Introduction
Cementum is a central component of periodontal
tissues with a very limited capacity for regeneration (1). This lack of regenerative potential of
an important functional periodontal tissue represents a major
challenge for dental clinicians. Periodontal ligament cells (PDLCs)
can differentiate towards both osteoblastic and cementogenic
lineage cells, which are responsible for bone and cementum
formation, respectively (2).
Cementum has many biochemical features in common with bone, but the
primary distinction is that cementum lacks vascularization and a
Haversian canal system (3).
Compared with bone, which undergoes continuous remodelling
throughout life, cementum is a more quiescent tissue (4). Cementoblasts express classical
osteogenic markers, including alkaline phosphatase, type I collagen
(COL1), runt-related transcription factor 2 (RUNX2) and
noncollagenous proteins including bone sialoprotein and osteocalcin
(1,5). Cementum protein 1 (CEMP1) and
cementum attached protein (CAP) are also specific markers of
cementum (6–8). There is debate as to whether or not
cementoblasts and osteoblasts have a common precursor (9), and the different properties of these
two cell types, including endogenous gene expression and the
response to the extracellular environment, remain subjects of
investigation.
The canonical Wnt/β-catenin pathway has a complex
role in mineral tissue development and regeneration (10,11),
with different functions depending on cell type and differentiation
stage. Wnt promotes bone formation by enhancing both the
proliferation and differentiation of bone marrow stromal cells
(BMSCs), however is reported to be down-regulated during the
terminal mineralisation stages (12). Wnt is also expressed by PDLCs and
has a role in cell proliferation (13). The impact of Wnt on cementoblasts
has been extensively investigated, however the results appear to be
contradictory: A study using an immortalised murine cementoblast
cell line (OCCM-30), demonstrated that the canonical Wnt signalling
pathway inhibited cementoblast differentiation (14), whereas another study, using primary
PDLCs cultured in osteogenic induction medium, demonstrated an
increase in cementogenic markers following the activation of the
canonical Wnt signalling (15).
Potentially, the differences observed in these studies are due to
the different cell types used (immortalised vs. primary cells), and
the local environment of PDLC differentiation. During chronic
periodontitis and orthodontic treatment, the cementum exhibits
severe damage and its regeneration is greatly restricted compared
with alveolar bone (16).
Local hypoxia may be an important factor, triggered
by inflammation and over-loading (17). Hypoxia inducible factor-1α
(HIF-1α), a principal mediator of hypoxia, interacts with the Wnt
signalling pathway in a complex pattern (18). It has been previously reported that
HIF-1α enhances Wnt signalling in undifferentiated cells and
promotes the proliferation of stem cells (19,20).
It has also been reported that hypoxia blocks Wnt/β-catenin
signalling by interfering with the function of the endoplasmic
reticulum, which prevents protein secretion in tumours (21). Furthermore, there is in vivo
evidence from neural stem cells suggesting that hypoxia activates
the transcription of β-catenin and the β-catenin signalling cascade
by increasing the expression of lymphoid enhancer-binding factor 1
(LEF-1) and T-cell factor 1 (TCF-1), which are the nuclear targets
of β-catenin (22). However, the
role of HIF-1α in the regulation of Wnt signalling in
differentiated cells remains unclear.
In the present study, primary human PDLCs in
osteogenic culture conditions were used to investigate the
interaction between hypoxia and Wnt signalling, and how this
affects cementogenesis. Overexpression of Wnt signalling molecules
was demonstrated to inhibit cementogenesis, and hypoxia rescued
this inhibition by blocking the canonical Wnt signalling pathway.
However, hypoxia and Wnt signalling promoted the osteogenic
differentiation of PDLCs.
Materials and methods
Isolation and culture of human PDLCs
(hPDLCs)
Isolation and culture of hPDLCs was performed
according to previously published protocols (23). Teeth were obtained from healthy
patients (18–25 years old) undergoing third molar extraction
surgery. Informed consent was provided by all patients involved and
the research protocol was approved by the Human Ethics Committees
of Queensland University of Technology (Brisbane, Australia).
Briefly, periodontal ligament tissues were separated from the
middle third of the root surface using a scalpel and were cultured
in a T25 flask in Dulbecco's modified Eagle's medium (DMEM; Thermo
Fisher Scientific, Inc., Waltham, MA, USA) supplemented with 10%
v/v foetal bovine serum (FBS; Thermo Fisher Scientific, Inc.) and
50 U/ml penicillin and 50 mg/ml streptomycin (Thermo Fisher
Scientific, Inc.) at 37°C in a humidified CO2 incubator.
The osteogenic medium was supplemented with 10−8 M
dexamethasone, 8 mM β-glycerol phosphate and 50 µg/ml ascorbic
acid. Following incubation for 5 days, the medium was changed and
the outgrown cells were passaged at ~80% confluence. Cells at
passages 2–5 were used for subsequent experiments.
Hypoxia-mimicking culture conditions
and activation of the Wnt signalling pathway
Dimethyloxalylglycine (DMOG, Sigma-Aldrich; Merck
Millipore, Darmstadt, Germany) was added into the culture medium of
the PDLCs at a final concentration of 1 mM to mimic a hypoxic
environment, as described previously (24). To activate the Wnt signalling
pathway, Wnt3a conditioned medium (Wnt-CM) was prepared using a
genetically modified murine cell line overexpressing L Wnt-3a (ATCC
CRL-2647; American Type Culture Collection, Manassas, VA, USA).
