Introduction
Unilateral ureteral obstruction (UUO), which results
in hydronephrosis, is characterized by decreased renal function,
increased interstitial fibrosis, tubular apoptosis and cellular
infiltration (1,2). Histological studies have suggested
that the mechanical stretch of the tubular epithelium and oxidative
stress are early stress factors, leading to tubular cell injury and
death (2,3). There is increasing evidence that the
mitochondrial pathway is involved in stretch-induced and oxidative
stress-enhanced tubular cell apoptosis (4,5).
Therefore, it appears that disorders of mitochondrial energy
metabolism may also be a primary factor underlying tubular cell
apoptosis in hydronephrosis.
As the kidney is a high energy-demanding organ, it
relies heavily on aerobic metabolism for the production of ATP by
oxidative phosphorylation within mitochondria. The reprogramming of
energy metabolism has been designated a ‘new’ hallmark for diverse
forms of chronic kidney disease (4). Mitochondrial β-F1-ATPase (ATP5B), the
catalytic subunit of the rate-limiting enzyme of oxidative
phosphorylation, and electron transfer flavoprotein β subunit
(ETFB), an electron acceptor of five isoforms of acyl-CoA
dehydrogenase, are metabolic markers, which have been shown to be
of value in several types of cancer (6) and multiple acylCoA dehydrogenation
deficiency (MADD) (7). However,
the expression and alterations in these two proteins, and their
underlying involvement of in hydronephrosis have not been reported
and remain to be elucidated.
In the present study, the mRNA and protein
expression levels of ATP5B and ETFB were investigated in cases of
hydronephrosis graded III and IV using the Society for Fetal
Urology (SFU) grading system, to identify the associations with
split renal function (SRF), and thereby evaluate whether ATP5B and
ETFB have functions in renal injury and the progression of
hydronephrosis.
Materials and methods
Patients
Following approval by the Ethics Committee of China
Medical University (Shenyang, China; no. 2012 PS81K), fresh kidney
specimens were prospectively collected from patients undergoing
surgery for unilateral hydronephrosis secondary to UUO. The
patients underwent surgery at Shengjing Hospital of China Medical
University between May 2012 and October 2013. No patients had a
urinary tract infection prior to surgery or any other associated
anomalies, including vesicoureteral reflux and ureterovesical
junction obstruction. A total of 20 control renal biopsy specimens
were obtained from patients undergoing nephrectomy for
nephroblastoma, and the tissues were confirmed histologically to be
unaffected.
SRF of patients
A technetium-99 m diethylenetriamine pentaacetic
acid renal scan (InfiniaVCII Hawkeye; GE Healthcare Life Sciences,
Chalfont UK) was used to evaluate the mean renal function. The
furosemide clearance half-time in diuretic renography was applied
to determine whether obstruction was present. A drainage clearance
half-time of >20 min was indicative of obstruction. The
differential glomerular filtration rate (GFR) of the affected
dilated kidney was determined to measure the SRF of the patient.
Clearance half-time and GFR were automatically determined by
Xeleris image processing system (GE Healthcare Life Sciences). It
detected the time-intensity curve and GFR was calculated
accordingly. Any cases of ‘supranormal function’ of the affected
kidney (SRF>55%) were omitted from the experimental group.
Tissue sample collection and RNA
extraction
Hydronephrotic tissues and normal tissues (10
mm3) were collected, respectively, and identified by
pathologists. Various histological changes within the obstructed
tissue were present, including visible dilated tubules
predominantly located in collecting tubules and other distal
tubules, flattened tubular epithelial cells, expansive Bowman
capsules and glomerular infiltration by inflammatory cells. In the
control group, no abnormalities in tumor cell infiltration were
found. Following confirmation, each tissue specimen was examined
via molecular analysis, including immunoblot analysis and reverse
transcription-quantitative polymerase chain reaction (RT-qPCR)
analysis. TRIzol reagent (Invitrogen; Thermo Fisher Scientific,
Inc., Waltham, MA, USA) was used for RNA extraction. Following
harvesting, the tissue was cut into small pieces on ice and 500 µl
TRIzol was added. Subsequently, the mixture was homogenized
intermittently on ice and processed according to the instructions
of the manufacturer. The RNA quality was assessed by measuring
A260/A280 using a spectrophotometer. An adequate quantity (≥3 µg)
and quality (A260/A280: 1.8–2.1) of RNA was extracted and used for
cDNA synthesis with the Takara RNA PCR kit (Takara Bio, Inc., Otus,
Japan).
