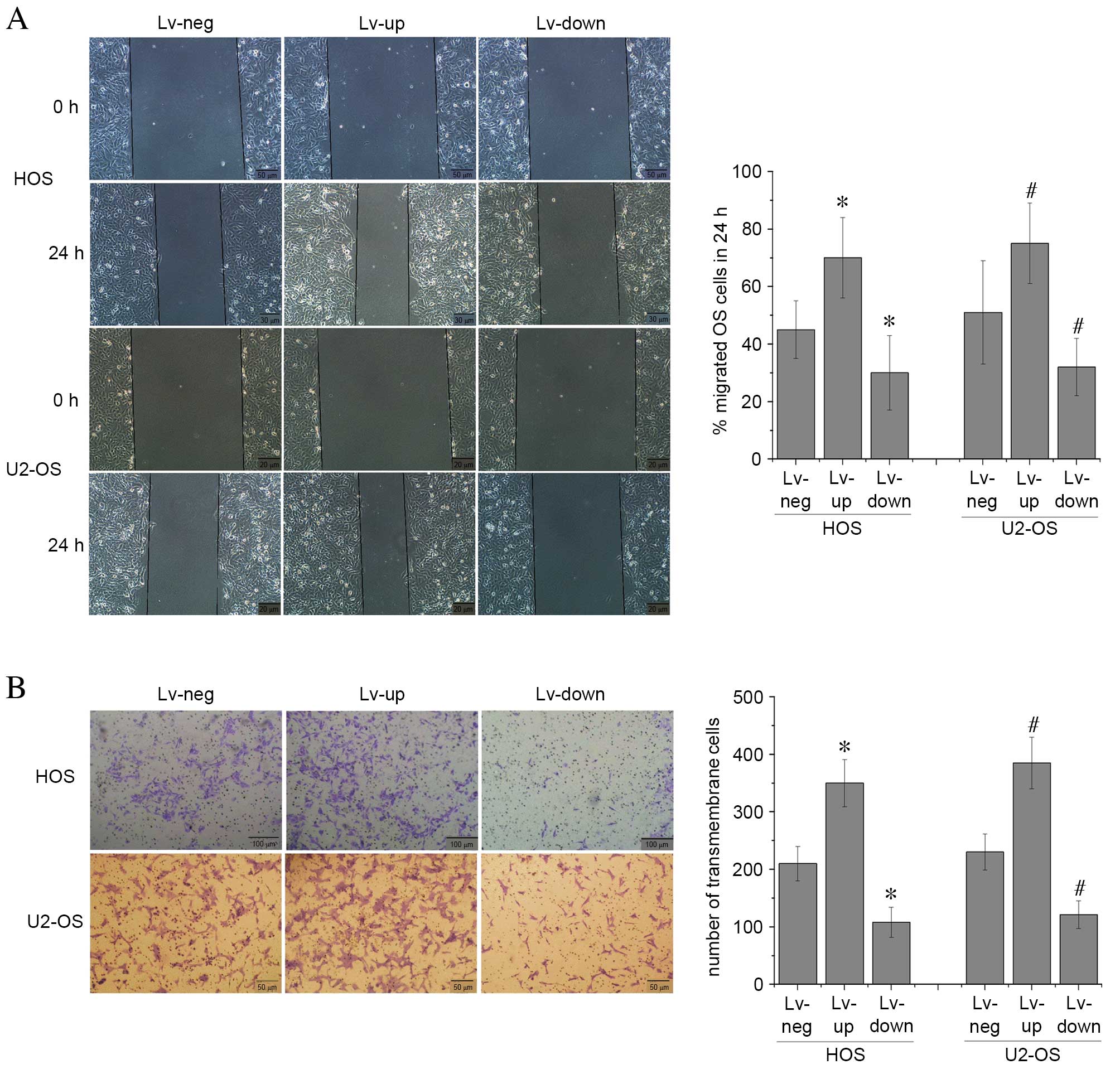
NF-YA alters the malignant phenotype
of OS cells in vitro
In order to investigate the effect of altered NF-YA
expression levels on the malignant phenotype of OS cells, cell
migration and invasion abilities were assessed by wound healing and
Transwell invasion assays, respectively, in U2-OS and HOS cells
transfected with Lv-up-NF-YA, Lv-down-NF-YA and Lv-neg. The results
revealed that OS cell migration (Fig.
4A) and invasion (Fig. 4B)
capabilities were significantly increased in cells with elevated
NF-YA levels when compared with cells transfected with Lv-neg. In
addition, these markers of a malignant phenotype were significantly
inhibited in U2-OS and HOS cells following transfection with
Lv-down-NR-YA compared with those transfected with Lv-neg (P=0.01;
Fig. 4). These findings suggest
that elevated NF-YA levels may promote a malignant phenotype in OS
cells in vitro.