Cells were cultured according to the supplier's guidelines in a T75
flask with ATCC-formulated DMEM, supplemented with 10% v/v FBS and
0.4 mg/ml G418 (Sigma-Aldrich; Merck Millipore) to select Wnt3a
positive cells. The conditioned medium (CM) was prepared by
splitting the cells 1:10 in 10 ml culture medium without G418 and
incubating for 4 days. The first batch of CM was removed and filter
sterilised, and 10 ml fresh culture medium added. The cells were
cultured for a further 3 days before a second batch of CM was
collected. The working CM consisted of a 1:1 mixture of the two
batches.
Cell proliferation assay
PDLCs were seeded in 96-well plates at
4×103 cells per well and cultured in either normal or
hypoxic medium (1 mM DMOG), with or without the addition of Wnt-CM.
On days 1 and 3, 20 µl of
3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT)
solution (0.5 mg/ml; Sigma-Aldrich; Merck Millipore) was added to
each well and incubated for 4 h at 37°C. The supernatants were
removed and replaced with 100 µl of dimethyl sulphoxide to
solubilise the MTT-formazan product. Absorbances were measured at a
wavelength of 495 nm using a microplate reader (Benchmark Plus;
Bio-Rad Laboratories, Inc., Hercules, CA, USA).
Reverse transcription-quantitative
polymerase chain reaction (RT-qPCR)
hPDLCs were seeded in 6-well plates cultured in
normoxic or hypoxic medium, with or without Wnt-CM, for 3 days.
Total RNA was extracted in 1 ml TRIzol® Reagent (Thermo
Fisher Scientific, Inc.) per well. Complementary DNA was
synthesised using a DyNAmo™ cDNA Synthesis Kit (Finnzymes; Thermo
Fisher Scientific, Inc.) following the manufacturer's protocols.
qPCR was performed on an ABI 7500 Real-Time PCR system (Applied
Biosystems; Thermo Fisher Scientific, Inc.) using SYBR Green
detection reagent (Thermo Fisher Scientific, Inc.) according to a
two-step protocol (initial denature at 95°C for 2 min, followed by
45 cycles of 5 sec at 95°C, 10 sec at 60°C and 15 sec at 72°C).
Transcription levels of COL1, RUNX2 and CEMP1 were assayed and
normalised against the housekeeping gene glyceraldehyde 3-phosphate
dehydrogenase (GAPDH), using the primers listed in Table I. Each reaction was performed in
triplicate and the mean cycle quantification (Cq) value of each
target gene was normalised against the Cq value of GAPDH, and the
relative expression calculated using the following formula:
2(−normalised average Cqs) ×104 (25).
 | Table I.Oligonucleotides sequences. |
Table I.
Oligonucleotides sequences.
| Gene | Forward primer
(5′→3′) | Reverse primer
(5′→3′) |
|---|
| CEMP1 |
GGGCACATCAAGCACTGACAG |
CCCTTAGGAAGTGGCTGTCCAG |
| COL1 |
CTGACTGGAAGAGCGGAGAG |
GAGTGGGGAACACACAGGTC |
| RUNX2 |
ACCAAGAAGGCACAGACAGAAGC |
AGGATTGTGTCTGCCTGGGATC |
| GAPDH |
TCAGCAATGCCTCCTGCAC |
TCTGGGTGGCAGTGATGGC |
Western blotting
Whole cell lysates for western blot analysis were
harvested in 250 µl cell lysis buffer (2 mM Tris-HCl, pH 7.5, 15 mM
NaCl, 0.1 mM Na2EDTA, 0.1 mM EGTA, 0.1% Triton, 0.25 mM
sodium pyrophosphate, 0.1 mM β-glycerophosphate, 0.1 mM
Na3VO4, 0.1 µg/ml leupeptin). Protein lysates
(15 µg per lane) were separated by 10% sodium dodecyl
sulphate-polyacrylamide gel electrophoresis, and transferred onto
nitrocellulose membranes (Pall Life Sciences, Port Washington, NY,
USA). Membranes were blocked for 1 h at room temperature in Odyssey
blocking buffer (cat. no. 927-40000; LI-COR, Inc., Lincoln, NE,
USA), then incubated overnight at 4°C with primary antibodies
against β-catenin (1:1,000, rabbit anti-human/rat; cat. no. 9581;
Cell Signaling Technology, Inc., Danvers, MA, USA); CEMP1 (1:1,000,
rabbit polyclonal antibody; cat. no. ab134231; Abcam, Cambridge,
UK); COL1 (1:1,000, rabbit anti-human/rat; cat. no. ab34710;
Abcam); RUNX2 (1:1,000, rabbit polyclonal antibody; cat. no.
sc-10758; Santa Cruz Biotechnology, Inc., Dallas, TX, USA); HIF-1α
(1:1,000, mouse monoclonal antibody; cat. no. sc-13515; Santa Cruz
Biotechnology, Inc.); and α-Tubulin (1:2,000, rabbit
anti-human/rat; cat. no. ab15246; Abcam). The membranes were
incubated with anti-mouse/rabbit fluorescently labelled secondary
antibodies (P/N 925-32211 or P/N 925-68070; LI-COR, Inc.) at
1:10,000 dilutions for 1 h at room temperature. Protein bands were
visualised using the Odyssey Infrared Imaging System (LI-COR,
Inc.). The relative intensity of protein bands compared with
α-Tubulin was quantified using Image J software version 1.47
(National Institutes of Health, Bethesda, MD, USA).
