Introduction
Glaucoma is an optic neuropathy, which has typical
structural and functional defects, and has long been the second
leading cause of blindness after cataracts (1), with almost 60,000,000 individuals
affected and 8,400,000 with bilateral blindness worldwide in 1979
(2). As a result of its
asymptomatic and chronic characteristics, glaucoma is difficult to
diagnose at an earlier stage. Glaucoma is divided into two major
forms: Open-angle glaucoma and angle-closure glaucoma, which are
associated with the death of a substantial number of retinal
ganglion cells in the inner retina and the loss of their axons in
the optic nerve (2). Glaucoma is
considered to be induced by an increase in intraocular pressure.
Trabecular meshwork cells can regulate aqueous humor outflow and
control intraocular pressure (3),
however, the degradation of these cells, for example in primary
open-angle glaucoma, leads to the loss of aqueous humor drainage
and results in a consequent increase in intraocular pressure
(4,5). Therefore, preventing the loss of
trabecular meshwork cells is pivotal for controlling glaucoma.
MicroRNAs (miRNAs) are small non-coding RNA
molecules, which guide argonaute proteins to their target RNAs and
modulate gene expression post-transcriptionally (6). They are generated from transcription
by RNA polymerase and are cleaved by ribonucleases, Drosha and
Dicer, from primary miRNA into precursor miRNA, and then to mature
miRNA (7). The regulatory
mechanism of miRNAs may vary among different target RNAs, cells and
conditions (8). miRNA regulation
is important in the altered gene expression, which occurs in
disease (9), and may be more
effective than gene methylation or histone modification (10). miRNAs are vital in trabecular
meshwork cell regulation, as revealed in previous studies. A
previous study showed that miR-200c significantly decreases
intraocular pressure via regulation of its targets; including Zinc
finger E-box binding homeobox 1 and 2 (11). Several miRNAs have been shown to be
aberrantly expressed in human trabecular meshwork (HTM) cells with
stress-induced premature senescence (12), of which miR-183 regulates integrin
β1 and thus modulating the senescence of HTM cells (13). Despite these previous studies
involving miRNAs, the association between miRNAs and HTM cell
proliferation and apoptosis remains to be elucidated.
miR-93 has been investigated in human colon cancer
stem cells and non-small cell lung cancer cells. It is
downregulated in the former cells, with its overexpression leading
to inhibited cell proliferation and colony formation (14), whereas the overexpression of miR-93
in the latter cells inhibits the expression of tumor suppressor
candidate 2 (15). In this
context, the present study hypothesized that miR-93 is involved in
the regulation of glaucoma trabecular meshwork (GTM) cell
apoptosis. To elucidate the role of miR-93 in GTM cell apoptosis, a
lentivirus containing the miR-93-specific inhibitor sponge and
expression vector were transfected into GTM cells to monitor the
effects on cell viability and apoptosis. In addition, the
expression of a possible associated factor in these processes was
examined to determine the functional mechanism of miR-93. The
results of these investigations may provide a basic understanding
of the role of miR-93 in regulating GTM cells.
Materials and methods
Cells
The HTM and GTM cells were provided by Zhongshan
Ophthalmic Center of Sun Yat-sen University (Guangzhou, China). The
cells were cultured in Dulbecco's modified Eagle's medium
(DMEM)/F12 supplemented with 20% fetal bovine serum in a humidified
atmosphere with 5% CO2 at 37°C. The cells were passaged when their
confluence reached 70% and were washed twice with D-Hanks
solution.
Lentivirus transfection
The lentiviral vectors specific for pre-miR-93 and
miR-93 sponge were constructed by GenePharma (Shanghai, China).
