Introduction
The renin-angiotensin system (RAS) is known to be a
regulator of blood pressure and fluid balance in organisms,
however, evidence suggests that it may also be important in the
regulation of follicular development, oocyte maturation, ovulation,
and steroidogenesis, in addition to the formation of the corpus
luteum (1). Angiotensin converting
enzyme (ACE), one of the most important components of RAS, is a
zinc metallopeptidase involved in the conversion of angiotensin I
to angiotensin II, and in the degradation of bradykinin (2). It is expressed in multiple tissues,
including the ovaries (2), and
exists in a membrane-anchored form on the surface of endothelial
and epithelial cells and as a circulating plasma form (3). Ovarian tissues contain
prorenin/renin, angiotensinogen, and ACE essential for the
production of angiotensin (2,4,5).
In vitro studies have demonstrated that ACE is responsible
for the angiogenesis of ovarian endothelium (6), steroidogenesis (7), resumption of meiosis (8), and follicular growth (9).
Polycystic ovary syndrome (PCOS) is a common
endocrine disorder present in 5–10% of women of reproductive age,
consisting of anovulation, hyperandrogenism, insulin resistance
(10,11), and long-term metabolic and
cardiovascular complications (12). Previous studies have evaluated
environmental and genetic factors that may contribute to the
etiology of PCOS (11,13). Although >70 candidate genes have
been investigated for a role in the development of PCOS and its
complications, the results have been inconclusive, indicating that
abnormalities in other genes may be key factors in the pathogenesis
of PCOS (14–16). Few studies have described the role
of ACE in human ovaries and the etiology of PCOS. It appears that
certain complications of PCOS may be associated with elevated
levels of angiotensin II and low bradykinin concentrations
(1,17). Individual variations in plasma ACE
concentration are associated with the insertion (I)/deletion (D)
polymorphism in the 287 bp DNA sequence situated in intron 16 of
the ACE gene (18). Previous
studies have indicated that receptors for angiotensin II are
present on steroidogenic cells responsible for the synthesis of
steroid hormones (1,2,4). It
has also been demonstrated that the ovarian RAS in women with PCOS
may be upregulated (4) and,
particularly, the presence of the D allele results in higher levels
of plasma ACE (18), which
subsequently leads to elevation of angiotensin II levels and
disorder of steroid hormone synthesis (19). Recent studies have investigated the
importance of the ACE gene I/D polymorphism in PCOS, insulin
resistance, and hyperandrogenism (20,21),
however, they have returned contradictory findings.
The aim of the present study was to analyze the
frequencies of the ACE gene I/D polymorphisms in a group of women
with PCOS compared with a control group consisting of healthy
women, and to determine the association between the ACE genetic
variants and the risk of metabolic and cardiovascular complications
in women with PCOS.
Materials and methods
Study subjects
Patients were recruited from the Department of
Infertility and Reproductive Medicine of Poznań University of
Medical Sciences between July 2012 and December 2013. A total of
138 patients were included in the study group. The diagnosis of
PCOS was confirmed according to the Rotterdam consensus criteria
(22). Other endocrinopathies and
associated disorders were excluded by measuring basal prolactin,
thyroid stimulating hormone and 17-hydroxyprogesterone levels.
Hyperandrogenism was identified based on the presence of hirsutism
as demonstrated by a Ferriman-Gallwey score (FG) (23) of >8 and/or presence of acne
and/or elevated androgen levels. Patients with a history of
endocrinopathies, including hyperprolactinemia and thyroid
diseases, and other metabolic disturbances, such as hyperlipidaemia
or diabetes mellitus were excluded.
The control group included 110 healthy volunteers
with no menstrual cycle irregularities, clinical or biochemical
hyperandrogenism, PCO on ultrasonography, history of
endocrinological or autoimmune disorders, and surgery to the pelvic
region.
The cardiovascular risk was estimated in the two
groups according to the criteria of the American Heart Association
(AHA) and the Androgen Excess and PCOS Society (AE-PCOS), which
considers the risk for type 2 diabetes mellitus, stroke, and
cardiovascular disease (CVD). The following criteria were used to
divide the patients in the study and control groups into at-risk
[metabolically unhealthy (MU)] and not at-risk [metabolically
healthy (MH)]. Patients were included in the at-risk group if any
of the following factors was present: i) Obesity, particularly
increased abdominal adiposity; ii) cigarette smoking; iii)
hypertension; iv) dyslipidemia, increased low-density lipoprotein
(LDL) cholesterol and/or non-high-density lipoprotein (HDL)
cholesterol; v) impaired glucose tolerance; vi) family history of
premature CVD, <55 and 65 years of age in male and female
relatives, respectively; and vii) metabolic syndrome (24,25).
