Introduction
Application of nucleic acid recombination
technology, particularly when conducted with stem cells, has
advanced gene therapy from bench to bedside (1,2).
Gene therapy is used in correcting inherited disorders, but also in
healing diverse acquired diseases, such as carcinoma, heart
failure, neurodegenerative and metabolic disorders and acquired
immune deficiency syndrome (3–8). An
increasing number of clinical trials have revealed that gene
transplantation- and stem cell-based bone tissue engineering are
effective therapeutic options for promoting osteogenesis in bone
and joint surgery (9–11).
Typically, bone regeneration is a complicated
process that involves a series of cellular signaling pathways that
are triggered or regulated by multiple growth factors and
biomolecules. The transforming growth factor-β (TGF-β) superfamily
comprises a group of multifunctional peptide growth factors
exerting a marked impact on the osteogenic potential of progenitor
cells. Initiation of canonical TGF-β/Smad signaling leads to
expression of osteogenic genes, which is followed by osteogenic
differentiation of various stem cells (12–14).
Among TGF-β growth factors, the subfamily of bone morphogenetic
proteins (BMPs) has been extensively studied and a number of
mediators in cartilage and bone formation have been identified
(15). As a member of the BMP
family, BMP-2 is important in the bone remodeling process (16,17).
It was demonstrated that BMP-2 had a role in bone defect healing
when delivered with a carrier substance (15,18)
and was able to induce bone synthesis within two weeks following
implantation of transfected cells (19). In addition, evidence has shown that
a short period of BMP-2 expression is sufficient to induce bone
regeneration (20), hence the
hypothesis that BMP-2 is one of the most active promoters for
differentiation of mesenchymal cells to osteoblasts in
vitro, in addition to being able to induce bone formation in
vivo (21).
TGF-β3, one of the three TGF-β isoforms, was
generally recognized to facilitate chondrogenic differentiation of
precursor cells (22,23), however, a previous study has also
shown it may have a dose-dependent inhibitory effect on
osteogenesis (24). By contrast,
previous studies have also demonstrated that mammalian TGF-β3 is a
regulator implicated in the early stages of osteoblastic
differentiation (25–27). Klar et al (28) observed that TGF-β3 signaling
elicited endochondral bone differentiation by regulating BMP
activity, and, thus, induction of bone formation. Furthermore, the
previous study reported that TGF-β3 stimulates bone synthesis via
upregulation of endogenous BMP-2. Therefore, the role of TGF-β3 in
bone formation is of considerable interest and remains to be
elucidated.
In a previous study, co-delivery of BMP-2 and TGF-β3
was demonstrated to be more effective than single gene-transfection
in promoting ossification of the annulus fibrosus (29). Therefore, the present study
simultaneously expressed BMP-2 and TGF-β3 genes in rBMSCs and
determined their expression status in vitro so as to
elucidate whether they can be synergistically expressed in
vivo. Further investigation into the effect on bone
differentiation and regeneration following delivery was also
conducted to elucidate possible underlying mechanisms of their
synergistic effect.
Materials and methods
Experimental animals
A total of two male and two female New Zealand white
rabbits (weight, 400–500 g; age, 4 weeks) were obtained from the
Animal Experimentation Center of Qingdao University (Qingdao,
China). Rabbits were housed individually in standard cages and
maintained under standard laboratory conditions (relative humidity,
50±10%; temperature 25±1°C; 12-h light/dark cycles), with access to
food twice a day and free access to water. Rabbits were sacrificed
by peritoneal injection with 10 ml/kg of 10% chloral hydrate.
All experiments were conducted in accordance with
the Guidance Suggestions for the Care and Use of Laboratory Animals
of the Ministry of Science and Technology of China (30). Animal procedures were approved by
the Medical Ethics Committee of Yantai Yuhuangding Hospital
(Yantai, China).
Isolation and culture of rBMSCs
BMSCs were isolated from the tibial and femoral
shafts of the rabbits. The ends of the femora were cut off at the
epiphysis, and the marrow was flushed out using 20 ml α-minimum
essential medium (α-MEM; Hyclone; GE Healthcare Life Sciences,
Logan, UT, USA) supplemented with 10% fetal bovine serum (FBS;
Gibco; Thermo Fisher Scientific, Inc., Waltham, MA, USA), 100 U/ml
of penicillin and 100 µg/ml of streptomycin (Sigma-Aldrich; Merck
Millipore, Darmstadt, Germany) with a 20-gauge needle. The
collected cells were collected into 25-cm2 cell culture
flasks (Nalge Nunc International, Penfield, NY, USA) containing 5
ml of the aforementioned medium. The medium was changed after 48 h
to remove non-adherent cells and then renewed every day. Cultures
were maintained in a humidified atmosphere of 5% CO2 at
37°C. Following reaching 70–80% confluence (after ~1 week), the
cells were harvested using 0.25% trypsin (Hyclone; GE Healthcare
Life Sciences) and cell concentration was adjusted to
1×106 cells/l. Following passage, the cells were plated
in flasks and cultured until third-passage rBMSCs (P3) were
obtained.