Prior to transfection, the GTM cells were transferred to 24-well
plates (1×105 cells/well) and cultured for 24 h. The
lentivirus suspension was added to the cells using Lipofectamine
3000 (Invitrogen; Thermo Fisher Scientific, Inc., Waltham, MA, USA)
according to the manufacturer's protocol, and blank lentivirus was
used in the control group. Following incubation for 24 h at 37°C,
the suspension in the wells was replaced with DMEM/F12 medium and
the cells were cultured for another 48 h at 37°C. The transfection
efficiency was monitored using a fluorescence-activated cell sorter
(BD FACSCanto II; BD Biosciences, San Jose, CA, USA), and the cells
were collected for further analyses.
Cell viability assay
The transfected GTM cells were adjusted to the
concentration of 5×104/ml, transferred to 96-well plates
(200 µl per well), and cultured at 37°C for 24, 48, 72 and 96 h.
Fresh medium containing 0.5 mg/ml methyl thiazolyl tetrazolium
(Sigma-Aldrich; Merck Millipore, Darmstadt, Germany) was added to
each well and the cells were incubated at 37°C for 4 h, following
which the reaction was terminated using 200 µl dimethyl sulfoxide.
The optical density at 570 nm was detected for each well using a
microplate reader (Molecular Devices LLC, Silicon Valley, CA,
USA).
Cell apoptosis assay
The transfected GTM cells were transferred to
24-well plates 48 h following transfection, at a density of
2×105 cells per well. Cell apoptosis was analyzed using
the annexin V-fluorescein isothiocyanate (FITC)/prodium iodide (PI)
dual staining method with an Annexin V:FITC Apoptosis Detection kit
II (BD Biosciences) according to the manufacturer's protocol. The
cells were then analyzed using the BD FACSCanto II. The percentages
of apoptotic cells (annexin V positive and PI negative) were
compared.
Reverse transcription-quantitative
polymerase chain reaction (RT-qPCR) analysis
The microRNAs of the HTM and GTM cells were
extracted using RNAiso for small RNAs (Takara Biotechnology Co.,
Ltd., Dalina, China) and reverse transcribed using a One Step
PrimeScript miRNA cDNA synthesis kit (Takara Biotechnology Co.,
Ltd.) according to the manufacturer's protocol. qPCR analysis was
performed with SYBR Green I Mastermix (Roche Diagnostics GmbH,
Mannheim, Germany) using a LightCycler 480 (Roche Diagnostics,
Basel, Switzerland). The total reaction volume was 10 µl which
consisted of 0.25 µM each primer and approximately 5 ng of template
cDNA. Accordingly, the thermocycling profiles were as follows:
Denaturation at 95°C for 5 min; 45 cycles of 95°C, 60°C for 20 sec
and 72°C for 20 sec, the melt curve protocol was 95°C for 5 sec,
65°C for 1 min and reheating to 97°C. Finally, the temperature was
reduced to 40°C and maintained for 10 sec. The specific primers for
miR-93 was synthesized as forward 5′-AGTCTCTGGCTGACTACATCACAG-3′
and reverse 5′-CTACTCACAAAACAGGAGTGGAATC-3′. U6 (forward
5′-CTCGCTTCGGCAGCACA-3′ and reverse 5′-AACGCTTCACGAATTTGCGT-3′) was
used as the internal control. Data were analyzed using the
2−ΔΔCq method (16).