Patient evaluation
Medical and family history were investigated for all
patients, and clinical examination was performed, including
measurement of body weight, height, waist circumference (WC) at the
midpoint between the lateral iliac crest and the lowest rib margin
at the end of normal expiration, waist to hip ratio (WHR), and hip
circumference (HC) measured at the widest level of the greater
trochanters. Body mass index (BMI) was calculated as weight in
kilograms divided by the square of height in meters
(kg/m2). According to the World Health Organization
categories, being overweight was defined as having a BMI from
25.0–29.9 kg/m2, and obesity was defined as BMI of ≥30.0
kg/m2 (26). All the
patients enrolled in the study were evaluated during the early
follicular phase of the menstrual cycle (days 3–5) subsequent to
discontinuation of antidiabetic and contraceptive agents for ≥3
months.
Biochemical and hormonal analysis
Biochemical parameters were measured in the Central
Laboratory of Poznań University of Medical Sciences (Poznań,
Poland), which is a certified facility meeting the criteria of ISO
9001. Blood samples for biochemical and hormonal analysis were
drawn from the antecubital vein between 8 and 10 AM following a
12-h overnight fast. Samples that were not analyzed the same day
were centrifuged and the plasma was aliquoted and stored in −70°C
until assayed.
The 75-g oral glucose tolerance test was performed
in all patients to measure glucose and insulin levels. Blood
samples were obtained at baseline and at 30-min intervals for 2 h.
Glucose level in venous blood was determined by means of the
enzymatic (hexokinase) method with Roche Diagnostics (Basel,
Switzerland) laboratory reagents on a Roche Hitachi 912 analyzer.
Insulin level was measured with the AxSYM Insulin assay
(microparticle enzyme immunoassay) from Abbott Laboratories
(Chicago, IL, USA).
Total serum cholesterol, HDL cholesterol, and
triglycerides (TG) levels were measured with appropriate Roche
Diagnostics reagents (Cholesterol CHOD-PAP, HDL-cholesterol Plus,
and Triglycerides GPO-PAP, respectively) on the Roche Hitachi 912
analyzer. LDL cholesterol levels were calculated using the
following formula: LDL cholesterol = total cholesterol - HDL
cholesterol - TG/5. The following definitions of normal levels were
used: Total cholesterol, 50.0–200.0 mg/dl; HDL cholesterol,
35.0–70.0 mg/dl; triglycerides, 50.0–150.0 mg/dl; and LDL
cholesterol, 35.0–130.0 mg/dl.
Visceral adipose index (VAI) in females was
calculated using the following formula: VAI = (WC/39.68+1.89 × BMI)
(TG/0.81) × (1.52/HDL) (27).
Molecular analysis
The molecular analysis was performed in the
Laboratory of Experimental Pharmacogenetics, Department of Clinical
Pharmacy and Biopharmacy, Poznań University of Medical Sciences
(Poznań, Poland). The ACE gene I/D polymorphism was assessed using
the polymerase chain reaction (PCR) method. DNA was isolated from
white blood cells with the QIAamp Blood Mini kit (Qiagen GmbH,
Hilden, Germany) and PCR was performed using LightSNiP ACE 289 bp
DeletionTib MolBiol GmbH (Berlin, Germany). The primers used in the
PCR reaction were as follows: Forward,
5′-CTGGAGACCACTCCCATCCTTTCT-3′ and reverse,
5′-GATGTGGCCATCACATTCGTCAGAT-3′ for ACE gene I/D polymorphism; and
an additional primer specific for the insertion,
5′-TGGGATTACAGGCGTGATACAG-3′. They were described by Rigat et
al (18) and Ueda et al
(28) and obtained from Tib
MolBiol GmbH. The PCR reaction protocol consisted of an initial
denaturation step at 95°C for 4 min, followed by 30 cycles of
amplification (30 sec at 95°C, 30 sec at 61°C, and 60 sec at 72°C).
The final extension step was 10 min at 72°C. The PCR products were
analyzed by agarose electrophoresis in the presence of ethidium
bromide and visualized in UV light using a documentation system (KS
4000; Syngen Biotech Molecular Biology Instruments, Wroclaw,
Poland). The following fragments were detected: Insertion/insertion
(II), 480 and 160 bp; insertion/deletion (ID), 480, 190, and 160
bp; and deletion/deletion (DD), 190 bp.
Statistical analysis
All statistical analyses were performed using the
Statistica version 10 PL software (StatSoft, Inc., Tulsa, OK, USA).
