Introduction
C-reactive protein (CRP) is an acute phase protein
synthesized primarily by the liver that is involved in the systemic
response to inflammation and is regulated by cytokines like
interleukin (IL)-6, IL-1β and tumor necrosis factor-α (1,2). In
clinical practice, it has been used as a non-specific marker of
inflammation and atherosclerotic cardiovascular disease. It is
unknown if CRP plays an active role as an etiologic factor in
cardiovascular disease. Some studies show that the effect of CRP on
atherogenesis may include interactions with other factors of
immunity and inflammation, such as the complement system, as well
as a direct effect of CRP on the cells involved in atherosclerotic
lesions (1,2). CRP has also shown value in predicting
disease risk and assisting decision making on treatment for a
series of diseases: Baseline CRP concentration has strong
predictive and prognostic values for future cardiovascular events
(3–5). The Centers for Disease Control and
Prevention and the American Heart Association reported that it is
reasonable to measure CRP as an adjunct to the measurement of
established risk factors in order to assess the risk of coronary
heart disease (5).
High-sensitivity CRP (hsCRP) typically refers to the
pentameric form of CRP, which is a cyclic structure comprised of 5
identical 21.5-kDa subunits (6).
Pentameric CRP (pCRP) has a recognition site that binds to
phosphocholine and an effector site that binds to complement
component C1q, Fcγ receptors or other putative CRP receptors
(7–9). In addition to pCRP, monomeric CRP
(mCRP) has been detected in the intimal region of blood vessels in
healthy human tissues and the atherosclerotic plaque, whereas pCRP
was not observed in healthy or diseased arteries (10–12).
The majority of previous studies have revealed the absence of pCRP
and mCRP in healthy tissues, including blood vessels (12–14).
Certain studies have indicated that pCRP and mCRP have opposing
functions (11,15–18).
The aim of the present study was to investigate the
conformations of human CRP in humans and transgenic rats. The
present study revealed, to the best of our knowledge for the first
time, that human CRP may be naturally present in other multimeric
isoforms, the trimer and tetramer. Notably, the appearance of these
additional isoforms appears to be age-associated. The existence of
different isoforms in different tissues may facilitate
understanding of the functional impact of CRP in cardiovascular
disease. In addition, when measuring CRP in the clinic, it may be
useful to analyze the conformational structures.
Materials and methods
Ethics statement
All animal experiments were performed with the
approval of the Animal Care Committee of the Universities of
Morehouse School of Medicine (Atlanta, GA, USA), and conformed to
the Guide for the Care and Use of Laboratory Animals produced by
the National Institutes of Health (Bethesda, MD, USA). The present
study meets the ARRIVE guidelines requirements for reporting
(www.nc3rs.org.uk/arrive-guidelines). Animals were
housed in the Center for Laboratory Animal Resources of Morehouse
School of Medicine. Transgenic CRP rats were maintained under a
12-h light-dark cycle with ad libitum access to water and a
standard rat chow diet (Laboratory Rodent Diet 5001; LabDiet, St.
Louis, MO, USA). The animal room temperature was maintained at
22±3°C, relative humidity was held at 30–70%, and air was exchanged
15 times/h.
The informed consent procedure of human experiments
applied at the First Affiliated Hospital of Xi'an Jiaotong
University (Xi'an, China) was in accordance with the approved
guidelines. The present study was approved by the Institutional
Review Board of Xi'an Jiaotong University, and written informed
consent was obtained from patients.
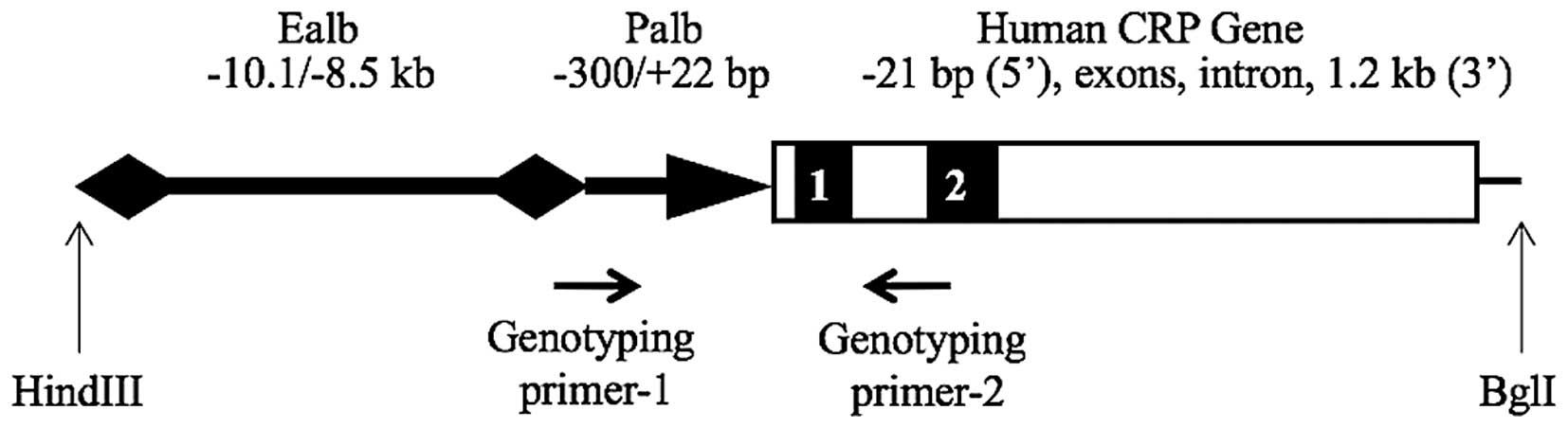
Generation and genotyping of
albumin-human CRP (ALB-hCRP) transgenic rats
A fragment containing 21 nucleotides upstream to the
transcription initiation site, exons and the intron, and 1.2 kb of
3′-flanking region of the human CRP gene, was amplified from human
genomic DNA purchased from the Coriell Institute for Medical
Research (Camden, NJ, USA) and cloned into a TA cloning vector
(Invitrogen; Thermo Fisher Scientific, Inc., Waltham, MA, USA).