Plasmid construction and transfection
of lentivirus vectors
The DNA fragment that encoded human BMP-2 or TGF-β3
that had been cloned into the pIRES vector, was provided by the
Central Laboratory at the Medical School of Qingdao University
(Qingdao, China). Corresponding lentivirus packaging plasmids were
produced by Shanghai GenePharma Co., Ltd. (Shanghai, China).
P3 rBMSCs were divided into four groups as follows:
i) Group I, negative controls, consisting of untransfected rBMSCs
or rBMSCs transfected with an empty vector (vehicle); ii) group II,
rBMSCs transfected with lentivirus carrying green fluorescent
protein (GFP)/BMP-2; iii) group III, rBMSCs transfected with
lentivirus carrying TGF-β3; iv) group IV, rBMSCs co-transfected
with BMP-2 and TGF-β3. The procedure of transfection was performed
as previously described (30).
Briefly, rBMSCs were seeded in 6-well culture plates and
sequentially infected with lentivirus (Shanghai GenePharma Co.,
Ltd.) encompassing the indicated genes [multiplicity of infection
(MOI) =20, 30, 40, 50, 55 and 60] or negative short hairpin RNA
(Lenti-shcontrol) at 80% confluency (~500,000 cells/well) using
Polybrene (8 µg/ml culture medium; Sigma-Aldrich; Merck Millipore).
The efficiency of transfection was estimated by detecting the
proportion of GFP-positive rBMSCs under a fluorescence
microscope.
Reverse transcription-polymerase chain
reaction (RT-PCR)
Following transfection with corresponding plasmids,
after one week, total RNA was isolated from cells using TRIzol
(Takara Bio, Inc., Otsu, Japan) according to the manufacturer's
protocols, and subsequently digested with RNase-free DNase I. The
concentration and quality of extracted RNA was evaluated by
calculating the absorbance at 260 nm (A260) and the
A260/280 ratio, respectively, using a spectrophotometer.
cDNA was generated by reverse transcription using 1 µg RNA as a
template, and RT-PCR was subsequently conducted. To evaluate BMP-2
and TGF-β3 expression, quantities of target genes were normalized
to that of the housekeeping gene GAPDH, which served as the
internal control. The sequences of forward and reverse primers
(synthesized at Sangon Biotech Co., Ltd., Shanghai, China) used in
the present study were as follows: Forward,
5′-CCAACCATGGATTCGTGGTG-3′ and reverse, 5′-GGTACAGCATCGAGATAGCA-3′
for BMP-2; forward, 5′-TGGCTGTTGAGAAGAGAGTCC-3′ and reverse,
5′-TGCTTCAGGGTTCAGAGTGTT-3′ for TGF-β3; and forward,
5′-GCCTGGAGAAAGCTGCTAAGTA-3′ and reverse,
5′-CGTTGTCATACCAGGAAATGAG-3′ for GAPDH. The amplification profile
was 95°C for 5 min, followed by 38 cycles (36 cycles for GAPDH) of
denaturation at 98°C for 10 sec, hybridization annealing at 62°C
(60°C for GAPDH) for 30 sec, and extension at 72°C for 45 sec,
followed by an extension cycle for 10 min at 72°C. PCR products
were visualized on 1.0% (w/v) agarose gels stained with ethidium
bromide. The band densities were quantified by detecting absorbance
values and analyzed using Quantity One software (version 4.6;
Bio-Rad Laboratories, Inc., Hercules, CA, USA) to measure mRNA
levels. The signals were normalized to GAPDH expression.
Experiments were performed in triplicate.
Osteogenic induction
To induce osteogenic differentiation, the rBMSCs
were cultured in α-MEM containing 10% FBS, 1% penicillin and
streptomycin, 50 µg/ml ascorbic acid (Sigma-Aldrich; Merck
Millipore), 10 mM β-glycerophosphate (Sigma-ldrich; Merck
Millipore), and 10 nM dexamethasone (Sigma Aldrich; Merck
Millipore). The culture medium was exchanged every 3 days.
Alizarin Red S staining
Staining was performed as described in our previous
study (30). Briefly, on day 21,
cells were fixed and stained with Alizarin Red S staining solution.
The stained monolayers were then washed 3 times with
phosphate-buffered saline (PBS) and visualized using phase
microscopy with an inverted microscope (DMI4000B, Leica
Microsystems GmbH, Wetzlar, Germany).
Alkaline phosphatase (ALP)
activity
The activity of ALP and total protein quantity were
assessed on days 0, 3, 7, 14 and 21. The lysates was determined by
LabAssay ALP colorimetric assay kit (Wako Pure Chemical Industries,
Ltd., Osaka, Japan). Total proteins were determined by BCA Protein
assay kit (Beyotime Institute of Biotechnology, Haimen, China)
following the standard protocol. The activity of ALP was calculated
as phosphorylated nitrophenol release in n/mol/h and was further
normalized to the cell protein input. Each sample was assessed in
triplicate.
