Introduction
The pathogenesis of spinal cord injury (SCI)
involves two phases: A primary trauma, which is pivotal for initial
tissue disruption, followed by a series of secondary cellular
processes that accentuate tissue damage beyond the original injury
site and that can lead to long-term functional spinal deficits and
disabilities (1). Although
considerable effort has been made to improve outcomes for patients
with SCI, advances in therapy for this disease have been limited,
and further efforts are necessary to improve the treatment of SCI.
Previous reports have suggested that hyperbaric oxygen (HBO)
therapy is beneficial for neurological recovery in SCI, and HBO has
become an important therapeutic approach in the treatment of
secondary SCI (2,3). However, the mechanism that underlies
this effect is not well understood. The inflammatory response is an
important source of secondary damage to neuronal tissue in the
spinal cord following SCI (4).
Receptor for advanced glycation end products (RAGE) binds diverse
ligands, including the high mobility group box-1 (HMGB1) and S100
calcium binding protein families (5,6).
Ligand binding to RAGE triggers a series of cellular signaling
events, including the activation of nuclear factor-κB (NF-κB),
which leads to pro-inflammatory cytokine production and causes
inflammation (7). Abnormal
upregulation and activation of RAGE is associated with diseases of
the central nervous system (CNS), including traumatic brain injury,
ischemic stroke and SCI (8–10).
Monocyte chemoattractant protein-1 (MCP-1) is a member of the
β-chemokine family that activates and recruits mononuclear
phagocytes, T cells and B cells, and is induced in response to
various CNS insults (11–13). However, previous studies have not
addressed the effect of HBO on the expression of RAGE and MCP-1
following SCI. In the present study, HBO-induced changes in RAGE
and MCP-1 expression levels were investigated in rats following
SCI, and the effect of HBO therapy on SCI recovery was
investigated.
Materials and methods
Animals
A total of 90 8-week-old adult Sprague-Dawley rats
(Center of Experimental Animals of Capital Medical University,
Beijing, China) weighing 220–250 g were maintained under
environmentally controlled conditions and subject to a 12 h
light/dark cycle, with food and water provided ad libitum.
The animals were acclimated to the facility for 7 days prior to
initiation of experimentation, then were divided into sham-operated
(SH), SCI, and SCI + HBO groups using the randomization table
method. Each group was further divided into five subgroups, each
containing six animals, that were evaluated at 12 h, and 1, 3, 7
and 14 days post-injury. All procedures and handling techniques
were in strict accordance with the Committee on the Ethics of
Animal Experiments of Capital Medical University (Beijing,
China).
Spinal cord injury
Traumatic spinal injury was induced using a
Multicenter Animal SCI Study Impactor weight drop device (14). Briefly, the rats were anesthetized
with 10% chloral hydrate (350 mg/kg) administered
intraperitoneally. The fur above the vertebral column was cleared
using clippers and cleaned with Betadine solution. A 20-mm midline
incision was made in the thoracic region, and the vertebral column
was exposed. A laminectomy was performed at the T-10 vertebra,
exposing the dorsal cord surface with the dura intact. The
vertebral column was stabilized with angled clamps on the T-8 and
T-12 vertebrae. A 10-g weight was dropped from 25 mm onto the T-10
segment, resulting in a moderate spinal cord injury. The impact rod
was removed immediately following the injury, and the muscles and
the incision were closed in layers. Following surgery, animals were
placed on a heating pad maintained at 37°C and monitored until
recovery from anesthesia, then returned to the cages. A single dose
of penicillin (0.8 mg/g) was administered daily by subcutaneous
injection until hematuria ceased. Manual bladder expression was
required daily until a reflex bladder was established. The
sham-operated rats received the equivalent surgical procedure, but
were not subjected to impact injury.
HBO therapy
Rats in the SCI + HBO group were placed in a
hyperbaric chamber for 6 h post-surgery and exposed to 2.0
atmospheres absolute (ATA) of 100% oxygen for 60 min once daily.
Following treatment, the chamber was flushed with 100% oxygen for 5
min, then the pressure was increased to 2.0 ATA for 10 min,
followed by slow decompression over 15 min to normobaric air (21%
oxygen). Rats in the SH and SCI groups were post-operatively
treated with normobaric air at 1.0 ATA.
Assessment of locomotor function
Recovery of locomotor function was assessed using an
open-field testing paradigm, the Basso-Beattie-Bresnahan (BBB)
Locomotor Rating Scale, which is based on a 21-point scale
originally developed in spinal cord-injured rats (15). This scale assesses 10 distinct
categories, ranging from limb movement to tail position, and
involves detailed observations of joint movement, stepping and
coordination. Uninjured animals exhibit a locomotor score of 21,
whereas animals that exhibit complete hind limb paralysis are
scored as 0. All animals were scored in an open field (120×120 cm)
for 5 min at 12 h and at 1, 3, 7 and 14 days post-injury.
Tissue sample collection
Terminally anesthetized (10% chloral hydrate by
intraperitoneal injection at a dose of 350 mg/kg) rats were
transcardially perfused with cold saline and 4% buffered
paraformaldehyde at the indicated time points post-surgery (12 h
and 1, 3, 7 and 14 days). The spinal cord containing the injured
center site was removed and divided into two segments: One was
fixed with 10% formaldehyde solution at 4°C for 1 week for
histological evaluation, and the remaining segment was preserved at
−80°C for molecular analyses.