qPCR analysis
The qPCR amplifications were performed in a 10 µl
reaction system with 5 µl SYBR Premix Ex Taqll (Tli RNaseH Plus;
Takara Bio, Inc.), 2 µl ddH20, 0.5 µl each primer and 2
µl cDNA. Reactions were performed in triplicate on a Light Cycler
(Roche Applied Science, Penzberg, Germany) with the primers as
follows: Human ATP5B, sense 5′-TCACCCAGGCTGGTTCAGA-3′ and antisense
5′-AGTGGCCAGGGTAGGCTGAT-3′; Human ETFB, sense
5-AGGAGGTACTGTCAAAGCTGC-3′ and antisense,
5′-TTTCATGGCCTTCCTCAGGG3′; Human β-actin, sense
5′AGAGCTACGAGCTGCCTGAC-3′ and antisense 5′-AGCACTGTGTTGGCGTACAG-3′.
The housekeeping gene. β-actin (cat. no. DR3783; Takara Bio, Inc.)
was used as an endogenous control. The amplification protocol
involved 5 sec denaturation at 95°C and 20 sec annealing at 56°C
for 45 cycles. The relative mRNA levels for each sample were
calculated using the 2−∆∆Cq method (8).
Immunoblot analysis
The tissue specimens (~50 mg) were minced into small
sections using surgical blades and sonicated in protein lysis
buffer. Proteins were quantified using the 2D Quant kit (GE
Healthcare Life Sciences) according to the manufacturer's
instructions. Briefly, 50 µg of total protein was
electrophoretically separated on a 12% SDS-PAGE gel. The proteins
were then transferred onto a PVDF membrane and blocked with 5%
non-fat milk in TBS with 0.1% Tween 20 (TBST) and shaken for 1 h.
The membrane was washed three times with TBST, each wash used 10 ml
TBST and lasted ~10 min. The membrane was probed with the following
polyclonal antibodies: Anti-ATP5B (1:2,000; monoclonal mouse
anti-human; cat. no. ab14730; Abcam, Cambridge, UK) and anti-ETFB
(1:500; polyclonal rabbit anti-human; cat. no. ab97036; Abcam).
β-actin (1:500; monoclonal mouse anti-human; cat. no. sc-8432;
Santa Cruz Biotechnology, Inc., Dallas, TX, USA) was used as a
loading control. The membranes were incubated with primary
antibodies overnight at 4°C. The protein expression was visualized
by incubation with goat anti-mouse (cat. no. sc-3793) and goat
anti-rabbit (cat. no. sc-3837) secondary antibody conjugated with
horseradish peroxidase (1:2,000; Santa Cruz Biotechnology, Inc.)
for 2 h at room temperature and then enhanced chemoluminescence
reagent (Pierce; Thermo Fisher Scientific, Inc.). The intensity of
protein staining was determined using ImageJ2x, a publicly
available Java-based image processing program (version 2.1.4.7;
National Institutes of Health, Bethesda, MA, USA). The relative
density of each protein was calculated by dividing the optical
density value of each protein by that of the loading control.
Statistical analysis
All statistical analyses were performed using SPSS
18.0 (SPSS, Inc., Chicago, IL, USA) software for Windows. All
values are presented as the mean ± standard error of the mean.