Protein extraction and Western
blotting analysis
Cultures were washed three times with PBS, and
sequentially harvested cells were pelleted by centrifugation at
8,000 × g for 15 min at room temperature. Cell pellets were
then resuspended in radioimmunoprecipitation assay lysis buffer
containing 1% phenylmethylsulfonyl fluoride protease inhibitor,
before samples were incubated on ice for 1 h. Lysates were
subjected to ultrasonication on ice for further lysing and cell
debris was removed by centrifugation at 16,000 × g for 10
min at 4°C. Following centrifugation, protein concentration was
determined using a QuantiPro BCA assay kit (Sigma-Aldrich; Merck
Millipore) and the protein supernatant was kept at −80°C for future
analysis.
For immunoblotting, proteins (~40 µg) were separated
on 8% SDS-PAGE and transferred to polyvinylidene difluoride
membranes at 60 V for 1 h at 4°C. The membranes were blocked with
milk and then incubated overnight at 4°C with primary antibodies
against mouse monoclonal BMP-2 (dilution, 1:1,000; Abcam,
Cambridge, MA, USA; cat no. ab6285), rabbit polyclonal TGF-β3
(dilution, 1:1,000; Abcam; cat no. ab15537), Runx2-C-terminal
region (dilution, 1:1,000; Aviva Systems Biology Corporation, San
Diego, CA, USA; cat no. ARP38453_P050), Osx (dilution, 1:200;
Beijing Biosynthesis Biotechnology Co., Ltd., Beijing, China; cat
no. bs-1110R), or mouse monoclonal β-actin (1:2,000; Abcam; cat no.
ab6276,), followed by rinsing 3 times with PBS with Tween 20 for 30
min, and subsequently incubated with secondary antibodies at room
temperature for 1 h. Secondary antibodies were horseradish
peroxidase (HRP)-conjugated goat anti-mouse IgG (dilution, 1:2,000;
Sigma-Aldrich; Merck Millipore; cat no. A0168) or HRP-conjugated
goat anti-rabbit IgG (dilution, 1:3,000; Sigma-Aldrich; Merck
Millipore; cat no. A0545). The membrane was washed with PBS
containing 0.05% Tween 20 three times, for 10 min each time, prior
to being developed using the Immobilon™ Western Chemiluminescent
HRP Substrate (Merck Millipore; cat. no. WBKLS0500).
Statistical analysis
Data are presented as the mean ± standard deviation.
Significance between various treatment samples was calculated using
the Student's t-test. All statistical analyses were conducted with
SPSS 19.0 software (IBM SPSS, Armonk, NY, USA). P<0.05 was
considered to indicate a statistically significant difference.
Results
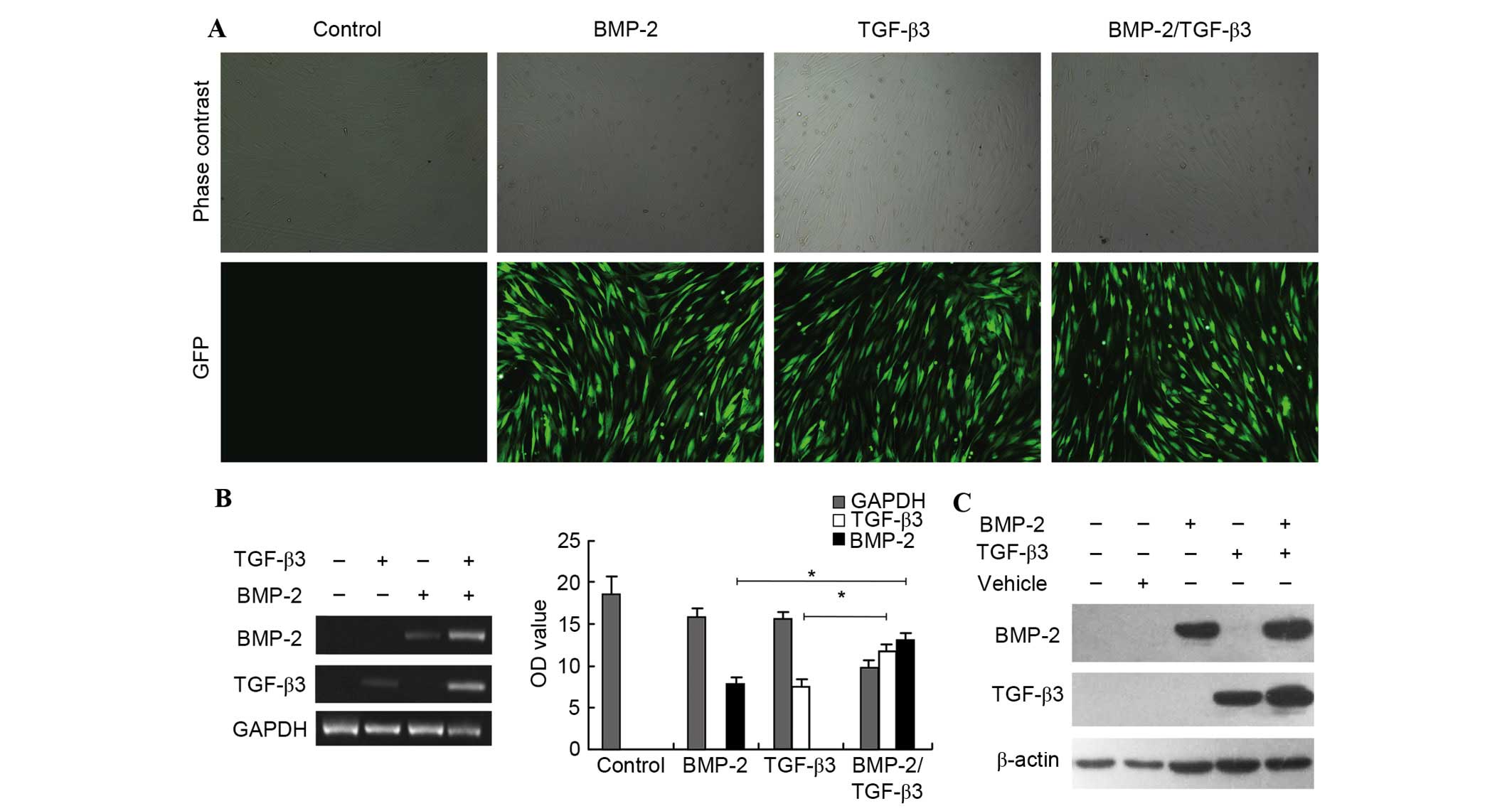
Marked high transfection efficiency
following co-transfection
To evaluate the efficiency of lentivirus-mediated
transfection, expression of a vector encoding GFP in rBMSCs was
visualized using fluorescence microscopy. GFP was expressed in
rBMSCs with high intensity and lasted stably, gradually reaching a
peak value at 72 h after transfection. Images from three random
fields were captured for each well and GFP-positive cells per
microscope field were counted. The ratio of GFP-positive cells
compared with total cells was defined as the transfection
efficiency. As presented in Fig.
1A, a robust transfection efficiency of >90% was observed in
each experimental group when cells were transfected with
lentiviral-mediated BMP-2, TGF-β3, or BMP-2/TGF-β3 genes at an MOI
of 40, 40 and 55, respectively. Furthermore, the incidence of
