Introduction
Acute leukemia is a clonal malignant hematopoietic
disorder that results from acquired genetic alterations and
epigenetic changes in normal hematopoietic stem/progenitor cells
(1). Since the use of all
trans-retinoic acid in acute promyelocytic leukemia, an increasing
number of therapeutic agents have been developed. Tyrosine-kinases
and proteasome inhibitors (2,3) were
used in leukemia and multiple myeloma respectively, and these
exhibited marked anti-tumor activities.
ZOL is used in metastatic bone disease, and
functions by accumulating in the bone and inhibiting osteoclastic
bone resorption (4). Preclinical
studies have suggested that ZOL had marked anti-tumor activities in
numerous types of solid and hematological malignancy by reducing
proliferation and inducing apoptosis, in addition to inhibiting
angiogenesis and tumor cell invasion (5–7). ZOL
also inhibited the prenylation of rat sarcoma (RAS) proteins by
inhibiting key enzymes, including farnesyl transferase and
geranylgeranyl transferase enzymes within the mevalonate pathway.
Blocking the prenylation of RAS proteins resulted in reduced
cellular proliferation and induced apoptosis of tumor cells
(8–10). ZOL alone exhibited marked
inhibitory effects on acute and chronic leukemic cell growth in
vitro and in vivo (11–13).
In chronic myeloid leukemia (CML) cells, previous studies indicated
that ZOL had anti-leukemic activity via suppression of the
proliferation and clonogenicity of imatinib-sensitive and
imatinib-resistant cells (11,14).
However, the underlying mechanism of the anti-leukemic activity of
ZOL in acute leukemia cells remains to be elucidated.
The present study revealed that ZOL inhibited cell
proliferation and induced cell apoptosis, with S phase cell cycle
arrest on HL-60 and HL-60/A cells. In addition, the present study
investigated the potential mechanism of apoptosis induced by ZOL,
which was accompanied by downregulation of B-cell lymphoma 2
(Bcl-2), upregulation of Bcl-2 associated X protein (Bax) and
cleaved poly (ADP-ribose) polymerase (PARP). These results
suggested that ZOL exerted an anti-leukemic effect via the
mitochondrial pathway, suggesting that ZOL may be useful as a novel
therapeutic agent in the treatment of leukemia.
Materials and methods
Cell culture and chemicals
HL-60 and HL-60/A cells were cultured in RPMI 1640
medium (Hyclone; GE Healthcare Life Sciences, Logan, UT, USA)
containing 10% fetal bovine serum (Hyclone; GE Healthcare Life
Sciences) at 37°C in 5% CO2. ZOL was obtained from
Sigma-Aldrich (Merck Millipore, Darmstadt, Germany).
Cell proliferation and viability
Cell proliferation was evaluated by WST-8 assay
using a Cell Counting Kit-8 (Dojindo Molecular Technologies, Inc.,
Rockville, MD, USA) according to the manufacturer's protocols.
Briefly, 1×104 cells were seeded into 96-well plates and
were then treated with increasing concentrations of ZOL (0, 0.025,
0.05, 0.1, 0.2, 0.4, 0.6, 0.8 and 1 mM) and incubated at 37°C in 5%
CO2 for 24, 48 and 72 h. Subsequently, 10 µl WST-8
solution was added to each well and incubated at 37°C for 4 h, and
the plates were read at a wavelength of 450 nm using a microplate
reader.
Measurement of apoptosis by flow
cytometry analysis and microscopic analysis
Apoptosis was evaluated using an Annexin V-propidium
iodide (Annexin V-PI) binding assay (Nanjing KeyGen Biotech Co.,
Ltd., Nanjing, China) according to the manufacturer's protocols.
HL-60 and HL-60/A cells were treated with 0, 0.2, 0.4 mM ZOL for
24, 48 and 72 h were collected and stained with Annexin V-PI for 15
min at 37°C in the dark. Apoptosis analysis was conducted using
flow cytometry and the data was analyzed using FlowJo software
version 7.6 (FlowJo, LLC., Ashland, OR, USA).