The distribution of variables was investigated using the
Shapiro-Wilk test and nonparametric tests. In particular, the
Mann-Whitney U test and the Kruskal-Wallis test were used for
non-normal distributions. Continuous variables are expressed as
medians (interquartile range (IQR), 25–75th percentile) unless
otherwise indicated. P<0.05 was considered to indicate a
statistically significant difference. Between-group differences and
differences among genotype groups were assessed using the
Mann-Whitney U test. χ2 analysis was used to compare the
distribution of genotypes and alleles for the ACE polymorphism
between the groups and to assess between-group differences for
non-continuous variables.
The study protocol was approved by the Ethics
Committee of Poznań University of Medical Sciences (Poznań,
Poland). Written consent was obtained from all the subjects
enrolled in the present study.
Results
The characteristics of the two groups are presented
in Table I. The median average age
in the control and study groups was 28.5 (26.0–31.0) and 27.0
(24.0–30.0) years, respectively (P=0.004). Although the subjects in
the control group were significantly older than those in the study
group, and it is known that older age is associated with more
severe metabolic disturbances, however, no characteristics, such as
high BMI and WC, elevated fasting insulin level, and presence of
metabolic syndrome or dyslipidemia, were more prevalent in the
controls compared with the study group subjects.
 | Table I.Characteristics of the control and
experimental groups. |
Table I.
Characteristics of the control and
experimental groups.
|
| Median (25–75) |
|
|---|
|
|
|
|
|---|
| Parameter | Control group
(n=110) | Study group
(n=138) | Pa |
|---|
| Age (years) | 28.5
(26.0–31.0) | 27.0
(24.0–30.0) | 0.004 |
| Body mass index
(kg/m2) | 20.5
(26.0–31.0) | 24.2
(20.9–28.8) | 0 |
| Hip circumference
(cm) | 87.9
(92.0–101.0) | 97.0
(92.0–108.0) | 0.26 |
| Waist circumference
(cm) | 69.5
(66.0–74.0) | 79.0
(70.0–89.5) | 0 |
| Waist to hip
ratio | 0.72
(0.7–0.76) | 0.78
(0.75–0.80) | 0 |
| Visceral adipose
index | 2.1
(1.45–3.65) | 2.55
(2.2–2.81) | 0.84 |
| Total cholesterol
(mg/dl) | 170 (156–180) | 188.4
(164.8–212.9) | <0.00001 |
| HDL cholesterol
(mg/dl) | 55 (45–63.5) | 60.2
(49.7–71.2) | <0.0013 |
| Tryglycerides
(mg/dl) | 87 (78–94) | 73.8
(57.9–107.0) | 0.01 |
| LDL cholesterol
(mg/dl) | 78 (72–88.5) | 106.0
(88.0–124.4) | 0.000001 |
| Fasting glucose
(md/dl) | 90 (84–96) | 88.1
(83.0–93.9) | 0.17 |
| Fasting insulin
(mU/ml) | 5.4 (4.6–8.02) | 7.6 (5.2–11.5) | 0.000005 |
| Systolic blood
pressure (mm/Hg) | 110 (100–120) | 115 (100–125) | 0.23 |
| Diastolic blood
pressure (mm/Hg) | 70 (60–80) | 70 (60–80) | 0.34 |
Anthropometric parameters, including BMI and WC were
significantly higher in the PCOS patients. Notably, there were no
differences in HC and WHR between the groups, and the present study
did not observe any significant differences in blood pressure
levels. Furthermore, none of the women suffered from hypertension.
Although abdominal and general obesity were more frequent in the
PCOS group, notably, the subjects in the control group tended to
have higher levels of TG and lower HDL cholesterol concentrations.
Nevertheless, the concentration of LDL cholesterol was
significantly higher in the PCOS group (P<0.0001). Although
abnormal glucose metabolism was not present in subjects from either
group, a tendency in the PCOS patients to have higher fasting
insulin levels was observed (Table
I).
The obtained results confirmed the observation that
the women in the PCOS group were significantly more predisposed to
having higher WC and BMI (P=0.000001; Table II). No women from the control
group were obese, as opposed to 26 (15.45%) of the women in the
study population. Although the median level of TG was lower in the
PCOS group, the number of patients with abnormal TG levels was
significantly higher (P=0.004).
 | Table II.Risk factors in the analyzed
groups. |
Table II.