Myeloperoxidase (MPO) activity
assay
MPO activity, an indicator of polymorphonuclear
leukocyte accumulation, was determined in the spinal cord tissue as
previously described (16). Each
tissue sample was weighed, then homogenized in homogenate medium
[0.5% (w/v) hexadecyltrimethyl-ammonium bromide dissolved in 10 mM
potassium phosphate buffer, pH 7] and centrifuged at 20,000 ×
g for 30 min at 4°C. An aliquot of the supernatant was
subsequently incubated with a solution of 1.6 mM
tetramethyl-benzidine and 0.1 mM H2O2. The
rate of change in absorbance was measured using a UV-2000 UV/Vis
spectrophotometer (Unico Shanghai Instrument Co. Ltd., Shanghai,
China) at 460 nm. MPO activity was defined as the quantity of
enzyme required to degrade 1 mmol H2O2 per
min at 37°C and was expressed as units of MPO/g wet tissue.
Immunohistochemical staining
Histopathological samples were fixed, embedded in
paraffin, and cut into 5-µm slices. Following deparaffinizing and
rehydrating, antigen retrieval was performed according to standard
protocols (17). Following
treatment with 3% H2O2 in methanol and normal
non-immune goat serum (Invitrogen; Thermo Fisher Scientific, Inc.,
Waltham, MA, USA) for 20 min at room temperature, the sections were
incubated overnight at 4°C with primary antibodies against RAGE
(1:1,000; catalog no. sc-365154; Santa Cruz Biotechnology, Inc.,
Dallas, TX, USA) or MCP-1 (1:2,000; catalog no. sc-28879; Santa
Cruz Biotechnology, Inc.). The sections were then incubated with
biotinylated-goat anti-rabbit immunoglobulin G (1:5,000; catalog
no. K500710; Dako, Glostrup, Denmark) for 50 min at room
temperature, then treated with streptavidin-peroxidase (Dako).
Phosphate-buffered saline was used in place of the primary antibody
in the negative control samples. Diaminobenzidine was used to
visualize peroxidase activity, and sections were counterstained
with hematoxylin. Five fields on each of the three slides per
animal were randomly selected for visualization by light
microscopy. Images were analyzed using Image-Pro Plus 6.0 software
(Media Cybernetics, Inc., Rockville, MD, USA).
Reverse transcription-quantitative
polymerase chain reaction (RT-qPCR)
Total RNA (5 µg) was extracted from frozen spinal
cord tissue using TRIzol reagent (Invitrogen; Thermo Fisher
Scientific, Inc.) and an extraction kit (Takara Bio, Inc., Otsu,
Japan), according to manufacturer protocols. First-strand cDNA
synthesis from 2 µg RNA was performed using Moloney Murine Leukemia
Virus reverse transcriptase (BIOER, Hangzhou, China), with a
temperature protocol of 42°C incubation for 60 min, followed by 10
min incubation at 70°C. qPCR was performed using the BioEasy SYBR
Green I Real Time PCR kit (BIOER) and a Line-Gene sequence detector
(BIOER). The amplification reaction consisted of 45 cycles of 95°C
for 20 sec, 60°C for 25 sec and 72°C for 30 sec. PCR products were
detected by the incorporation of SYBR Green during the reaction and
verified by generating an amplification curve and by gel
electrophoresis. The primer sequences for RAGE, MCP-1 and
glyceraldehyde-3-phosphate dehydrogenase (GAPDH) are provided in
Table I. Amplification products
were quantified relative to the level of GAPDH, using the
2−ΔΔCq method (18).
 | Table I.Sequences of primers for reverse
transcription-quantitative polymerase chain reaction. |
Table I.
Sequences of primers for reverse
transcription-quantitative polymerase chain reaction.
| Target gene | Sequence
(5′-3′) |
|---|
| RAGE |
F-AGAAACCGGTGATGAAGGACAA |
|
|
R-TCGTTTTCGCCACAGGATGG |
| MCP-1 |
F-TCTGGGCCTGTTGTTCACAGT |
|
|
R-TGCTGCTGGTGATTCTCTTGTAGT |
| GAPDH |
F-GCAAGTTCAACGGCACAG |
|
|
R-CGCCAGTAGACTCCACGAC |
Western blot analysis
Frozen spinal cord tissue was homogenized in
ice-cold isolation buffer containing 250 mmol/l sucrose, 10 mmol/l
triethanolamine, 1 mg/ml leupeptin and 0.1 mg/ml
phenylmethylsulfonyl fluoride. Homogenates were centrifuged at
15,000 × g for 10 min at 4°C, and the protein concentration
in the supernatant was measured using a bicinchoninic acid protein
quantitation kit (Beijing Sunbio Biotech Co., Ltd., Beijing,
China). Total protein (50 µg) in each sample was resolved by 12%
sodium dodecyl sulfate-polyacrylamide gel electrophoresis and
transferred to polyvinylidene difluoride membranes. Membranes were
blocked in 5% skimmed milk powder for 2 h at room temperature, then
incubated overnight at 4°C with primary antibodies against RAGE
(1:1,000; catalog no. sc-365154; Santa Cruz Biotechnology, Inc.),
MCP-1 (1:2,000; catalog no. sc-28879; Santa Cruz Biotechnology,
Inc.), or actin (1:2,000; catalog no. sc-1616R; Santa Cruz
Biotechnology, Inc.) as indicated. Membranes were washed, then
incubated with horseradish peroxidase-conjugated secondary
antibodies (catalog nos. sc-2004 and sc-2005; Santa Cruz
Biotechnology, Inc.) diluted to 1:10,000 for 2 h at 37°C, and
immunoreactive protein was visualized using an enhanced
chemiluminescence western blotting detection system (BestBio Inc.,
Shanghai, China). The film was scanned (Konica Minolta Medical
Imaging, Inc., Wayne, NJ, USA), and protein expression was
quantified from 2 blots using Adobe Photoshop CS2 (Adobe Systems
Inc., San Jose, CA, USA) and LabWorks (UVP, Inc., Upland, CA,
USA).
