Introduction
Idiopathic pulmonary fibrosis (IPF) is a chronic,
irreversible and progressive lung disease of unknown origin, marked
by progressive dyspnoea, and ultimately, respiratory failure and
mortality (1). Idiopathic
pulmonary fibrosis occurs more commonly in older individuals and
men, cases are predominantly observed in male ex-smokers, it has a
mean survival of 3–5 years from the time of diagnosis (2,3).
Research over the past decade aiming to determine an effective
treatment to slow disease progression and improve survival, has
lead to unsatisfactory results.
Epithelial-mesenchymal transition (EMT) is an
essential mechanism and process in embryonic development and tissue
repair by which differentiated epithelial cells undergo a
phenotypic conversion and acquire a mesenchymal phenotype,
including a change from epithelial morphology and physiology, the
loss of cell-cell adhesion, and enhanced migratory and invasion
capacity (4,5). However, EMT also contributes to the
progression of disease, including organ fibrosis and cancer
(6–8). A previous study determined that EMT
has been observed in pulmonary epithelial cells and the lung in
vivo (9). A number of
myofibroblasts are derived from lung epithelial cells by
epithelial-mesenchymal transition (10). However, the molecular mechanisms
underlying IPF are largely unknown, the epithelial-mesenchymal
transition is likely responsible for the enhanced synthesis of
abnormal matrix observed in pulmonary fibrosis. The present study
aimed to investigate transforming growth factor-β1 (TGF-β1)-induced
EMT and whether melatonin prevents EMT in the A549 cell line.
Melatonin is secreted from the pineal gland, it
regulates circadian rhythms, sleep and immune system activity. In
addition, melatonin is a powerful and effective free radical
scavenger in oxidative stress and inflammation (11). As oxidative stress is important in
epithelial-mesenchymal transition (EMT) and fibrosis (12,13),
melatonin may protect cells, tissues, and organs against oxidative
damage and partially reverse EMT by reducing the oxidative stress.
Previous studies have also indicated that melatonin exerts an
anti-fibrotic effect at tissue level in liver, lung and heart
disease (14–16). However, the exact underlying
mechanism requires further elucidation.
From the guidelines on IPF treatment by the National
Clinical Guideline Centre (UK) in 2013, the limitations of current
pharmacological therapies for IPF demonstrate the importance of
other forms of treatment, including lung transplantation and best
supportive care, such as oxygen therapy, pulmonary rehabilitation
and palliation of symptoms (17).
Research into treatment of IPF has produced limited results.
Pirfenidone and nintedanib have been demonstrated to reduce
functional decline and disease progression with equivalent efficacy
and an acceptable safety profile, and have also resulted in
improved survival (18,19). However, the treatment outcomes do
not match expectations. The present study hypothesizes that
melatonin may be a potential therapeutic candidate for IPF and
aimed to investigate underlying mechanisms.
Materials and methods
Reagents
RPMI 1640 and fetal bovine serum (FBS) were obtained
from (Abcam, Cambridge, UK), penicillin-streptomycin (5,000 U/ml
penicillin; 5,000 U/ml streptomycin), were obtained from Gibco
(Thermo Fisher Scientific, Inc., Waltham, MA, USA). Dimethyl
sulfoxide (DMSO), paraformaldehyde, Triton X-100, Hoechst 33342 and
MTT were purchased from Sigma-Aldrich (St. Louis, MO, USA).
Melatonin was purchased from J&K Scientific Ltd. (Beijing,
China). Anti-β-actin, anti-E-cadherin, anti-vimentin,
anti-N-cadherin, anti-phosphorylated (p)-β-catenin, anti-p-Smad2
and anti-p-Smad3 antibodies were obtained from Santa Cruz
Biotechnology, Inc. (Dallas, TX, USA). ECL kit was purchased from
Thermo Fisher Scientific, Inc. All reagents used were trace element
analysis grade and all water used was glass distilled.
Cell culture
The A549 human alveolar epithelial cell line was
obtained from the American Type Cell Collection (Manassas, VA,
USA). Cells were cultured in RPMI 1640 with 10% FBS, 100 U/ml
penicillin and 100 µg/ml streptomycin. All cells were maintained at
37°C and 5% CO2 in a humidified atmosphere.
Cell viability assay
Cellular viability was assessed using the MTT assay.
Cells were plated in 96-well plates containing 200 µl culture
medium at a concentration of 5,000 cells/well with ≥90% viability.
Cells were cultured with various concentrations of melatonin (0,
0.25, 0.5, 1, 1.25, 1.5, 1.75 and 2 mM) for 24 h. Following
incubation, MTT dye (20 µl; 5 mg/ml) was added to the cells for 4 h
followed by incubation with DMSO for 10 min. Absorbance was
measured at a wavelength of 570 nm using a microplate reader
(UVM340; Asys-Hitech GmbH, Eugendorf, Austria). The cell viability
was determined as the ratio of signal between the treated and
control cultures. Melatonin alone at 1 to 2 mM suppressed cell
viability in a dose-dependent manner, however, above this
concentration the cell inhibition rate was almost unchanged.