Hoechst 33342 (Sigma-Aldrich; Merck Millipore) was
used to examine nuclear fragmentation of apoptotic cells. Cells
were harvested and stained with Hoechst 33342 (10 µg/ml) for 15 min
and then slides were viewed using a fluorescence microscope. The
nuclei of normal cells, and those that had undergone apoptosis,
were counted in ten random areas per coverslip, with at least 100
cells counted. The data collected were from three independent
experiments.
Cell cycle analysis
Cells were collected, fixed and resuspended in
phosphate-buffered saline containing 100 µg/ml RNaseA, 0.2% Triton
X-100, and 50 µg/ml PI. The cell cycle was analyzed by flow
cytometry and the data was analyzed using Modfit LT for Mac Version
2.0 software (BD Biosciences, Franklin Lakes, NJ, USA).
Western blot analysis
HL-60 and HL-60/A cellular proteins were isolated
using a lysis buffer. Protein concentrations were measured using
Bradford's method. Proteins (40 µg) were separated on 8 and 12%
SDS-PAGE gels and transferred to nitrocellulose membranes (EMD
Millipore, Billerica, MA, USA). Membranes were blocked with 5%
bovine serum albumin (MP Biomedicals, Santa Ana, CA, USA) for 1 h
at room temperature and then incubated with rabbit primary
antibodies against Bcl-2 (1:1,000; catalog no. 4223), Bax (1:1,000;
catalog no. 5023), PARP (1:1,000; catalog no. 9532) and β-actin
(1:2,000; catalog no. 4970), obtained from Cell Signaling
Technology, Inc. (Danvers, MA, USA). Subsequently, the membranes
were incubated with a horseradish peroxidase-conjugated goat
anti-rabbit IgG (1:5,000; catalog no. SA00001-2; ProteinTech Group,
Inc., Chicago, IL, USA) and detected using enhanced
chemiluminescence reagents (Sigma-Aldrich; Merck Millipore)
according to the manufacturer's protocols.
Colony formation assay
HL-60 and HL-60/A cells were incubated in
methylcellulose culture in triplicate as described previously
(15). Briefly, 1 ml culture
mixture containing 2×103 cells, 0.9% methylcellulose
(R&D Systems, Inc., Minnneapolis, MN, USA), and various
concentrations of ZOL (0, 0.2 and 0.4 mM) was plated and incubated
at 37°C in 5% CO2 for two weeks. The colonies (>40 cells) were
evaluated by direct counting under an inverted microscope.
Statistical analysis
SPSS software version 16.0 (SPSS, Inc., Chicago, IL,
USA) was used to analyze data, which are presented as the mean ±
standard deviation. One-way analysis of variance was performed to
compare groups, followed by Fisher's post hoc test. P<0.05 was
considered to indicate a statistically significant difference.
Results
ZOL inhibits cell proliferation in AML
cells in a dose- and time-dependent manner
The structure of ZOL is presented in Fig. 1A. Initially, the anti-proliferative
effect of ZOL in AML cells was detected by the CCK-8 assay.
Compared with HL-60 cells, HL-60/A cells were resistant to
adriamycin (data not shown). Subsequently, HL-60 and HL-60/A cells
were exposed to a series of concentrations (0, 0.025, 0.05, 0.1,
0.2, 0.4, 0.6, 0.8, 1.0 mM) of ZOL for 24, 48 and 72 h. As
presented in Fig. 1B and C, the
cell viability inhibition was greater at 72 h compared with 24 and
48 h. ZOL inhibited the proliferation of HL-60 and HL-60/A cells in
a dose- and time-dependent manner. The half maximal inhibitory
concentration value at 48 and 72 h was 1.10±0.08 and 0.41±0.03 mM
for HL-60 cells, and 1.56±0.20 and 0.25±0.02 mM for HL-60/A cells,
respectively.