suspended cells increased in the group undergoing co-transfection
compared with single-gene transfected counterparts, indicating
decreased proliferation of rBMSCs when co-transfected. However, it
did not affect the transfection rate. This demonstrated that the
present study efficiently delivered BMP-2 and TGF-β3 into rBMSCs
together, which has been technically challenging to this point.
BMP-2 and TGF-β3 were mutually
upregulated in co-transfected rBMSCs
The expression status of corresponding exogenous
genes was detected based on RT-PCR analysis, 5 days after
transfection. The results indicated that expression levels of BMP-2
and TGF-β3 in lentivirus-treated rBMSCs were markedly increased
compared with those of untreated cells. Notably, expression of
BMP-2 in rBMSCs was significantly increased when co-expressed with
TGF-β3, in comparison to that in rBMSCs transfected with BMP-2
(P=0.019). Similarly, increased TGF-β3 mRNA level was exacerbated
by BMP-2 (P=0.021; Fig. 1B),
indicating that BMP-2 and TGF-β3 were expressed in rBMSCs when
cultured in vitro.
This RT-PCR result was consistent with results
obtained by assessing BMP-2 and TGF-β3 protein expression levels
using Western blotting. It was demonstrated that BMP-2 or TGF-β3
transfection significantly facilitated upregulation of the
corresponding gene, and higher expression levels of BMP-2 and
TGF-β3 proteins were observed in co-transfected rBMSCs than in
cells transfected with a single gene (Fig. 1C), consistent with the PCR results.
The data collectively suggested that BMP-2 and TGF-β3
synergistically induced expression of the other, with a possible
association indicated by the mutual role they play in the
bone-forming process. Following this, the present study next
assessed the alteration of osteogenesis in post-co-expressed
rBMSCs.
TGF-β3 enhanced osteogenic function of
BMP2
To further investigate the osteogenic function of
rBMSCs undergoing BMP-2 and/or TGF-β3 delivery, expression levels
of Runx2, and Osx, the representative early osteogenic-specific
markers, were estimated. As expected, BMP-2 and TGF-β3
overexpression markedly upregulated Runx-2 and Osx expression
levels (Fig. 2A). Notably, rBMSCs
demonstrated increased expression of Runx2 and Osx when
co-transfected with BMP-2 and TGF-β3, compared with those
transfected with BMP-2 alone, which indicated that TGF-β3 enhanced
osteogenic differentiation capacity for BMP-2 in rBMSCs.
 | Figure 2.Mutual effect of BMP-2 and TGF-β3 on
osteogenic differentiation. (A) Increased expression levels of
Runx2 and Osx, which are early markers for osteogenic
differentiation in osteogenic cultures, were observed in cells
transfected with BMP-2 and TGF-β3. (B) ALP activity of rabbit BMSCs
osteogenic cultures was analyzed at the indicated time points. Data
are presented as the mean ± standard deviation. *P<0.05 and
**P<0.01 vs. indicated groups. (C) Alizarin Red S staining was
performed to visualize mineral deposition at day 21
post-transfection (magnification ×200). Marginal mineralized
nodules were observed in negative control groups, however a
markedly higher density of nodules were detected in BMSCs
undergoing gene transfection, particularly in co-delivered cells.