Treatment of cells with melatonin (from 0.25 to 1.0 mM) did not
markedly enhance the inhibition of cell viability, compared with
the control cells. Thus, 0.5 mM was used as the treated
concentration in all subsequent experiments.
Western blotting
The A549 cell lysates were homogenized in a
radioimmunoprecipitation buffer (Thermo Fisher Scientific, Inc.)
comprised of 50 mM Tris-HCl (pH 7.5), 150 mM NaCl, 1% Triton X-100,
0.1% sodium dodecyl sulfate (SDS), 0.5% deoxycholic acid and 0.02%
sodium azide. Protein concentrations were determined using the
Pierce BCA Protein assay kit (Thermo Fisher Scientific, Inc.)
according to manufacturer's protocols. Equal quantities of protein
from each sample were separated electrophoretically using 10%
SDS-PAGE and transferred to a polyvinylidene fluoride membrane. The
membranes were blocked with 5% non-fat milk overnight at 4°C The
primary antibodies used in the present study were as follows: Mouse
monoclonal anti-β-actin (1:400; cat. no. sc-47778), mouse
monoclonal anti-N-cadherin (1:400; cat. no. sc-121905), mouse
monoclonal anti-vimentin (1:400; cat. no. sc-373717), mouse
monoclonal anti-E-cadherin (1:400; cat. no. sc-52327), mouse
monoclonal anti-p-β-catenin (1:400; cat. no. sc-101651), mouse
monoclonal anti-p-Smad2 (1:400; cat. no. sc-101801 2), and mouse
monoclonal anti-p-Smad3 (1:400; cat. no.sc-130218). The membranes
were then washed with TBST on a shaker twice at room temperature
for 10 min each timefollowed by TBS for 10 min. The membranes were
then incubated with horseradish peroxidase-conjugated rabbit
anti-mouse IgG secondary antibodies (1:400; Santa Cruz
Biotechnology, Inc.; cat. no. sc-358920) overnight. Detection was
performed using an enhanced chemiluminescence kit (Thermo Fisher
Scientific, Inc.) and the images were captured by X-ray film. The
relative quantities of various proteins were analyzed and the
results were quantified by Quantity One software V4.62 (Bio-Rad,
Hercules, CA, USA).
Immunofluorescence experiments
The protein expression levels of E-cadherin in A549
cells was examined using immunofluorescence. The cells were seeded
in 6-well plates, washed with ice-cold phosphate-buffered saline
(PBS) and fixed with 4% paraformaldehyde for 30 min at 4°C.
Following washing with PBS three times, the cells were incubated
with 1% Triton X-100 for 10 min. The cells were subsequently
incubated mouse monoclonal anti-E-cadherin (1:400) overnight.
Tetramethylrhodamine-conjugated goat anti-mouse IgG (1:100; Santa
Cruz Biotechnology, Inc.; cat. no. sc-2086) was incubated with the
cells for 30 min at room temperature. Finally, Hoechst 33342 was
added to the cells for 15 min and following three times with PBS,
cells were visualized under fluorescence microscopy.
Reverse transcription-polymerase chain
reaction (RT-PCR)
Total RNA from A549 cells was isolated using TRIzol
reagent (Invitrogen; Thermo Fisher Scientific, Inc.). The RNA
concentration was measured using absorbance at a wavelength 260 nm.
Total RNA (2 µg) was reverse transcribed to cDNA using a
PrimeScript RT reagent kit (Takara Biotechnology, Co., Ltd.,
Dalian, China). The reverse transcription program was as follows:
15 min at 37°C and 5 sec at 85°C. The sequences of WNT1 gene was
obtained from the GenBank database, and specific primers were
designed over an exon-exon junction with Primer Premier 5.0 (Jiran
Biotechnology, Co., Ltd., Shanghai, China). The primers used for
amplification were as follows: Forward,
5′-CGGAGTCAACGGATTTGGTCGTAT-3′ and reverse,
5′-AGCCTTCTCCATGGTGGTGAAGAC-3′ for GAPDH; and forward,
5′-TACCTCCAGTCACACTCCCC-3′ and reverse, 5′-CCATGGCAGGAGAATAGGAA-3′
for WNT1. For PCR, the cDNA templates were initially heat-denatured
at 94°C for 3 min; followed by 35 cycles of 94°C for 1 min,
annealing at 60°C for 1 min, and extension at 72°C for 1 min; with
a final extension cycle at 72°C for 10 min. The appropriate
negative control reactions were performed to demonstrate the
absence of DNA contamination. Products were analyzed on ethidium
bromide-stained 2% agarose gels and photographed under ultraviolet
light.
Statistical analysis
Statistical analysis was performed using SPSS
software (version 18.0; SPSS, Inc., Chicago, IL, USA). The data are
expressed as the mean ± standard error. The statistical
significance of the differences was calculated using Student's
t-test and one-way analysis of variance and Student-Neuman-Keuls
test. P≤0.05 was considered to indicate a statistically significant
difference.