Statistical analysis
Data are presented as the mean ± standard deviation
of 3 independent experiments (with each experiment containing 3
technical replicates). Analysis was performed using SPSS software
version 22.0 (SPSS Inc., Chicago, IL, USA). Nonparametric Wilcoxon
test was carried out to distinguish the differences between
different groups. Comparison tests were performed as indicated.
P<0.05 was considered to indicate a statistically significant
difference.
Results
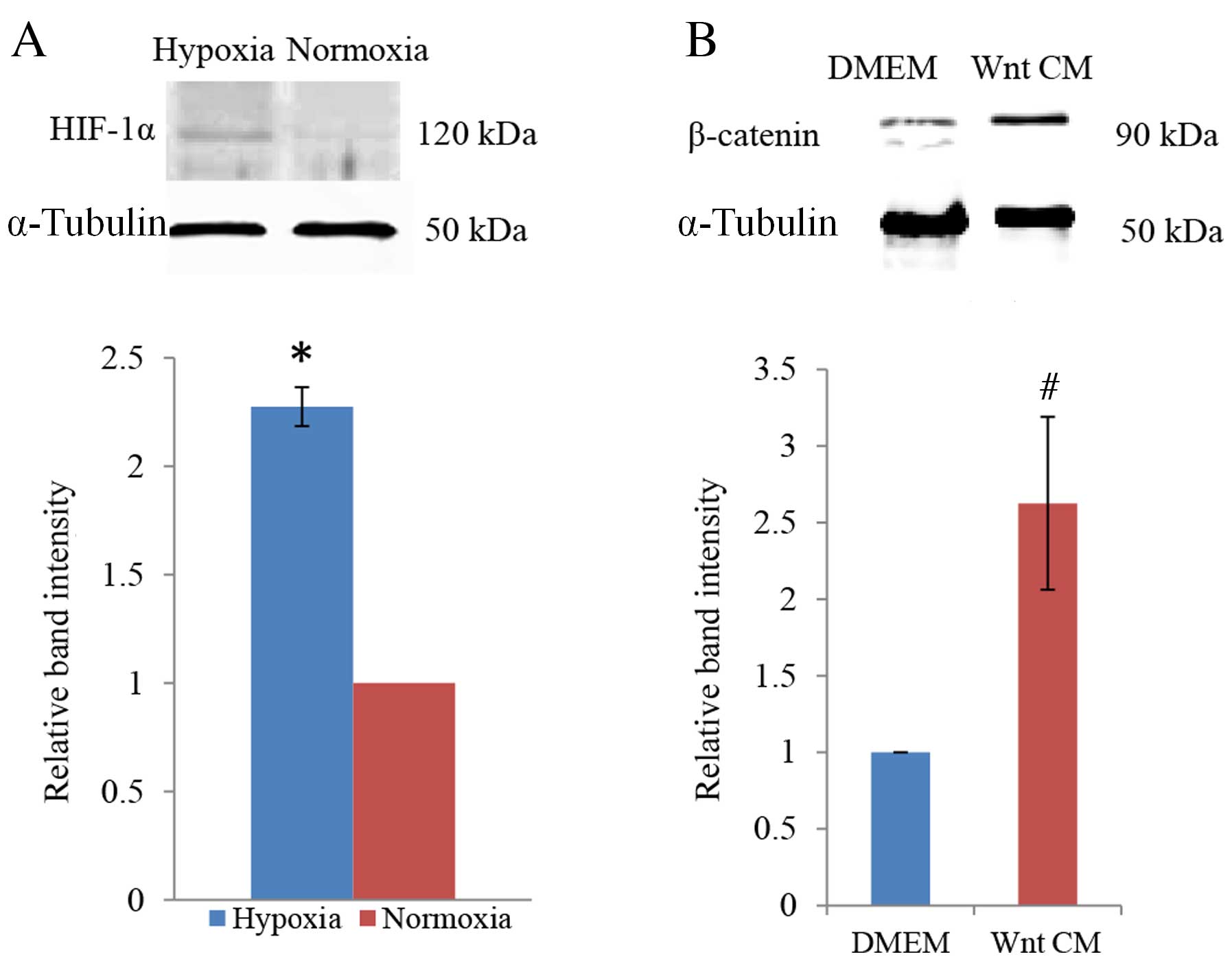
Confirmation of cellular hypoxia
To determine whether PDLCs responded to the
hypoxia-mimicking culture conditions of DMOG, protein expression
levels of HIF-1α were examined by western blot analysis. This
revealed a distinct increase in HIF-1α expression in cells exposed
to 1 mM DMOG, with densitometric quantification of the bands
demonstrating a statistically significant increase compared with
the normoxic control condition (P=0.011; Fig. 1A). This confirmed that DMOG
generates hypoxia-like culture conditions for PDLCs. β-catenin was
used to examine the efficiency of Wnt signalling induction by
Wnt-CM. Compared with culture in DMEM, a significant increase in
β-catenin protein expression was observed when PDLCs were cultured
in Wnt-CM (P=0.014; Fig. 1B).