Western blot analysis
The online tool microRNA.org
(www.microrna.org/microrna/home.do) was used to predict
the target gene for miR-93, and was used to select the specific
primary antibody for the present study. Protein samples of the
infected GTM cells were prepared using RIPA lysis buffer (Beyotime
Institute of Biotechnology, Inc., Shanghai, China). The protein
contents were quantified using the BCA Protein Assay kit (Pierce;
Thermo Fisher Scientific, Inc.). A protein sample of 20 µg was
loaded in each lane for separation of the samples by 12% sodium
dodecyl sulfate-polyacrylamide gel electrophoresis, following which
they were transferred onto a polyvinylidene fluoride membrane. The
membrane was blocked in 5% skim milk for 2 h at room temperature
and incubated with anti-NFE2L2 (monoclonal; rabbit anti-human;
1:1,000; cat. no. ab62352) or anti-GAPDH (internal reference;
monoclonal; rabbit anti-human; 1:10,000; cat. no. ab181602) primary
antibodies (Abcam, Cambridge, UK) at 4°C overnight. Following
washing with a buffer containing 0.1% Tween-20, 20 mM Tris-HC and
137 mM NaCl (TBST), the membrane was incubated in horseradish
peroxidase-conjugated secondary antibody (polyclonal; goat
anti-rabbit; 1:10,000; cat. no. ab97051) for 1 h at room
temperature. Signals were developed using ECL Plus western blotting
substrate (Thermo Fisher Scientific, Inc.) and the intensity of
bands was analyzed using the ChemiDoc XRS system (Bio-Rad
Laboratories, Inc., Hercules, CA, USA).
Statistical analysis
All experiments were repeated in triplicate, and
results are presented as the mean ± standard deviation. Statistical
analysis was performed using SPSS 19.0 software (IBM SPSS, Armonk,
NY, USA) using one-way analysis of variance. P<0.05 was
considered to indicate a statistically significant difference.
Results
miR-93 is upregulated in GTM
cells
Prior to investigating the functions of miR-93, the
expression levels of miR-93 in HTM and GTM cells were compared
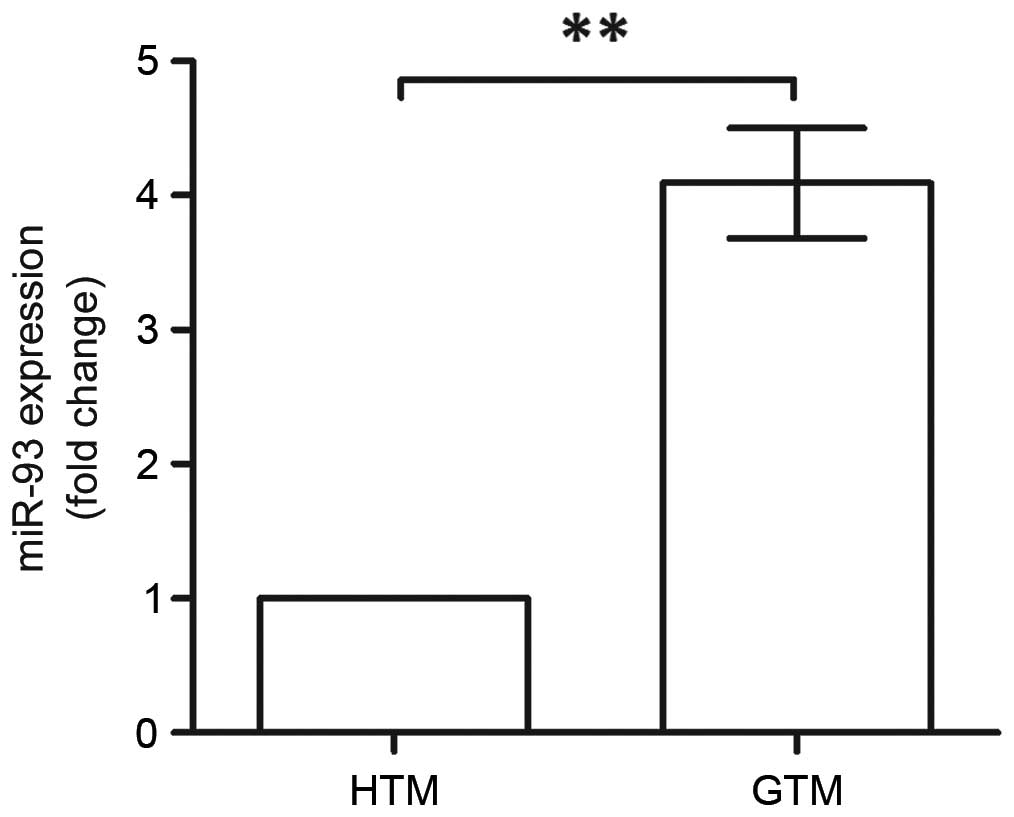
using RT-qPCR analysis (Fig. 1).