Statistical analysis
Data are presented as the mean ± standard deviation
of 3 replicates. One-way analysis of variance was used to compare
the mean responses between the treatments, followed by least
significant difference or Student-Newman-Keuls post hoc
tests. P<0.05 was considered to indicate a statistically
significant difference. Analyses were performed using SPSS (v15.0;
SPSS Inc., Chicago, IL, USA).
Results
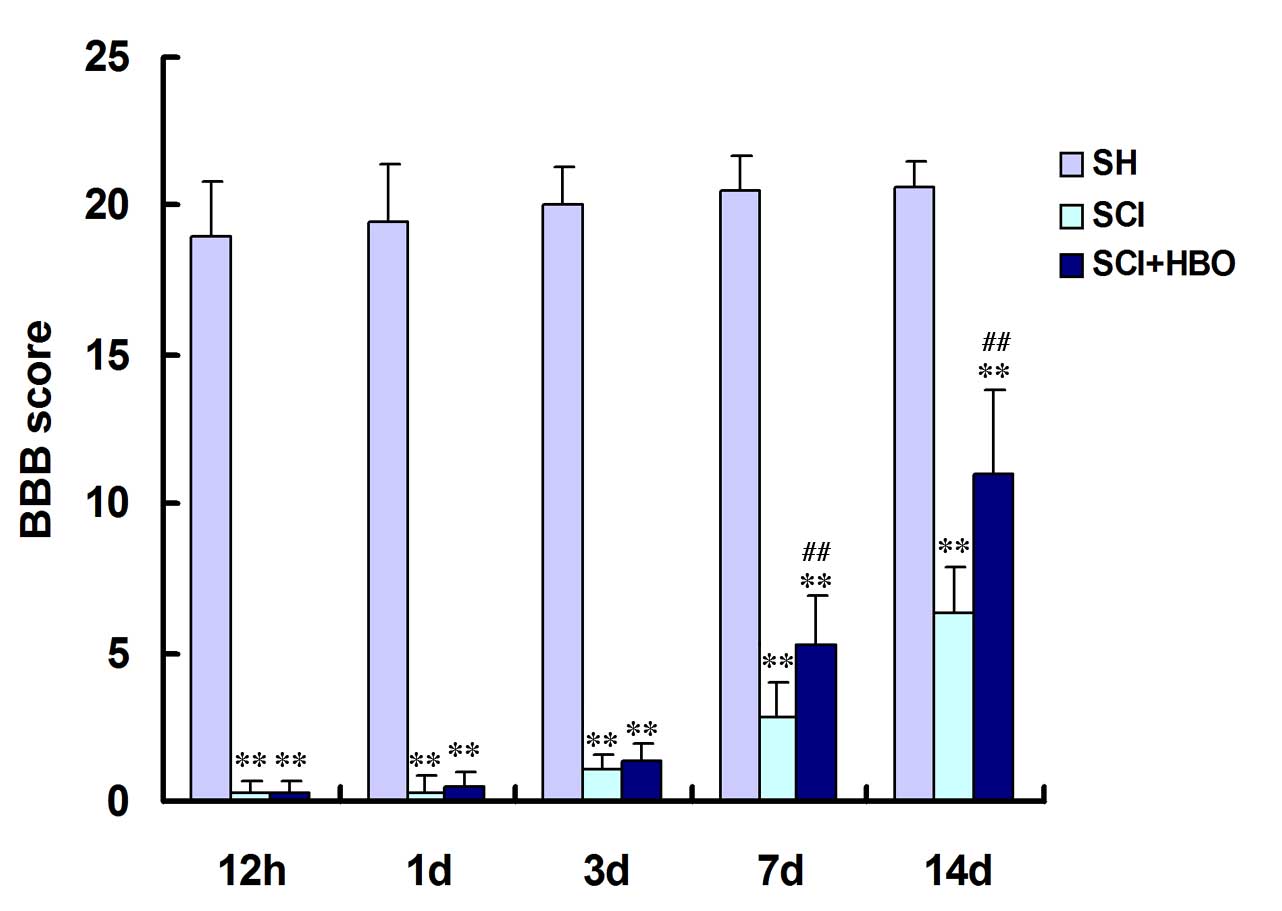
HBO promotes recovery of locomotor
function following SCI
To evaluate the extent of locomotor function, BBB
locomotor scores were calculated for control and experimental
animals. As demonstrated in Fig.
1, the mean BBB scores of the SCI and SCI + HBO groups were
significantly lower than those of the SH group at every time point
(P<0.01). BBB scores in rats in the SCI and SCI + HBO groups
improved from day 3, though the rats in the SCI + HBO group
improved more rapidly; statistical analysis indicated a
significantly greater increase in the BBB score in the SCI + HBO
group compared with the SCI group on days 7 and 14 (P=0.006 and
P=0.001, respectively; Fig. 1),
indicating that HBO therapy promotes locomotor function in rats
with SCI.