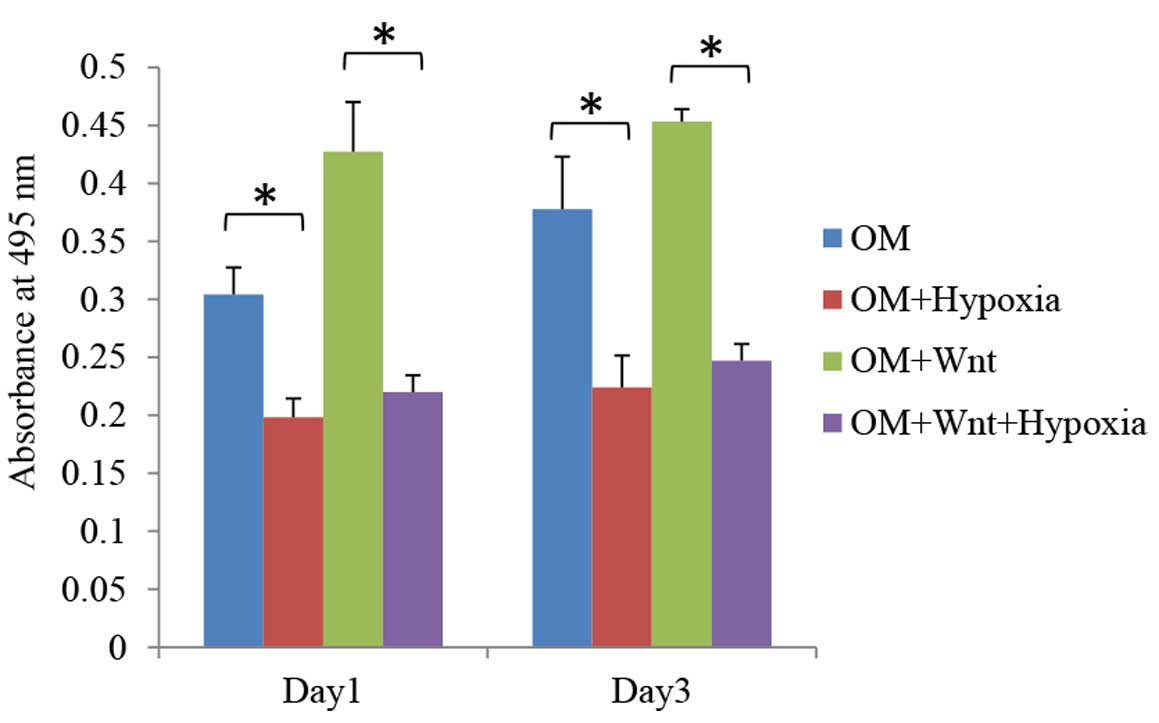
Effects of hypoxia and Wnt on cell
proliferation
The proliferation rate of PDLCs cultured in normoxic
and hypoxia-like conditions with/without Wnt-CM was determined by
MTT assay. A significantly higher rate of proliferation was
observed in the normoxia plus Wnt-CM condition compared with
hypoxia plus Wnt-CM condition on days 1 and 3 (P=0.038 and P=0.029,
respectively; Fig. 2), indicating
that the effect of Wnt signalling on cell proliferation was
inhibited by hypoxia. When cultured in osteogenic medium, hypoxic
conditions significantly inhibited the proliferation rates of PDLCs
in Wnt stimulated and non-stimulated groups on days 1 and 3
(P<0.05; Fig. 3).
Hypoxia combined with Wnt3a
conditioned medium promotes osteogenic differentiation of
PDLCs
RT-qPCR and western blot analysis were performed to
determine the effects of hypoxia and Wnt signalling on osteogenic
differentiation of PDLCs. Cells were cultured under normoxic and
hypoxia-like conditions, with and without Wnt-CM. There were no
significant differences between the protein (Fig. 4A) or mRNA expression levels
(Fig. 4B) of COL1 and RUNX2 in
PDLCs under normoxic and hypoxic conditions. However, when cells
were cultured in Wnt-CM and hypoxic conditions, there was a
significant up-regulation of the protein and mRNA expression levels
of COL1 (P=0.021 and P=0.019, respectively; Fig. 4A and B, respectively) and RUNX2
(P=0.031 and P=0.029, respectively; Fig. 4A and B, respectively) compared with
the normoxic cultures. In this context, as COL1 and RUNX2 are
markers of osteogenic differentiation, hypoxic cells in which Wnt
signalling has been induced appear to have a stronger osteogenic
capacity than normoxic cells.