The expression of miR-93 was significantly upregulated in the GTM
cells, which was almost four times higher, compared with that in
the HTM cells (P<0.01). This indicated the association between
miR-93 and glaucoma; therefore, it was necessary to reveal the
roles of miR-93 in GTM.
miR-93 inhibits viability and promotes
apoptosis of GTM cells
To investigate the role of miR-93 in GTM cells,
miR-93 was inhibited and overexpressed by transfection with its
specific inhibitor sponge and expression vector, respectively.
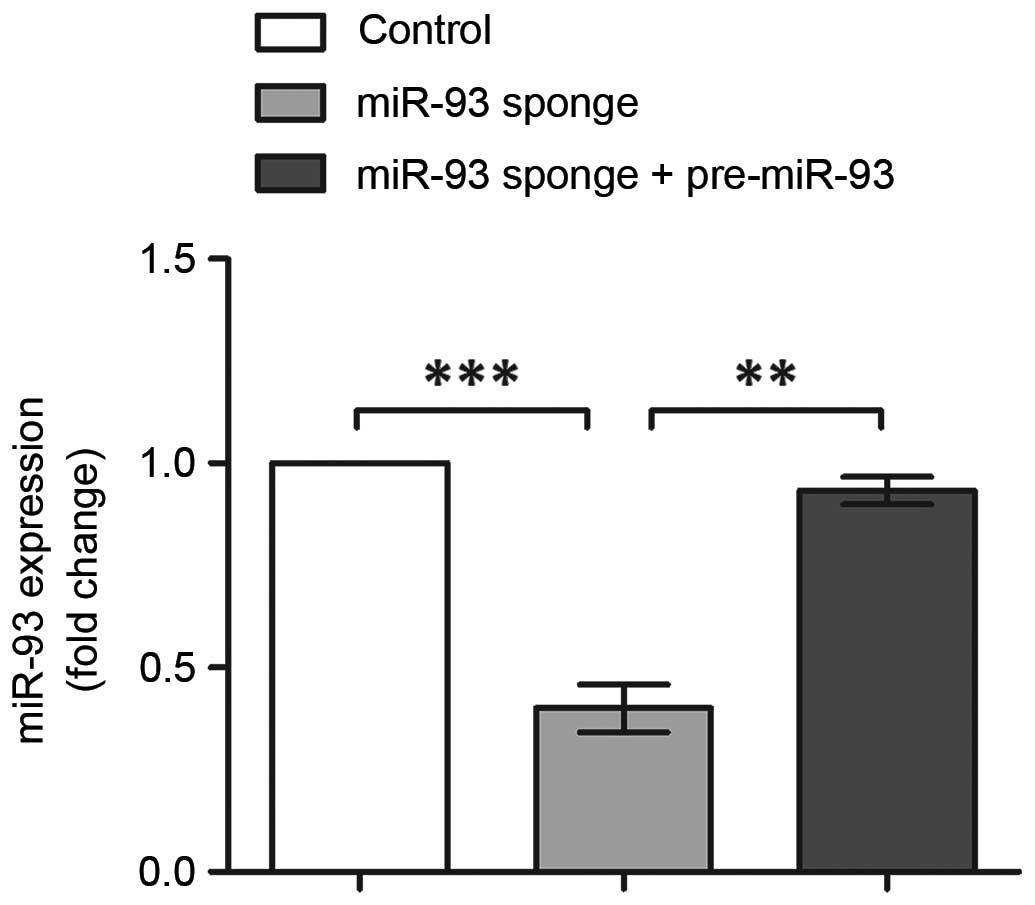
Alterations in the expression of miR-93 were analyzed using RT-qPCR
analysis (Fig. 2). The results
indicated the successful inhibition and overexpression of miR-93,
with its expression levels significantly reduced following miR-93
sponge transfection (P<0.001), and recovered to the original
level following co-transfection with the miR-93 sponge and
pre-miR-93 vector (P<0.01). Therefore, these three groups of
transfected cells were suitable for use in the following
experiments.