Risk factors in the analyzed
groups.
| Risk factor | Control
group(n=110) | Study
group(n=138) | P |
|---|
| Cigarette smoking
(n, %) | 28 (25.5%) | 48 (28.57%) | 0.56a |
| Family history of
premature CVD (n, %) | 0 (0%) | 0 (0%) |
|
| Metabolic syndrome
(n, %) | 0 (0%) | 15 (8.9 %) | 0.001a |
| Diabetes or fasting
glucose ≥5.6 mmol//l | 0 (0%) | 0 (0%) |
|
| High blood pressure
(≥130/85 mmHg) | 0 (0%) | 0 (0%) |
|
| High triglycerides
(≥1.7 mmol/l) | 0 (0%) | 12 (7.1%) | 0.004a |
| Low HDL cholesterol
(<1.04 mmol/l) | 37 (33.6%) | 42 (25%) | 0.12a |
| Increased WC
(>80 cm) | 16 (14.5%) | 74 (44%) |
0.00001a |
| Impaired fasting
glucose (>5.6 mmo/l) | 12 (10.9%) | 11(6,54%) | 0.19a |
| Fasting insulin
>5.4 (mU/ml) | 54 (49.1%) | 124 (73.8%) |
0.00001a |
| Fasting insulin
>10.4 (mU/ml) | 9 (8.2%) | 59 (35.1%) |
0.00001a |
| Diabetes
mellitus | 0 (0%) | 0 (0%) |
|
| Body mass index
(kg/m2) |
|
|
|
|
Underweight (<18.50) | 3 (2.7%) | 5 (3.0%) | 0.9b |
| Normal
weight (18.50–24.99) | 94 (85.4%) | 92 (54.7%) | 0.0001b |
|
Abnormal weight
(>24.99) | 13 (11.8%) | 71 (42.3%) |
0.00001b |
|
Overweight (25.00–29.99) | 13 (11.8%) | 45 (26.8%) | 0.003b |
| Class I
obesity (30.00–34.99) | 0 (0%) | 15 (8.9%) | 0.0013b |
| Class
II obesity (35.00–39.99) | 0 (0%) | 10 (5.95%) | 0.009b |
| Class
III obesity (≥40.00) | 0 (0%) | 1 (0.6%) | 0.4b |
ACE genotypes were subsequently analyzed. The II,
ID, and DD genotype frequencies were 29.1, 44.5, and 26.4% in the
controls and 5.0, 37.7, and 57.3% in the women with PCOS,
respectively. In the control group, the II genotype was observed
significantly more often than in the PCOS group (P<0.00001),
whereas the DD genotype was presented significantly more often in
the PCOS group (P<0.002; Table
III). The genotype distribution complied with the
Hardy-Weinberg equilibrium (P>0.05). The frequency of the I
allele was 51.4% in the control group, but only 23.9% in the study
group, whereas the D allele was present in 48.6% of the controls
and 76.09% of the PCOS patients (P<0.00001).
 | Table III.Distributions of ACE-1 genotypes and
I and D alleles in the study and control groups. |
Table III.
Distributions of ACE-1 genotypes and
I and D alleles in the study and control groups.
| ACE-1 | Control group
(n=110) | Study group
(n=138) | Pa |