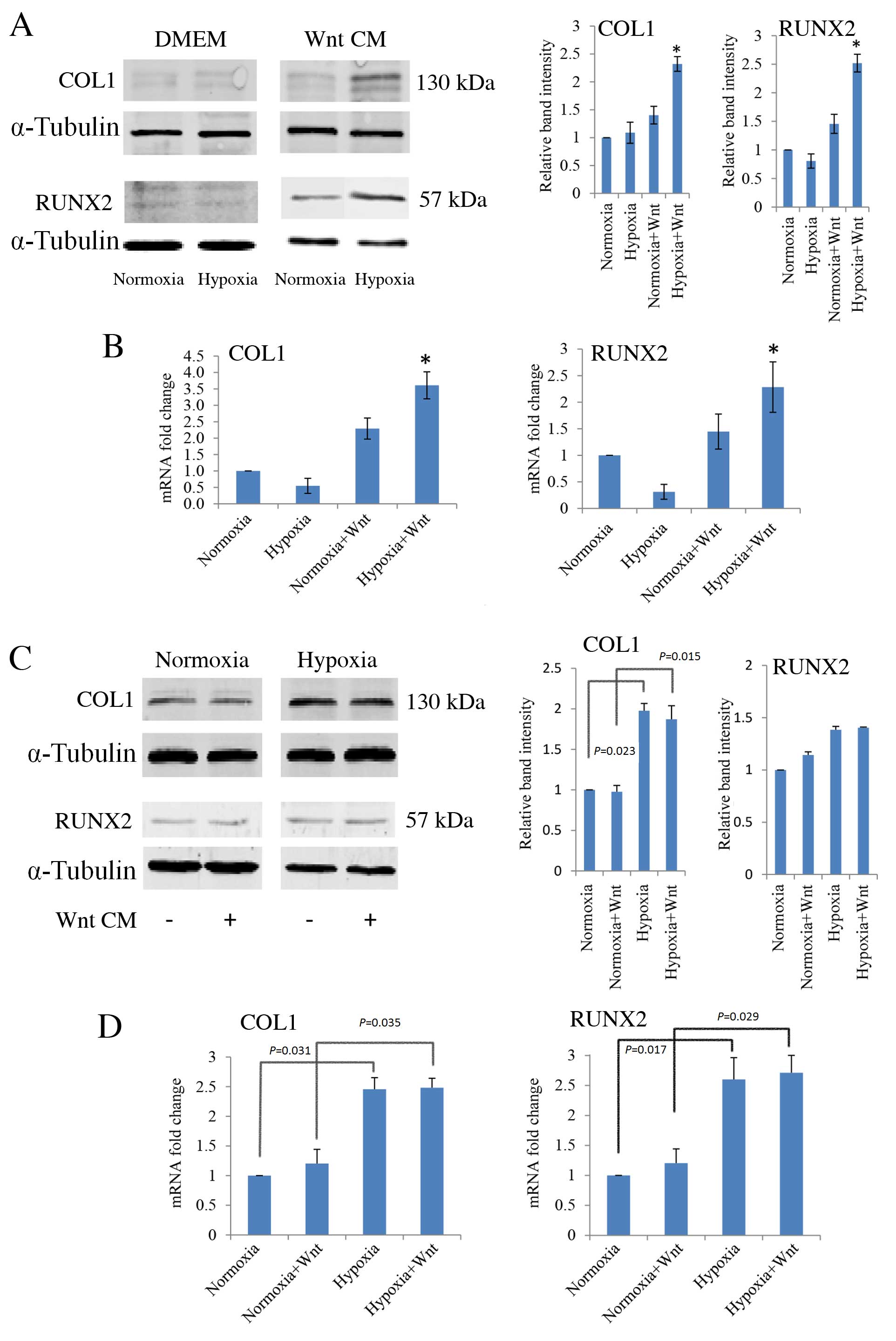 | Figure 4.Effect of hypoxia and Wnt on the
expression of osteogenic markers, COL1 and RUNX2, in PDLCs cultured
in DMEM and osteogenic medium, in normoxic and hypoxic (1 mM
dimethyloxalylglycine) conditions, with or without Wnt-CM. (A)
Western blot analysis with quantification relative to α-tubulin,
and (B) RT-qPCR analysis, with quantification relative to GAPDH, of
COL1 and RUNX2 expression levels in PDLCs cultured in DMEM or
Wnt-CM. (C) Western blot analysis, with quantification relative to
α-tubulin, and (D) RT-qPCR analysis, with quantification relative
to GAPDH, of COL1 and RUNX2 expression levels in PDLCs cultured in
osteogenic medium. *P<0.05 vs. normoxia group. PDLC, periodontal
ligament cells; DMEM, Dulbecco's modified Eagle's medium; CM,
conditioned medium; COL1, type I collagen; RUNX2, runt-related
transcription factor 2; GAPDH, glyceraldehyde 3-phosphate
dehydrogenase. |
Furthermore, when cells were cultured in osteogenic
medium, COL1 and RUNX2 were expressed and their expression was
further increased in response to hypoxia compared with normoxic
levels, with and without Wnt-CM (P<0.05; Fig. 4C and D).
Wnt signalling inhibits cementogenic
differentiation of PDLCs
The cementum specific marker, CEMP1, was used to
evaluate cementogenic differentiation of PDLCs. PDLCs were cultured
in normal growth medium or in osteogenic medium to induce
differentiation, then protein expression levels were analysed by
western blot and mRNA expression levels by RT-qPCR. Wnt-CM
stimulation inhibited the protein expression levels of CEMP1 in
undifferentiated PDLCs compared with the levels without Wnt-CM
stimulation in normoxic and hypoxic conditions (P=0.039 and
P=0.042, respectively; Fig. 5A).
mRNA expression levels of CEMP1 were also decreased by Wnt-CM in
undifferentiated PDLCs in normoxic and hypoxic conditions compared
with the levels without Wnt-CM stimulation (P=0.034 and P=0.018,
respectively; Fig. 5B).
Additionally, an increase in protein expression levels of β-catenin
in PDLCs following osteogenic differentiation was observed (data
not shown). When cultured in osteogenic medium, CEMP1 protein
expression levels were significantly higher in hypoxic conditions
compared with normoxia, both without and with Wnt (P=0.014 and
P=0.025, respectively; Fig. 5C).
This observation was further confirmed by analysis of mRNA
transcription levels (Fig. 5D).