ZOL reduces colony formation capacity
in AML cells
In order to identify the effect of ZOL on colony
formation in AML cells, HL-60 and HL-60/A cells treated with
various concentrations of ZOL (0, 0.2 and 0.4 mM) were incubated in
methylcellulose culture for two weeks. Results demonstrated that
ZOL blocked colony formation in the two types of cells, suggesting
that ZOL significantly inhibited the colony formation capacity of
AML cells (Fig. 1D).
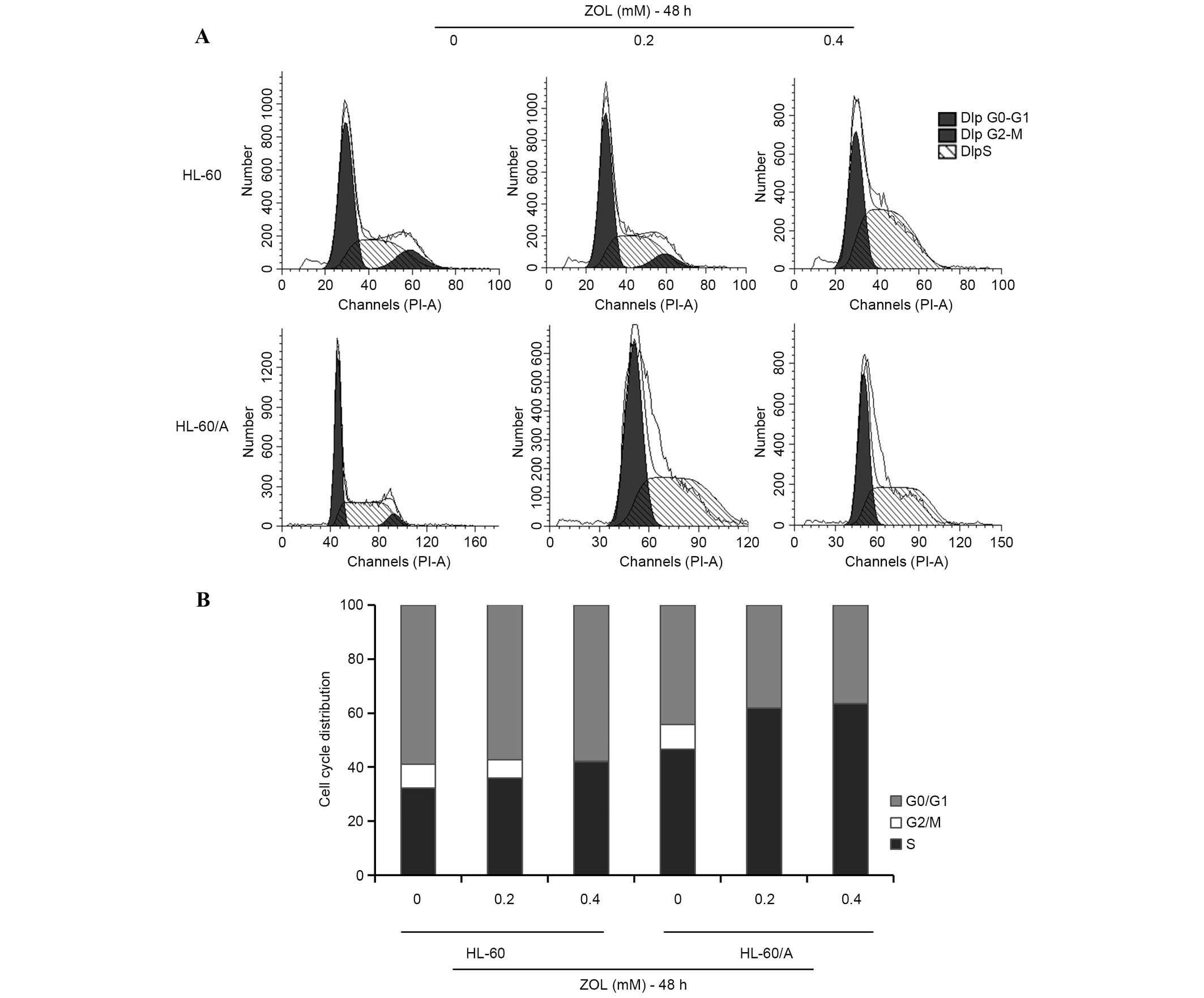
ZOL induces cell cycle arrest in S
phase
In order to determine whether ZOL induces cell cycle
arrest, the effect of ZOL on the AML cell cycle distribution was
analyzed using PI staining. Following treatment of cells with
various concentrations of ZOL (0, 0.2 and 0.4 mM) for 48 h, the
proportion of cells in S phase increased (Fig. 2A). Treatment of HL-60 and HL-60/A
cells with 0.2 mM ZOL for 48 h resulted in an increase in the
proportion of S phase cells from 32.14±1.78 to 41.23±2.31% in HL-60
cells and from 46.56±1.34 to 61.87±14.18% in HL-60/A cells
(Fig. 2B).