Results
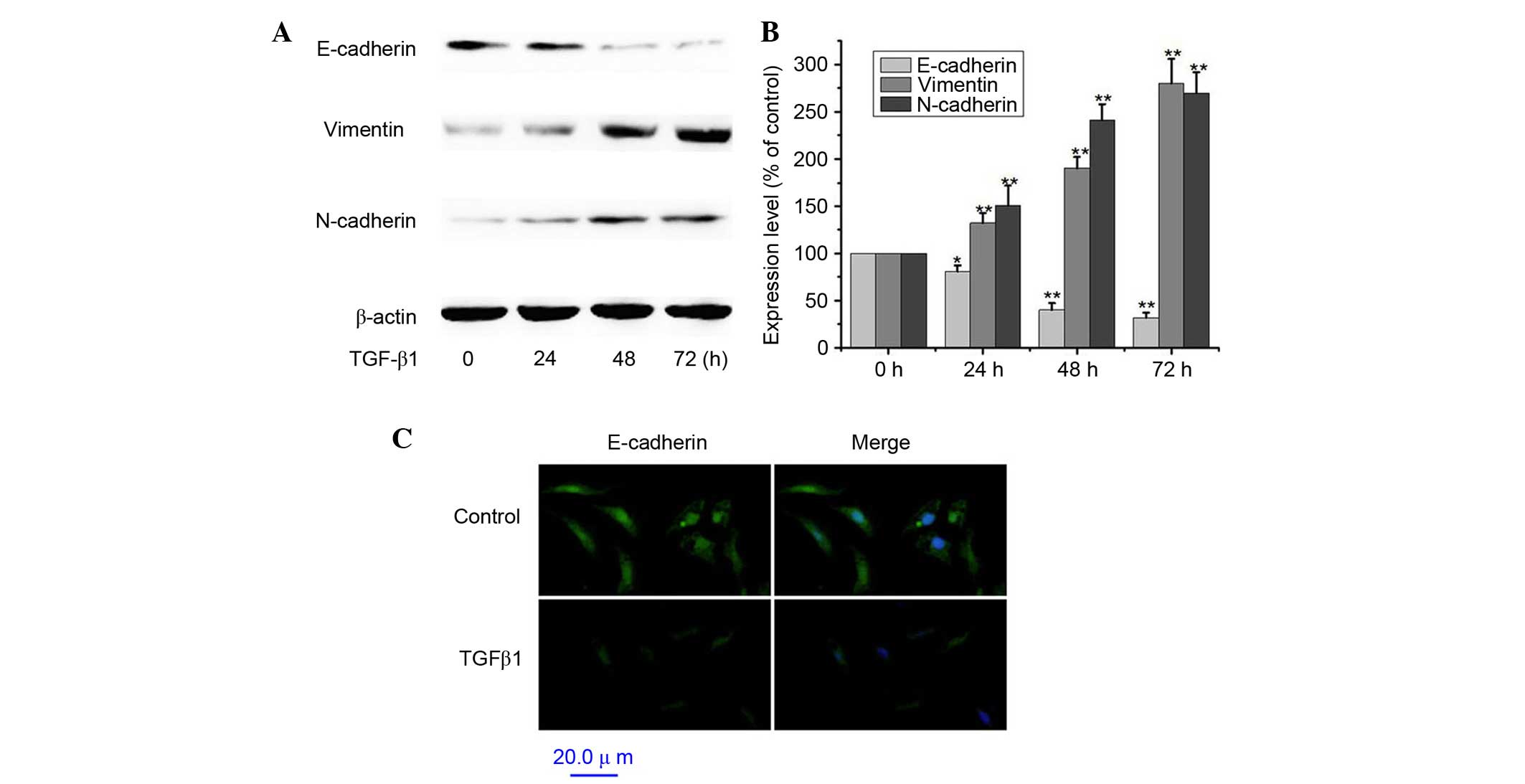
TGF-β1 induces EMT in A549 cells
A549 cells were treated with 5 ng/ml TGF-β1 for 0,
24, 48 and 72 h (20). To assess
the TGF-β1-induced EMT, A549 cells served as an in vitro
model system for investigating the expression of E-cadherin,
vimentin and N-cadherin. Exposure of A549 cells to 5 ng/ml TGF-β1
for 12–72 h significantly decreased protein expression levels of
the epithelial cell marker E-cadherin (P<0.01 at 72 h) and
significantly increased the expression levels of the mesenchymal
markers vimentin and N-cadherin compared with non-treated cells
(Fig. 1A and B; P<0.01 at 72
h). Similarly, treatment with TGF-β1 resulted in decreased
expression of E-cadherin by immunofluorescence (Fig. 1C). Thus, these results indicated
that TGF-β1-treated A549 cells lost their epithelial
characteristics and gained a mesenchymal phenotype. Therefore,
TGFβ1 positively induced the EMT of A549 cells.