NF-YA alters the malignant phenotype
of OS by regulating FASN expression
To investigate whether NF-YA facilitates the
malignant phenotype of OS cells by activating FASN, the migration
and invasion capabilities of U2-OS and HOS cells infected with
Lv-neg, Lv-up-NF-YA, and co-infected with Lv-down-FASN and
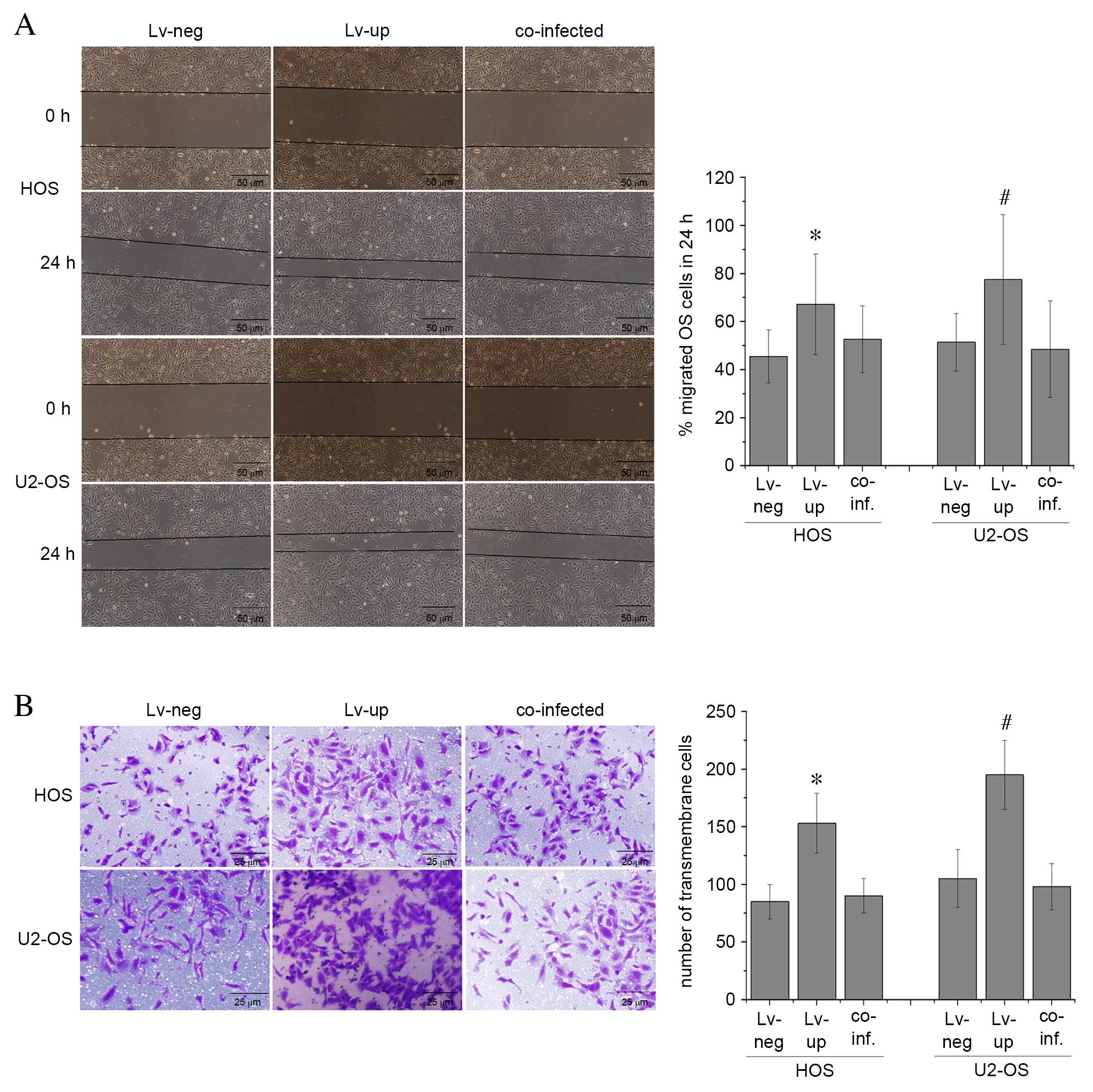
Lv-up-NF-YA were assessed. The proportion of migrated HOS and U2-OS
cells was significantly higher following transfection with
LV-up-NF-YA when compared with cells transfected with LV-neg
(P=0.02 and P=0.01, respectively; Fig.
