Introduction
Adolescent idiopathic scoliosis (AIS) is
characterized by deformity of the spine, which develops without a
known cause, predominantly in previously healthy individuals during
the adolescent growth spurt (1,2). It
affects between 2 and 4% of the global population, and is more
prevalent in girls (gender ratio, 8:1) (3–5).
Since there is currently no early preventive treatment available,
and a significant proportion of patients with AIS require intensive
brace therapy or invasive surgical correction (2,6,7),
improved understanding regarding the etiopathogenesis of AIS is
required. Several suggestions have been proposed regarding the
etiology of AIS, including neuromuscular, genetic, mechanical,
growth-related or developmental hypotheses; however, at present, no
single key factor has been identified (8,9).
The potential role of local changes to deep
paravertebral muscles in the development of AIS has been the
subject of several analyses. Numerous histochemical studies have
reported significant differences in fiber size and the proportion
of muscle fiber types between the convex and concave sides of the
scoliotic curve (10–12). Therefore, muscle imbalance and
asymmetry of paravertebral muscles in patients with AIS may be
considered to serve an important role in the development of spinal
deformity (10–13).
The asymmetric expression of several molecules in
bilateral paravertebral muscles has also been suggested to be
involved in the pathogenesis of AIS. Among the various molecular
hypotheses, melatonin deficiency (14,15)
and dysfunctional melatonin signaling (16,17)
have received major attention. The expression of melatonin
receptors 1A/1B (MTNR1A/MTNR1B; also referred as MT1 and MT2
receptors) in paravertebral muscles was previously investigated by
Qiu et al (18), and MTNR1B
expression was shown to be asymmetric. At the protein level,
Acaroglu et al (19)
demonstrated an asymmetric distribution of calmodulin (CALM1) in
the paravertebral muscles of patients with AIS. This finding is of
particular interest, since CALM1 not only regulates the contractile
properties of muscle fibers by regulating calcium transport through
the cellular membranes (20), but
also acts as a neurotransmitter in the regulation of melatonin
secretion (21). Crosstalk between
estrogens and the melatonin signaling pathway has previously been
reported (22), and the existence
of an anomaly specific to the estrogen system has been suggested in
patients with scoliosis (23). In
addition, the expression levels of estrogen receptor 2 (ESR2) have
previously been investigated in the paravertebral muscles of
patients with AIS; the results indicated that ESR2 exhibited
asymmetrical expression, albeit not unidirectional (24).
Although the hypothesis that asymmetric molecular
alterations to the melatonin signaling pathway in paravertebral
muscles are associated with AIS appears promising, it is based on
single studies in which data were obtained by different
methodological approaches. In order to verify and provide more
evidence for this hypothesis, the present study examined the
relative mRNA expression levels of MTNR1A, MTNR1B, ESR2 and CALM1
in deep paravertebral muscles from patients with AIS using reverse
transcription-quantitative polymerase chain reaction (RT-qPCR).
Materials and methods
Patient characteristics
The present study recruited 18 patients with AIS and
10 control subjects in University Hospital Kralovske Vinohrady
(Prague, Czech Republic) between March 2012 and April 2015. The AIS
group consisted of 15 female and 3 male patients that were
undergoing posterior instrumentation and fusion for treatment of
AIS. The mean age was 17.2±5.5 years (range, 11–31 years), and all
patients had relatively severe spinal curves, with a mean Cobb
angle measurement of 50.5°±8.7° (range, 31°-70°). All but one
subject had thoracic convexity to the right. The control group
comprised 6 female and 4 male patients without scoliosis (including
4 cases of thoracolumbar trauma with burst fractures, 4 disc
herniations and 2 cases of degenerative stenosis) undergoing
posterior surgery. The mean age was 32.7±11.9 years (range, 12–55
years). Written informed consent was obtained from each
patient/guardian prior to the enrollment. The present study was
approved by the ethics committee of the 3rd Faculty of Medicine,
Charles University and University Hospital Kralovske Vinohrady.
Muscle biopsy
Muscle biopsies (size, ~10×5×5 mm) were obtained
during surgery. The biopsies were taken bilaterally from the
multifidus muscle at the apex of the scoliotic curve between the
9th and 12th thoracic vertebral levels in patients with AIS, and
bilaterally from the same region in individuals of the control
group.
Muscle tissues were snap-frozen in isopentane
(2-methylbutane; Sigma-Aldrich; Merck Millipore, Darmstadt,
Germany) and were maintained in liquid nitrogen. From each tissue
block, 3 µm cryosections were examined by routine hematoxylin-eosin
staining to ensure the quality and the relevance of the harvested
tissue. Muscle samples were subsequently stored at −80°C.