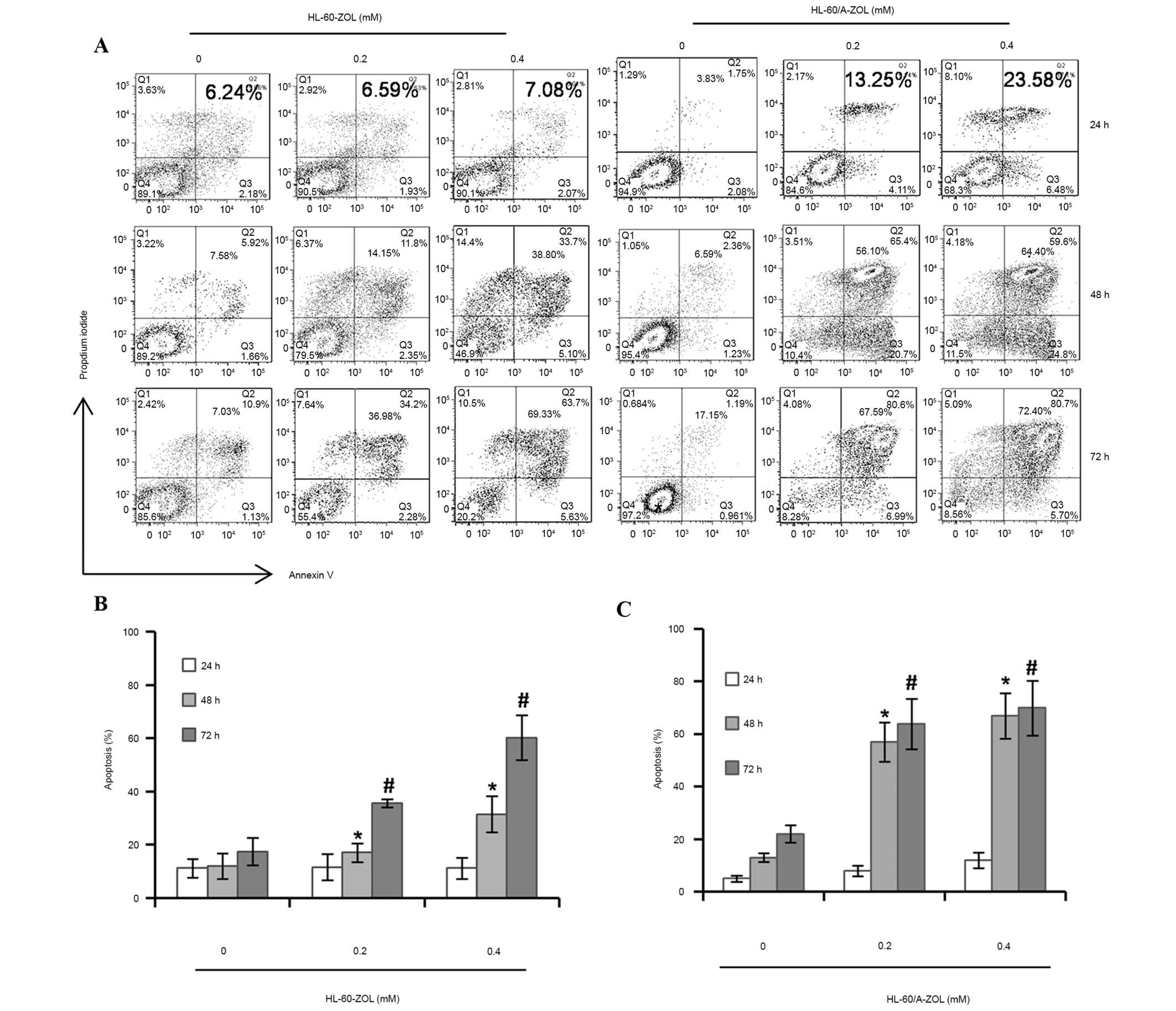
ZOL induces apoptosis in AML
cells
The Annexin V-PI double staining assay demonstrated
that ZOL induced apoptosis of AML cells in a dose- and
time-dependent manner (Fig. 3A).