|---|
| Genotype |
|
|
|
| II | 32 (29.1%) | 7 (5.07%) | <0.00001 |
| ID | 49 (44.5%) | 52 (37.68%) | 0.274 |
| DD | 29 (26.4%) | 79 (57.25%) | <0.002 |
| Allele |
|
|
|
| I | 113 (51.4%) | 66 (23.91%) | <0.00001 |
| D | 107 (48.6%) | 210 (76.09%) | <0.00001 |
The association between the ACE gene I/D
polymorphism and the development of metabolic disturbances was
investigated. The distribution of the alleles and genotypes were
associated with presence of metabolic disturbances in the groups
formed according to the AHA and AE-PCOS criteria. The two groups
were divided according to the presence of metabolic risk factors,
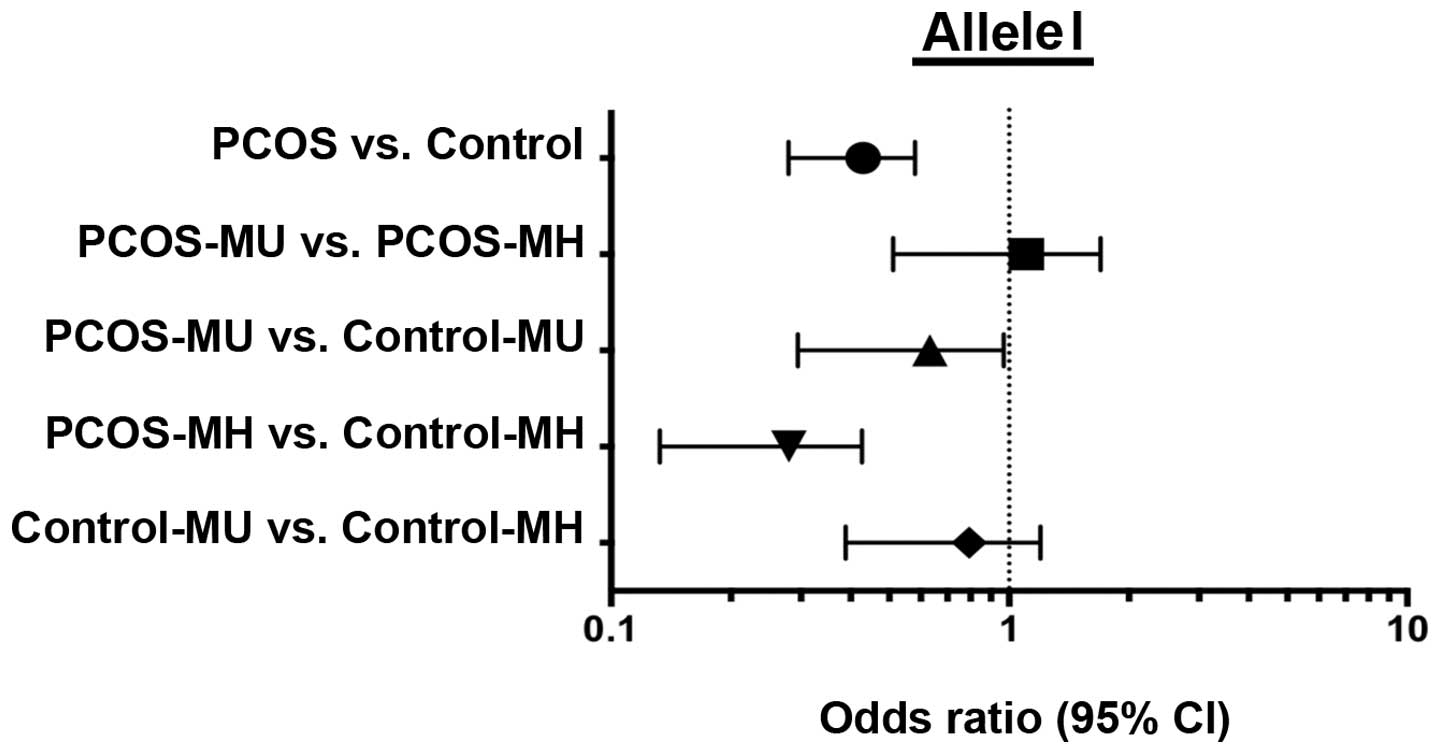
and it was subsequently observed that there was a significantly
higher prevalence of the D allele in all the PCOS subgroups
compared with the control subgroups, irrespective of the presence
of metabolic risk factors [odds ratio (OR), 3.27; P=0.0001;
Fig. 1]. There was no significant
difference in the frequency of the D allele, which predisposes to
heart disease, between the MU control subgroup (Control-MU) and the
metabolically healthy control group (Control-MH; Fig. 1). By contrast, the cardioprotective
I allele was encountered significantly less often in the PCOS
subgroups than in the controls (OR, 3.27; P=0.0001; Fig. 2).
The distributions of the DD and II genotypes
presented in Figs. 3 and 4, respectively, confirmed that the DD
genotype, which is known to increase the risk of CVD, was
significantly more frequent among the PCOS patients (OR, 3.87;
P=0.0003), whereas the cardioprotective II genotype occurred in
this group less frequently (OR, 0.4; P=0.06). There was no
difference in the distribution of ID genotype (data not
shown).
Discussion
Although the present study demonstrates the
conclusion of numerous previous reports that metabolic syndrome is
more frequent in women with PCOS than in the healthy population
(29,30), the prevalence of metabolic syndrome
in the study population (8.9%) is low compared to the results of
Apridonidze et al (29) and
the data obtained in women who participated in the National Health
and Nutrition Examination Survey III survey (30). In fact, this frequency is only
slightly higher than the estimated overall frequency of 6% in women
between 20 and 29 years old (30).