Control cells were stem cells normally cultured without
osteogenesis induction treatment, while BMSC indicates cells
undergoing induction but without exogenous gene transfection.
BMP-2, bone morphogenetic protein 2; TGF-β3, transforming growth
factor β; Runx-2, Runt-related transcription factor 2; Osx,
Osterix; ALP, alkaline phosphatase; BMSCs, bone marrow-derived
mesenchymal stem cells. |
Following lentivirus infection, ALP activities were
measured to examine the mechanism by which BMP-2 and/or TGF-β3
overexpression affects the osteogenic differentiation process.
Compared with the negative control, ALP activities in rBMSCs
transfected with BMP-2 and TGF-β3 gradually increased with time. As
presented in Fig. 2B, ALP activity
in the BMP-2-transfected rBMSCs was higher than that in TGF-β3
transfected stem cells (P=0.0353 and P=0.023 at days 3 and 7,
respectively; P<0.01 at days 14 and 21) possibly due to a more
robust osteogenic activity of BMP-2. However, when co-transfected
with BMP-2 and TGF-β3, rBMSCs presented significantly increased ALP
activities at all time points compared with rBMSCs transfected with
BMP2. (P=0.0187 at day 3; P<0.01 at days 7, 14 and 21). The
capacity of TGF-β3 in osteogenic differentiation may be elevated by
BMP-2, and BMP-2 mediated ossification was in turn enhanced by
TGF-β3 delivery.
In addition, osteogenic capabilities were
characterized by examining the mineralization of the extracellular
matrix using Alizarin Red S staining after 21 days of culture. As
hypothesized, marginal mineralized nodules were observed in
negative control groups with or without osteogenic introduction
(Fig. 2C). However, in agreement
with data from the Western blotting, although there was no marked
difference in the density of mineralized nodule areas between BMP-2
and TGF-β3 overexpressed rBMSCs, the proportion of mineralized
nodules was notably increased in rBMSCs incubated with the BMP-2
and TGF-β3 encapsulated lentivirus.
The results of the present study demonstrated that
when acting together, BMP-2 and TGF-β3 increased promotion of
osteogenic differentiation compared with when functioning
individually.
Discussion
Stem cells have been extensively introduced to the
field of clinical bioengineering, resulting from their ability to
self-renew and differentiate into multiple types of cell. Research
focus has shifted to the application of mesenchymal stem cells
(MSCs) for therapeutic models primarily as MSCs may be favorably
isolated from bone marrow aspiration and expand >20 population
doublings without a loss of their potency of differentiation, with
no untoward reaction in allogeneic MSC transplantation (31,32).
BMSCs are particularly promising in orthopedic surgery due to their
osteoinductive potential (33,34).
Notably, osteogenic differentiation of BMSCs, coupled with
maintenance of cell phenotypes following differentiation, requires
induction of multiple growth factors and specific microenvironments
(21,35,36).
Transforming growth factors are known to be
associated with the coordination of diverse physiological
processes, including cellular proliferation and differentiation,
embryogenesis, the immune response, and wound healing (37,38).
The TGF-β superfamily principally comprises the TGF-β subfamily
(with three isoforms) (39), and
the decapentaplegic Vg-related subfamily including BMPs. Numerous
previous studies have demonstrated the osteogenic importance of
BMP-2, a growth factor that belongs to the BMP subfamily (13–18,40).
BMP-2 is currently used as an intervention in spondylodesis, bone
defects and osteoporosis (41–43).
BMP-2 regulates osteoblast differentiation and later bone formation
via a classical TGF-β/BMP linear signaling cascade. BMP-2 is
secreted from mesenchymal cells, and then interacts with BMP
receptors on the cell membrane, and a subsequent phosphorylation of
the Smad transducer occurs. Activated Smad then translocates into
the nucleus and BMP and TGF-β signals converge to modulate the
transcription of numerous osteoblast-specific target genes, namely,
the early osteogenic markers ALP, Runx2 and Osx, specifically
expressed in developing bones and essential in osteoblast
differentiation and bone formation (44–46).
TGF-β3 was formerly reported to be an inductive part
of the chondrogenic differentiation of progenitor cells (22,23).
Exposure of murine induced pluripotent stem cells to TGF-β3 in the
presence of retinoic acid resulted in bone deposition on ceramic
scaffolds implanted in mice (27).
Toom et al (47) described
an increased level of TGF-β2 and TGF-β3 during bone formation and
remodeling, indicating the implication of TGF-β3 in bone formation
in heterotopic ossification. Scaffolds infused with BMP-2 and
TGF-β3 enhanced bone formation in vivo and improved
treatment of the orthotopic defect region, which was consistent
with the data from Oest et al (48). A previous study also suggested that
craniofacial osteogenesis relied on tight modulation of TGF-β3
levels in zebrafish embryos (49).