Two-tailed Student's t-test was used for analyzing significant
differences from the control group. The receiver operating
characteristic (ROC) curve was used to determine the cut-off values
of ATP5B and ETFB at the optimum sensitivity and specificity.
Correlation analysis was used for the comparisons between ATP5B,
ETFB and other variables. Bivariate analysis was presented as
Pearson or Spearman correlation coefficient. P<0.05 was
considered to indicate a statistically significant difference.
Results
Clinical parameters of patients with
hydronephrosis
The clinical and pathological characteristics of the
patients are presented in Table I.
In the present study, boys were more frequently affected with
hydronephrosis, compared with girls, which has also been reported
previously (9). The left kidney
was more commonly involved than the right kidney (18:2,
respectively). The mean age at pyeloplasty was ~2 years. Grade III
hydronephrosis was present in six children, and grade IV
hydronephrosis was present in 14 children. The AP pelvic diameters
and SRF in the study group are listed. A radionuclide scan was not
performed in children from the control group; therefore, the SRF
was not evaluated in this group.
 | Table I.Summary of the clinical parameters of
all patients included in the present study. |
Table I.
Summary of the clinical parameters of
all patients included in the present study.
| Clinical
parameter | Hydronephrosis | Normal control |
|---|
| Gender, n
(male/female) | 20 (14/6) | 20 (12/8) |
| Age at the time of
surgery (years) | 2.16±2.88 | 2.56±1.09 |
| Clinical diagnosis
(SFU grading) |
|
|
| Grade
III | 6 | – |
| Grade
IV | 14 | – |
| Laterality
(left/right) | 18/2 | – |
| Split renal function
(%) | 35.00±17.72 | – |
| AP at prepyeloplasty
(mm) | 37.35±11.40 | – |
Transcription levels of ATP5B and
ETFB
Compared with the normal kidney tissues, the kidney
tissues with hydronephrosis showed increased mRNA levels of ATP5B,
by 2.57-fold (P<0.05; Table
II). The mRNA expression of ETFB did not differ between the
normal kidneys and those in the hydronephrosis group did not differ
in (P>0.05) (Table III).
 | Table II.Relative mRNA level of ATP5B in the
two tissue groups. |
Table II.
Relative mRNA level of ATP5B in the
two tissue groups.
| Tissue section | ATP5B average Cq
value | β-actin average Cq
value | ∆Cq | ∆∆Cq | Fold-change of gene
(vs. normal) |
|---|
| Hydronephrosis | 24.050±1.582 | 24.412±3.095 | −0.362 | −1.359 | 2.57 |
| Normal | 24.600±1.470 | 23.603±0.878 | 0.997 | 0 | 1 |
 | Table III.Relative mRNA level of ETFB in the two
tissue groups. |
Table III.
Relative mRNA level of ETFB in the two
tissue groups.