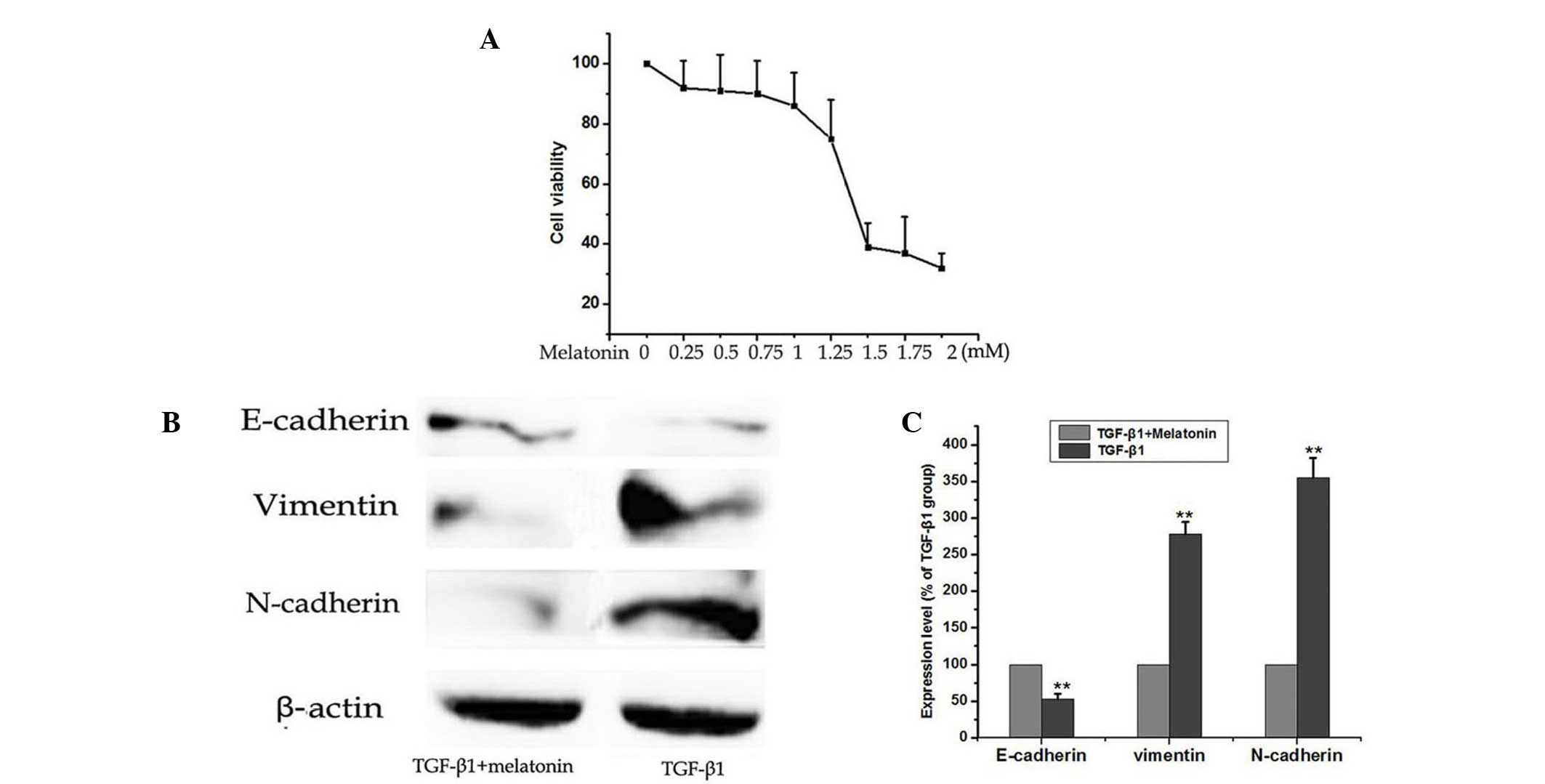
Melatonin inhibits TGF-β1-induced EMT
in A549 cells
The MTT assay was used to test the effects of
melatonin on cell viability in A549 cells. Melatonin alone (1–2 mM)
suppressed cell viability in a dose-dependent manner (Fig. 2A). However, the treatment of the
cells with doses of melatonin between 0.25 and 1.0 mM did not
significantly enhance the inhibition of cell viability compared
with the control cells. Above 2 mM, the cell inhibition rate was
almost unchanged. To detect whether melatonin inhibited TGF-β1
mediated EMT, A549 cells were cultured with 5 ng/ml TGF-β1 with or
without 0.5 mM melatonin for 48 h and the protein expression levels
of E-cadherin, vimentin and N-cadherin were detected by western
blotting. As demonstrated in the results of Fig. 2, the TGF-β1-induced EMT was
attenuated by co-treating the A549 cells with 0.5 mM melatonin.
This resulted in significant reduction of N-cadherin and vimentin,
and increased expression of E-cadherin. Treatment with melatonin
(0.5 mM) alone did not inhibit the cell viability as shown in
Fig. 2A; however, it markedly
inhibited the TGF-β1-induced EMT in A549 cells. (Fig. 2B and C). These results indicated
that melatonin supplementation reversed TGF-β1-induced EMT in A549
cells.