RNA extraction and preparation of cDNA
by RT
Total RNA was isolated from cryosections of the
samples using TRIzol® reagent (Invitrogen; Thermo Fisher
Scientific, Inc., Waltham, MA, USA) according to the manufacturer's
protocol. cDNA was prepared by RT from 10 µl RNA in a 20 µl
reaction volume. The reaction mixture consisted of 50 mM Tris-HCl,
pH 8.3; 75 mM KCl; 3 mM MgCl2; 10 mM dithiotreitol; 0.5
mM each dNTP; 12.5 mM random hexamers and 50 units MMLV Reverse
Transcriptase (Gibco; Thermo Fisher Scientific, Inc.) according to
the manufacturer's protocol. RT included an incubation period at
37°C for 60 min.
RT-qPCR
RT-qPCR analyses were performed using
LightCycler® 480 Instrument II (Roche Diagnostics
Deutschland GmbH, Mannheim, Germany). RT-qPCR analysis of the
housekeeping gene β2-microglobulin was conducted using a
hydrolyzation probe in order to evaluate the amount and
amplificability of cDNA. The primers were designed as reported by
Bijwaard et al (25) and
are presented in Table I.
 | Table I.Primers and probes used in the
present study, including those used for confirmatory analysis of
melatonin receptor gene expression (Metabion International AG,
Planegg, Germany). |
Table I.
Primers and probes used in the
present study, including those used for confirmatory analysis of
melatonin receptor gene expression (Metabion International AG,
Planegg, Germany).
| Gene | Sequences |
|---|
|
β2-microglobulin | F:
5′TGACTTTGTCACAGCCCAAGATA3′ |
|
| R:
5′AATCCAAATGCGGCATCTTC3′ |
|
| Probe:
5′TGATGCTGCTTACATGTCTCGATCCCA3′ |
| Confirmatory
analysis |
|
| MTNR1A | F:
5′-CGGTGTATCGGAACAAGAAGCT |
|
| R:
5′-AGGTCTGCCACCGCTAAGC |
|
| TaqMan probe:
5′-6-Fam-TCACCACAAAGATGTTTCCT |
|
|
GCGTTCCT-Tamra-3′ |
| MTNR1B | F:
5′-CGGAACGCAGGTAATTTGTT |
|
| R:
5′-TAATGGCGATGGCAGTGATA |
Relative mRNA expression levels of CALM1 (id. Hs
003000085_s1), ESR2 (id. Hs 01100353_m1), MTNR1A (id. Hs
00195567_m1) and MTNR1B (id. Hs 001737947_m1) were evaluated by
qPCR using TaqMan Master Mix II, and primers and probes contained
in TaqMan assays (cat. no. 4331182; Applied Biosystems; Thermo
Fisher Scientific, Inc.), according to the manufacturer's protocol,
including the thermocycling conditions.
For confirmation of the observed low or absent
expression levels of melatonin receptors, the results of the TaqMan
assays were re-analyzed using another set of primers and a TaqMan
probe for MTNR1A (26) using
TaqMan Master Mix II, and a set of primers with SYBR Green
detection [Go Taq® qPCR Master Mix (Promega Corporation,
Madison, WI, USA)] for MTNR1B (27) (Table
I). The program for confirmatory assay for MTNR1B consisted of
initial denaturation at 95°C for 3 min, followed by 55 cycles at
95°C for 20 sec, and 60°C for 60 sec (single fluorescence
measurement) followed by melting curves analysis at 95°C for 15 sec
and 65°C for 10 sec and 97°C (continuous fluorescence measurement;
ramping rate, 0.1) Four archive samples of invasive ductal breast
carcinoma were obtained from the archives of the Department of
Pathology and Molecular Medicine, University Hospital Motol
(Prague, Czech Republic) with known expression of both melatonin
receptors (28) were co-analyzed
by both methods, in order to ensure functionality of the TaqMan
assays and/or primers and probes used in the present study.
All analyses were performed in duplicate and the
mean values were obtained for further analysis. Representative
amplification curves obtained for the genes of interest are
presented in Fig. 1.