Notably, the expression of CEMP1 is negatively associated with
β-catenin expression, an active element of canonical Wnt
signalling. These results suggest that Wnt signalling inhibits
cementogenesis (Fig. 5A and B),
whereas hypoxia promotes cementogenesis by down-regulating Wnt
signalling (Fig. 5C and D).
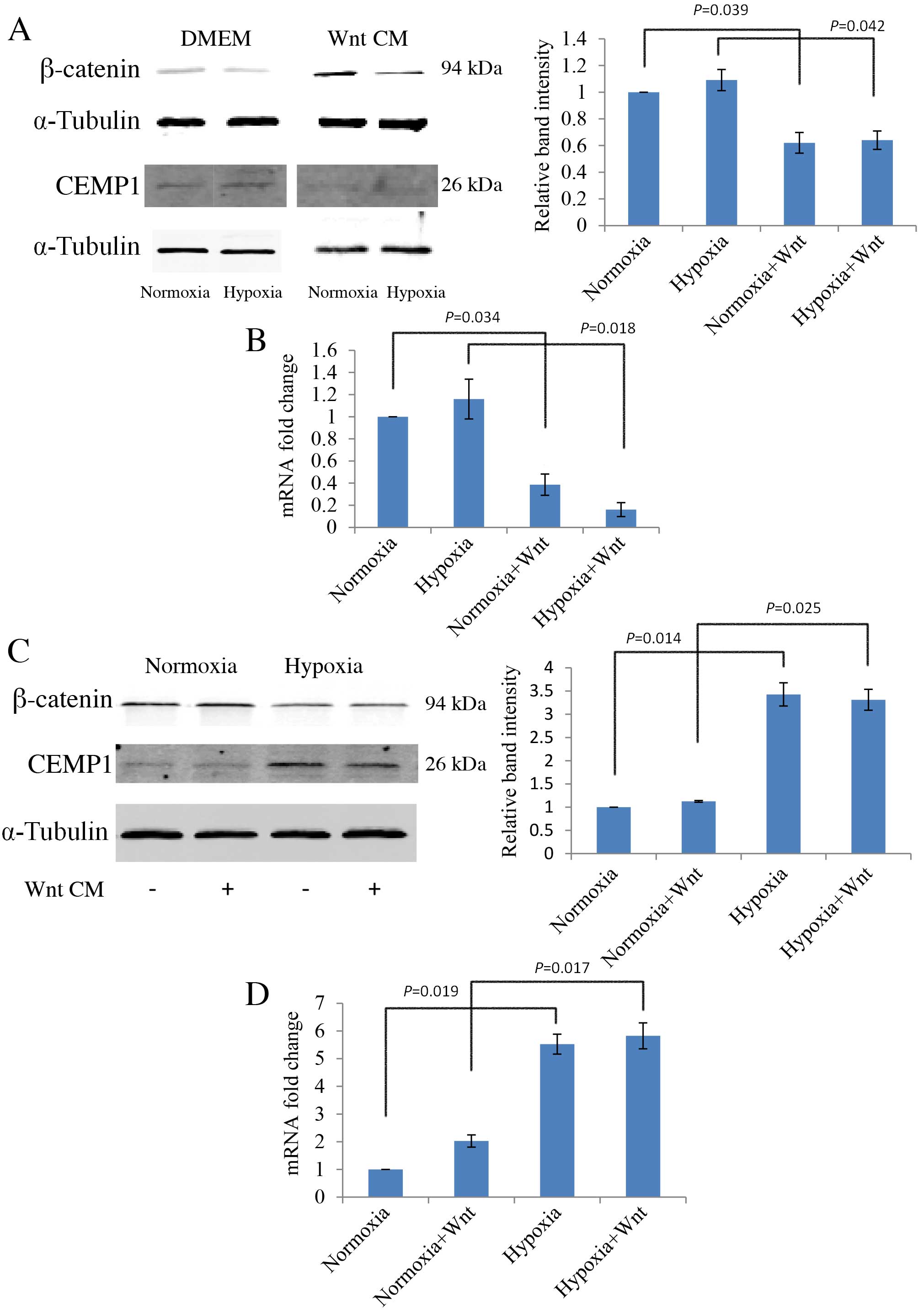 | Figure 5.Effect of hypoxia and Wnt on the
expression of cementogenic marker, CEMP1, in PDLCs in normoxic and
hypoxic (1 mM dimethyloxalylglycine) conditions, with or without
Wnt-CM. (A) Western blot analysis of CEMP1 protein expression
levels in DMEM, with quantification relative to α-Tubulin. (B)
RT-qPCR analysis of CEMP1 mRNA expression levels in DMEM, with
quantification relative to GAPDH. (C) Western blot analysis of
CEMP1 protein expression levels in OM, with quantification relative
to α-Tubulin. (D) RT-qPCR of CEMP1 mRNA expression levels in OM,
with quantification relative to GAPDH. PDLC, periodontal ligament
cells; OM, osteogenic medium; DMEM, Dulbecco's modified Eagle's
medium; CM, conditioned medium; CEMP1, cementum protein 1; GAPDH,
glyceraldehyde 3-phosphate dehydrogenase. |
Discussion
Cementum consists of the cellular intrinsic fibre
cementum (CIFC) layer, located at the tip of the root, and the
acellular extrinsic fibre cementum (AEFC) layer, located at the
upper two-thirds of the root (9).