This fragment was then released by restriction enzymes and inserted
into a vector downstream of the mouse albumin enhancer/promoter
element. The construct was verified by restriction enzyme mapping
and DNA sequencing. The plasmid ALB-hCRP contained a 3.5-kb mouse
albumin enhancer element (−8.5 to −12 kb) and a 0.3 kb promoter
element (−300 to +22) as well as the human CRP gene. A 4.3-kb
fragment (Fig. 1) was released
from the ALB-hCRP construct by HindIII/BglI digestion, purified
with agarose gel electrophoresis and a QIAquick Polymerase Chain
Reaction (PCR) Purification kit (Qiagen, Inc., Valencia, CA, USA).
To generate transgenic founders, fertilized eggs from Sprague
Dawley (SD) rats were microinjected with the transgene fragment;
this was performed by the University of Michigan Transgenic Animal
Model Core Facility (Ann Arbor, MI, USA). Transgenic founders were
identified by genotyping with the primers presented in Fig. 1, and mated with non-transgenic rats
to produce F1 progeny (19,20).
Rats were anesthetized with isoflurane (Laser Animal Health,
Sydney, Australia) and a 0.2-cm tail biopsy was excised and
collected. Genomic DNA was extracted from the tail biopsies with
DNeasy Blood & Tissue Kit (catalog no. 69506; Qiagen GmbH,
Hilden, Germany) and PCR was performed in 20-µl reactions,
containing 5 ng genomic DNA, 5 µM each primer (forward,
5′-ACATACGCAAGGGATTTAGTC-3′; reverse, 5′-AACAGCTTCTCCATGGTCAC-3′),
5 mM dNTP, 2 µl 10X PCR buffer and 1 unit Taq polymerase
(catalog no. 180384042; Thermo Fisher Scientific, Inc.).
Amplification was performed for 35 cycles of 94°C for 30 sec, 55°C
for 30 sec and 72°C for 1 min, using a GeneAmp® PCR
System 9600 (Applied Biosystems; Thermo Fisher Scientific, Inc.).
PCR products were then visualized on 1% agarose gels following
ethidium bromide staining.
Samples
Rats were anesthetized using isoflurane. Blood
samples were collected from anesthetized transgenic rats and three
human patients who volunteered for this research from the Inpatient
Cardiology Department of the First Affiliated Hospital of Xi'an
Jiaotong University (Table I) in
10-ml tubes without anticoagulant. Samples were allowed to clot for
1 h at room temperature, and then centrifuged at 2,220 × g at 4°C
for 10 min to separate the serum. All 12 rats were sacrificed with
70% CO2 following anesthesia at the same time at 9, 14
and 37 weeks of age, as the 9- and 37-week-old rats belonged to
separate generations. Liver, pancreas, kidney, aorta, heart and
muscle were collected from transgenic rats. Harvested tissues (30
mg) were snap frozen in liquid nitrogen and homogenized with a
Teflon glass homogenizer in extraction medium (protease inhibitor
cocktail (catalog no. P8340-5; Sigma-Aldrich; Merck Millipore,
Darmstadt, Germany) diluted 1:30 in tissue protein extraction
reagent (catalog no. 78510, Thermo Fisher Scientific, Inc.).
Following centrifugation at 13,200 × g at 4°C for 10 min, an
aliquot of the supernatant was used to determine the protein
concentrations using the bicinchoninic acid assay and a
BioPhotometer (Eppendorf, Hamburg, Germany).
 | Table I.The characteristics of human
participants. |
Table I.
The characteristics of human
participants.
|
| Patient |
|---|
|
|
|
|---|
| Characteristic | a | b | c |
|---|
| Gender | Male | Male | Male |
| Age (year) | 65 | 60 | 62 |
| Diagnosis | Myocardial
infarction | Coronary
disease | Congenital heart
disease |
| Levels of
high-sensitivity C-reactive protein (µg/ml) | 32.2 | 25.8 | 0.0 |
Enzyme-linked immunosorbent assay
(ELISA) for human CRP
The concentration of human CRP in the samples was
measured by ELISA using a commercial hsCRP ELISA kit (catalog no.