These previous studies indicate TGF-β3 may serve as a promoter to
accelerate and induce bone formation. However, the osteogenic
function of TGF-β3 in BMSCs remains to be elucidated.
The present study was the first, to the best of our
knowledge, to succeed in delivering BMP-2 and TGF-β3 together into
rBMSCs. The RT-PCR and Western blotting results demonstrated that
BMP-2/TGF-β3 co-transfected rBMSCs expressed markedly elevated
quantities of BMP-2 and TGF-β3 proteins, compared with individual
gene transfected rBMSCs, indicating that overexpression of TGF-β3
ex vivo stimulated the secretion of BMP-2, and vice versa.
This was partly consistent with a previous study suggesting that
the expression of BMP-2 was positively influenced in a
time-dependent manner in vivo when pretreated with TGF-β3
(28). It was also suggested that
TGF-β3 elicited bone formation via increasing endogenous BMP-2
levels, and was involved in reprogramming progenitor cells into
active secreting osteoblasts (28). In addition, a notable, but as yet
unreported, observation is that the addition of TGF-β3 increased
the osteogenic effect exerted by BMP-2 in vitro, suggesting
that Runx-2 and Osx, which are characteristic of early stage bone
formation, were markedly upregulated in rBMSCs with BMP-2 and
TGF-β3 co-expression. Therefore, it was assumed that although
TGF-β3 did not exert a marked impact on the osteogenic
differentiation of BMSCs, it was involved due to increasing the
quantity of BMP-2.
TGF-β participates in a wide array of processes
involved in matrix release and deposition, such as collagen
synthesis, including wound healing, angiogenesis, and fibrotic
disease. According to Kovacevic et al (50), TGF-β3 delivered with a
fibrin/heparin composite gel to the healing rotator cuff enthesis
resulted in enhanced structural and material properties, and the
addition of TGF-β3 could expedite healing tendon-bone repair, with
an accumulation of osteoconductive calcium-phosphate matrix at the
tendon-bone site, associated with new bone formation. This
suggested another hypothesis regarding whether TGF-β3 promoted bone
development by inducing matrix deposition.
The present study conducted a time-dependent
measurement of ALP activity, however no investigation into
time-dependent Runx-2 and Osx release, or expression levels of
BMP-2 and TGF-β3 was conducted. The present study also expected to
determine whether an interaction existed between BMP-2 and TGF-β3,
or if TGF-β3 collaborated with BMP-2 via a TGF-β/BMP signaling
pathway. The precise mechanism remains to be elucidated.
In conclusion, the present study, was the first, to
the best of our knowledge, to successfully deliver BMP-2 and TGF-β3
into BMSCs. The results of the present study demonstrated that
combining TGF-β3 with BMP-2 was able to promote the process of bone
formation more markedly in vitro, providing a promising
clinical strategy in the field of skeletal regeneration and in
fracture healing. Future work in the present laboratory would
involve research into time-dependent Runx-2 and Osx release, and
time-dependent expression levels of BMP-2 and TGF-β3. Thus, the
mechanism involved in the interplay between BMP-2 and TGF-β3, and
their reciprocal roles in osteogenesis, may be elucidated.
Acknowledgements
The present study was supported by the National
Natural Science Foundation of China (grant no. 81301570) and the
Shandong Natural Science Foundation in China (grant no.
2013ZRCQ018).
References
|
1
|
Kaufmann KB, Büning H, Galy A, Schambach A
and Grez M: Gene therapy on the move. EMBO Mol Med. 5:1642–1661.
2013. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Wilson JM: Gendicine: The first commercial
gene therapy product. Hum Gene Ther. 16:1014–1015. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Cronin M, Stanton R, Francis K and Tangney
M: Bacterial vectors for imaging and cancer gene therapy: A review.
Cancer Gene Ther. 19:731–740. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Lam P, Khan G, Stripecke R, Hui K,
Kasahara N, Peng K and Guinn B: The innovative evolution of cancer
gene and cellular therapies. Cancer Gene Ther. 20:141–149. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Jessup M, Greenberg B, Mancini D, Cappola
T, Pauly DF, Jaski B, Yaroshinsky A, Zsebo KM, Dittrich H and
Hajjar RJ: Calcium Upregulation by Percutaneous Administration of
Gene Therapy in Cardiac Disease (CUPID) Investigators: Calcium
upregulation by percutaneous administration of gene therapy in
cardiac disease (CUPID): A phase 2 trial of intracoronary gene
therapy of sarcoplasmic reticulum Ca2+-ATPase in patients with
advanced heart failure. Circulation. 124:304–313. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
LeWitt PA, Rezai AR, Leehey MA, Ojemann
SG, Flaherty AW, Eskandar EN, Kostyk SK, Thomas K, Sarkar A,
Siddiqui MS, et al: AAV2-GAD gene therapy for advanced Parkinson's
disease: A double-blind, sham-surgery controlled, randomised trial.