CIFC is continuously deposited at the tip of the root to compensate
for normal physiological occlusive abrasion; however, it is the
AEFC that predominantly contributes to periodontal attachment.
Therefore, regenerated cementum would ideally resemble the AEFC
(26). However, compared with the
CIFC, the regenerative capacity of the AEFC is significantly lower
(26). Cementum regeneration must
also include reattachment of the periodontal ligament to the
cementum. From a biochemical perspective, CIFC and bone share
certain common features but are distinct tissues; as opposed to
bone, CIFC has no lamellar organization, blood vessels or nerve
innervation. The CIFC and AEFC are both formed by cementoblasts,
however, the specific mechanisms resulting in the production of
these distinct types of cementum are of significant research
interest. The cementoblasts that form the CIFC become embedded
within the matrix they secrete, and a resemblance to bone formation
is apparent. However, the reason for the lack of embedded
cementoblasts within the AEFC, which resembles the tooth enamel
formed by non-embedded ameloblasts, remains unclear. These are
properties that distinguish cementum tissues from bone, and also
CIFC and AEFC within the cementum.
It has been proposed that hypoxia maintains the
stem-like properties of PDLCs by enhancing the expression of
pluripotency markers (27). In the
present study, an in vitro model was used to demonstrate
that Wnt signalling inhibits PDLC differentiation towards a
cementoblast lineage, instead promoting differentiation towards an
osteoblastic lineage. Therefore, the regeneration of periodontal
tissue cannot be realized by simply activating Wnt signalling
(15). To improve the
understanding the specific function of Wnt signalling and hypoxia
in the regeneration of CIFC and AEFC, a site-specific in
vivo model should be established to investigate the
regeneration of CIFC and AEFC as separate phenomena.
Previous studies have demonstrated that the Wnt
signalling pathway promotes cementogenesis in PDLCs cultured in
osteogenic media (15). In the
present study, PDLCs were cultured in non-osteogenic medium,
revealing that Wnt signalling inhibits cementogenic differentiation
of naive PDLCs. However, when PDLCs were cultured in osteogenic
medium Wnt signalling was spontaneously up-regulated and any
further stimulation of Wnt by the addition of Wnt3a conditioned
medium had limited effects on β-catenin activation, and the
expression of cementogenic marker, CEMP1. The association between
hypoxia and the Wnt signalling pathway has been intensively
investigated, resulting in a numerous contradictory conclusions.
For example, it has been proposed that hypoxia can activate
canonical Wnt signalling by up-regulating the expression of LEF-1
and TCF-1 in embryonic stem cells, thereby increasing proliferation
(19). Hypoxia normally inhibits
the formation of β-catenin-TCF-4 complex and transcriptional
activity, however, in a certain microenvironment, HIF-1α can
compete with TCF-4 for direct binding of β-catenin to promote cell
survival and tumourigenesis (28).
Another study suggests that HIF-1α can inhibit β-catenin signalling
by interfering with human arrest defective 1, which would otherwise
acetylate and activate β-catenin (29). The results of the present study
indicated that in PDLCs, hypoxia inhibits β-catenin, as a marker of
Wnt signalling, which contributes to the understanding of the cell-
and tissue-specific effects of hypoxia and Wnt signalling.
Unpublished data from our lab has also demonstrated that
undifferentiated PDLCs have low intrinsic Wnt signalling and that
CEMP1 expression decreases in spite of reduced β-catenin activity
under hypoxia-like conditions. By contrast, PDLCs have relatively
high Wnt signalling expression following osteogenic induction (data
not shown). In this context, hypoxia inhibited β-catenin expression
and promoted the expression of CEMP1. The role of Wnt signalling in
cementogenesis as demonstrated in the present study is to inhibit
cementogenic differentiation.
The Wnt signalling pathway has been considered as a
therapeutic target for bone and mineral tissue regeneration.
However, the results of the current and previous studies suggest
that Wnt signalling has widely varying effects depending on the
tissue, and in terms of regeneration of specific mineralised
tissues, it is necessary to initially establish the precise
function of Wnt signalling in the local environment.
PDLCs can differentiate to osteoblasts and
cementoblasts, thus, the present study suggests that cementogenic
and osteogenic differentiation of PDLCs may originate from the
different local environments of the periodontal tissues. For
example, an infection of the root surface may lead to an increased
inflammatory response and induce hypoxic conditions. This, in turn,
may affect the capacity of naive PDLCs to differentiate, resulting
in inhibition of cementogenesis following periodontal treatment.
Hypoxia increases CEMP1 expression in differentiated PDLCs, which
may be crucial for cementum regeneration.
References
|
1
|
Diekwisch TG: The developmental biology of
cementum. Int J Dev Biol. 45:695–706. 2001.PubMed/NCBI
|
|
2
|
Freeman E: Peridontium. Ten Cate A.R.:
Mosby-Year Book, Inc; St. Louis, MO, USA: 1994
|
|
3
|
Cool SM, Forwood MR, Campbell P and
Bennett MB: Comparisons between bone and cementum compositions and
the possible basis for their layered appearances. Bone. 30:386–392.
2002. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Dimitriou R, Jones E, McGonagle D and
Giannoudis PV: Bone regeneration: Current concepts and future
directions. BMC Med. 9:662011. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Hirata A, Sugahara T and Nakamura H:
Localization of runx2, osterix, and osteopontin in tooth root
formation in rat molars. J Histochem Cytochem. 57:397–403. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Thomas HF: Root formation. Int J Dev Biol.