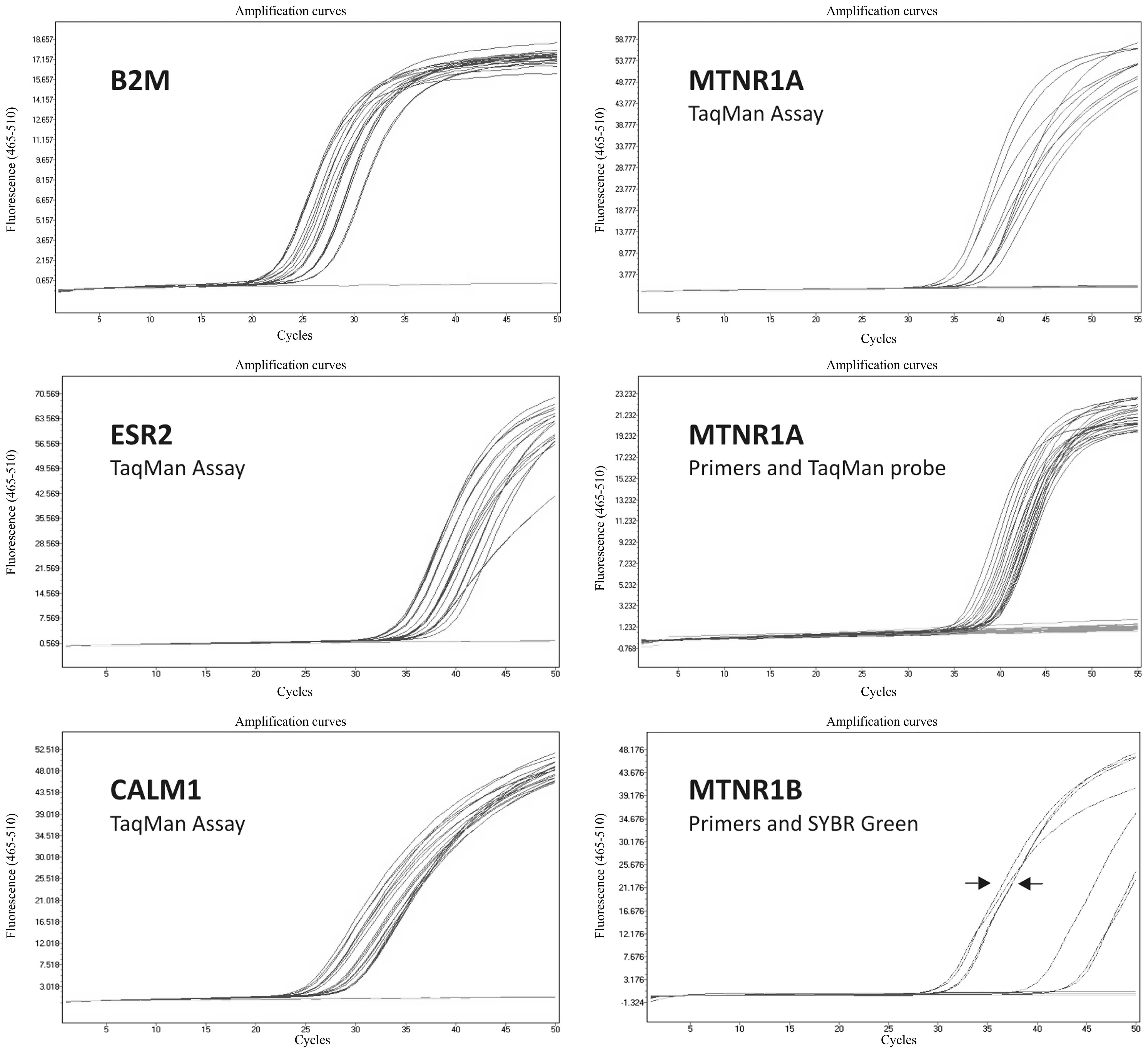 | Figure 1.Representative RT-qPCR amplification
plots in semi-logarithmic view for B2M housekeeping gene, and for
ESR2, CALM1 and MTNR1A using TaqMan assays. Furthermore, the
results of the confirmatory analysis of MTNR1A expression using
primers and a TaqMan probe, and of MTNR1B expression using primers
and SYBR Green are presented. Arrows represent cases of invasive
ductal breast carcinoma with known MTNR1B mRNA expression (Cq
values around the 30th cycle, compared with the studied samples
where Cq values are around the 40th cycle or are completely
negative). X-axes indicate RT-qPCR cycle number, Y-axes indicate
fluorescence intensity. RT-qPCR, reverse transcription-quantitative
polymerase chain reaction; B2M, β2-microglobulin; ESR2, estrogen
receptor 2; CALM1, calmodulin; MTNR1A, melatonin receptor 1A;
MTNR1B, melatonin receptor 1B. |
Evaluation of RT-qPCR results
The mRNA expression levels of CALM1, ESR2, MTNR1A
and MTNR1B were calculated according to the ∆Cq relative
quantification method (29,30),
which is based on the expression levels of a target gene versus the
reference housekeeping gene. Briefly, fluorescence was measured
continually during the melting curve cycle and a melting curve
analysis was performed. Data were analyzed using
LightCycler® 480 Software, version 1.5 (Roche
Diagnostics Deutschland GmbH). Results were obtained as
quantification cycle (Cq) values. The software determines a
threshold based on the baseline fluorescent signal, and the data
point that meets the threshold is considered the Cq value, which is
inversely proportional to the starting number of template copies.