| Tissue section | ETFB average Cq
value | β-actin average Cq
value | ∆Cq | ∆∆Cq | Fold-change of
gene(vs. normal) |
|---|
| Hydronephrosis | 23.923±1.220 | 24.412±3.095 | −0.489 | −0.346 | 1.27 |
| Normal | 23.460±0.813 | 23.603±0.878 | −0.143 | 0 | 1 |
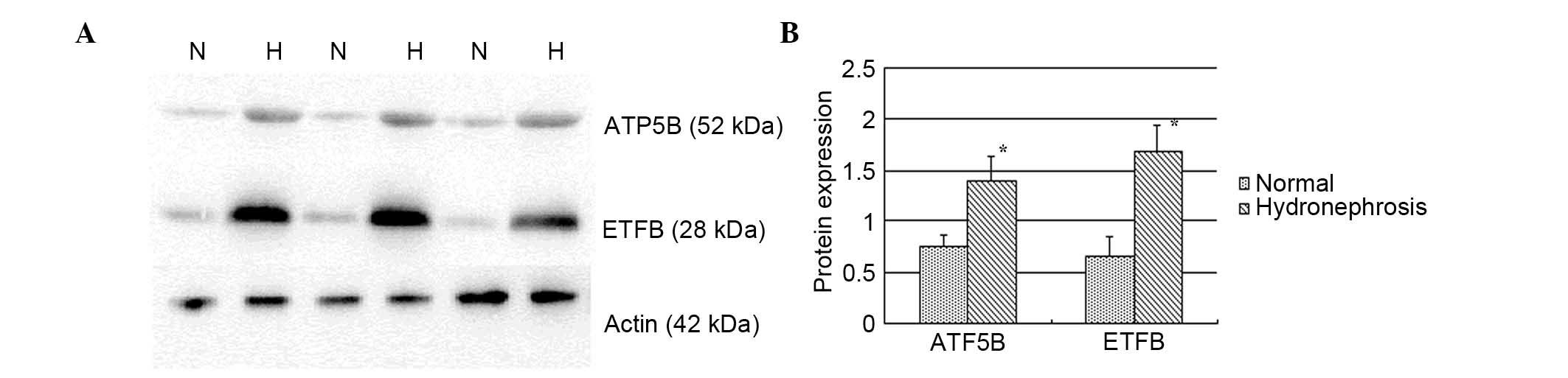
Results of immunoblot analysis
Higher protein expression levels of ATP5B and ETFB
were observed in the hydronephrosis group (Fig. 1), which were upregulated in the
hydronephrotic kidney by 1.87- and 2.58-fold, respectively
(P<0.05).
Correlations between ATP5B, ETFB and
SRF
A negative correlation was found between the ATP5B
protein and SRF (r=−0.458; P=0.042). The correlation was even more
marked between ETFB protein and SRF (r=−0.753; P<0.001)
(Fig. 2). No statistically
significant correlations were found between the mRNA expression of
ATP5B and ETFB and SRF. In addition, no significant correlation was
found and the age of the patients, parenchymal thickness, GFR and
initial AP diameter (length) of the pelvis.
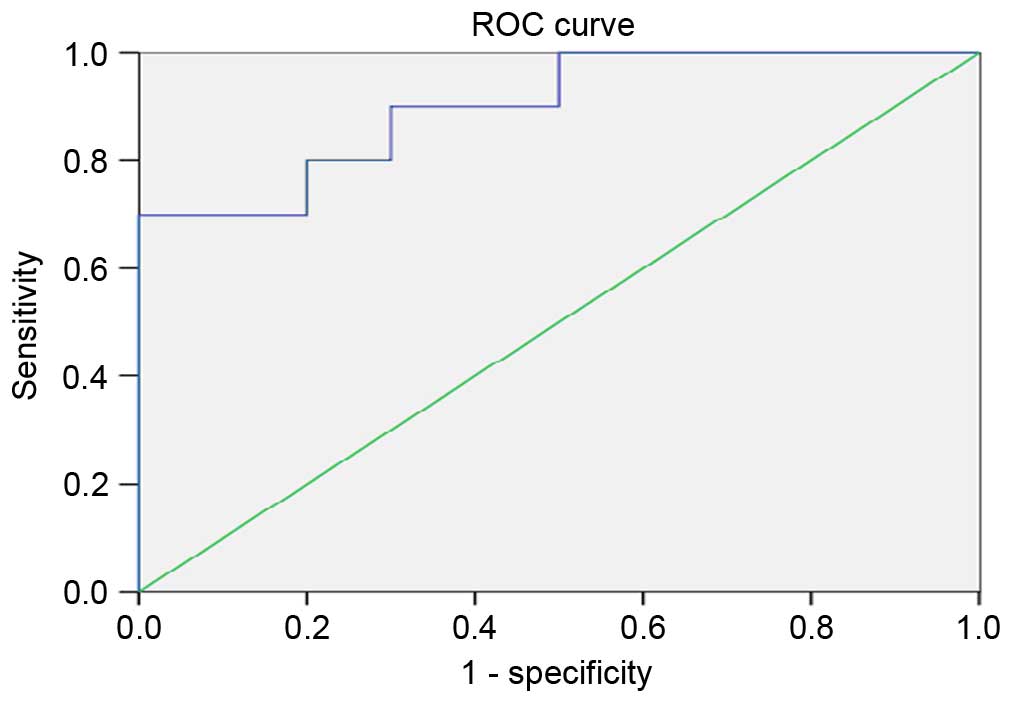
ROC analyses were performed to define the diagnostic
profile of ATP5B and ETFB in identifying children with abnormal SRF
(<45%) among all examined children. In the analysis, it was
found that only ETFB showed a suitable diagnostic profile, with the
area under the curve (AUC) for the ETFB protein being 0.900 (95%
confidence interval, 0.766–1.034) with an optimal cutoff value of
1.59 (sensitivity, 90%; specificity, 70%; Fig. 3).