Flow cytometry analysis indicated that the total apoptosis rates
were 11.57±4.94 and 16.18±4.00% in HL-60 cells treated with ZOL at
the concentrations of 0.2 and 0.4 mM for 24 h and increased to
35.58±1.49 and 60.24±8.50% at 72 h, respectively (Fig. 3B). However, in HL-60/A cells
treated with ZOL, the total apoptosis rates were 13.05±4.22 and
22.49±5.12% at the concentrations of 0.2 and 0.4 mM for 24 h and
significantly increased to 65.14±6.11 and 71.24±7.98% for 72 h
respectively (P<0.05; Fig. 3B).
Subsequently, AML cells were stained with Hoechst 33342 dye
following exposure to ZOL for 48 h and observed under a
fluorescence microscope. The results demonstrated that the nuclei
of untreated cells were round in shape, whilst the nuclei of cells
treated with ZOL were condensed or ruptured (Fig. 4A). The total apoptosis rates were
15.01±4.22 (0.2 mM) and 27.35±5.78% (0.4 mM) in HL-60 cells and
43.38±7.35 (0.2 mM) and 61.68±8.1% (0.4 mM) in HL-60/A cells
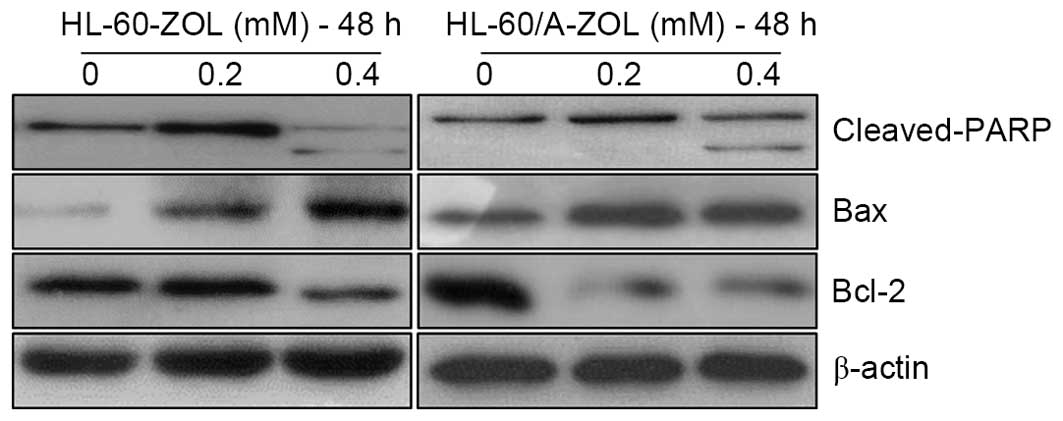
(Fig. 4B and C). In order to
determine the apoptotic mechanism of ZOL, the expression levels of
apoptosis-associated proteins were measured using the western
blotting method. Treatment of cells with 0.4 mM ZOL for 48 h
induced the expression of cleaved PARP and Bax, as well as
decreased the expression of Bcl-2. The upregulation of cleaved PARP
and Bax, and the downregulation of Bcl-2 are presented in Fig. 5.