Lancet Neurol. 10:309–319. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Elsner M, Terbish T, Jörns A, Naujok O,
Wedekind D, Hedrich HJ and Lenzen S: Reversal of diabetes through
gene therapy of diabetic rats by hepatic insulin expression via
lentiviral transduction. Mol Ther. 20:918–926. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Herrera-Carrillo E and Berkhout B: Bone
marrow gene therapy for HIV/AIDS. Viruses. 7:3910–3936. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Tian K, Qi M, Wang L, Li Z, Xu J, Li Y,
Liu G, Wang B, Huard J and Li G: Two-stage therapeutic utility of
ectopically formed bone tissue in skeletal muscle induced by
adeno-associated virus containing bone morphogenetic protein-4
gene. J Orthop Surg Res. 10:862015. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Tseng SS, Lee MA and Reddi AH: Nonunions
and the potential of stem cells in fracture-healing. J Bone Joint
Surg Am. 1(90): Suppl. S92–S98. 2008. View Article : Google Scholar
|
|
11
|
Wang Y, Zeng B and Li X: Expression of
human calcitonin by microencapsulated recombinant myoblasts.
Biotechnol Lett. 28:1453–89. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Johnsen SA, Subramaniam M, Katagiri T,
Janknecht R and Spelsberg TC: Transcriptional regulation of Smad2
is required for enhancement of TGFbeta/Smad signaling by TGFbeta
inducible early gene. J Cell Biochem. 87:233–241. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Kobayashi T, Liu X, Wen FQ, Kohyama T,
Shen L, Wang XQ, Hashimoto M, Mao L, Togo S, Kawasaki S, et al:
Smad3 mediates TGF-beta1-induced collagen gel contraction by human
lung fibroblasts. Biochem Biophys Res Commun. 339:290–295. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Guo L, Zhao RC and Wu Y: The role of
microRNAs in self-renewal and differentiation of mesenchymal stem
cells. Exp Hematol. 39:608–616. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Wang EA, Rosen V, D'Alessandro JS, Bauduy
M, Cordes P, Harada T, Israel DI, Hewick RM, Kerns KM and LaPan P:
Recombinant human bone morphogenetic protein induces bone
formation. Proc Natl Acad Sci USA. 87:2220–2224. 1990. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Hay E, Hott M, Graulet AM, Lomri A and
Marie PJ: Effects of bone morphogenetic protein-2 on human neonatal
calvaria cell differentiation. J Cell Biochem. 72:81–93. 1999.
View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Noshi T, Yoshikawa T, Dohi Y, Ikeuchi M,
Horiuchi K, Ichijima K, Sugimura M, Yonemasu K and Ohgushi H:
Recombinant human bone morphogenetic protein-2 potentiates the in
vivo osteogenic ability of marrow/hydroxyapatite composites. Artif
Organs. 25:201–208. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Wilson JM: Adenoviruses as gene-delivery
vehicles. N Engl J Med. 334:1185–1187. 1996. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Laurencin CT, Attawia MA, Lu LQ, Borden
MD, Lu HH, Gorum WJ and Lieberman JR: Poly
(lactide-co-glycolide)/hydroxyapatite delivery of BMP-2-producing
cells: A regional gene therapy approach to bone regeneration.
Biomaterials. 22:1271–1277. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Huang Z, Ren PG, Ma T, Smith RL and
Goodman SB: Modulating osteogenesis of mesenchymal stem cells by
modifying growth factor availability. Cytokine. 51:305–10. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Cohen MM Jr: Bone morphogenetic proteins
with some comments on fibrodysplasia ossificans progressiva and
NOGGIN. Am J Med Genet. 109:87–92. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Guo Q, Liu C, Li J, Zhu C, Yang H and Li
B: Gene expression modulation in TGF-β3-mediated rabbit bone marrow
stem cells using electrospun scaffolds of various stiffness. J Cell
Mol Med. 19:1582–1592. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Hara ES, Ono M, Pham HT, Sonoyama W,
Kubota S, Kubota S, Takigawa M, Matsumoto T, Young MF, Olsen BR and
Kuboki T: Fluocinolone acetonide Is a potent synergistic factor of
TGF-β3-associated chondrogenesis of bone marrow-derived mesenchymal
stem cells for articular surface regeneration. J Bone Miner Res.
30:1585–1596. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Eduardo K, Hong L and Mao JJ: Inhibition
of osteogenic differentiation of human mesenchymal stem cells.
Wound Repair Regen. 15:413–9421. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Shi Y and Massagué J: Mechanisms of
TGF-beta signaling from cell membrane to the nucleus. Cell.
113:685–700. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Moioli EK, Hong L, Guardado J, Clark PA
and Mao JJ: Sustained Release of TGFbeta3 from PLGA microspheres
and its effect on early osteogenic differentiation of human
mesenchymal stem cells tissue eng. 12:537–546. 2006.PubMed/NCBI
|
|
27
|
Li F and Niyibizi C: Cells derived from
murine induced pluripotent stem cells (iPSC) by treatment with
members of TGF-beta family give rise to osteoblasts differentiation
and form bone in vivo. BMC Cell Biol. 13:352012. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Klar RM, Duarte R, Dix-Peek T and
Ripamonti U: The induction of bone formation by the recombinant
human transforming growth factor-β3. Biomaterials. 35:2773–2788.