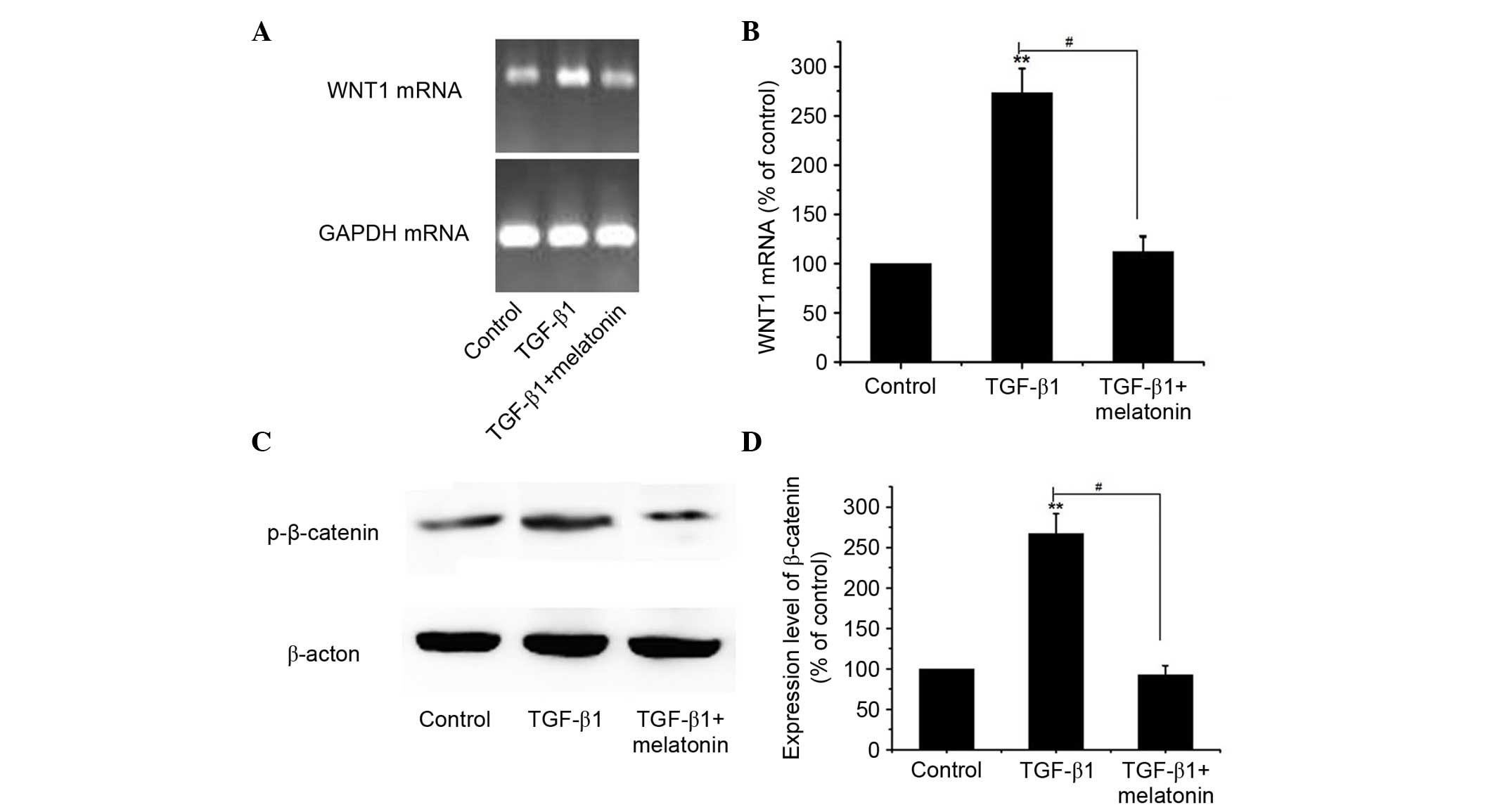
Melatonin inhibits TGF-β1-induced EMT
via suppression of Wnt/β-catenin signaling
The Wnt signaling pathway has previously been
associated with the development and progression of cancer due to
EMT-like transition (21). Thus,
the present study aimed to investigate the effect of melatonin on
the Wnt/β-catenin signaling pathway. A549 cells were cultured with
5 ng/ml TGF-β1 with or without 0.5 mM melatonin for 48 h, and the
expression levels of WNT1 and p-β-catenin were detected by RT-PCR
and western blotting. As demonstrated in Fig. 3, TGF-β1 alone resulted in
significantly increased mRNA and protein expression levels of WNT1
and p-β-catenin compared with the control. Notably, it was observed
that melatonin markedly inhibited TGF-β1-induced upregulation of
WNT1 and p-β-catenin compared with the treatment with TGF-β1 alone
(P<0.01). These results indicate that the anti-EMT effect of
melatonin treatment may partially be mediated via the inactivation
of the Wnt/β-catenin signaling pathway.