961CRP01H-96; Helica Biosystems, Inc., Santa Ana, CA, USA). Samples
were measured in duplicate, and experiments were repeated more than
three times.
Western blot analysis
Total proteins (20 µg) were denatured at 70°C for 10
min, mixed with NativePAGE Sample Buffer (Thermo Fisher Scientific,
Inc.), electrophoresed on Novex 4–12% Tris Glycine Midi Protein
Gels (Thermo Fisher Scientific, Inc.) and transferred to
polyvinylidene difluoride membranes. Membranes were blocked with 5%
milk for 1 h and incubated with anti-human CRP antibodies
overnight: Mouse anti-CRP (1:500; clone, C6; catalog no. M86284M;
Meridian Life Science, Inc., Cincinnati, OH, USA), mouse anti-CRP
(1:500; clone, C5; catalog no. M86005M; Meridian Life Science,
Inc.) and mouse anti-CRP (1:500; polyclonal; catalog no. ab52687;
Abcam, Cambridge, MA, USA). The membranes were washed 3 times with
TBST (1% serum albumin in 50 mM Tris-HCl, pH 7.4, containing 0.05%
Tween-20) at room temperature and incubated with a horseradish
peroxidase-conjugated goat anti-mouse IgG secondary antibody
(1:2,000; catalog no. sc-2005; Santa Cruz Biotechnology, Inc.,
Dallas, TX, USA) at room temperature for 1 h. Membranes were washed
three times, visualized with SuperSignal West Pico
Chemilluminescent substrate (Thermo Fisher Scientific, Inc.) for 5
min and exposed to X-ray film. Densitometry was performed using
Image J 2.1.4.7 software (National Institutes of Health).
Statistical analysis
Data are expressed as the mean ± standard deviation.
Differences between groups were analyzed using ANOVA for more than
two variables. Where ANOVA revealed a significance, post hoc
comparisons were made by means of Tukey's range test. Statistical
analysis was performed using SAS 9.3 software; SAS Institute, Inc.,
Cary, NC, USA. P<0.05 was considered to indicate a statistically
significant difference.
Results
Characterization of transgenic
rats
A total of nine founders were identified. Transgenic
founders were bred with wild-type SD rats to establish transgenic
lines. The offspring of these breeding pairs containing the
ALB-hCRP transgene were identified by PCR using primers presented
in Fig. 1 and genomic DNA obtained
from tail biopsies. In addition, human hsCRP ELISA was performed on
the founders to identify the transgenic lines with detectable
levels of human CRP in the blood (Fig.
2). Lines were selected for use in subsequent experiments on
the basis of this ELISA. Male heterozygous offspring were included
in the present study.
Multimeric isoforms of human CRP in
the blood of transgenic rats
Blood was collected from the tail veins of 11 human
CRP transgenic rats from the 4 independent transgenic founder
lines. In the non-reducing western blot analysis it was observed
that CRP was detected as a series of bands, rather than as a
pentamer only; in addition to bands the size of pCRP, three
additional bands were observed, with sizes consistent with
tetrameric, trimeric and dimeric CRP (Fig. 3A). The CRP band patterns were
reproducible using different CRP-specific antibodies (Fig. 3B) and were observed to be identical
between serum and plasma (Fig.
3C). mCRP was not observed in these experiments (Fig. 3). Notably, the appearance of the
trimer and tetramer appeared to be associated with age, as these
non-traditional multimeric isoforms were observed only in the 14-
and 37-week-old rats, and not in the 9-week-old rats (Fig. 3). The fractional composition of
different CRP multimeric isoforms is presented for each age group
(Fig. 3A and Table II). To examine the
cross-reactivity of antibodies for human and rat CRP, a 38-week-old
wild-type control was included (lane 12, Fig. 3A), in which only the pentamer and
dimer were observed.