As presented in Tables I and
II, the predominant indicators of
metabolic syndrome in PCOS women are increased BMI and deranged
biochemical profile. Despite the presence of numerous women with
obesity, there were no subjects with hypertension in either of the
groups, and no tendency to exhibit higher blood pressure was
detected, which may be associated with the young age of the
participants. These results contradict the previous findings of
Chen et al (31) who
indicated that PCOS and accompanying hyperandrogenemia in young
women with PCOS were associated with elevated systolic and
diastolic blood pressure independently of age and presence of
insulin resistance, obesity, and dyslipidemia. Notably, the PCOS
patients in the present study had healthier lipid profiles compared
with the controls. The higher levels of HDL cholesterol and the
lower levels of TG in the study group suggest that there may be a
different mechanism underlying the development of metabolic
disturbances in women with PCOS compared with healthy women. This
finding is in disagreement with the results of Legro et al
(32) who observed that PCOS
patients more frequently exhibited low HDL cholesterol levels and
hypertriglyceridemia. Cardoso et al (33), who investigated the association
between the ACE gene D/I polymorphism and lipid profile,
demonstrated that, in the general population, women with the DD
genotype had the highest mean values of triglyceride levels, while
women with the II genotype had the highest mean values of HDL
cholesterol (33). The subjects in
the PCOS group in the present study had higher concentrations of
HDL cholesterol and lower TG levels, and the majority of them had
the DD genotype. Low serum HDL cholesterol is a known risk factor
of CVD, independently of serum LDL cholesterol levels (34). Therefore, the finding of the
present study and the results of Apridonidze et al (29) who also suggested that the presence
of PCOS by itself confers an increased risk of metabolic syndrome,
indicate that this disturbance may have an alternative
pathogenesis.
The role of ACE in RAS is crucial as it is
responsible for the regulation of blood pressure, angiogenesis of
ovarian endothelium, growth of follicles, steroidogenesis, and
inflammation. ACE is expressed in multiple tissues, including the
ovaries, and contributes to the genesis of numerous disorders
(6,7). More than 76 polymorphisms in the ACE
gene have been identified, with the ACE I/D polymorphism being the
most common. The frequency of this polymorphism in women with PCOS
requires further elucidation, and the association between the ACE
I/D polymorphism and PCOS and its complications has not yet been
confirmed.
The present study has demonstrated that women with
PCOS exhibit a significantly higher prevalence of the D allele,
which predisposes them to CVD and hypertension, and they are less
likely to have the protective I allele. This finding contradicts
the previous results of Sun et al (19) who did not observe differences in
genotypic distribution between patients with PCOS and controls.
Numerous possibilities may account for this discrepancy. First, the
previous study predominantly aimed to investigate whether ACE-1
polymorphism has an impact on the pathogenesis of PCOS, rather than
on the development of metabolic disturbances. The smaller sample
size in the present study may be another factor. In this regard,
the study of Deepika et al (35) which was performed in a large group
of patients, confirmed the results of the present study that the DD
genotype occurs significantly more often and the II genotype less
often in PCOS patients compared with healthy controls. However, in
this previous study the frequencies of the D and I alleles did not
vary significantly between the groups.
Notably, although the control group was older, and
age is a risk factor in metabolic disorders, metabolic syndrome was
more frequently observed in the PCOS group. Deepika et al
(35) suggested that the DD
genotype in women with PCOS may be a risk factor for the early
onset of symptoms, including hyperandrogenism, insulin resistance,
obesity, and enhanced RAS activity. This may confirm the findings
of the present study that indicate metabolic risk factors were
present more frequently in the PCOS group and in the subgroups with
the II genotype. Finally, the finding that MU subjects had
significantly higher fasting insulin levels is in line with the
results of a previous study that demonstrated an association
between the presence of acanthosis, a marker for insulin
resistance, in women with D alleles and DD genotypes (35). The present study determined that
there is an association between the ACE I/D polymorphism and the
presence and intensity of metabolic disturbances in PCOS women.
Future studies should aim detect patients at higher risk of
developing metabolic complications in order to alter their
lifestyle and diet. Metabolic improvement during the early years of
genetically at-risk individuals may attenuate the risk and
progression of CVD.
Acknowledgements
The authors would like to thank Editage (www.editage.com) for their English language editing.
The present study was funded by the Grant for Young Scientists by
the Poznań University of Medical Sciences (Poznań, Poland)
Glossary
Abbreviations
Abbreviations:
|
ACE
|
angiotensin converting enzyme
|
|
CVD
|
cardiovascular disease
|
|
HC
|
hip circumference
|
|
I/D
|
insertion/deletion
|
|
MH
|
metabolically healthy
|
|
MU
|
metabolically unhealthy
|
|
OGTT
|
oral glucose tolerance test
|
|
PCOS
|
polycystic ovary syndrome
|
|
RAS
|
renin-angiotensin system
|
|
WC
|
waist circumference
|
|
WHR
|
waist to hip ratio
|
|
VAI
|
visceral adipose index
|
References
|
1
|
Yoshimura Y: The ovarian renin-angiotensin
system in reproductive physiology. Front Neuroendocrinol.