HBO reduces neutrophil infiltration
following SCI
The effect of HBO therapy on neutrophil infiltration
was assessed via MPO activity assays at multiple time points
following SCI. The MPO activity was significantly increased in the
SCI and SCI + HBO groups compared with the SH group at each
post-operative time point (Fig.
2). However, the MPO activity of the SCI + HBO group was
significantly lower than the SCI group at all time points from day
1 (Fig. 2), suggesting that HBO
therapy reduces neutrophil infiltration following SCI.
HBO suppresses the expression of RAGE
and MCP-1 following SCI
Immunohistochemistry was then used to detect RAGE
and MCP-1 expression and localization. As demonstrated in Fig. 3A, RAGE and MCP-1 were predominantly
expressed in the grey matter. RAGE- and MCP-1-positive cells were
detectable in the SH group at all time points, however, the
proportion of RAGE- and MCP-1-positive cells was significantly
higher following SCI, with or without HBO therapy, with the
exception of MCP-1 on day 14 (Fig.
3B). However, the proportion of RAGE- and MCP-1-positive cells
was significantly lower in the SCI + HBO group compared with the
SCI group at all time points from day 1, with the exception of
MCP-1 on day 14 (Fig. 3B).
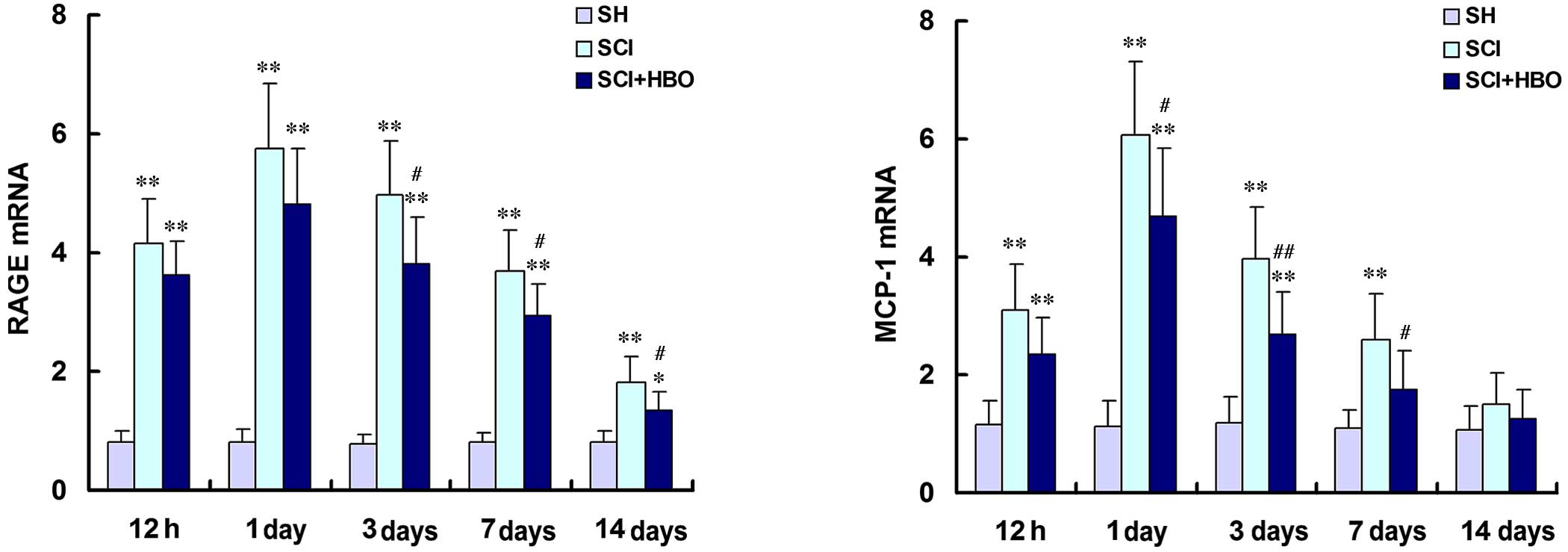
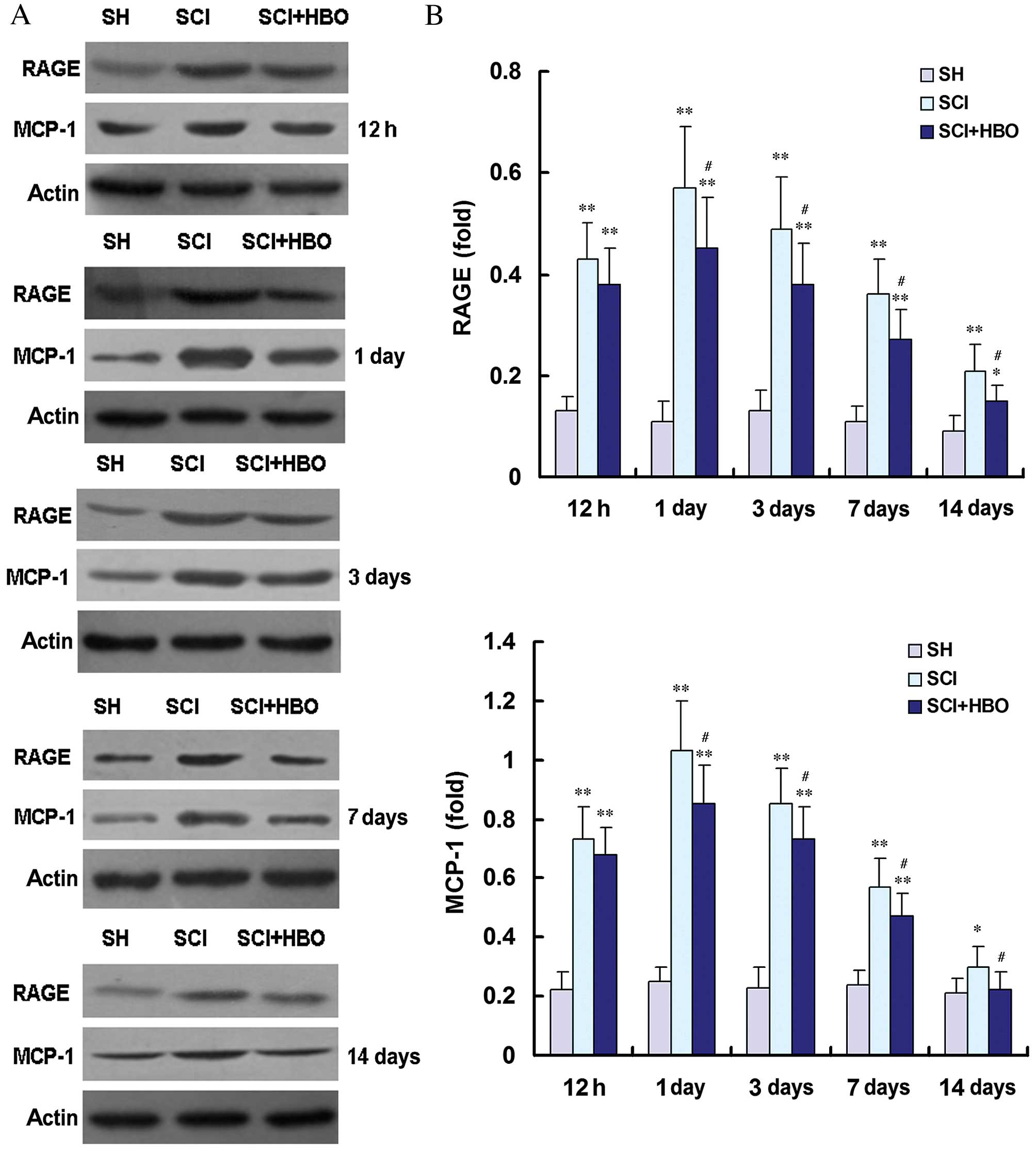
RAGE and MCP-1 mRNA and protein expression levels
were then assessed by RT-qPCR (Fig.
4) and western blot (Fig. 5),
respectively. RAGE and MCP-1 mRNA and protein expression levels
were consistently low in the SH group (Figs. 4 and 5, respectively). Following SCI, RAGE and
MCP-1 mRNA and protein expression levels were significantly
increased at 12 h compared with the SH group, reaching their
maximum level at the 1 day time point (Figs. 4 and 5, respectively). RAGE and MCP-1 mRNA and
protein levels were significantly higher in the SCI group compared
with the SH group at 12 h, and days 1, 3 and 7 post-operatively
(all P<0.01; Figs. 4 and