Discussion
Obstructive nephropathy is a major cause of renal
failure, particularly in infants and children. Despite numerous
clinical and experimental studies over several decades, the
cellular and molecular mechanisms responsible for the progression
of hydronephrosis remain to be fully elucidated (10).
In post-UUO, mechanical stretch of the tubular
epithelium and oxidative stress are considered to be of importance
and act as early stress factors leading to tubular cell injury.
Studies (11,12) have demonstrated that stretch
induces caspase-dependent apoptosis in tubular epithelial cells via
the intrinsic mitochondrial pathway, and the mitochondrial release
of proapoptotic factors into the cytosol leads to apoptosome
assembly, the activation of caspase-9 and the cleavage of effector
caspases.
Oxidative stress, which develops from an imbalance
between increased free radical production and reduced antioxidant
defenses, is considered to be an important pathogenic mechanism in
obstructive nephropathy. Perturbations in cellular oxidant handling
affect downstream cellular signaling and, in the kidney, promote
renal cell apoptosis and senescence, decreased regenerative ability
of cells and fibrosis. These characteristics may be caused, at
least in part, by the gradual loss of renal energy through the
development of mitochondrial dysfunction and result in increasing,
oxidative stress (13).
Therefore, aerobic metabolism relies on ATP synthase
along the electron transport chain (ETC) within mitochondria, which
is vital for renal cellular function, yet potentially damaging in
the long-term (4). The ATP5B gene
encodes a β subunit of mitochondrial ATP synthase, and the ETFB
gene provides instructions for producing the β subunit of an enzyme
called electron transfer flavoprotein associated with ETC (Gene
Ontology). These genes are responsible for maintaining
mitochondrial membrane potential and ATP generation. ATP5B is
associated with transmembrane transporter and transporter activity,
and produces ATP from ADP by electron transport complexes of the
respiratory chain. As for ETFB, it serves as a specific electron
acceptor for several dehydrogenases, and transfers the electrons to
the primary mitochondrial respiratory chain. The direct and
indirect links between ATP5B, ETFB and mitochondria energy
metabolism are emerging in multiple areas of investigation. The
contribution of ATP5B in the regulation of types of cancer and of
ETFB in the mutation of MADD have been investigated extensively
(6,7). To the best of our knowledge, no
previous study has investigated the clinical effect of these
metabolic markers in hydronephrosis.
In order to obtain further information about the
association in the present study, RT-qPCR analysis and
immunoblot-based molecular biology were used to investigate the
differential expression in gene and protein levels between the
hydronephrotic kidney tissues and the normal kidney tissues. The
resulting data indicated that ATP5B and ETFB were well represented
in the kidney, and they are upregulated, compared with those in the
normal kidney, revealed by immunoblotting In addition, the results
of the RT-qPCR analysis revealed the results of the gene levels
were consistent with those of protein levels in ATP5B only. The
inconsistency between the mRNA and protein expression for ETFB may
be caused by posttranscriptional regulation.