18:247–291. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Van Sande ME, Scharpé SL, Neels HM and Van
Camp KO: Distribution of angiotensin converting enzyme in human
tissues. Clin Chim Acta. 147:255–260. 1985. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Soubrier F, Wei L, Hubert C, Clauser E,
Alhenc-Gelas F and Corvol P: Molecular biology of the angiotensin I
converting enzyme: II. Structure-function. Gene polymorphism and
clinical implications. J Hypertens. 11:599–604. 1993. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Palumbo A, Pourmotabbed G, Carcangiu ML,
Andrade-Gordon P, Roa L, DeCherney A and Naftolin F:
Immunohistochemical localization of renin and angiotensin in the
ovary: Comparison between normal women and patients with
histologically proven polycystic ovarian disease. Fertil Steril.
60:280–284. 1993. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Johnson MC, Vega M, Vantman D, Troncoso JL
and Devoto L: Regulatory role of angiotensin II on progesterone
production by cultured human granulosa cells. Expression of
angiotensin II type-2 receptor. Mol Hum Reprod. 3:663–668. 1997.
View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Plendl J, Neumüller C, Vollmar A, Auerbach
R and Sinowatz F: Isolation and characterization of endothelial
cells from different organs of fetal pigs. Anat Embryol (Berl).
194:445–456. 1996. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Acosta TJ, Berisha B, Ozawa T, Sato K,
Schams D and Miyamoto A: Evidence for a local
endothelin-angiotensin-atrial natriuretic peptide systemin bovine
mature follicles in vitro: Effects on steroid hormones and
prostaglandin secretion. Biol Reprod. 61:1419–1425. 1999.
View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Stefanello JR, Barreta MH, Porciuncula PM,
Arruda JN, Oliveira JF, Oliveira MA and Gonçalves PB: Effect of
angiotensin II with follicle cells and insulin-like growth factor-I
or insulin on bovine oocyte maturation and embryo development.
Theriogenology. 66:2068–2076. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Nielsen AH, Hagemann A, Svenstrup B,
Nielsen J and Poulsen K: Angiotensin II receptor density in bovine
ovarian follicles relates to tissue renin and follicular size. Clin
Exp Pharmacol Physiol. 21:463–469. 1994. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Azziz R, Carmina E, Dewailly D,
Diamanti-Kandarakis E, Escobar-Morreale HF, Futterweit W, Janssen
OE, Legro RS, Norman RJ, Taylor AE, et al: The androgen excess and
PCOS society criteria for the polycystic ovary syndrome: The
complete task force report. Fertil Steril. 91:456–488. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Diamanti-Kandarakis E: Polycystic ovarian
syndrome: Pathophysiology, molecular aspects and clinical
implications. Expert Rev Mol Med. 10:e32008. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Cho LW, Randeva HS and Atkin SL:
Cardiometabolic aspects of polycystic ovarian syndrome. Vasc Health
Risk Manag. 3:55–63. 2007.PubMed/NCBI
|
|
13
|
Diamanti-Kandarakis E, Piperi C, Spina J,
Argyrakopoulou G, Papanastasiou L, Bergiele A and Panidis D:
Polycystic ovary syndrome: The influence of environmental and
genetic factors. Hormones (Athens). 5:17–34. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Pérez MS, Cerrone GE, Benencia H, Márquez
N, De Piano E and Frechtel GD: Polymorphism in CYP11alpha and CYP17
genes and the etiology of hyperandrogenism in patients with
polycystic ovary syndrome. Medicina (B Aires). 68:129–134. 2008.(In
Spanish). PubMed/NCBI
|
|
15
|
Mlinar B, Pfeifer M, Vrtacnik-Bokal E,
Jensterle M and Marc J: Decreased lipin 1 beta expression in
visceral adipose tissue is associated with insulin resistance in
polycystic ovary syndrome. Eur J Endocrinol. 159:833–839. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Christopoulos P, Mastorakos G, Gazouli M,
Panidis D, Deligeoroglou E, Katsikis I, Papadias K,
Diamandi-Kandarakis E and Creatsas G: Genetic variants in TCF7L2
and KCNJ11 genes in a Greek population with polycystic ovary
syndrome. Gynecol Endocrinol. 24:486–490. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Giacchetti G, Sechi LA, Rilli S and Carey
RM: The renin-angiotensin-aldosterone system, glucose metabolism
and diabetes. Trends Endocrinol Metab. 16:120–126. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Rigat B, Hubert C, Alhenc-Gelas F, Cambien
F, Corvol P and Soubrier F: An insertion/deletion polymorphism in
the angiotensin I-converting enzyme gene accounting for half the
variance of serum enzyme levels. J Clin Invest. 86:1343–1346. 1990.