The average Cq value for β2-microglobulin was subtracted from the
average Cq value of the gene of interest to yield the ΔCq
value.
Statistical analysis
Results are presented as the mean ± standard
deviation. Differences between the numeric variables of two groups
were evaluated using the Mann-Whitney U test. Correlations between
numeric variables were assessed according to the Spearman's rank
correlation analysis. Statistical analysis was considered using JMP
IN 5.1 software (SAS Institute, Cary, NC, USA). P<0.05 was
considered to indicate a statistically significant difference.
Results
mRNA expression of CALM1 and ESR2
The results of the RT-qPCR analysis of the mRNA
expression levels of CALM1 and ESR2 in the specific subgroups are
summarized in Table II. For mRNA
expression of both CALM1 and ESR2 no statistically significant
differences were observed when comparing the AIS and control groups
(P=0.09 and 0.47, respectively), as well as when comparing the
concave and convex samples from the patients with AIS AIS (P=0.53
and 0.75, respectively). In addition, no statistically significant
correlation was observed between the value of the Cobb angle and
the mRNA expression levels of the studied genes (data not
shown).
 | Table II.Results of the reverse
transcription-quantitative polymerase chain reaction analysis of
the relative mRNA expression levels (ΔCq) of CALM1 and ESR2 in the
subgroups of patients with AIS and control individuals. |
Table II.
Results of the reverse
transcription-quantitative polymerase chain reaction analysis of
the relative mRNA expression levels (ΔCq) of CALM1 and ESR2 in the
subgroups of patients with AIS and control individuals.
| Group | n | ΔCq CALM1 | ΔCq ESR2 |
|---|
| AIS | 36 | 3.69±0.70 | 13.39±1.32 |
| Convex
side | 18 | 3.79±0.53 | 12.93±1.42 |
| Concave
side | 18 | 3.60±0.82 | 13.86±1.01 |
| Control | 20 | 3.51±0.70 | 12.48±1.18 |
| Left
side | 10 | 3.50±0.73 | 12.39±1.35 |
| Right
side | 10 | 3.52±0.66 | 12.57±0.98 |
mRNA expression of MTNR1A and
MTNR1B
In general, for MTNR1A and MTNR1B, the observed mRNA
expression levels were very weak or absent in patients with AIS, as
well as in control individuals.
MTNR1A mRNA expression near the detection limit was
observed in 9/18 samples from the concave side of the curve in
patients with AIS (mean Cq value, 37.06±1.43) and in 11/18 convex
scoliosis samples (mean Cq value, 37.18±1.19). Similarly in the
control group, MTNR1A mRNA expression was weakly detectable in a
small proportion of samples (3/10 from the left and 2/10 from the
right side; mean Cq values, 38.55±0.17 and 39.77±0.55,
respectively). The remaining samples were completely negative for
MTNR1A mRNA expression. The results of the mRNA expression analysis
of MTNR1B were completely negative in both AIS and control groups,
as determined using TaqMan assay and have not been presented.
The results of the present study were confirmed by
repeating the analysis using different sets of primers and probes.
The confirmatory analysis for MNTR1A revealed similar results as
the TaqMan assay. MNTR1A mRNA expression near the detection limit
was observed in 10/18 AIS samples from the convex and concave sides
(mean Cq values, 38.10±4.50 and 37.87±2.37, respectively).
Furthermore, MNTR1A mRNA expression near the detection limit was
observed in 1/10 control samples from the left side and in 2/10
control samples from the right side (mean Cq values, 38.32 and
37.31±3.00, respectively).
Using MTNR1B primers and SYBR Green, MTNR1B mRNA
expression was detected in 10/18 concave and convex AIS samples
(mean Cq values, 46.13±3.28 and 45.21±4.56, respectively). In the
control group, mRNA expression of MTNR1B was detected in 4/10
left-sided and 2/10 right-sided samples (mean Cq values, 45.49±2.78
and 47.54±2.45, respectively). The remaining samples were
completely negative for MTNR1B expression.