Discussion
Over the past half century, progress has been made
regarding the treatment of AML, due to the development of molecular
targeted therapy and the development of chemotherapy and
hematopoietic stem cell transplantation. Long-term disease-free
survival can be achieved, however certain patients will relapse
following remission. Relapse is the predominant reason for
treatment failure. The cause of relapse remains controversial, with
minimal residual disease and multiple drug resistance as key
factors. Recently, molecular targeted therapy has become an area of
interest, with regards to overcoming the problems presented by
relapse (16).
ZOL, a third-generation nitrogen-containing
bisphosphonate, is used for the treatment of cancer-induced bone
disease in solid tumors and multiple myeloma. Accumulating evidence
has demonstrated that ZOL has marked anti-tumor activities in a
variety of cancer cells. The first study to assess the
anti-leukemic effect of ZOL investigated primary Philadelphia
chromosome-positive CML cells (11). Chuah et al (14) later demonstrated that ZOL was
effective in inhibiting the proliferation and clonogenicity of
imatinib-sensitive and -resistant CML cells, regardless of the
mechanism of resistance. The present study indicated that ZOL
inhibited the proliferation of AML cells in a dose- and
time-dependent manner, and this was consistent with results
obtained in previous studies (12,17).
To investigate the anti-proliferative efficacy on cell lines
refractory to other anti-cancer therapeutic agents, the current
study evaluated the ability of ZOL to suppress the growth of
HL-60/A cells. ZOL was demonstrated to have a marked effect in
HL-60/A cells. The present study also investigated the effect of
ZOL on colony formation activity in HL-60 and HL-60/A cells, and it
was demonstrated that 0.2 mM ZOL inhibited the colony formation
activities of HL-60 and HL-60/A cells. Previous studies
demonstrated that adriamycin-resistant tumor cells were associated
with the overexpression of multidrug resistance (MDR-1) and
activation of Akt/mammalian target of rapamycin signaling.
Knockdown of MDR-1, however, reversed drug resistance (18). Adriamycin-resistant gastric cancer
cells (SGC-7901/ADR) also exhibited activation of the Wnt/β-catenin
signaling pathway. The proton pump inhibitor pantoprazole could
block SGC7901/ADR cell invasiveness (19). The present study demonstrated that
ZOL overcomes adriamycin-induced drug resistance, indicating a
novel application in leukemia therapy.
The manner by which ZOL affects the cell cycle
remains to be elucidated. Forsea et al (20) indicated melanoma cells treated with
ZOL were arrested in S phase, however, Chuah et al (14) demonstrated that various CML cells
treated with ZOL were resistant to imatinib-induced S phase arrest.
In addition, ZOL altered the cell cycle distribution of the BV173
leukemia cell line from S phase to the boundary of G2/M
phase (11), and this effect was
tumor protein 53-independent. The present study demonstrated that
with an increase of therapeutic agent concentration, and length of
exposure time, the cell cycle could be arrested in the S phase,
indicating that the anti-proliferative effect of ZOL was achieved
by the induction of S-phase cell cycle arrest.
Apoptosis is defined as programmed cell death and
associated signaling pathways include the mitochondria-mediated
pathway and the death receptor-mediated pathway. The
apoptosis-associated Bcl-2 protein family regulates the
mitochondria-mediated pathway. The increase in the Bax/Bcl-2 ratio
results in an increase in the permeability of the mitochondrial
outer membrane and induces the release of high molecular weight
pro-apoptotic effectors from the mitochondrial inner membrane to
the cytoplasm (21). The present
study investigated the effect of ZOL on cell apoptosis using the
Annexin V-PI assay. The results indicated that ZOL significantly
induced apoptosis of HL-60 and HL-60/A cells compared with the
control group. As presented in Fig
3B, following exposure to 0.2 mM ZOL for 48 and 72 h, the
apoptotic rate was determined to be 17.04 and 35.58% for HL-60
cells, and 76.2 and 75.14% for HL-60/A cells. Similar results were
observed in Hoechst 333342 staining. In order to investigate the
mechanism of the pro-apoptotic effect of ZOL, the effect of ZOL on
apoptosis-associated proteins, such as Bax, Bcl-2 and cleaved PARP
were investigated, and the levels of these proteins were
demonstrated to be consistent. Thus, the proapoptotic effect of ZOL
may be associated with the mitochondrial-mediated pathway of cell
apoptosis. Ottewell et al (22) indicated that ZOL and doxorubicin
induced cleavage of caspase 8 resulting in activation of the
mitochondria-independent pathway, which was consistent with results
observed in the present study.