5A), with a migration rate of 67.2±21% in HOS and 77.5±27% in
U2-OS cells when transfected with Lv-up-NF-YA, compared with
45.5±11% of HOS and 51.4±12% of U2-OS cells in the Lv-neg group.
Notably, this effect was abolished in the co-transfected cells,
with migration rates of 52.6±14% in HOS and 48.5±20% in U2-OS cells
following co-transfection with Lv-down-FASN and Lv-up-NF-YA. No
statistically significant difference in cell migration was observed
between co-transfected U2-OS and HOS cells when compared with
Lv-neg-transfected cells (P=0.07 and P=0.06, respectively; Fig. 5A). The number of HOS and U2-OS
cells that had traversed the membrane was significantly higher when
transfected with LV-up-NF-YA compared with LV-neg (P=0.01 and
P=0.01, respectively; Fig. 5B),
with 153±26 transmembrane HOS cells/high power field, ×400 (Hp) and
195±30 transmembrane U2-OS cells/Hp transfected with Lv-up-NF-YA,
compared with 85±15 transmembrane HOS cells/Hp and 105±25
transmembrane U2-OS cells/Hp in the Lv-neg group. However, in the
co-infected group, there were 90±15 transmembrane HOS cells/Hp and
98±20 transmembrane U2-OS cells/Hp, which was not significantly
different when compared with HOS and U2-OS cells transfected with
Lv-neg (P=0.01 and P=0.01, respectively; Fig. 5B). These results suggest that the
effect of OS tumor cell migration and invasion mediated by NF-YA
was reversed by silencing FASN. This indicates that NF-YA may
promote the malignant phenotype of OS cells, in part, by regulating
FASN expression.
Discussion
NF-Y is a ubiquitous protein, composed of 3
subunits, NF-YA, NF-YB and NF-YC, whose genes are highly conserved
from yeast to mammals (20). While
previous studies have aimed to understand the biological role of
NF-Y, recent findings have revealed that NF-Y is involved in
malignant tumor development (21–23).
Clinically, elevated NF-Y genes indicate a poor prognosis in
various cancers, such as breast and lung cancers (24). In the present study, expression of
NF-YA was higher in OS cells compared with osteoblastic HOB cells.
In addition, the malignant phenotype was inhibited in OS cells
in vitro when NF-YA expression was silenced, while ectopic
NF-YA expression promoted a malignant phenotype. These findings
suggest that NF-YA may be involved in mediating the malignant
phenotype of OS. However, the cellular and molecular mechanisms of
this effect remain unclear.
FASN is an important enzyme for endogenous
lipogenesis in mammals and is responsible for catalyzing the
synthesis of long-chain fatty acids (25). Previous studies have demonstrated
elevated FASN expression in a variety of human tumors, but low
expression levels in normal tissues (26–28).
Due to this increased expression in malignant tumor tissues, FASN
may represent a novel diagnostic marker and therapeutic target. In
addition, previous studies have revealed that inhibition of FASN
promotes apoptosis, and reduces cell growth and metastasis in
vitro and in vivo (29,30).
FASN has been previously demonstrated to be overexpressed in OS
tissues, while inhibition of FASN suppresses the malignant
phenotype by inhibiting the phosphoinositide 3-kinase/Akt
serine/threonine kinase 1/nuclear factor kappa
κ-light-chain-enhancer of activated B cells signaling pathway
(17,18). However, the molecular signaling
pathways resulting from upregulated FASN expression in OS remain to
be fully elucidated. NF-Y is an important nuclear transcription
factor that regulates the expression of numerous genes (31–33).
Previous studies have demonstrated that NF-Y expression is a key
component in the regulation of FASN expression by promoter activity
in primary hepatocytes (34). In
the present study, ectopic NF-YA expression was demonstrated to
upregulate FASN expression in OS cells, while knockdown of NF-YA
expression resulted in inhibition of FASN expression. In addition,
the effect of NF-YA on OS cell migration and invasion was reversed
by silencing FASN. These results suggest that NF-YA promotes cell
proliferation, invasion and migration in OS, in part, via
regulation of FASN expression.
The findings of the present study indicate that
increased expression of NF-YA may promote a malignant phenotype in
OS cells via modulation of FASN expression. However, further in
vivo experiments are necessary to establish whether inhibition
of NF-YA may represent a novel strategy in the management of
OS.
Acknowledgements
The present study was supported by grants from The
National Natural Science Foundation of China (grant no. 81260400)
and The Natural Science Foundation of Jiangxi Province (grant no.
20114BAB205093).