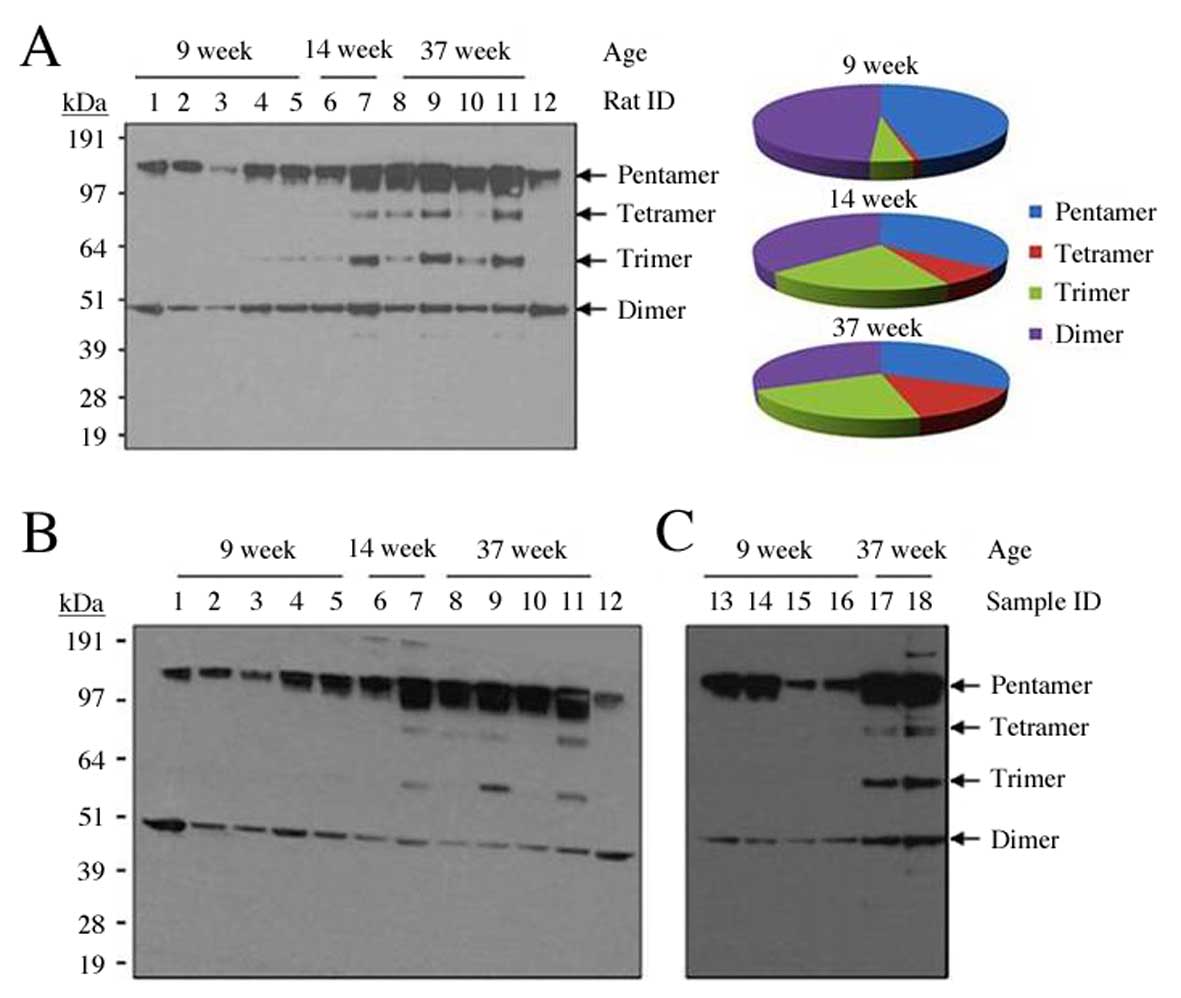 | Figure 3.Detection of multimeric isoforms of
CRP in the serum of transgenic rats at various ages, using 3
different anti-human CRP antibodies. (A) Detection of human CRP
isoforms by non-reducing western blotting with anti-human CRP
monoclonal antibody, catalog no. M86284M (Meridian Life Sciences,
Inc.), and fractional composition of CRP multimeric isoforms for
each age group following densitometric analysis. (B) The membrane
was then stripped and re-blotted with anti-human CRP antibody,
catalog no. ab52687 (Abcam). A total of 12 rats were analyzed, 11
transgenic rats from 4 transgenic lines and one wild-type control
littermate (rat 12) at 38 weeks old. Rats 1, 2, 5 and 11 belong to
transgenic line 519; rats 3, 4, 6, 7 and 10 belong to line 539; rat
8 belongs to line 538; rat 9 belongs to line 514. (C) Detection of
human CRP isoforms by non-reducing western blotting with anti-human
CRP antibody, catalog no. M86005M (Meridian Life Sciences, Inc), in
the plasma (samples 13, 15 and 17) and serum (samples 14, 16 and
18) of three transgenic rats (line 519, F1 offspring). Samples 13,
14, 15 and 16 were from two 9-week old transgenic rats, whereas
samples 17 and 18 were from a 37-week old transgenic rat. CRP,
C-reactive protein. |
 | Table II.Human C-reactive protein
concentrations in the serum of transgenic rats at various ages,
quantified from western blot analysis using the M86284M antibody
(Meridian Life Sciences, Inc.) and analyzed using Image J
software. |
Table II.