The present study used two independent sensitive
detection techniques to determine the mRNA expression levels of
MTNR1A and MTNR1B. The results indicate that MTNR1A and MTNR1B
expression was absent in a marked proportion of AIS and control
samples. In the remaining samples, expression was near the
detection limit, with Cq values ranging between 37 and 47; however,
the majority of these results should also be interpreted as
negative, since Cq values >40 approach the sensitivity limits of
the RT-qPCR system (31,32).
No statistically significant differences were
revealed when comparing the mRNA expression levels of MTNR1A and
MTNR1B between the studied subgroups. In addition, no correlation
was observed between the expression levels of melatonin receptors
and the value of the Cobb angle (data not shown).
Discussion
The hypothesis that melatonin deficiency and
dysfunctional melatonin signaling have roles in the
etiopathogenesis of AIS remains controversial. Early experiments,
which revealed the development of scoliosis after pinealectomy in
chickens (33,34), and the prevention of
experimentally-induced scoliosis development following
intramuscular implantation of the pineal body into pinealectomised
chickens (33), led to the
hypothesis that defects in the neurohormonal system of the pineal
body may serve a major role in the development of AIS. Recently,
however, major concerns have been raised regarding the scientific
validity and limitations of the melatonin-deficient experimental
animal models used for studying the etiopathogenesis of AIS in
humans (35). Furthermore, the
hypothesis that melatonin deficiency is a causative factor in the
etiology of AIS has not been supported by data from several studies
in human patients with AIS, as reviewed by Girardo et al
(36).
The involvement of melatonin receptors, particularly
MTNR1B, in the development of AIS has been suggested by a few
reports published by a single center, which have reported that the
protein and mRNA expression levels of MTNR1B are significantly
reduced in cultured osteoblasts from girls with AIS compared with
controls (37,38). Furthermore, functional abnormality
of the melatonin signaling pathway, resulting in an abnormal
proliferative and differentiative response of cultured growth plate
chondrocytes (GPCs) to melatonin, alongside significantly reduced
MTNR1B mRNA expression, has been detected in AIS GPCs compared with
in controls (39). However, at
present, these associations have not been replicated and validated.
Another group investigated the paravertebral muscles in patients
with AIS, and demonstrated that the mRNA expression levels of
MTNR1B were higher on the concave side of the scoliotic curve
compared with on the convex side, as determined by endpoint PCR;
however, MTNR1A mRNA expression exhibited no significant difference
(18). The present study did not
observe significant differences in MTNR1A mRNA expression, nor in
MTNR1B expression, as determined using RT-qPCR. Notably, RT-qPCR is
a more precise method for quantitative gene expression analysis,
which displays a dynamic range compared with quantification by
endpoint PCR (40). Furthermore,
in a marked proportion of patients with AIS and control
individuals, the expression of MTNR1B was undetectable, even when
employing two different approaches, and the remaining positive
samples had Cq values that reached the sensitivity limits of the
RT-qPCR system.
The pathogenic role of melatonin receptors in AIS
has been further questioned, since no significant association has
been reported between mutations in any known melatonin-related
receptors and AIS (41). Although
an MTNR1B gene polymorphism has been revealed to be associated with
AIS in Chinese patients (42), it
was not confirmed in the Japanese population (43) nor by a recently published
meta-analysis (44).
Among other molecules involved in the melatonin
signaling pathway, the role of estrogen receptors and CALM1 have
attracted the attention of several investigators. Previous
experimental studies have demonstrated that although selective
estrogen receptor modulators, and CALM1 antagonists, do not prevent
the occurrence of scoliotic deformities, they can decrease the rate
of progression of the deformity in experimental animals (45–47).
Conversely, replication studies and a meta-analysis did not confirm
the previously suggested association between estrogen receptor gene
polymorphisms and AIS predisposition or curve severity in humans
(48–51). In the paravertebral muscles of
patients with AIS, the expression of ESR2 was investigated in a
previous study (24). The observed
asymmetry of ESR2 expression, although not unidirectional, could
not be supported by the present results. The present study observed
no significant difference in the expression of ESR2 between the
convex and concave sides of the scoliotic curve, or between
patients with AIS and non-scoliotic controls. Similar results were
observed with regards to the mRNA expression levels of CALM1 in
paravertebral muscles. Therefore, the present study provided no
support for the results of two previous studies, which reported the
asymmetric distribution of CALM1 (at the protein level) in the
paravertebral muscles of patients with AIS; one study reported that
its expression was higher at the convex side and lower at the
concave side (19), whereas the
other study indicated the opposite (52).