In conclusion, the present study demosntrated that
ZOL inhibited proliferation and induced apoptosis in HL-60 and
HL-60/A cells, and its anti-leukemic activity was more
predominantly observed in HL-60/A cells, suggesting that ZOL could
overcome adriamycin resistance, providing a novel approach and
possible therapeutic strategy for the treatment of leukemia.
Acknowledgements
The present study was supported by the Science and
Technology Project of Guangdong (grant no. 2011B080701008 to X.-F.
Liu and no. 2013B021800079 to Dr D.-J. Lin) and the Medical
Research Foundation of Guangdong Province (grant no. B2013134 to Dr
R.-F. Fan). The authors would like to thank the members of Quentin
Liu's laboratory and the Medical Research Center, Third Affiliated
Hospital, Sun Yat-sen University for their critical comments and
technical support.
Glossary
Abbreviations
Abbreviations:
|
AML
|
acute myeloid leukemia
|
|
ZOL
|
zoledronic acid
|
|
HL-60/A
|
adriamycin-resistant HL-60
|
|
CML
|
chronic myeloid leukemia
|
|
MDR-1
|
multidrug resistance
|
References
|
1
|
Focosi D: Acute myeloid leukaemia. Lancet.
369:3672007. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Waller CF: Imatinib mesylate. Recent
Results Cancer Res. 201:1–25. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Moreau P, Richardson PG, Cavo M, Orlowski
RZ, San Miguel JF, Palumbo A and Harousseau JL: Proteasome
inhibitors in multiple myeloma: 10 years later. Blood. 120:947–959.
2012. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Kiper HD, Kaymaz B Tezcanli, Gokbulut AA,
Selvi N, Avci CB, Kosova B, Iskender G, Yandim MK, Gunduz C, Sahin
F, et al: STAT pathway in the regulation of zoledronic acid-induced
apoptosis in chronic myeloid leukemia cells. Biomed Pharmacother.
67:527–532. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Jagdev SP, Coleman RE, Shipman CM, Rostami
HA and Croucher PI: The bisphosphonate, zoledronic acid, induces
apoptosis of breast cancer cells: Evidence for synergy with
paclitaxel. Br J Cancer. 84:1126–1134. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Stresing V, Fournier PG, Bellahcéné A,
Benzaïd I, Mönkkönen H, Colombel M, Ebetino FH, Castronovo V and
Clézardin P: Nitrogen-containing bisphosphonates can inhibit
angiogenesis in vivo without the involvement of farnesyl
pyrophosphate synthase. Bone. 48:259–266. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Tassone P, Tagliaferri P, Viscomi C,
Palmieri C, Caraglia M, D'Alessandro A, Galea E, Goel A, Abbruzzese
A, Boland CR and Venuta S: Zoledronic acid induces
antiproliferative and apoptotic effects in human pancreatic cancer
cells in vitro. Br J Cancer. 88:1971–1978. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Goffinet M, Thoulouzan M, Pradines A,
Lajoie-Mazenc I, Weinbaum C, Faye JC and Séronie-Vivien S:
Zoledronic acid treatment impairs protein geranyl-geranylation for
biological effects in prostatic cells. BMC Cancer. 6:602006.
View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Iguchi T, Miyakawa Y, Yamamoto K, Kizaki M
and Ikeda Y: Nitrogen-containing bisphosphonates induce S-phase
cell cycle arrest and apoptosis of myeloma cells by activating MAPK
pathway and inhibiting mevalonate pathway. Cell Signal. 15:719–727.