GTM cell viability was monitored during the 96 h
time period following transfection (Fig. 3A). The viability of the GTM cells
in the control group gradually decreased from 48 h
post-transfection. By contrast, the cells with inhibited miR-93
showed marked promotion of cell viability, which was significant
higher, compared with that in the control group (P<0.01). The
results also showed that cell viability was inhibited when miR-93
was overexpressed (P<0.05), which indicated that miR-93 was
capable of inhibiting GTM cell viability. At 96 h
post-transfection, the percentages of apoptotic cells were also
examined using annexin V-FITC/PI staining (Fig. 3B). Compared with the control group,
cell apoptosis was inhibited when miR-93 was suppressed
(P<0.001). However, in the cells overexpressing miR-93, the
effects of the miR-93 sponge was abrogated, with the percentage of
apoptotic cells significantly increased (P<0.001), and an
increase in cells in the late stage of apoptosis. Taken together,
these results indicated that miR-93 induced the apoptosis of GTM
cells and inhibited their viability, suggesting it is an important
regulator in glaucoma.
 | Figure 3.Inhibition of miR-93 leads to the
promotion of cell viability and suppression of apoptosis in GTM
cells. (A) Cell viability indicated by the OD at 570 nm was
monitored following the transfection of GTM cells with miR-93
sponge and pre-miR-93 for 24, 48, 72 and 96 h. At 96 h
post-transfection, the inhibition of miR-93 led to a significant
increase in cell viability, compared with the control group
(**P<0.01). At 96 h post-transfection, the viability of cells
co-transfected with the miR-93 sponge and pre-miR-93 were
significantly decreased, compared with those transfected with the
miR-93 sponge alone (#P<0.05). (B) GTM cell apoptosis
was inhibited by suppressing miR-93 and promoted by co-transfection
with pre-miR-93. The histogram shows the percentages of apoptotic
cells in Q3 (***P<0.001). miR-93, microRNA-93; pre-miR-93,
miR-93 precursor; GTM, glaucoma trabecular meshwork; OD, optical
density; PE, phycoerythrin; FITC, fluorescein isothiocyanate. |
miR-93 suppresses the expression of
NFE2L2
The present study subsequently investigated the
regulatory mechanism of miR-93. As a previous study reported that
NFE2L2 is a target gene of miR-93 during breast carcinogenesis
(17), the protein expression
levels of NFE2L2 were analyzed in the transfected GTM cell groups
in the present study (Fig. 4). The
results indicated that the expression of NFE2L2 was promoted in the
GTM cells with inhibited miR-93 (P<0.01) and was suppressed when
miR-93 was overexpressed in the cells (P<0.01). These results
suggested that miR-93 inhibited NFE2L2 in the GTM cells, which may
be the possible functional pathway underlying the effect of miR-93
on regulating glaucoma.
Discussion
In the present study, miR-93 was found to be
upregulated in GTM cells. The inhibition of miR-93 resulted in the
promotion of cell viability and suppression of cell apoptosis,
whereas the overexpression of miR-93 abrogated these effects.
miR-93 was further found to suppress the expression of NFE2L2,
which provides a possible clue to the functional mechanism of
miR-93 in regulating GTM cells.
NFE2L2 is a regulator mediating the transcription
process of cytoprotective genes, which has been well-characterized
in previous studies of oxidative stress. Oxidative stress, together
with mitochondria impairment and pathogenic events, contributes to
a complex network of mechanisms leading to glaucoma (18,19).