View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Sun J, Fan H, Che Y, Cao Y, Wu X, Sun HX,
Liang F, Yi L and Wang Y: Association between ACE gene I/D
polymorphisms and hyperandrogenism in women with polycystic ovary
syndrome (PCOS) and controls. BMC Med Genet. 10:642009. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Celik O, Yesilada E, Hascalik S, Celik N,
Sahin I, Keskin L and Ozerol E: Angiotensin-converting enzyme gene
polymorphism and risk of insulin resistance in PCOS. Reprod Biomed
Online. 20:492–498. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Karabulut A, Turgut S and Turgut G:
Angiotensin converting enzyme gene insertion/deletion polymorphism
in patients with polycystic ovary syndrome. Gynecol Endocrinol.
26:393–398. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Amsterdam ESHRE/ASRM-Sponsored 3rd PCOS
Consensus Workshop Group, . Consensus on women's health aspects of
polycystic ovary syndrome (PCOS). Hum Reprod. 27:14–24. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Ferriman D and Gallwey JD: Clinical
Assessment of body hair growth in women. J Clin Endocrinol Metab.
21:1440–1447. 1961. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Amato MC, Guarnotta V, Forti D, Donatelli
M, Dolcimascolo S and Giordano C: Metabolically healthy polycystic
ovary syndrome (MH-PCOS) and metabolically unhealthy polycystic
ovary syndrome (MU-PCOS): A comparative analysis of four simple
methods useful for metabolic assessment. Hum Reprod. 28:1919–1928.
2013. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Grundy SM, Cleeman JI, Daniels SR, Donato
KA, Eckel RH, Franklin BA, Gordon DJ, Krauss RM, Savage PJ, Smith
SC Jr, et al: Diagnosis and management of the metabolic syndrome:
An American heart association/national heart, lung and blood
institute scientific statement. Circulation. 112:2735–2752. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Obesity, . Preventing and managing the
global epidemic. Report of a WHO consultation. World Health Organ
Tech Rep Ser. 894:i-xii.1–253. 2000.
|
|
27
|
Amato MC, Giordano C, Galia M, Criscimanna
A, Vitabile S, Midiri M and Galluzzo A: AlkaMeSy Study Group:
Visceral adiposity index: A reliable indicator of visceral fat
function associated with cardiometabolic risk. Diabetes Care.
33:920–922. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Ueda S, Heeley RP, Lees KR, Elliott HL and
Connell JM: Mistyping of the human angiotensin-converting enzyme
gene polymorphism: Frequency, causes and possible methods to avoid
errors in typing. J Mol Endocrinol. 17:27–30. 1996. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Apridonidze T, Essah PA, Iuorno MJ and
Nestler JE: Prevalence and characteristics of the metabolic
syndrome in women with polycystic ovary syndrome. J Clin Endocrinol
Metab. 90:1929–1935. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Ford ES, Giles WH and Dietz WH: Prevalence
of the metabolic syndrome among US adults: Findings from the third
national health and nutrition examination survey. JAMA.
287:356–359. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Chen MJ, Yang WS, Yang JH, Chen CL, Ho HN
and Yang YS: Relationship between androgen levels and blood
pressure in young women with polycystic ovary syndrome.
Hypertension. 49:1442–1447. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Legro RS, Urbanek M, Kunselman AR, Leiby
BE and Dunaif A: Self-selected women with polycystic ovary syndrome
are reproductively and metabolically abnormal and undertreated.
Fertil Steril. 78:51–57. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Cardoso RL, Nogueira AR, Salis LH, Urményi
TP, Silva R, Moura-Neto RS, Pereira BB, Rondinelli E and Souza e
Silva NA: The association of ACE gene D/I polymorphism with
cardiovascular risk factors in a population from Rio de Janeiro.
Braz J Med Biol Res. 41:512–518. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Feig JE, Hewing B, Smith JD, Hazen SL and
Fisher EA: High-density lipoprotein and atherosclerosis regression:
Evidence from preclinical and clinical studies. Circ Res.
114:205–213. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Deepika ML, Reddy KR, Rani VU, Balakrishna
N, Latha KP and Jahan P: Do ACE I/D gene polymorphism serve as a
predictive marker for age at onset in PCOS? J Assist Reprod Genet.
30:125–130. 2013. View Article : Google Scholar : PubMed/NCBI
|