5, respectively). On day 14, the
expression of RAGE mRNA (P<0.0001; Fig. 4), RAGE protein (P<0.0001;
Fig. 5) and MCP-1 protein
(P=0.021; Fig. 5) was increased in
the SCI group compared with the SH group. However, the expression
levels of RAGE and MCP-1 mRNA (Fig.
4) and protein (Fig. 5) were
significantly lower in the SCI + HBO group on days 3 and 7
post-surgery compared with the SCI group. RAGE mRNA expression
levels were significantly reduced in the SCI + HBO group compared
with the SCI group from day 3 (P<0.05; Fig. 4) as were protein expression levels
from day 1 (P<0.05; Fig. 5B).
MCP-1 mRNA expression levels were significantly reduced in the SCI
+ HBO group compared with the SCI group on days 1, 3 and 7
(P<0.05; Fig. 4) as were
protein expression levels on days 1, 3, 7 and 14 (P<0.05;
Fig. 5). These data suggest that
HBO therapy suppresses the expression of RAGE and MCP-1 following
SCI.
Discussion
Numerous studies have demonstrated that mechanical
injury following SCI results in secondary injuries, including
inflammatory responses, hemorrhage, ischemia, excessive free
radical generation, vascular dysregulation, and immune cell
infiltration (19,20). Specifically, inflammation following
SCI is important for the regulation of remyelination and in
neuronal and glial cell death (21,22).
Therefore, the inhibition of the inflammatory response is an
important factor in neuroprotection and for promoting the recovery
of locomotor function.
HBO therapy is a clinical therapy that involves
administering 100% oxygen at a pressure higher than atmospheric
pressure at sea level for a prescribed amount of time (23). HBO therapy is a well established
treatment for acute and chronic SCI (3,24–26).
Exposure to HBO decreases the expression levels of superoxide
dismutase, glutathione peroxidase and nitric oxide synthase,
relieves secondary inflammatory responses, inhibits apoptosis
following injury and promotes the regeneration of nerve tissue
(3,24–26).