References
|
1
|
Salah S, Ahmad R, Sultan I, Yaser S and
Shehadeh A: Osteosarcoma with metastasis at initial diagnosis:
Current outcomes and prognostic factors in the context of a
comprehensive cancer center. Mol Clin Oncol. 2:811–816.
2014.PubMed/NCBI
|
|
2
|
Lewis VO: What's new in musculoskeletal
oncology. J Bone Joint Surg Am. 91:1546–1556. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Iwamoto Y, Tanaka K, Isu K, Kawai A,
Tatezaki S, Ishii T, Kushida K, Beppu Y, Usui M, Tateishi A, et al:
Multiinstitutional phase II study of neoadjuvant chemotherapy for
osteosarcoma (NECO study) in Japan: NECO-93J and NECO-95J. J Orthop
Sci. 14:397–404. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Cho Y, Jung GH, Chung SH, Kim JY, Choi Y
and Kim JD: Long-term survivals of stage IIb osteosarcoma: A
20-year experience in a single institution. Clin Orthop Surg.
3:48–54. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Bölling T, Schuller P, Distelmaier B,
Schuck A, Ernst I, Gosheger G, Winkelmann W, Dirksen U, Jürgens H,
Kronholz HL, et al: Perioperative high-dose rate brachytherapy
using a bendy applicator (flab): Treatment results of 74 patients.
Anticancer Res. 28:3885–3890. 2008.PubMed/NCBI
|
|
6
|
Lourda M, Trougakos IP and Gonos ES:
Development of resistance to chemotherapeutic drugs in human
osteosarcoma cell lines largely depends on up-regulation of
Clusterin/Apolipoprotein J. Int J Cancer. 120:611–622. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Schwartz CL, Gorlick R, Teot L, Krailo M,
Chen Z, Goorin A, Grier HE, Bernstein ML and Meyers P: Children's
Oncology Group: Multiple drug resistance in osteogenic sarcoma:
INT0133 from the Children's Oncology Group. J Clin Oncol.
25:2057–2062. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Chou AJ and Gorlick R: Chemotherapy
resistance in osteosarcoma: Current challenges and future
directions. Expert Rev Anticancer Ther. 6:1075–1085. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Dean M, Fojo T and Bates S: Tumour stem
cells and drug resistance. Nat Rev Cancer. 5:275–284. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Dolfini D, Gatta R and Mantovani R: NF-Y
and the transcriptional activation of CCAAT promoters. Crit Rev
Biochem Mol Biol. 47:29–49. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Mojsin M, Topalovic V, Vicentic J
Marjanovic and Stevanovic M: Transcription factor NF-Y inhibits
cell growth and decreases SOX2 expression in human embryonal
carcinoma cell line NT2/D1. Biochemistry (Mosc). 80:202–207. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Garipov A, Li H, Bitler BG, Thapa RJ,
Balachandran S and Zhang R: NF-YA underlies EZH2 upregulation and
is essential for proliferation of human epithelial ovarian cancer
cells. Mol Cancer Res. 11:360–369. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Bungartz G, Land H, Scadden DT and Emerson
SG: NF-Y is necessary for hematopoietic stem cell proliferation and
survival. Blood. 119:1380–1389. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Zhang HT, Zhang D, Zha ZG and Hu CD:
Transcriptional activation of PRMT5 by NF-Y is required for cell
growth and negatively regulated by the PKC/c-Fos signaling in
prostate cancer cells. Biochim Biophys Acta. 1839:1330–1340. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Ma H, Yue X, Gao L, Liang X, Yan W, Zhang
Z, Shan H, Zhang H, Spear BT and Ma C: ZHX2 enhances the
cytotoxicity of chemotherapeutic drugs in liver tumor cells by
repressing MDR1 via interfering with NF-YA. Oncotarget.
6:1049–1063. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Shi Z, Chiang CI, Labhart P, Zhao Y, Yang
J, Mistretta TA, Henning SJ, Maity SN and Mori-Akiyama Y:
Context-specific role of SOX9 in NF-Y mediated gene regulation in
colorectal cancer cells. Nucleic Acids Res. 43:6257–6269. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Liu ZL, Wang G, Peng AF, Luo QF, Zhou Y
and Huang SH: Fatty acid synthase expression in osteosarcoma and
its correlation with pulmonary metastasis. Oncol Lett. 4:878–882.
2012.PubMed/NCBI
|
|
18
|
Liu ZL, Mao JH, Peng AF, Yin QS, Zhou Y,
Long XH and Huang SH: Inhibition of fatty acid synthase suppresses
osteosarcoma cell invasion and migration via downregulation of the
PI3K/Akt signaling pathway in vitro. Mol Med Rep. 7:608–612.