In the present study, the levels of ATP5B and ETFB
were significantly elevated in patients who had developed an
obstructed kidney and undergone pyeloplasty. The mechanism
underlying this increase in the patients remains to be elucidated.
As it has been documented that cell apoptosis is important in renal
damage in hydronephrosis, and the number of apoptotic cells
increases with an increasing degree of hydronephrosis (14), the present study hypothesized that
the ATP5B and ETFB metabolic markers are essential in cellular
energetic metabolism and the execution of cell apoptosis. Higher
levels of ATP5B and ETFB are molecularly and functionally
integrated to provide energy and promote the execution of apoptosis
more effectively, and function in cell injury to the obstructed
kidneys. Furthermore, in our previous animal model study, it was
found that ATP5B was continuously upregulated and ETFB was
continuously downregulated with obstruction persisting in the
obstructed kidney, whereas in advanced obstruction, no significant
differences were found in the expression of ETFB, compared with the
sham group (Data submission). Based on data from animals and
patients, the present study hypothesized that the patients in the
present study, who had undergone pyeloplasty at SFU grades III and
IV are in relatively early obstruction. If obstruction is relieved
at this time-point, it may contribute to the long-term recovery of
renal function and avoid irreversible kidney fibrosis. This
hypothesis is consistent with the observations in the postoperative
follow-up in the present study (data not shown). Additionally,
based on data from Hirokawa et al (15,16),
ETFB is involved in the mechanoregulation of fibroblast cell number
and the knock down of ETFB can reduce transforming growth
factor-β-induced mRNA expression of α-smooth muscle actin, and
affect the tissue remodeling and/or fibrotic processes. The
upregulation of ETFB may be an attempt to promote and aggravate
renal fibrosis in the early stages.
The present study further evaluated the usefulness
of ATP5B and ETFB in renal injury caused by obstructive
nephropathy. By calculating the correlations of ATP5B and ETFB with
the primary clinical features of the affected kidney, significant
negative correlations were found between the protein levels of
ATP5B, ETFB and the SRF, suggesting the value for ATP5B and ETFB in
predicting SRF in congenital hydronephrosis. That is, the higher
the expression, the lower the SRF. No significant correlations with
the age of the patients, GFR, renal thickness or the
anterior-posterior diameter (length) of the pelvis were found (data
not shown). The present study hypothesized that ATP5B and ETFB
proteins may indicate the degree of renal injury, and may represent
a measurable index of renal injury, reflected by SRF, to assess
whether the injury may progress to chronic kidney disease. This
hypothesis was confirmed by data from the ROC analyses, which
showed a useful diagnostic profile for the ETFB protein in
detecting kidney injury in children with hydronephrosis with SRF
<45% (AUC, 0.900). These results suggest that the ETFB protein
may be useful in identifying kidney injury.
As circulating proteins also contribute to urinary
levels, whether urinary levels can also reliably measure renal
injury and the impairment of renal function requires further
investigation. For this, the expression levels of ETFB in the
plasma or urine, and their diagnostic implications on renal injury,
require assessment.
In conclusion, the results of the present pilot
study preliminary demonstrated that the children in the cohort with
hydronephrosis had increased gene and protein levels of ATP5B and
protein levels of ETFB, based on protein in renal tissues. The
protein expression levels of the markers correlated negatively with
SFR in the radionuclide scan, and the ETFB protein was indicated as
potentially being more useful in identifying kidney injury. The
results of the present study suggested that increasing levels of
metabolic markers were associated with worsening renal injury.
ATP5B and ETFB may be novel diagnostic or prognostic markers, and
require further detailed investigation
Acknowledgements
This study was supported by the National Nature
Science Foundation of China (grant no. 81370772).