Previous studies have demonstrated that HBO relieves secondary
inflammatory responses by decreasing the expression of NF-κB,
HMGB1, Toll-like receptor 2, interleukin-1β (IL-1β), and tumor
necrosis factor-α (TNF-α) following SCI (27,28).
In addition, Chen et al (29) reported that exposure to HBO
attenuates inflammation, as indicated by reduced expression of
MCP-1 and other inflammatory cytokines in a traumatic brain injury
model. However, the effect of HBO therapy on the expression of RAGE
and MCP-1 following SCI remains unclear. Following SCI, the
inflammatory response becomes intense and is most destructive
within the initial few days following injury (30). Thus, in the present study, the
anti-inflammatory and neuroprotective effects of HBO were measured
during the early phase of SCI (12 h-14 days). Application of HBO
following SCI was revealed to inhibit the infiltration of
neutrophils, suppress the expression of inflammatory cytokines
(including RAGE and MCP-1), and promote the recovery of locomotor
function. To the best of our knowledge, this is the first study to
examine the effect of HBO therapy on RAGE and MCP-1 expression
following SCI.
Neutrophils are critical cellular components of the
inflammatory response and the first inflammatory cell type to reach
damaged tissue (31). Activated
neutrophils promote tissue repair by inducing the phagocytosis of
necrotic tissue in the wound area, while simultaneously promoting
the release of elastase via a respiratory burst and producing large
amounts of reactive oxygen species (ROS) (32). These ROS induce two effects: i)
They attack the polyunsaturated fatty acids on the cell membrane,
causing lipid peroxidation and disrupting the cell osmotic balance;
and ii) they activate complement systems, resulting in a positive
feedback loop in which neutrophils are further activated to produce
more ROS (22,32). These downstream effects expand the
inflammatory response and exacerbate secondary injury (31,32).
The results of the present study reveal that HBO therapy
significantly inhibits the activity of neutrophils following SCI,
which reduces secondary injury and provides a neuroprotective
effect.
RAGE, a transmembrane protein and member of the
immunoglobulin superfamily, is expressed in endothelial cells,
neurons, macrophages and monocytes (33). RAGE is involved in various
inflammatory mechanisms and participates in numerous diseases,
including CNS disorders, by binding to diverse ligands. In
particular, several studies have reported that RAGE was upregulated
following SCI in rats and mice (10,34).
In confirmatory studies using RAGE-deficient animals, RAGE was
demonstrated to be involved in various pathophysiological processes
of SCI (35). In the present
study, RAGE expression was demonstrated to be significantly
increased from 12 h following SCI and persisted for 14 days. RAGE
expression was further observed to be significantly decreased in
animals with SCI that were treated with HBO therapy compared with
untreated animals with SCI. HBO administration following SCI was
also demonstrated to reduce neutrophil infiltration and increase
the BBB scores of injured rats. Therefore, it is surmised that
decreased expression of RAGE as a result of HBO therapy may
contribute to a reduced inflammatory response and an improvement in
the restoration of locomotor function. Following binding to ligands
including HMGB1 and S100β, RAGE triggers the activation of NF-κB,
induces the production of pro-inflammatory cytokines, including
IL-1β, IL-6 and TNF-α, and causes inflammation (36). The expression of RAGE is also
controlled by NF-κB transcription factor (7). Therefore, RAGE is upregulated in
environments that are rich in RAGE ligands (37). Previous studies by this group have
demonstrated that HBO therapy decreases the expression of NF-κB and
HMGB1 following SCI (27,28). These results may, therefore,
partially explain why HBO therapy dramatically relieves RAGE
expression following SCI.
MCP-1 is a potent chemoattractant with a significant
role in recruiting lymphocytes and monocytes into inflammatory
sites. In the CNS, MCP-1 is produced by a variety of cells,
including reactive astrocytes, neurons, activated microglia and
endothelial cells (38). Several
recent studies have reported upregulation of MCP-1 expression
following SCI in rats (39,40).
Inhibition of MCP-1 also attenuates leukocyte infiltration and
tissue destruction in SCI (41).
In the present study, the expression of MCP-1 was significantly
increased following SCI, beginning at 12 h, peaking at day 1, and
gradually declining to control levels by day 14; these findings are
consistent with those of previous studies (42). Furthermore, HBO therapy
significantly decreased the expression of MCP-1 in animals with SCI
that were treated with HBO therapy compared with untreated animals
in the SCI group. HBO therapy-mediated attenuation of neutrophil
infiltration was also observed. Therefore, is it proposed that HBO
therapy inhibits the expression of MCP-1 and that this change in
MCP-1 is important in reducing the inflammatory response following
SCI. This method may, therefore, be beneficial in promoting the
recovery of locomotor function in rats with SCI. The question of
how HBO therapy influences MCP-1 expression following SCI requires
further investigation.
In conclusion, exposure to HBO following SCI has
been demonstrated to reduce secondary inflammatory responses by
decreasing the expression of RAGE and MCP-1, resulting in a
significant restoration of locomotor function. These results are
preliminary, however, they provide an important insight into the
molecular mechanism by which HBO therapy promotes locomotor
recovery in SCI rats, and may be useful for improving the clinical
application of HBO in human patients with SCI. Further study will
be required to elucidate the mechanism by which HBO therapy
influences the expression of RAGE and MCP-1 following SCI.