2014. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Haschtmann D, Ferguson SJ and Stoyanov JV:
BMP-2 and TGF-β3 do not prevent spontaneous degeneration in rabbit
disc explants but induce ossification of the annulus fibrosus. Eur
Spine J. 21:1724–1733. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
He T, Wang Y, Xiang J and Zhang H: In vivo
tracking of novel SPIO-Molday ION rhodamine-B™-labeled human bone
marrow-derived mesenchymal stem cells after lentivirus-mediated
COX-2 silencing: A preliminary study. Curr Gene Ther. 14:136–145.
2014. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Alhadlaq A and Mao JJ: Mesenchymal stem
cells: Isolation and therapeutics. Stem Cells Dev. 13:436–448.
2004. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Parekkadan B and Milwid JM: Mesenchymal
stem cells as therapeutics. Annu Rev Biomed Eng. 12:87–1174. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Bianco P, Riminucci M, Gronthos S and
Robey PG: Bone marrow stromal stem cells: Nature, biology, and
potential applications. Stem Cells. 19:180–192. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Derubeis AR and Cancedda R: Bone marrow
stromal cells (BMSCs) in bone engineering: Limitations and recent
advances. Ann. Biomed. Eng. 32:160–165. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Hu H, Hilton MJ, Tu X, Yu K, Ornitz DM and
Long F: Sequential roles of hedgehog and wnt signaling in
osteoblast development. Development. 132:49–60. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Forriol F and Shapiro F: Bone development:
Interaction of molecular components and biophysical forces. Clin
Orthop Relat Res. 432:14–33. 2005. View Article : Google Scholar
|
|
37
|
Gordon KJ and Blobe GC: Role of
transforming growth factor-beta superfamily signaling pathways in
human disease. Biochim Biophys Acta. 1782:197–228. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Herpin A, Lelong C and Favrel P:
Transforming growth factor-beta-related proteins: An ancestral and
widespread superfamily of cytokines in metazoans. Dev Comp Immunol.
28:461–485. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Assoian RK, Komoriya A, Meyers CA, Miller
DM and Sporn MB: Transforming growth factor-beta in human
platelets. Identification of a major storage site, purification and
characterization. J Biol Chem. 258:7155–7160. 1983.PubMed/NCBI
|
|
40
|
Ripamonti U: Soluble osteogenic molecular
signals and the induction of bone formation. Biomaterials.
27:807–822. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Carreira AC, Lojudice FH, Halcsik E,
Navarro RD, Sogayar MC and Granjeiro JM: Bone morphogenetic
proteins: Facts, challenges, and future perspectives. J Dent Res.
93:335–345. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Burkus JK, Sandhu HS, Gornet MF and
Longley MC: Use of rhBMP-2 in combination with structural cortical
allografts: Clinical and radiographic outcomes in anterior lumbar
spinal surgery. J Bone Joint Surg Am. 87:1205–1212. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Meisel HJ, Schnöring M, Hohaus C, Minkus
Y, Beier A, Ganey T and Mansmann U: Posterior lumbar interbody
fusion using rhBMP-2. Eur Spine J. 17:1735–1744. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Rahman MS, Akhtar N, Jamil HM, Banik RS
and Asaduzzaman SM: TGF-b/BMP signaling and other molecular events:
Regulation of osteoblastogenesis and bone formation. Bone Research.
3:150052015. View Article : Google Scholar : PubMed/NCBI
|
|
45
|
ten Dijke P, Miyazono K and Heldin CH:
Signaling inputs converge on nuclear effectors in TGF-beta
signaling. Trends Biochem Sci. 25:64–70. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Kusanagi K, Inoue H, Ishidou Y, Mishima
HK, Kawabata M and Miyazono K: Characterization of a bone
morphogenetic protein-responsive Smad-binding element. Mol Biol
Cell. 11:555–565. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
47
|
Toom A, Arend A, Gunnarsson D, Ulfsparre
R, Suutre S, Haviko T and Selstam G: Bone formation zones in
heterotopic ossifications: Histologic findings and increased
expression of bonemorphogenetic protein 2 and transforming growth
factors beta2 and beta3. Calcif Tissue Int. 80:259–267. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
48
|
Oest ME, Dupont KM, Kong HJ, Mooney DJ and
Guldberg RE: Quantitative assessment of scaffold and growth
factor-mediated repair of critically sized bone defects. J Orthop
Res. 25:941–950. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
49
|
Cheah FS, Winkler C, Jabs EW and Chong SS:
Tgfbeta3 regulation of chondrogenesis and osteogenesis in zebrafish
is mediated through formation and survival of a subpopulation of
the cranial neural crest. Mech Dev. 127:329–344. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
50
|
Kovacevic D, Fox JA, Bedi A, Ying L, Deng
XH, Warren RF and Rodeo AS: Calcium-phosphate matrix with or
without TGF-β3 improves tendon-bone healing after rotator cuff
repair. Am J Sports Med. 39:811–819. 2011. View Article : Google Scholar : PubMed/NCBI
|