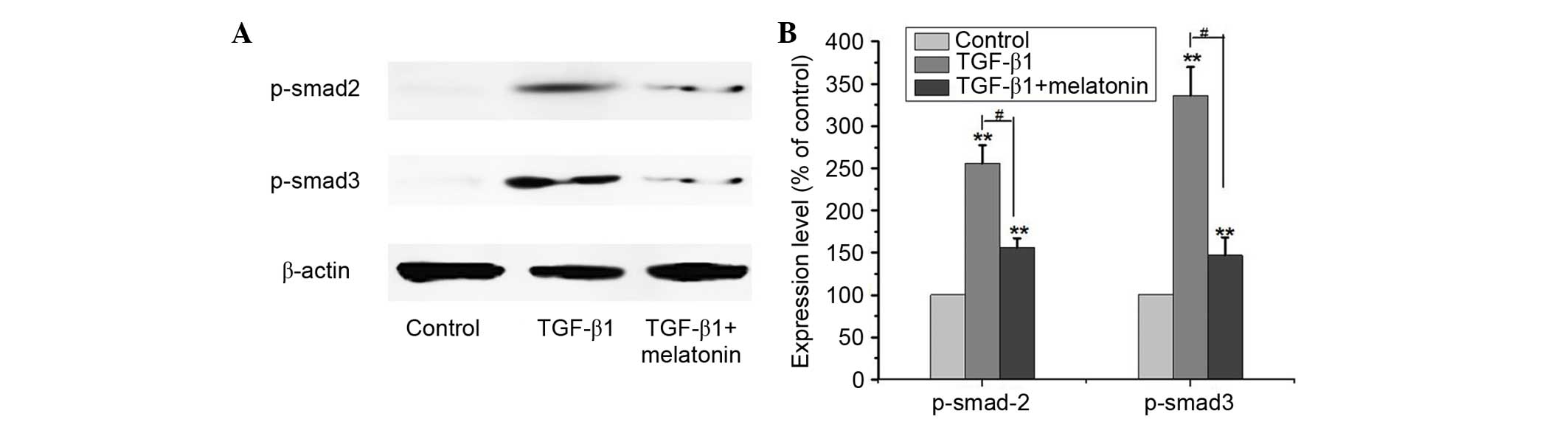
Melatonin inhibits TGF-β1-induced EMT
via suppression of Smad signaling
TGF-β1 has been regarded as a ‘master switch’ in the
regulation of EMT and demonstrated to signal primarily via the
Smad2/3 pathway (22). Thus, the
present study aimed to investigate whether melatonin treatment of
A549 cells inhibited the expression of Smad2/3 in blocking EMT.
Cells were cultured with 5 ng/ml TGF-β1 with or without 0.5 mM
melatonin for 48 h, and the protein expression levels of p-Smad2/3
were detected by western blotting. As presented in Fig. 4, TGF-β1 alone resulted in
significantly increased expression levels of p-Smad2/3 compared
with the control (P<0.01). Melatonin markedly inhibited the
upregulation of p-Smad2/3 when used with TGF-β1 compared with
treatment with TGF-β1 alone (P<0.01). These results indicate
that the anti-EMT effect of melatonin may partially be mediated via
inactivation of the Smad signaling pathway.
Discussion
Idiopathic pulmonary fibrosis (IPF) has a low
incidence (4.6–16.3/100,000), and a high mortality rate. It is
characterised by an interstitial fibrotic process (23). Over 10 years, the mortality of
pulmonary fibrosis has increased year by year (24). IPF patients require pharmacological
and non-pharmacological management strategies, however, numerous
attempts to elucidate therapeutic agents have not been successful.
Understanding of the pathobiology of IPF has increased, however,
the causative factors require further elucidation and the disease
pathogenesis is incompletely understood. Current concepts suggest
that alveolar epithelial cell injury characterized by
proliferation, migration, and activation of fibroblasts, and
secretion of excessive quantities of extracellular matrix
components, result in scarring of the lung, architectural
distortion, and irreversible loss of function (25). A previous study demonstrated that
EMT is essential in the pathogenesis of lung fibrosis (26). The present study aimed to
investigate the therapeutic potential and possible mechanisms of
action of melatonin in lung fibrosis.
Melatonin is predominantly produced by the pineal
gland, however it is also produced by other organs, including the
cerebellum and ovaries. It has numerous physiological functions,
such as sleep improvement, antioxidative effects, and endothelial
function, and does not have toxic or mutagenic effects (27,28).
A previous study demonstrated that melatonin is closely associated
with expression of TLR4 and TLR4-mediated inflammation, which is
key in the development of EMT (29). Melatonin may inhibit TLR4-mediated
inflammation (30). Reactive
oxygen species (ROS) have also been demonstrated to be important in
early EMT, and increase EMT in developmental and pathological EMT
(31). The present study
demonstrated that melatonin inhibited TGFβ1-induced EMT in the A549
cell line. Inflammation and oxidative stress are important in the
process of epithelial-mesenchymal transition and lung fibrosis. As
a potent antioxidant, melatonin may prevent the development of
atherosclerosis, hyperlipidemia and other consequences of aging
(32). Melatonin may have a
protective effect against ROS and EMT. The current study also
indicated that the Wnt/β-catenin and Smad2/3 signaling pathways are
involved in EMT in the A549 cell lines. Melatonin inhibits the
Wnt/β-catenin and Smad signaling pathways and ameliorates the
TGF-β1-induced EMT in A549 cells.