Human C-reactive protein
concentrations in the serum of transgenic rats at various ages,
quantified from western blot analysis using the M86284M antibody
(Meridian Life Sciences, Inc.) and analyzed using Image J
software.
|
| Mean ± standard
deviation | P-value |
|---|
|
|
|
|
|---|
| Isoform | 9 week | 14 week | 37 week | 9 vs. 14 week | 9 vs. 37 week | 14 vs. 37 week |
|---|
| Pentamer | 77.49±9.34 | 84.88±4.01 | 89.30±8.97 | 0.855 | 0.555 | 0.948 |
| Tetramer | 1.38±0.04 | 18.35±2.56 | 40.65±9.54 | 0.474 | 0.019 | 0.319 |
| Trimer | 7.83±1.29 | 50.62±8.10 | 63.57±12.30 | 0.143 | 0.020 | 0.811 |
| Dimer | 82.91±4.51 | 88.97±6.43 | 90.02±5.39 | 0.4999 | 0.256 | 0.979 |
To assess the specificity of the CRP-specific
antibodies used in the western blotting experiments, three
antibodies were purchased from two companies and compared using
serum and plasma from transgenic rats (Fig. 3). The results were identical with
different antibodies. M86284M (Meridian Life Science, Inc.) and
ab52687 (Abcam) cross-reacted with the rat endogenous CRP, which
revealed only pentamer and dimer in the blood of the wild-type rat
(Lane 12, Fig. 3A and B). The
bands of tetramer and trimer were unique in transgenic rats
compared with the wild-type rats, indicating that these two
multimeric isoforms were specifically derived from transgenic rats.
All three anti-human CRP antibodies used in western blotting
consistently detected trimers and tetramers in the transgenic rats
(Fig. 3). This multimeric
phenomenon was observed in all three CRP transgenic lines (Fig. 3).
Multimeric isoforms of human CRP in
various tissues of transgenic rats
In addition to serum and plasma, six tissues of the
ALB-hCRP transgenic rats were analyzed for human CRP protein
expression levels: Aorta, liver, kidney, pancreas, heart and
skeletal muscle. mCRP was not observed in the tissues, as for the
blood. Similar to the observations in blood, CRP was present in a
series of bands in all tissues analyzed using non-reducing western
blotting (Fig. 4A). Notably, the
appearance of trimers and tetramers again appeared age-associated,
as these non-traditional multimeric isoforms were observed only in
the 37-week-old rats and not in the 9-week-old rats. It is possible
that the inter-subunit disulfide bond may give rise to additional
oligomeric bands (21).
Furthermore, the additional bands may represent a homogenous
protein complex formed by dissociated CRP subunits or a
heterogenous protein complex formed by dissociated CRP subunits and
other plasma or tissue proteins (22,23).
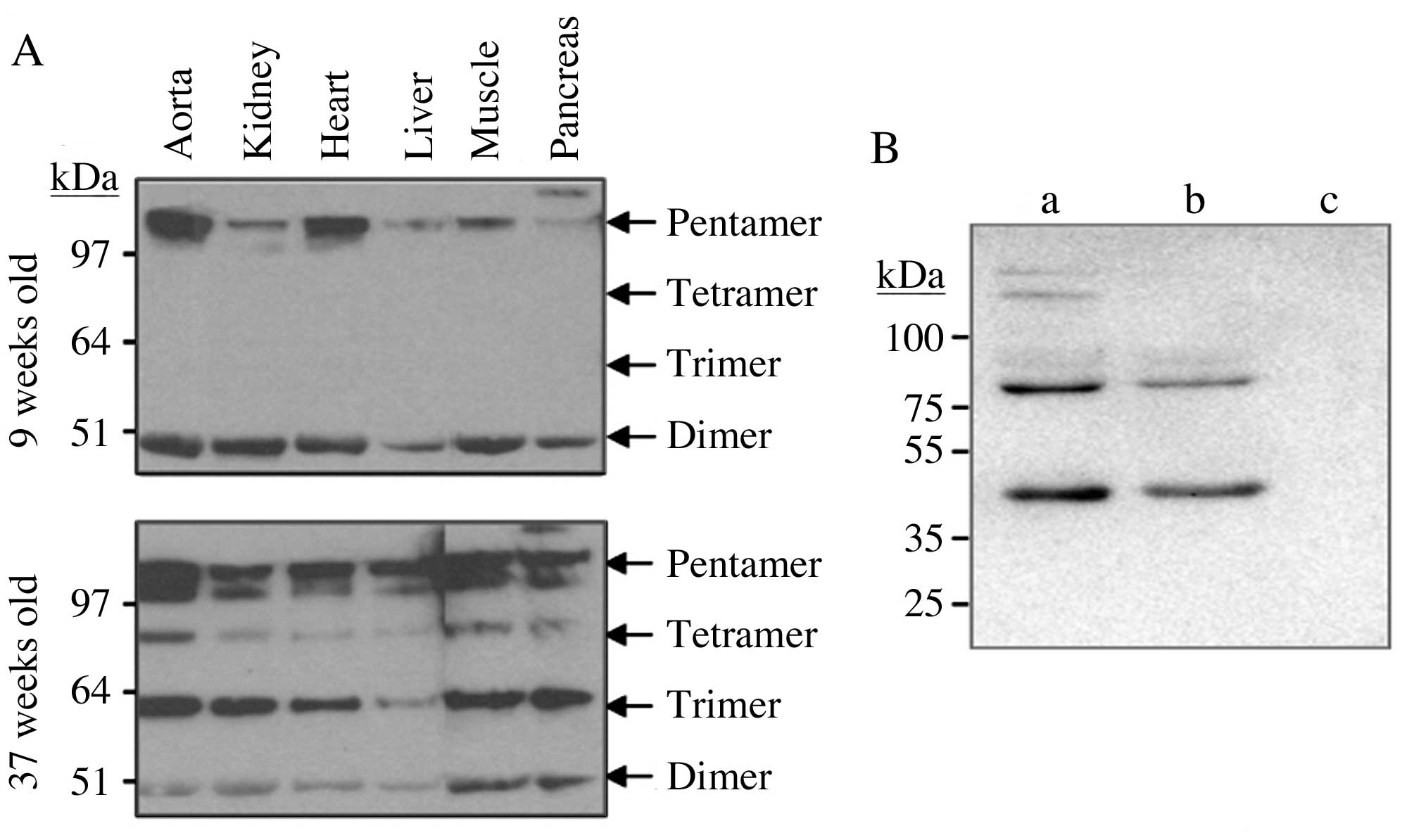 | Figure 4.Multimeric isoforms of CRP in various
tissues of transgenic rats and in human blood. (A) Human CRP
isoforms were analyzed in the tissues of transgenic rats by
non-reducing western blotting with an anti-human CRP monoclonal
antibody (M86284M, Meridian Life Sciences, Inc.). The tissues were
perfused to eliminate blood prior to processing. As for blood,
dimer, trimer, tetramer and pentamer isoforms were detected in all
six tissues, with the trimer and tetramer isoforms appearing only
in the 37-week-old rats. (B) Three human subjects were recruited,
among which individuals a and b were patients with coronary heart
disease and individual c was a male with congenital heart disease
who was recruited as a control. Non-reducing western blotting was
performed using the M86284M monoclonal antibody (Meridian Life
Sciences, Inc.). CRP was detected in pentamer, tetramer and dimer
isoforms in patients a and b, and was undetectable in individual c.
CRP, C-reactive protein. |
Multimeric isoforms of CRP in the
blood of human patients
Three human patients were recruited, among whom,
individuals a and b were patients with coronary heart disease, and