References
|
1
|
Beattie MS, Hermann GE, Rogers RC and
Bresnahan JC: Cell death in models of spinal cord injury. Prog
Brain Res. 137:37–47. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Nie H, Xiong L, Lao N, Chen S, Xu N and
Zhu Z: Hyperbaric oxygen preconditioning induces tolerance against
spinal cord ischemia by upregulation of antioxidant enzymes in
rabbits. J Cereb Blood Flow Metab. 26:666–674. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Dayan K, Keser A, Konyalioglu S, Erturk M,
Aydin F, Sengul G and Dagci T: The effect of hyperbaric oxygen on
neuroregeneration following acute thoracic spinal cord injury. Life
Sci. 90:360–364. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Nakamura M, Houghtling RA, MacArthur L,
Bayer BM and Bregman BS: Differences in cytokine gene expression
profile between acute and secondary injury in adult rat spinal
cord. Exp Neurol. 184:313–325. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Fritz G: RAGE: A single receptor fits
multiple ligands. Trends Biochem Sci. 36:625–632. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Xie J, Mendez JD, Méndez-Valenzuela V and
Aguilar-Hernández MM: Cellular signalling of the receptor for
advanced glycation end products (RAGE). Cell Signal. 25:2185–2197.
2013. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Lin L, Park S and Lakatta EG: RAGE
signaling in inflammation and arterial aging. Front Biosci
(Landmark Ed). 14:1403–1413. 2009. View
Article : Google Scholar : PubMed/NCBI
|
|
8
|
Gao TL, Yuan XT, Yang D, Dai HL, Wang WJ,
Peng X, Shao HJ, Jin ZF and Fu ZJ: Expression of HMGB1 and RAGE in
rat and human brains after traumatic brain injury. J Trauma Acute
Care Surg. 72:643–649. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Muhammad S, Barakat W, Stoyanov S,
Murikinati S, Yang H, Tracey KJ, Bendszus M, Rossetti G, Nawroth
PP, Bierhaus A and Schwaninger M: The HMGB1 receptor RAGE mediates
ischemic brain damage. J Neurosci. 28:12023–12031. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Chen KB, Uchida K, Nakajima H, Yayama T,
Hirai T, Guerrero A Rodriguez, Kobayashi S, Ma WY, Liu SY, Zhu P
and Baba H: High-mobility group box-1 and its receptors contribute
to proinflammatory response in the acute phase of spinal cord
injury in rats. Spine (Phila Pa 1976). 36:2122–2129. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Kwon BK, Stammers AM, Belanger LM,
Bernardo A, Chan D, Bishop CM, Slobogean GP, Zhang H, Umedaly H,
Giffin M, et al: Cerebrospinal fluid inflammatory cytokines and
biomarkers of injury severity in acute human spinal cord injury. J
Neurotrauma. 27:669–682. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Liu SQ, Ma YG, Peng H and Fan L: Monocyte
chemoattractant protein-1 level in serum of patients with acute
spinal cord injury. Chin J Traumatol. 8:216–219. 2005.PubMed/NCBI
|
|
13
|
Galasso JM, Liu Y, Szaflarski J, Warren JS
and Silverstein FS: Monocyte chemoattractant protein-1 is a
mediator of acute excitotoxic injury in neonatal rat brain.
Neuroscience. 101:737–744. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Allen EA, Erhardt EB and Calhoun VD: Data
visualization in the neurosciences: Overcoming the curse of
dimensionality. Neuron. 74:603–608. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Basso DM, Beattie MS and Bresnahan JC: A
sensitive and reliable locomotor rating scale for open field
testing in rats. J Neurotrauma. 12:1–21. 1995. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Yang L, Jones NR, Blumbergs PC, Van Den
Heuvel C, Moore EJ, Manavis J, Sarvestani GT and Ghabriel MN:
Severity-dependent expression of pro-inflammatory cytokines in
traumatic spinal cord injury in the rat. J Clin Neurosci.
12:276–284. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Anik I, Kokturk S, Genc H, Cabuk B, Koc K,
Yavuz S, Ceylan S, Ceylan S, Kamaci L and Anik Y:
Immunohistochemical analysis of TIMP-2 and collagen types I and IV
in experimental spinal cord ischemia-reperfusion injury in rats. J
Spinal Cord Med. 34:257–264. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(−Delta Delta C(T)) Method. Methods. 25:402–408. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Kwon BK, Tetzlaff W, Grauer JN, Beiner J
and Vaccaro AR: Pathophysiology and pharmacologic treatment of
acute spinal cord injury. Spine J. 4:451–464. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Sinescu C, Popa F, Grigorean VT, Onose G,
Sandu AM, Popescu M, Burnei G, Strambu V and Popa C: Molecular
basis of vascular events following spinal cord injury. J Med Life.
3:254–261. 2010.PubMed/NCBI
|
|
21
|
Miron VE, Boyd A, Zhao JW, Yuen TJ, Ruckh
JM, Shadrach JL, van Wijngaarden P, Wagers AJ, Williams A, Franklin
RJ and ffrench-Constant C: M2 microglia and macrophages drive
oligodendrocyte differentiation during CNS remyelination. Nat
Neurosci. 16:1211–1218. 2013. View
Article : Google Scholar : PubMed/NCBI
|
|
22
|
Xue H, Zhang XY, Liu JM, Song Y, Liu TT
and Chen D: NDGA reduces secondary damage after spinal cord injury
in rats via anti-inflammatory effects. Brain Res. 1516:83–92. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Tibbles PM and Edelsberg JS:
Hyperbaric-oxygen therapy. N Engl J Med. 334:1642–1648. 1996.