Melatonin markedly inhibits TGF-β1-induced
activation of EMT and has a protective effect in lung fibrosis,
this indicates that melatonin may be a promising therapeutic agent
for fibrosis of the lung. However, further studies on suppression
of pulmonary fibrosis by melatonin are required, including animal
experiments, research into side effects and assessment of the
effect of melatonin on experimentally-induced lung fibrosis.
Furthermore, there are potential limitations in the present study.
Animal experiments were not conducted and the direct impact of
melatonin on pulmonary fibrosis was not assessed, however, they may
be investigated in the future. IPF is closely associated with
TGF-β1-induced EMT, while melatonin could inhibit TGF-β1-induced
epithelial-mesenchymal transition in the A549 cell line. These
studies have opened up a new road for the treatment of pulmonary
fibrosis and provide a theoretical basis for clinical research.
References
|
1
|
Costabel U: The changing treatment
landscape in idiopathic pulmonary fibrosis. Eur Respir Rev.
24:65–68. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Renzoni E, Srihari V and Sestini P:
Pathogenesis of idiopathic pulmonary fibrosis: Review of recent
findings. F1000Prime Rep. 6:692014. View
Article : Google Scholar : PubMed/NCBI
|
|
3
|
Kraan JW, van den Blink B, van den Toorn
LM, Bresser P, van Beek FT, Grutters JC and Wijsenbeek MS:
Idiopathic pulmonary fibrosis: New insights. Ned Tijdschr Geneeskd.
159:A81482015.(In Dutch). PubMed/NCBI
|
|
4
|
Mezni I, Galichon P, Bacha MM, Sfar I,
Hertig A, Goucha R, Xu-Dubois YC, Abderrahim E, Gorgi Y, Rondeau E
and Abdallah TB: The epithelial-mesenchymal transition and fibrosis
of the renal transplant. Med Sci (Paris). 31:68–74. 2015.(In
French). View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Gonzalez DM and Medici D: Signaling
mechanisms of the epithelial-mesenchymal transition. Sci Signal.
7:re82014. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Long H, Xiang T, Qi W, Huang J, Chen J, He
L, Liang Z, Guo B, Li Y, Xie R and Zhu B: CD133+ ovarian cancer
stem-like cells promote non-stem cancer cell metastasis via CCL5
induced epithelial-mesenchymal transition. Oncotarget. 6:5846–5859.
2015. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Park JH and Yoon J: Schizandrin inhibits
fibrosis and epithelial-mesenchymal transition in transforming
growth factor-β1-stimulated AML12 cells. Int Immunopharmacol.
25:276–284. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
He L, Lou W, Ji L, Liang W, Zhou M, Xu G,
Zhao L, Huang C, Li R, Wang H, et al: Serum response factor
accelerates the high glucose-induced Epithelial-to-Mesenchymal
Transition (EMT) via snail signaling in human peritoneal
mesothelial cells. PLoS One. 9:e1085932014. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Kim KK, Kugler MC, Wolters PJ, Robillard
L, Galvez MG, Brumwell AN, Sheppard D and Chapman HA: Alveolar
epithelial cell mesenchymal transition develops in vivo during
pulmonary fibrosis and is regulated by the extracellular matrix.
Proc Natl Acad Sci USA. 103:13180–13185. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Namba T, Tanaka KI, Ito Y, Hoshino T,
Matoyama M, Yamakawa N, Isohama Y, Azuma A and Mizushima T:
Induction of EMT-like phenotypes by an active metabolite of
leflunomide and its contribution to pulmonary fibrosis. Cell Death
Differ. 17:1882–1895. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Hong RT, Xu JM and Mei Q: Melatonin
ameliorates experimental hepatic fibrosis induced by carbon
tetrachloride in rats. World J Gastroenterol. 15:1452–1458. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Milara J, Navarro R, Juan G, Peiró T,
Serrano A, Ramón M, Morcillo E and Cortijo J:
Sphingosine-1-phosphate is increased in patients with idiopathic
pulmonary fibrosis and mediates epithelial to mesenchymal
transition. Thorax. 67:147–156. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Zhou CF, Zhou DC, Zhang JX, Wang F, Cha
WS, Wu CH and Zhu QX: Bleomycin-induced epithelial-mesenchymal
transition in sclerotic skin of mice: Possible role of oxidative
stress in the pathogenesis. Toxicol Appl Pharmacol. 277:250–258.