individual c was a patient with congenital heart disease as
control. A hsCRP ELISA was performed prior to western blotting
analysis. The CRP levels in the blood were 32.2 µg/ml in patient a,
25.8 µg/ml in patient b and undetectable in individual c.
Non-reducing western blotting was performed using the M86284M
monoclonal antibody (Meridian Life Sciences, Inc.). CRP was
detected in pentamer, tetramer and dimer isoforms (Fig. 4B).
Discussion
The present study demonstrated that CRP may be
present in various conformational isoforms in addition to
pentamers. As the conformation may be critically associated with
the functionality of CRP (12,15,16,24,25)
the presence of these isoforms in various tissues indicted that CRP
conformation should be considered when analyzing the pathological
roles of CRP in cardiovascular disease and when using hsCRP as a
marker for clinical diagnosis.
There are various possibilities that may result in
the differences in the detection of CRP trimers and tetramers
between young and older rats. First, the detection of additional
bands in older rats may be due to a greater concentration of CRP,
which may enable the detection of other isoforms besides the
pentamer. Second, older rats may have certain physiological
conditions that alter the aggregation of mCRP or the degradation of
pCRP. For example, calcium and sodium concentration may be involved
in CRP aggregation (8,26–30),
and has been associated with diseases of the elderly. It is
estimated that almost 60% of dietary calcium is absorbed during
childhood and early adulthood; beyond 35 years old, the absorption
rate typically decreases to 20%. The possibility that pCRP
dissociated in non-reducing gels during the western blotting
procedure performed in the present study has been eliminated by
using extreme denaturing conditions in denaturing gel western
blotting, in which trimers were still observed (data not shown).
The local calcium concentration in the gel during electrophoresis
is unknown. However, even if calcium dictates the formation of the
novel multimeric isoforms of CRP, as calcium levels vary in human
patients and among different tissues, the discovery of other CPR
isoforms may be important. The mechanisms underlying the
age-associated appearance of CRP trimers and tetramers remain to be
elucidated.
Conformation of CRP may be critically associated
with its function. Previous studies have demonstrated that mCRP and
pCRP have opposing functions: mCRP induced interleukin-8 secretion
by neutrophils (24) and human
coronary artery endothelial cells (16), increased neutrophil-endothelial
cell adhesion (25) and inhibited
neutrophil apoptosis (31). By
contrast, native pentameric CRP failed to affect neutrophil
apoptosis (31). The accumulation
of mCRP but not pCRP has been reported in human atherosclerosis
(12). Therefore, mCRP may be the
active isoform that contributes to atherogenesis by modulating
monocyte behavior (32). The
functional roles of trimeric and tetrameric CRP in different
tissues remain unknown. It has been recently reported that CRP may
be present in isoforms greater than pentamers (33). Taken together, these results
suggest that CRP may exist in multiple conformational isoforms
beyond the well-defined pentamers and monomers.
The association between increased concentrations of
hsCRP and future cardiovascular events has been well established.
The current ELISA-based hsCRP assays distinguish pentameric
molecules from other multimeric CRP isoforms; furthermore, these
serum-based ELISA assays do not provide information on CRP
concentration and conformation in other tissues. At the
transcriptional and protein levels, CRP is expressed at varying
levels in different human tissues (13,34–36).