Under quiescent conditions, NFE2L2 localizes in the cytoplasm
anchored by Kelch ECH associating protein 1 (KEAP1). When the cells
are stimulated by chemical signals, NFE2L2 escapes from KEAP1 and
is translocated to the nucleus, where it activates the expression
of target genes to enhance cell survival (20). To be specific, NFE2L2 prevents
chromium (VI)-induced cell apoptosis and oxidative stress via
promoting the expression of cytoprotective genes, including heme
oxygenase 1 and NAD(P)H: quinone oxidoreductase 1 (21). It also assists in resistance
against nitric oxide-dependent toxicity, thus preventing motor
neuron apoptosis (22). In tumor
cells, NFE2L2 functions as an inhibitor of apoptosis, with its
silencing leading to the suppression of tumor growth (23). In the present study, the level of
NFE2L2 was promoted when miR-93 was inhibited, which was
accompanied by reduced GTM cell apoptosis. This result is
concordant with those of existing reports that NFE2L2 acts as an
anti-apoptotic factor (24–26),
and suggested that NFE2L2 was involved in the regulation of GTM
cell apoptosis.
As mentioned above, miRNAs execute their functions
via regulating target genes post-transcriptionally. miR-93 can
regulate vascular endothelial growth factor (VEGF) (27) and the phosphatase and tensin
homolog/Akt pathway (28), thus
modulating cell performance and disease progression. It also
inhibits tumor growth in human colorectal cancer by suppressing the
cell cycle-associated factor, cyclin B1, and cell
proliferation-associated factors, Erb-B2 receptor tyrosine kinase
2, cyclin-dependent kinase inhibitor 1A and VEGF (29). In the present study, miR-93 was
found to inhibit the protein expression of NFE2L2. Although miR-93
was predicted to target the sequence ‘CACUUU’ of NFE2L2
using the online tool, microRNA.org
(data not shown), whether NFE2L2 is directly bound and
regulated by miR-93 remains to be elucidated. It is possible that
other modulators regulated by miR-93 caused the observed
downregulation of NFE2L2, which requires further investigation.
However, based on the results of the present study, it was clear
that miR-93 did function in GTM cells via regulating NFE2L2. The
affected NFE2L2 may have inhibited the expression of its target
genes, which contributed to the effects of miR-93 as a promoter of
cell apoptosis in the GTM cells.
The dynamic and aberrant expression of miRNAs has
been observed in various types of disease. The miR-23b/27b/24-1
cluster is downregulated in prostate cancer tissues, which can
serve as a marker of progression and a tumor suppressor of this
specific disease (30). miR-93 is
dynamically expressed during neural stem cell differentiation
(31). Similarly, in the present
study, miR-93 was found to be upregulated in GTM cells, which was
associated with its regulation of GTM cells by inhibiting cell
viability and promoting cell apoptosis, possibly by suppressing the
expression of NFE2L2. These specific expression profiles may
provide tools for cell isolation, target gene modulation in
specific stages and cell types, and gene therapy (32). As for glaucoma, gene therapy is
promising owing to the modification and improvement of transgene
vector delivery methods, including canaloplasty, which are under
investigation (33,34). Further investigations on the
therapeutic targets of glaucoma are essential for the future
implementation of gene therapy for glaucoma. It appears that miR-93
and its possible target, NFE2L2, discussed in the present study are
significant as potential therapeutic targets for treating
glaucoma.
In conclusion, the upregulation of miR-93 in GTM
cells was associated with its regulatory functions on inhibiting
cell viability and inducing cell apoptosis, which were possibly
enabled by suppressing NFE2L2. These results elucidated the roles
of miR-93 and its regulatory mechanism in GTM cells, providing
fundamental information and potential therapeutic targets for
treating glaucoma.