View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Topuz K, Colak A, Cemil B, Kutlay M,
Demircan MN, Simsek H, Ipcioglu O, Kucukodaci Z and Uzun G:
Combined hyperbaric oxygen and hypothermia treatment on oxidative
stress parameters after spinal cord injury: An experimental study.
Arch Med Res. 41:506–512. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Liu X, Zhou Y, Wang Z, Yang J, Gao C and
Su Q: Effect of VEGF and CX43 on the promotion of neurological
recovery by hyperbaric oxygen treatment in spinal cord-injured
rats. Spine J. 14:119–127. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Lu PG, Feng H, Yuan SJ, Zhang RW, Li M, Hu
R, Liu ZS and Yin J: Effect of preconditioning with hyperbaric
oxygen on neural cell apoptosis after spinal cord injury in rats. J
Neurosurg Sci. 57:253–258. 2013.PubMed/NCBI
|
|
27
|
Yang J, Liu X, Zhou Y, Wang G, Gao C and
Su Q: Hyperbaric oxygen alleviates experimental (spinal cord)
injury by downregulating HMGB1/NF-κB expression. Spine (Phila Pa
1976). 38:E1641–E1648. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Tan J, Zhang F, Liang F, Wang Y, Li Z,
Yang J and Liu X: Protective effects of hyperbaric oxygen treatment
against spinal cord injury in rats via toll-like receptor 2/nuclear
factor-κB signaling. Int J Clin Exp Pathol. 7:1911–1919.
2014.PubMed/NCBI
|
|
29
|
Chen X, Duan XS, Xu LJ, Zhao JJ, She ZF,
Chen WW, Zheng ZJ and Jiang GD: Interleukin-10 mediates the
neuroprotection of hyperbaric oxygen therapy against traumatic
brain injury in mice. Neuroscience. 266:235–243. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Leskovar A, Moriarty LJ, Turek JJ,
Schoenlein IA and Borgens RB: The macrophage in acute neural
injury: Changes in cell numbers over time and levels of cytokine
production in mammalian central and peripheral nervous systems. J
Exp Biol. 203:1783–1795. 2000.PubMed/NCBI
|
|
31
|
Tjoa T, Strausbaugh HJ, Maida N, Dazin PF,
Rosen SD and Noble-Haeusslein LJ: The use of flow cytometry to
assess neutrophil infiltration in the injured murine spinal cord. J
Neurosci Methods. 129:49–59. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Carlson SL, Parrish ME, Springer JE, Doty
K and Dossett L: Acute inflammatory response in spinal cord
following impact injury. Exp Neurol. 151:77–88. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Stern D, Yan SD, Yan SF and Schmidt AM:
Receptor for advanced glycation endproducts: A multiligand receptor
magnifying cell stress in diverse pathologic settings. Adv Drug
Deliv Rev. 54:1615–1625. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Kawabata H, Setoguchi T, Yone K, Souda M,
Yoshida H, Kawahara K, Maruyama I and Komiya S: High mobility group
box 1 is upregulated after spinal cord injury and is associated
with neuronal cell apoptosis. Spine (Phila Pa 1976). 35:1109–1115.
2010. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Guo JD, Li L, Shi YM, Wang HD, Yuan YL,
Shi XX and Hou SX: Genetic ablation of receptor for advanced
glycation end products promotes functional recovery in mouse model
of spinal cord injury. Mol Cell Biochem. 390:215–223. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Li H, Wu W, Sun Q, Liu M, Li W, Zhang XS,
Zhou ML and Hang CH: Expression and cell distribution of receptor
for advanced glycation end-products in the rat cortex following
experimental subarachnoid hemorrhage. Brain Res. 1543:315–323.
2014. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Schmidt AM, Yan SD, Yan SF and Stern DM:
The multiligand receptor RAGE as a progression factor amplifying
immune and inflammatory responses. J Clin Invest. 108:949–955.
2001. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Che X, Ye W, Panga L, Wu DC and Yang GY:
Monocyte chemoattractant protein-1 expressed in neurons and
astrocytes during focal ischemia in mice. Brain Res. 902:171–177.
2001. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Stammers AT, Liu J and Kwon BK: Expression
of inflammatory cytokines following acute spinal cord injury in a
rodent model. J Neurosci Res. 90:782–790. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Sandhir R, Gregory E, He YY and Berman NE:
Upregulation of inflammatory mediators in a model of chronic pain
after spinal cord injury. Neurochem Res. 36:856–862. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Zhang X, Chen C, Ma S, Wang Y and Su X:
Inhibition of monocyte chemoattractant peptide-1 decreases
secondary spinal cord injury. Mol Med Rep. 11:4262–4266.
2015.PubMed/NCBI
|
|
42
|
McTigue DM, Tani M, Krivacic K, Chernosky
A, Kelner GS, Maciejewski D, Maki R, Ransohoff RM and Stokes BT:
Selective chemokine mRNA accumulation in the rat spinal cord after
contusion injury. J Neurosci Res. 53:368–376. 1998. View Article : Google Scholar : PubMed/NCBI
|