2013.PubMed/NCBI
|
|
19
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(−Delta Delta C(T)) Method. Methods. 25:402–408. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Hackenberg D, Wu Y, Voigt A, Adams R,
Schramm P and Grimm B: Studies on differential nuclear
translocation mechanism and assembly of the three subunits of the
Arabidopsis thaliana transcription factor NF-Y. Mol Plant.
5:876–888. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Ying S, Dunnebier T, Si J and Hamann U:
Estrogen receptor alpha and nuclear factor Y coordinately regulate
the transcription of the SUMO-conjugating UBC9 gene in MCF-7 breast
cancer cells. PLoS One. 8:e756952013. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Belluti S, Basile V, Benatti P, Ferrari E,
Marverti G and Imbriano C: Concurrent inhibition of enzymatic
activity and NF-Y-mediated transcription of Topoisomerase-IIα by
bis-DemethoxyCurcumin in cancer cells. Cell Death Dis. 4:e7562013.
View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Lützner N, De-Castro Arce J and Rösl F:
Gene expression of the tumour suppressor LKB1 is mediated by Sp1,
NF-Y and FOXO transcription factors. PLoS One. 7:e325902012.
View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Yamanaka K, Mizuarai S, Eguchi T, Itadani
H, Hirai H and Kotani H: Expression levels of NF-Y target genes
changed by CDKN1B correlate with clinical prognosis in multiple
cancers. Genomics. 94:219–227. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Uemoto Y, Abe T, Tameoka N, Hasebe H,
Inoue K, Nakajima H, Shoji N, Kobayashi M and Kobayashi E:
Whole-genome association study for fatty acid composition of oleic
acid in Japanese Black cattle. Anim Genet. 42:141–148. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Bian Y, Yu Y, Wang S and Li L:
Up-regulation of fatty acid synthase induced by EGFR/ERK activation
promotes tumor growth in pancreatic cancer. Biochem Biophys Res
Commun. 463:612–617. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Bauerschlag DO, Maass N, Leonhardt P,
Verburg FA, Pecks U, Zeppernick F, Morgenroth A, Mottaghy FM, Tolba
R, Meinhold-Heerlein I and Bräutigam K: Fatty acid synthase
overexpression: Target for therapy and reversal of chemoresistance
in ovarian cancer. J Transl Med. 13:1462015. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Grube S, Dunisch P, Freitag D, Klausnitzer
M, Sakr Y, Walter J, Kalff R and Ewald C: Overexpression of fatty
acid synthase in human gliomas correlates with the WHO tumor grade
and inhibition with Orlistat reduces cell viability and triggers
apoptosis. J Neurooncol. 118:277–287. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Agostini M, Almeida LY, Bastos DC, Ortega
RM, Moreira FS, Seguin F, Zecchin KG, Raposo HF, Oliveira HC,
Amoêdo ND, et al: The fatty acid synthase inhibitor orlistat
reduces the growth and metastasis of orthotopic tongue oral
squamous cell carcinomas. Mol Cancer Ther. 13:585–595. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Zaytseva YY, Harris JW, Mitov MI, Kim JT,
Butterfield DA, Lee EY, Weiss HL, Gao T and Evers BM: Increased
expression of fatty acid synthase provides a survival advantage to
colorectal cancer cells via upregulation of cellular respiration.
Oncotarget. 6:18891–18904. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Xu H, Fu J, Ha SW, Ju D, Zheng J, Li L and
Xie Y: The CCAAT box-binding transcription factor NF-Y regulates
basal expression of human proteasome genes. Biochim Biophys Acta.
1823:818–825. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Lu YH, Dallner OS, Birsoy K,
Fayzikhodjaeva G and Friedman JM: Nuclear Factor-Y is an adipogenic
factor that regulates leptin gene expression. Mol Metab. 4:392–405.
2015. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Hida M, Hamanaka R, Okamoto O, Yamashita
K, Sasaki T, Yoshioka H and Matsuo N: Nuclear factor Y (NF-Y)
regulates the proximal promoter activity of the mouse collagen
α1(XI) gene (Col11a1) in chondrocytes. In Vitro Cell Dev Biol Anim.
50:358–366. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Teran-Garcia M, Adamson AW, Yu G, Rufo C,
Suchankova G, Dreesen TD, Tekle M, Clarke SD and Gettys TW:
Polyunsaturated fatty acid suppression of fatty acid synthase
(FASN): Evidence for dietary modulation of NF-Y binding to the Fasn
promoter by SREBP-1c. Biochem J. 402:591–600. 2007. View Article : Google Scholar : PubMed/NCBI
|