References
|
1
|
Kingman S: Glaucoma is second leading
cause of blindness globally. Bull World Health Organ. 82:887–888.
2004.PubMed/NCBI
|
|
2
|
Quigley HA: Glaucoma. Lancet.
377:1367–1377. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Gasiorowski JZ and Russell P: Biological
properties of trabecular meshwork cells. Exp Eye Res. 88:671–675.
2009. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Mozaffarieh M, Grieshaber MC and Flammer
J: Oxygen and blood flow: Players in the pathogenesis of glaucoma.
Mol Vis. 14:224–233. 2008.PubMed/NCBI
|
|
5
|
Tektas OY and Lütjen-Drecoll E: Structural
changes of the trabecular meshwork in different kinds of glaucoma.
Exp Eye Res. 88:769–775. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Hausser J and Zavolan M: Identification
and consequences of miRNA-target interactions-beyond repression of
gene expression. Nat Rev Genet. 15:599–612. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Asirvatham AJ, Magner WJ and Tomasi TB:
miRNA regulation of cytokine genes. Cytokine. 45:58–69. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Wu L and Belasco JG: Let me count the
ways: Mechanisms of gene regulation by miRNAs and siRNAs. Mol Cell.
29:1–7. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Georges M, Coppieters W and Charlier C:
Polymorphic miRNA-mediated gene regulation: Contribution to
phenotypic variation and disease. Curr Opin Genet Dev. 17:166–176.
2007. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Das J, Podder S and Ghosh TC: Insights
into the miRNA regulations in human disease genes. BMC Genomics.
15:10102014. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Luna C, Li G, Huang J, Qiu J, Wu J, Yuan
F, Epstein DL and Gonzalez P: Regulation of trabecular meshwork
cell contraction and intraocular pressure by miR-200c. PLoS One.
7:e516882012. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Li G, Luna C, Qiu J, Epstein DL and
Gonzalez P: Alterations in microRNA expression in stress-induced
cellular senescence. Mech Ageing Dev. 130:731–741. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Li G, Luna C, Qiu J, Epstein DL and
Gonzalez P: Targeting of integrin beta1 and kinesin 2alpha by
microRNA 183. J Biol Chem. 285:5461–5471. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Yu XF, Zou J, Bao ZJ and Dong J: miR-93
suppresses proliferation and colony formation of human colon cancer
stem cells. World J Gastroenterol. 17:4711–4717. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Du L, Schageman JJ, Subauste MC, Saber B,
Hammond SM, Prudkin L, Wistuba II, Ji L, Roth JA, Minna JD and
Pertsemlidis A: miR-93, miR-98, and miR-197 regulate expression of
tumor suppressor gene FUS1. Mol Cancer Res. 7:1234–1243. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(−Delta Delta C(T)) Method. Methods. 25:402–408. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Singh B, Ronghe AM, Chatterjee A, Bhat NK
and Bhat HK: MicroRNA-93 regulates NRF2 expression and is
associated with breast carcinogenesis. Carcinogenesis.
34:1165–1172. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Izzotti A, Saccà SC, Longobardi M and
Cartiglia C: Mitochondrial damage in the trabecular meshwork of
patients with glaucoma. Arch Ophthalmol. 128:724–730. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Saccà SC and Izzotti A: Focus on molecular
events in the anterior chamber leading to glaucoma. Cell Mol Life
Sci. 71:2197–2218. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Kensler TW, Wakabayashi N and Biswal S:
Cell survival responses to environmental stresses via the
Keap1-Nrf2-ARE pathway. Annu Rev Pharmacol Toxicol. 47:89–116.