The locally-produced CRP within tissues may exert its functions
locally or systemically via the bloodstream. The recently
identified CRP promoter mutation in cancers with enhanced CRP
expression may support the functional importance of CRP in
regulating local inflammation (37). It may be useful to determine
whether the CRP levels and conformation within tissues is important
in the pathogenesis of diseases; however, it is unclear if a
polymorphism exists regarding CRP conformation in humans, and if
the CRP conformational alterations are associated with disease
pathogenesis and diagnosis.
In conclusion, the results of the present study
suggested that CRP may exist in multiple multimeric isoforms. These
results indicated that it may be beneficial to investigate CRP
conformation, in addition to the CRP concentration currently
measured as hsCRP in the clinic.
Glossary
Abbreviations
Abbreviations:
|
CRP
|
C-reactive protein
|
|
ELISA
|
enzyme-linked immunosorbent assay
|
|
hsCRP
|
high-sensitivity C-reactive
protein
|
|
pCRP
|
pentameric C-reactive protein
|
|
mCRP
|
monomeric C-reactive protein
|
References
|
1
|
Nakou ES, Liberopoulos EN, Milionis HJ and
Elisaf MS: The role of C-reactive protein in atherosclerotic
cardiovascular disease: An overview. Curr Vasc Pharmacol.
6:258–270. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Bansal T, Pandey A, Deepa D and Asthana
AK: C-reactive proten (CRP) and its association with periodontal
disease: A brief review. J Clin Diagn Res. 8:ZE21–ZE24.
2014.PubMed/NCBI
|
|
3
|
Kones R: Rosuvastatin, inflammation,
C-reactive protein, JUPITER, and primary prevention of
cardiovascular disease-a perspective. Drug Des Devel Ther.
4:383–413. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Yeh ET and Willerson JT: Coming of age of
C-reactive protein: Using inflammation markers in cardiology.
Circulation. 107:370–371. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Li Q, Kang T, Tian X, Ma Y, Li M, Richards
J, Bythwood T, Wang Y, Li X, Liu D, et al: Multimeric stability of
human C-reactive protein in archived specimens. PLoS One.
8:e580942013. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Kilpatrick JM and Volanakis JE: Molecular
genetics, structure, and function of C-reactive protein. Immunol
Res. 10:43–53. 1991. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Bodman-Smith KB, Melendez AJ, Campbell I,
Harrison PT, Allen JM and Raynes JG: C-reactive protein-mediated
phagocytosis and phospholipase D signalling through the
high-affinity receptor for immunoglobulin G (FcgammaRI).
Immunology. 107:252–260. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Okemefuna AI, Nan R, Miller A, Gor J and
Perkins SJ: Complement factor H binds at two independent sites to
C-reactive protein in acute phase concentrations. J Biol Chem.
285:1053–1065. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Peisajovich A, Marnell L, Mold C and Du
Clos TW: C-reactive protein at the interface between innate
immunity and inflammation. Expert Rev Clin Immunol. 4:379–390.
2008. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Diehl EE, Haines GK III, Radosevich JA and
Potempa LA: Immunohistochemical localization of modified C-reactive
protein antigen in normal vascular tissue. Am J Med Sci. 319:79–83.
2000. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Molins B, Peña E, Vilahur G, Mendieta C,
Slevin M and Badimon L: C-reactive protein isoforms differ in their
effects on thrombus growth. Arterioscler Thromb Vasc Biol.
28:2239–2246. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Eisenhardt SU, Habersberger J, Murphy A,
Chen YC, Woollard KJ, Bassler N, Qian H, von Zur Muhlen C,
Hagemeyer CE, Ahrens I, et al: Dissociation of pentameric to
monomeric C-reactive protein on activated platelets localizes
inflammation to atherosclerotic plaques. Circ Res. 105:128–137.
2009. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Ji SR, Ma L, Bai CJ, Shi JM, Li HY,
Potempa LA, Filep JG, Zhao J and Wu Y: Monomeric C-reactive protein
activates endothelial cells via interaction with lipid raft
microdomains. FASEB J. 23:1806–1816. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Mihlan M, Blom AM, Kupreishvili K, Lauer
N, Stelzner K, Bergström F, Niessen HW and Zipfel PF: Monomeric
C-reactive protein modulates classic complement activation on
necrotic cells. FASEB J. 25:4198–4210. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Khreiss T, József L, Potempa LA and Filep
JG: Opposing effects of C-reactive protein isoforms on
shear-induced neutrophil-platelet adhesion and neutrophil
aggregation in whole blood. Circulation. 110:2713–2720. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Khreiss T, József L, Potempa LA and Filep
JG: Conformational rearrangement in C-reactive protein is required
for proinflammatory actions on human endothelial cells.
Circulation. 109:2016–2022. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Potempa LA, Zeller JM, Fiedel BA,
Kinoshita CM and Gewurz H: Stimulation of human neutrophils,
monocytes, and platelets by modified C-reactive protein (CRP)
expressing a neoantigenic specificity. Inflammation. 12:391–405.