2014. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Cho YA, Noh K, Jue SS, Lee SY and Kim EC:
Melatonin promotes hepatic differentiation of human dental pulp
stem cells: Clinical implications for the prevention of liver
fibrosis. J Pineal Res. 58:127–135. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Drobnik J, Slotwinska D, Olczak S, Tosik
D, Pieniazek A, Matczak K, Koceva-Chyla A and Szczepanowska A:
Pharmacological doses of melatonin reduce the glycosaminoglycan
level within the infarcted heart scar. J Physiol Pharmacol.
62:29–35. 2011.PubMed/NCBI
|
|
16
|
Zhao H, Wu QQ, Cao LF, Qing HY, Zhang C,
Chen YH, Wang H, Liu RY and Xu DX: Melatonin inhibits endoplasmic
reticulum stress and epithelial-mesenchymal transition during
bleomycin-induced pulmonary fibrosis in mice. PLoS One.
9:e972662014. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
National Clinical Guideline Centre (UK), .
Diagnosis and management of suspected idiopathic pulmonary
fibrosis: Idiopathic pulmonary fibrosis [Internet]. National
Institute for Health and Care Excellence: Clinical Guidelines.
2013.
|
|
18
|
Inomata M, Nishioka Y and Azuma A:
Nintedanib: Evidence for its therapeutic potential in idiopathic
pulmonary fibrosis. Core Evid. 10:89–98. 2015.PubMed/NCBI
|
|
19
|
Aravena C, Labarca G, Venegas C, Arenas A
and Rada G: Pirfenidone for idiopathic pulmonary fibrosis: A
systematic review and meta-analysis. PLoS One. 10:e01361602015.
View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Johansson J, Tabor V, Wikell A, Jalkanen S
and Fuxe J: TGF-β1-Induced epithelial-mesenchymal transition
promotes monocyte/macrophage properties in breast cancer cells.
Front Oncol. 5:32015. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Wu Y, Ginther C, Kim J, Mosher N, Chung S,
Slamon D and Vadgama JV: Expression of Wnt3 activates Wnt/β-catenin
pathway and promotes EMT-like phenotype in trastuzumab-resistant
HER2-overexpressing breast cancer cells. Mol Cancer Res.
10:1597–1606. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Borthwick LA, Gardner A, De Soyza A, Mann
DA and Fisher AJ: Transforming growth factor-β1 (TGF-β1) driven
epithelial to mesenchymal transition (EMT) is accentuated by tumour
necrosis factor α (TNFα) via crosstalk between the SMAD and NF-κB
pathways. Cancer Microenviron. 5:45–57. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Noth I, Zhang Y, Ma SF, Flores C, Barber
M, Huang Y, Broderick SM, Wade MS, Hysi P, Scuirba J, et al:
Genetic variants associated with idiopathic pulmonary fibrosis
susceptibility and mortality: A genome-wide association study.
Lancet Respir Med. 1:309–317. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Singh VK, George M Patricia and Gries CJ:
Pulmonary hypertension is associated with increased post-lung
transplant mortality risk in patients with chronic obstructive
pulmonary disease. J Heart Lung Transplant. 34:424–429. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Tzouvelekis A, Bonella F and Spagnolo P:
Update on therapeutic management of idiopathic pulmonary fibrosis.
Ther Clin Risk Manag. 11:359–370. 2015.PubMed/NCBI
|
|
26
|
Choi SH, Hong ZY, Nam JK, Lee HJ, Jang J,
Yoo RJ, Lee YJ, Lee CY, Kim KH, Park S, et al: A hypoxia-induced
vascular endothelial-to-mesenchymal transition in development of
radiation-induced pulmonary fibrosis. Clin Cancer Res.
21:3716–3726. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Longatti P, Perin A, Rizzo V, Comai S,
Giusti P and Costa CV: Ventricular cerebrospinal fluid melatonin
concentrations investigated with an endoscopic technique. J Pineal
Res. 42:113–118. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Rodella LF, Favero G, Foglio E, Rossini C,
Castrezzati S, Lonati C and Rezzani R: Vascular endothelial cells
and dysfunctions: Role of melatonin. Front Biosci (Elite Ed).
5:119–129. 2013.PubMed/NCBI
|
|
29
|
Li JG, Lin JJ, Wang ZL, Cai WK, Wang PN,
Jia Q, Zhang AS, Wu GY, Zhu GX and Ni LX: Melatonin attenuates
inflammation of acute pulpitis subjected to dental pulp injury. Am
J Transl Res. 7:66–78. 2015.PubMed/NCBI
|
|
30
|
Li H, Li Y, Liu D and Liu J: LPS promotes
epithelial-mesenchymal transition and activation of TLR4/JNK
signaling. Tumour Biol. 35:10429–10435. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Kim YM and Cho M: Activation of NADPH
oxidase subunit NCF4 induces ROS-mediated EMT signaling in HeLa
cells. Cell Signal. 26:784–796. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Duan W, Yang Y, Yi W, Yan J, Liang Z, Wang
N, Li Y, Chen W, Yu S, Jin Z and Yi D: New role of JAK2/STAT3
signaling in endothelial cell oxidative stress injury and
protective effect of melatonin. PLoS One. 8:e579412013. View Article : Google Scholar : PubMed/NCBI
|