In conclusion, the present study does not support
the hypothesis that the expression of molecules involved in the
melatonin signaling pathway (MTNR1A, MTNR1B, ESR2 and CALM1) in the
paravertebral muscles is associated with development of AIS.
Acknowledgements
The present study was supported by a grant from the
Ministry of Health of the Czech Republic (no. IGA NT/13693-4).
References
|
1
|
Lonstein JE: Adolescent idiopathic
scoliosis. Lancet. 344:1407–1412. 1994. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Altaf F, Gibson A, Dannawi Z and Noordeen
H: Adolescent idiopathic scoliosis. BMJ. 346:f25082013. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Weinstein SL: Adolescent idiopathic
scoliosis: Prevalence and natural history. Instr Course Lect.
38:115–128. 1989.PubMed/NCBI
|
|
4
|
Brooks HL, Azen SP, Gerberg E, Brooks R
and Chan L: Scoliosis: A prospective epidemiological study. J Bone
Joint Surg Am. 57:968–972. 1975. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Rogala EJ, Drummond DS and Gurr J:
Scoliosis: Incidence and natural history. A prospective
epidemiological study. J Bone Joint Surg Am. 60:173–176. 1978.
View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Weinstein SL and Ponseti IV: Curve
progression in idiopathic scoliosis. J Bone Joint Surg Am.
65:447–455. 1983. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Weinstein SL, Dolan LA, Wright JG and
Dobbs MB: Effects of bracing in adolescents with idiopathic
scoliosis. N Engl J Med. 369:1512–1521. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Dayer R, Haumont T, Belaieff W and
Lascombes P: Idiopathic scoliosis: Etiological concepts and
hypotheses. J Child Orthop. 7:11–16. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
de Séze M and Cugy E: Pathogenesis of
idiopathic scoliosis: A review. Ann Phys Rehabil Med. 55:128–138.
2012.(In English, French). View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Meier MP, Klein MP, Krebs D, Grob D and
Müntener M: Fiber transformations in multifidus muscle of young
patients with idiopathic scoliosis. Spine (Phila Pa 1976).
22:2357–2364. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Mannion AF, Meier M, Grob D and Müntener
M: Paraspinal muscle fibre type alterations associated with
scoliosis: An old problem revisited with new evidence. Eur Spine J.
7:289–293. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Green RJ: Histochemistry and
ultrastructure of the paraspinal muscles in idiopathic scoliosis
and in control subjects. Med Lab Sci. 38:197–216. 1981.PubMed/NCBI
|
|
13
|
Ford DM, Bagnall KM, McFadden KD,
Greenhill BJ and Raso VJ: Paraspinal muscle imbalance in adolescent
idiopathic scoliosis. Spine (Phila Pa 1976). 9:373–376. 1984.
View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Sadat-Ali M, al-Habdan I and al-Othman A:
Adolescent idiopathic scoliosis. Is low melatonin a cause? Joint
Bone Spine. 67:62–64. 2000.PubMed/NCBI
|
|
15
|
Machida M, Dubousset J, Yamada T and
Kimura J: Serum melatonin levels in adolescent idiopathic scoliosis
prediction and prevention for curve progression-a prospective
study. J Pineal Res. 46:344–348. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Azeddine B, Letellier K, Wang da S,
Moldovan F and Moreau A: Molecular determinants of melatonin
signaling dysfunction in adolescent idiopathic scoliosis. Clin
Orthop Relat Res. 462:45–52. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Moreau A, Wang DS, Forget S, Azeddine B,
Angeloni D, Fraschini F, Labelle H, Poitras B, Rivard CH and
Grimard G: Melatonin signaling dysfunction in adolescent idiopathic
scoliosis. Spine (Phila Pa 1976). 29:1772–1781. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Qiu Y, Wu L, Wang B, Yu Y and Zhu Z:
Asymmetric expression of melatonin receptor mRNA in bilateral
paravertebral muscles in adolescent idiopathic scoliosis. Spine
(Phila Pa 1976). 32:667–672. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Acaroglu E, Akel I, Alanay A, Yazici M and
Marcucio R: Comparison of the melatonin and calmodulin in
paravertebral muscle and platelets of patients with or without
adolescent idiopathic scoliosis. Spine (Phila Pa 1976).
34:E659–E663. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Cheung WY: Calmodulin plays a pivotal role
in cellular regulation. Science. 207:19–27. 1980. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Xia Z and Storm DR: Calmodulin-regulated
adenylyl cyclases and neuromodulation. Curr Opin Neurobiol.