References
|
1
|
Peters CA and Chevalier RL: Congenital
urinary obstruction: Pathophysiology and clinical
evaluationCampbell's Urology. Kavoussi LR, Novick AC, Partin AW and
Peters CA: 10th. Saunders; Philadelphia, PA: pp. 3030–3047.
2012
|
|
2
|
Klahr S and Morrissey J: Obstructive
nephropathy and renal fibrosis. Am J Physiol Renal Physiol.
283:F861–F875. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Mizuguchi Y, Chen J, Seshan SV, Poppas DP,
Szeto HH and Felsen D: A novel cell-permeable antioxidant peptide
decreases renal tubular apoptosis and damage in unilateral ureteral
obstruction. Am J Physiol Renal Physiol. 295:F1545–F1553. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Small DM, Coombes JS, Bennett N, Johnson
DW and Gobe GC: Oxidative stress, anti-oxidant therapies and
chronic kidney disease. Nephrology (Carlton). 17:311–321. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Zhang G, Oldroyd SD, Huang LH, Yang B, Li
Y, Ye R and El Nahas AM: Role of apoptosis and Bcl-2/Bax in the
development of tubulointerstitial fibrosis during experimental
obstructive nephropathy. Exp Nephrol. 9:71–80. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Hjerpe E, Brage S Egyhazi, Carlson J,
Stolt M Frostvik, Schedvins K, Johansson H, Shoshan M and
Avall-Lundqvist E: Metabolic markers GAPDH, PKM2, ATP5B and
BEC-index in advanced serous ovarian cancer. BMC Clin Pathol.
13:302013. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Schiff M, Froissart R, Olsen RK, Acquaviva
C and Vianey-Saban C: Electron transfer flavoprotein deficiency:
Functional and molecular aspects. Mol Genet Metab. 88:153–158.
2006. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(−Delta Delta C(T)) Method. Methods. 25:402–408. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Kajbafzadeh AM, Elmi A, Talab SS, Emami H,
Esfahani SA and Saeedi P: Urinary and serum carbohydrate antigen
19-9 as a biomarker in ureteropelvic junction obstruction in
children. J Urol. 183:2353–2360. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Chevalier RL: Obstructive nephropathy:
Towards biomarker discovery and gene therapy. Nat Clin Pract
Nephrol. 2:157–168. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Green DR and Kroemer G: The
pathophysiology of mitochondrial cell death. Science. 305:626–629.
2004. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Power RE, Doyle BT, Higgins D, Brady HR,
Fitzpatrick JM and Watson RW: Mechanical deformation induced
apoptosis in human proximal renal tubular epithelial cells is
caspase dependent. J Urol. 171:457–461. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Manucha W, Carrizo L, Ruete C, Brady HR,
Fitzpatrick JM and Watson RW: Angiotensin II type I antagonist on
oxidative stress and heat shock protein 70 (HSP 70) expression in
obstructive nephropathy. Cell Mol Biol (Noisy-le-grand).
51:547–555. 2005.PubMed/NCBI
|
|
14
|
Yang Y, Ji S, Wang C and Hou Y: Apoptosis
of renal tubular cells in congenital hydronephrosis. Chin Med J
(Engl). 114:502–505. 2001.PubMed/NCBI
|
|
15
|
Hirokawa S, Shimanuki T, Kitajima H,
Nishimori Y and Shimosaka M: Knockdown of electron transfer
flavoprotein β subunit reduced TGF-β-induced α-SMA mRNA expression
but not COL1A1 in fibroblast-populated three-dimensional collagen
gel cultures. J Dermatol Sci. 68:179–186. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Hirokawa S, Shimanuki T, Kitajima H,
Nishimori Y and Shimosaka M: Identification of ETFB as a candidate
protein that participates in the mechanoregulation of fibroblast
cell number in collagen gel culture. J Dermatol Sci. 64:119–126.
2011. View Article : Google Scholar : PubMed/NCBI
|