2003. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Oades GM, Senaratne SG, Clarke IA, Kirby
RS and Colston KW: Nitrogen containing bisphosphonates induce
apoptosis and inhibit the mevalonate pathway, impairing Ras
membrane localization in prostate cancer cells. J Urol.
170:246–252. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Kuroda J, Kimura S, Segawa H, Kobayashi Y,
Yoshikawa T, Urasaki Y, Ueda T, Enjo F, Tokuda H, Ottmann OG and
Maekawa T: The third-generation bisphosphonate Zoledronic Acid
synergistically augments the anti-Ph+ leukemia activity of imatinib
mesylate. Blood. 102:2229–2235. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Fiegl M, Juergens M, Hiddemann W and
Braess J: Cytotoxic activity of the third-generation bisphosphonate
zoledronic acid in acute myeloid leukemia. Leuk Res. 31:531–539.
2007. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Ohtsuka Y, Manabe A, Kawasaki H, Hasegawa
D, Zaike Y, Watanabe S, Tanizawa T, Nakahata T and Tsuji K:
RAS-blocking bisphosphonate zoledronic acid inhibits the abnormal
proliferation and differentiation of juvenile myelomonocytic
leukemia cells in vitro. Blood. 106:3134–3141. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Chuah C, Barnes DJ, Kwok M, Corbin A,
Deininger MW, Druker BJ and Melo JV: Zoledronate inhibits
proliferation and induces apoptosis of imatinib-resistant chronic
myeloid leukaemia cells. Leukemia. 19:1896–1904. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Ebihara Y, Tsuji K, Lyman SD, Sui X,
Yoshida M, Muraoka K, Yamada K, Tanaka R and Nakahata T:
Synergistic action of Flt3 and gp130 signalings in human
hematopoiesis. Blood. 90:4363–4368. 1997.PubMed/NCBI
|
|
16
|
Hatzimichael E, Georgiou G, Benetatos L
and Briasoulis E: Gene mutations and molecularly targeted therapies
in acute myeloid leukemia. Am J Blood Res. 3:29–51. 2013.PubMed/NCBI
|
|
17
|
Liu SS, Wang XP, Li XB, Liang JY, Liu LL,
Lu Y, Zhong XY and Chen YX: Zoledronic acid exerts antitumor
effects in NB4 acute promyelocytic leukemia cells by inducing
apoptosis and S phase arrest. Biomed Pharmacother. 68:1031–1036.
2014. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Wang L, Chen B, Lin M, Cao Y, Chen Y, Chen
X, Liu T and Hu J: Decreased expression of nucleophosmin/B23
increases drug sensitivity of adriamycin-resistant Molt-4 leukemia
cells through mdr-1 regulation and Akt/mTOR signaling.
Immunobiology. 220:331–340. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Zhang B, Yang Y, Shi X, Liao W, Chen M,
Cheng AS, Yan H, Fang C, Zhang S, Xu G, et al: Proton pump
inhibitor pantoprazole abrogates adriamycin-resistant gastric
cancer cell invasiveness via suppression of Akt/GSK-β/β-catenin
signaling and epithelial-mesenchymal transition. Cancer Lett.
356:704–712. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Forsea AM, Müller C, Riebeling C, Orfanos
CE and Geilen CC: Nitrogen-containing bisphosphonates inhibit cell
cycle progression in human melanoma cells. Br J Cancer. 91:803–810.
2004.PubMed/NCBI
|
|
21
|
Wong WW and Puthalakath H: Bcl-2 family
proteins: The sentinels of the mitochondrial apoptosis pathway.
IUBMB Life. 60:390–397. 2008. View
Article : Google Scholar : PubMed/NCBI
|
|
22
|
Ottewell PD, Woodward JK, Lefley DV, Evans
CA, Coleman RE, Holen I and Penelope D: Anticancer mechanisms of
doxorubicin and zoledronic acid in breast cancer tumor growth in
bone. Mol Cancer Ther. 8:2821–2832. 2009. View Article : Google Scholar : PubMed/NCBI
|