39:231–237. 1995.PubMed/NCBI
|
|
7
|
Huang X, Bringas P Jr, Slavkin HC and Chai
Y: Fate of HERS during tooth root development. Dev Biol. 334:22–30.
2009. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Cao Z, Zhang H, Zhou X, Han X, Ren Y, Gao
T, Xiao Y, de Crombrugghe B, Somerman MJ and Feng JQ: Genetic
evidence for the vital function of Osterix in cementogenesis. J
Bone Miner Res. 27:1080–1092. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Bosshardt DD: Are cementoblasts a
subpopulation of osteoblasts or a unique phenotype? J Dent Res.
84:390–406. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Westendorf JJ, Kahler RA and Schroeder TM:
Wnt signaling in osteoblasts and bone diseases. Gene. 341:19–39.
2004. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Krishnan V, Bryant HU and Macdougald OA:
Regulation of bone mass by Wnt signaling. J Clin Invest.
116:1202–1209. 2006. View
Article : Google Scholar : PubMed/NCBI
|
|
12
|
Li X, Liu P, Liu W, Maye P, Zhang J, Zhang
Y, Hurley M, Guo C, Boskey A, Sun L, et al: Dkk2 has a role in
terminal osteoblast differentiation and mineralized matrix
formation. Nat Genet. 37:945–952. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Rooker SM, Liu B and Helms JA: Role of Wnt
signaling in the biology of the periodontium. Dev Dyn. 239:140–147.
2010.PubMed/NCBI
|
|
14
|
Nemoto E, Koshikawa Y, Kanaya S, Tsuchiya
M, Tamura M, Somerman MJ and Shimauchi H: Wnt signaling inhibits
cementoblast differentiation and promotes proliferation. Bone.
44:805–812. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Han P, Wu C, Chang J and Xiao Y: The
cementogenic differentiation of periodontal ligament cells via the
activation of Wnt/β-catenin signalling pathway by Li+ ions released
from bioactive scaffolds. Biomaterials. 33:6370–6379. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Grzesik WJ and Narayanan AS: Cementum and
periodontal wound healing and regeneration. Crit Rev Oral Biol Med.
13:474–484. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Ng KT, Li JP, Ng KM, Tipoe GL, Leung WK
and Fung ML: Expression of hypoxia-inducible factor-1α in human
periodontal tissue. J Periodontol. 82:136–141. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Semenza GL: HIF-1 and human disease: One
highly involved factor. Genes Dev. 14:1983–1991. 2000.PubMed/NCBI
|
|
19
|
Mazumdar J, O'Brien WT, Johnson RS,
LaManna JC, Chavez JC, Klein PS and Simon MC: O2 regulates stem
cells through Wnt/β-catenin signalling. Nat Cell Biol.
12:1007–1013. 2010. View
Article : Google Scholar : PubMed/NCBI
|
|
20
|
Grayson WL, Zhao F, Izadpanah R, Bunnell B
and Ma T: Effects of hypoxia on human mesenchymal stem cell
expansion and plasticity in 3D constructs. J Cell Physiol.
207:331–339. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Verras M, Papandreou I, Lim AL and Denko
NC: Tumor hypoxia blocks Wnt processing and secretion through the
induction of endoplasmic reticulum stress. Mol Cell Biol.
28:7212–7224. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Varela-Nallar L, Rojas-Abalos M, Abbott
AC, Moya EA, Iturriaga R and Inestrosa NC: Chronic hypoxia induces
the activation of the Wnt/β-catenin signaling pathway and
stimulates hippocampal neurogenesis in wild-type and APPswe-PS1ΔE9
transgenic mice in vivo. Front Cell Neurosci. 8:172014. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Zhou Y, Wu C and Xiao Y: The stimulation
of proliferation and differentiation of periodontal ligament cells
by the ionic products from Ca7Si2P2O16 bioceramics. Acta Biomater.
8:2307–2316. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Jaakkola P, Mole DR, Tian YM, Wilson MI,
Gielbert J, Gaskell SJ, von Kriegsheim A, Hebestreit HF, Mukherji
M, Schofield CJ, et al: Targeting of HIF-alpha to the von
Hippel-Lindau ubiquitylation complex by O2-regulated prolyl
hydroxylation. Science. 292:468–472. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Bookout AL and Mangelsdorf DJ:
Quantitative real-time PCR protocol for analysis of nuclear
receptor signaling pathways. Nucl Recept Signal. 1:e0122003.
View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Bosshardt DD and Selvig KA: Dental
cementum: The dynamic tissue covering of the root. Periodontol
2000. 13:41–75. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Zhou Y, Fan W and Xiao Y: The effect of
hypoxia on the stemness and differentiation capacity of PDLC and
DPC. Biomed Res Int. 2014:8906752014. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Kaidi A, Williams AC and Paraskeva C:
Interaction between beta-catenin and HIF-1 promotes cellular
adaptation to hypoxia. Nat Cell Biol. 9:210–217. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Lim JH, Chun YS and Park JW:
Hypoxia-inducible factor-1alpha obstructs a Wnt signaling pathway
by inhibiting the hARD1-mediated activation of beta-catenin. Cancer
Res. 68:5177–5184. 2008. View Article : Google Scholar : PubMed/NCBI
|