1988. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Schwedler SB, Amann K, Wernicke K, Krebs
A, Nauck M, Wanner C, Potempa LA and Galle J: Native C-reactive
protein increases whereas modified C-reactive protein reduces
atherosclerosis in apolipoprotein E-knockout mice. Circulation.
112:1016–1023. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Li Q, Ma Y, Li W, Xu W, Ma L, Fu G, Tian
X, Wang Y, Li X, Bythwood T, et al: A promoter that drives gene
expression preferentially in male transgenic rats. Transgenic Res.
23:341–349. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Li Q, Xu W, Cui Y, Ma L, Richards J, Li W,
Ma Y, Fu G, Bythwood T, Wang Y, et al: A prelimininary exploration
on DNA methylation of transgene across generations in transgenic
rats. Sci Rep. 5:82922015. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Baltz ML, de Beer FC, Feinstein A, Munn
EA, Milstein CP, Fletcher TC, March JF, Taylor J, Bruton C, Clamp
JR, et al: Phylogenetic aspects of C-reactive protein and related
proteins. Ann N Y Acad Sci. 389:49–75. 1982. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Hammond DJ Jr, Singh SK, Thompson JA,
Beeler BW, Rusiñol AE, Pangburn MK, Potempa LA and Agrawal A:
Identification of acidic pH-dependent ligands of pentameric
C-reactive protein. J Biol Chem. 285:36235–36244. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Wang MY, Ji SR, Bai CJ, El Kebir D, Li HY,
Shi JM, Zhu W, Costantino S, Zhou HH, Potempa LA, et al: A redox
switch in C-reactive protein modulates activation of endothelial
cells. FASEB J. 25:3186–3196. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Khreiss T, József L, Potempa LA and Filep
JG: Loss of pentameric symmetry in C-reactive protein induces
interleukin-8 secretion through peroxynitrite signaling in human
neutrophils. Circ Res. 97:690–697. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Zouki C, Haas B, Chan JS, Potempa LA and
Filep JG: Loss of pentameric symmetry of C-reactive protein is
associated with promotion of neutrophil-endothelial cell adhesion.
J Immunol. 167:5355–5361. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Motie M, Brockmeier S and Potempa LA:
Binding of model soluble immune complexes to modified C-reactive
protein. J Immunol. 156:4435–4441. 1996.PubMed/NCBI
|
|
27
|
Wu Y, Ji SR, Wang HW and Sui SF: Study of
the spontaneous dissociation of rabbit C-reactive protein.
Biochemistry (Mosc). 67:1377–1382. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Ji SR, Wu Y, Zhu L, Potempa LA, Sheng FL,
Lu W and Zhao J: Cell membranes and liposomes dissociate C-reactive
protein (CRP) to form a new, biologically active structural
intermediate: mCRP(m). FASEB J. 21:284–294. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Kresl JJ, Potempa LA and Anderson BE:
Conversion of native oligomeric to a modified monomeric form of
human C-reactive protein. Int J Biochem Cell Biol. 30:1415–1426.
1998. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Okemefuna AI, Stach L, Rana S, Buetas AJ,
Gor J and Perkins SJ: C-reactive protein exists in an NaCl
concentration-dependent pentamer-decamer equilibrium in
physiological buffer. J Biol Chem. 285:1041–1052. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Khreiss T, József L, Hossain S, Chan JS,
Potempa LA and Filep JG: Loss of pentameric symmetry of C-reactive
protein is associated with delayed apoptosis of human neutrophils.
J Biol Chem. 277:40775–40781. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Zhao J and Shi XH: Study of the
interaction of the C-reactive protein monomer with the U937
monocyte. Cell Mol Biol Lett. 15:485–495. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Asztalos BF, Horan MS, Horvath KV,
McDermott AY, Chalasani NP and Schaefer EJ: Obesity associated
molecular forms of C-reactive protein in human. PLoS One.
9:e1092382014. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Beri A, Contractor T, Khasnis A and Thakur
R: Statins and the reduction of sudden cardiac death:
Antiarrhythmic or anti-ischemic effect? Am J Cardiovasc Drugs.
10:155–164. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Juma WM, Lira A, Marzuk A, Marzuk Z, Hakim
AM and Thompson CS: C-reactive protein expression in a rodent model
of chronic cerebral hypoperfusion. Brain Res. 1414:85–93. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Peyrin-Biroulet L, Gonzalez F, Dubuquoy L,
Rousseaux C, Dubuquoy C, Decourcelle C, Saudemont A, Tachon M,
Béclin E, Odou MF, et al: Mesenteric fat as a source of C reactive
protein and as a target for bacterial translocation in Crohn's
disease. Gut. 61:78–85. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Wang MY, Zhou HH, Zhang SC, Hui F, Zhu W,
Su HX, Guo HY, Li XW, Ji SR and Wu Y: Recurrent mutations at
C-reactive protein gene promoter SNP position −286 in human
cancers. Cell Res. 24:505–508. 2014. View Article : Google Scholar : PubMed/NCBI
|