2007. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
He X, Lin GX, Chen MG, Zhang JX and Ma Q:
Protection against chromium (VI)-induced oxidative stress and
apoptosis by Nrf2. Recruiting Nrf2 into the nucleus and disrupting
the nuclear Nrf2/Keap1 association. Toxicol Sci. 98:298–309. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Vargas MR, Pehar M, Cassina P, Beckman JS
and Barbeito L: Increased glutathione biosynthesis by Nrf2
activation in astrocytes prevents p75NTR-dependent motor neuron
apoptosis. J Neurochem. 97:687–696. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Singh A, Boldin-Adamsky S, Thimmulappa RK,
Rath SK, Ashush H, Coulter J, Blackford A, Goodman SN, Bunz F,
Watson WH, et al: RNAi-mediated silencing of nuclear factor
erythroid-2-related factor 2 gene expression in non-small cell lung
cancer inhibits tumor growth and increases efficacy of
chemotherapy. Cancer Res. 68:7975–7984. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Narasimhan M, Mahimainathan L, Rathinam
ML, Riar AK and Henderson GI: Overexpression of Nrf2 protects
cerebral cortical neurons from ethanol-induced apoptotic death. Mol
Pharmacol. 80:988–999. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Son YO, Pratheeshkumar P, Roy RV, Hitron
JA, Wang L, Zhang Z and Shi X: Nrf2/p62 signaling in apoptosis
resistance and its role in cadmium-induced carcinogenesis. J Biol
Chem. 289:28660–28675. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Bhakkiyalakshmi E, Shalini D, Sekar TV,
Rajaguru P, Paulmurugan R and Ramkumar KM: Therapeutic potential of
pterostilbene against pancreatic beta-cell apoptosis mediated
through Nrf2. Br JPharmacol. 171:1747–1757. 2014. View Article : Google Scholar
|
|
27
|
Long J, Wang Y, Wang W, Chang BH and
Danesh FR: Identification of microRNA-93 as a novel regulator of
vascular endothelial growth factor in hyperglycemic conditions. J
Biol Chem. 285:23457–23465. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Fu X, Tian J, Zhang L, Chen Y and Hao Q:
Involvement of microRNA-93, a new regulator of PTEN/Akt signaling
pathway, in regulation of chemotherapeutic drug cisplatin
chemosensitivity in ovarian cancer cells. FEBS Lett. 586:1279–1286.
2012. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Yang IP, Tsai HL, Hou MF, Chen KC, Tsai
PC, Huang SW, Chou WW, Wang JY and Juo SH: MicroRNA-93 inhibits
tumor growth and early relapse of human colorectal cancer by
affecting genes involved in the cell cycle. Carcinogenesis.
33:1522–1530. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Goto Y, Kojima S, Nishikawa R, Enokida H,
Chiyomaru T, Kinoshita T, Nakagawa M, Naya Y, Ichikawa T and Seki
N: The microRNA-23b/27b/24-1 cluster is a disease progression
marker and tumor suppressor in prostate cancer. Oncotarget.
5:7748–7759. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Lattanzi A, Gentner B, Corno D, Di Tomaso
T, Mestdagh P, Speleman F, Naldini L and Gritti A: Dynamic activity
of miR-125b and miR-93 during murine neural stem cell
differentiation in vitro and in the Subventricular Zone Neurogenic
Niche. PLoS One. 8:e674112013. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Gentner B, Visigalli I, Hiramatsu H,
Lechman E, Ungari S, Giustacchini A, Schira G, Amendola M,
Quattrini A, Martino S, et al: Identification of hematopoietic stem
cell-specific miRNAs enables gene therapy of globoid cell
leukodystrophy. Sci Transl Med. 2:58ra842010. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Tian B and Kaufman PL: A potential
application of canaloplasty in glaucoma gene therapy. Transl Vis
Sci Technol. 2pii(2)2013.
|
|
34
|
Aktas Z, Tian B, McDonald J, Yamamato R,
Larsen C, Kiland J, Kaufman PL and Rasmussen CA: Application of
canaloplasty in glaucoma gene therapy: Where are we? J Ocul
Pharmacol Ther. 30:277–282. 2014. View Article : Google Scholar : PubMed/NCBI
|