7:391–396. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Letellier K, Azeddine B, Parent S, Labelle
H, Rompré PH, Moreau A and Moldovan F: Estrogen cross-talk with the
melatonin signaling pathway in human osteoblasts derived from
adolescent idiopathic scoliosis patients. J Pineal Res. 45:383–393.
2008. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Marty-Poumarat C, Scattin L, Marpeau M, de
Loubresse C Garreau and Aegerter P: Natural history of progressive
adult scoliosis. Spine (Phila Pa 1976). 32:1227–1234; discussion
1235. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Rusin B, Kotwicki T, Głodek A,
Andrusiewicz M, Urbaniak P and Kotwicka M: Estrogen receptor 2
expression in back muscles of girls with idiopathic
scoliosis-relation to radiological parameters. Stud Health Technol
Inform. 176:59–62. 2012.PubMed/NCBI
|
|
25
|
Bijwaard KE, Aguilera NS, Monczak Y,
Trudel M, Taubenberger JK and Lichy JH: Quantitative real-time
reverse transcription-PCR assay for cyclin D1 expression: Utility
in the diagnosis of mantle cell lymphoma. Clin Chem. 47:195–201.
2001.PubMed/NCBI
|
|
26
|
Toma CD, Svoboda M, Arrich F, Ekmekcioglu
C, Assadian O and Thalhammer T: Expression of the melatonin
receptor (MT) 1 in benign and malignant human bone tumors. J Pineal
Res. 43:206–213. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Adi N, Mash DC, Ali Y, Singer C, Shehadeh
L and Papapetropoulos S: Melatonin MT1 and MT2 receptor expression
in Parkinson's disease. Med Sci Monit. 16:BR61–BR67.
2010.PubMed/NCBI
|
|
28
|
Lai L, Yuan L, Cheng Q, Dong C, Mao L and
Hill SM: Alteration of the MT1 melatonin receptor gene and its
expression in primary human breast tumors and breast cancer cell
lines. Breast Cancer Res Treat. 118:293–305. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Morse DL, Carroll D, Weberg L, Borgstrom
MC, Ranger-Moore J and Gillies RJ: Determining suitable internal
standards for mRNA quantification of increasing cancer progression
in human breast cells by real-time reverse transcriptase polymerase
chain reaction. Anal Biochem. 342:69–77. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(−Delta Delta C(T)) Method. Methods. 25:402–408. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Burns MJ and Valdivia H: Modelling the
limit of detection in real-time quantitative PCR. Eur Food Res
Technol. 226:1513–1524. 2008. View Article : Google Scholar
|
|
32
|
Bustin SA, Benes V, Garson JA, Hellemans
J, Huggett J, Kubista M, Mueller R, Nolan T, Pfaffl MW, Shipley GL,
et al: The MIQE guidelines: Minimum information for publication of
quantitative real-time PCR experiments. Clin Chem. 55:611–622.
2009. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Machida M, Dubousset J, Imamura Y, Iwaya
T, Yamada T and Kimura J: An experimental study in chickens for the
pathogenesis of idiopathic scoliosis. Spine (Phila Pa 1976).
18:1609–1615. 1993. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Dubousset J, Queneau P and Thillard M:
Experimental scoliosis induced by pineal and diencephalic lesions
in young chickens: Its relation with clinical findings. Orthop
Trans. 7:7–12. 1983.
|
|
35
|
Man GC, Wang WW, Yim AP, Wong JH, Ng TB,
Lam TP, Lee SK, Ng BK, Wang CC, Qiu Y and Cheng CY: A review of
pinealectomy-induced melatonin-deficient animal models for the
study of etiopathogenesis of adolescent idiopathic scoliosis. Int J
Mol Sci. 15:16484–16499. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Girardo M, Bettini N, Dema E and
Cervellati S: The role of melatonin in the pathogenesis of
adolescent idiopathic scoliosis (AIS). Eur Spine J. 20:(Suppl 1).
S68–S74. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Man GC, Wong JH, Wang WW, Sun GQ, Yeung
BH, Ng TB, Lee SK, Ng BK, Qiu Y and Cheng JC: Abnormal melatonin
receptor 1B expression in osteoblasts from girls with adolescent
idiopathic scoliosis. J Pineal Res. 50:395–402. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Yim AP, Yeung HY, Sun G, Lee KM, Ng TB,
Lam TP, Ng BK, Qiu Y, Moreau A and Cheng JC: Abnormal skeletal
growth in adolescent idiopathic scoliosis is associated with
abnormal quantitative expression of melatonin receptor, MT2. Int J
Mol Sci. 14:6345–6358. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Wang WW, Man GC, Wong JH, Ng TB, Lee KM,
Ng BK, Yeung HY, Qiu Y and Cheng JC: Abnormal response of the
proliferation and differentiation of growth plate chondrocytes to
melatonin in adolescent idiopathic scoliosis. Int J Mol Sci.
15:17100–17114. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Schmittgen TD, Zakrajsek BA, Mills AG,
Gorn V, Singer MJ and Reed MW: Quantitative reverse
transcription-polymerase chain reaction to study mRNA decay:
Comparison of endpoint and real-time methods. Anal Biochem.
285:194–204. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Shyy W, Wang K, Gurnett CA, Dobbs MB,
Miller NH, Wise C, Sheffield VC and Morcuende JA: Evaluation of
GPR50, hMel-1B and ROR-alpha melatonin-related receptors and the
etiology of adolescent idiopathic scoliosis. J Pediatr Orthop.
30:539–543. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Qiu XS, Tang NL, Yeung HY, Lee KM, Hung
VW, Ng BK, Ma SL, Kwok RH, Qin L, Qiu Y and Cheng JC: Melatonin
receptor 1B (MTNR1B) gene polymorphism is associated with the
occurrence of adolescent idiopathic scoliosis. Spine (Phila Pa
1976). 32:1748–1753. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Takahashi Y, Matsumoto M, Karasugi T,
Watanabe K, Chiba K, Kawakami N, Tsuji T, Uno K, Suzuki T, Ito M,
et al: Lack of association between adolescent idiopathic scoliosis
and previously reported single nucleotide polymorphisms in MATN1,
MTNR1B, TPH1 and IGF1 in a Japanese population. J Orthop Res.
29:1055–1058. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Yang M, Wei X, Yang W, Li Y, Ni H, Zhao Y,
Chen Z, Bai Y and Li M: The polymorphisms of melatonin receptor 1B
gene (MTNR1B) (rs4753426 and rs10830963) and susceptibility to
adolescent idiopathic scoliosis: A meta-analysis. J Orthop Sci.
20:593–600. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Demirkiran G, Dede O, Yalcin N, Akel I,
Marcucio R and Acaroglu E: Selective estrogen receptor modulation
prevents scoliotic curve progression: Radiologic and
histomorphometric study on a bipedal C57Bl6 mice model. Eur Spine
J. 23:455–462. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Akel I, Kocak O, Bozkurt G, Alanay A,
Marcucio R and Acaroglu E: The effect of calmodulin antagonists on
experimental scoliosis: A pinealectomized chicken model. Spine
(Phila Pa 1976). 34:533–538. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
47
|
Akel I, Demirkiran G, Alanay A, Karahan S,
Marcucio R and Acaroglu E: The effect of calmodulin antagonists on
scoliosis: Bipedal C57BL/6 mice model. Eur Spine J. 18:499–505.
2009. View Article : Google Scholar : PubMed/NCBI
|
|
48
|
Yang M, Li C and Li M: The estrogen
receptor α gene (XbaI, PvuII) polymorphisms and susceptibility to
idiopathic scoliosis: A meta-analysis. J Orthop Sci. 19:713–721.
2014. View Article : Google Scholar : PubMed/NCBI
|
|
49
|
Takahashi Y, Matsumoto M, Karasugi T,
Watanabe K, Chiba K, Kawakami N, Tsuji T, Uno K, Suzuki T, Ito M,
et al: Replication study of the association between adolescent
idiopathic scoliosis and two estrogen receptor genes. J Orthop Res.
29:834–837. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
50
|
Ogura Y, Takahashi Y, Kou I, Nakajima M,
Kono K, Kawakami N, Uno K, Ito M, Minami S, Yanagida H, et al: A
replication study for association of 5 single nucleotide
polymorphisms with curve progression of adolescent idiopathic
scoliosis in Japanese patients. Spine (Phila Pa 1976). 38:571–575.
2013. View Article : Google Scholar : PubMed/NCBI
|
|
51
|
Ogura Y, Takahashi Y, Kou I, Nakajima M,
Kono K, Kawakami N, Uno K, Ito M, Minami S, Yanagida H, et al: A
replication study for association of 53 single nucleotide
polymorphisms in a scoliosis prognostic test with progression of
adolescent idiopathic scoliosis in Japanese. Spine (Phila Pa 1976).
38:1375–1379. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
52
|
Zhao Y and Qiu GX: Expression of
calmodulin and nNOS in the paraspinal muscles in idiopathic
scoliosis. Zhonghua Yi Xue Za Zhi. 84:1358–1361. 2004.(In Chinese).
PubMed/NCBI
|















