Introduction
Arachidonic acid (AA) can be metabolized into
hydroxyeicosatetraenoic acids (HETEs) by ω-hydroxylases, and
epoxyeicosatrienoic acids (EETs) by epoxygenases, respectively
(1). Of the HETEs, 20-HETE is the
most important isoform and is predominantly generated by the
cytochrome P450 (CYP) 4A and 4F families, which have ω-hydroxylase
activities in the kidney and liver. EETs, including 8, 9-EET, 11,
12-EET and 14, 15-EET, are formed by the CYP2C and 2J families
which have epoxygenase activity in the kidney and liver (2). Furthermore, EETs are rapidly degraded
to the biologically less active metabolites,
dihydroxyeicosatrienoic acids (DiHETEs) via soluble epoxide
hydrolase (sEH) (3). 20-HETE has
paradoxical functions in the regulation of blood pressure, with a
prohypertensive effect by vasoconstriction and an anti-hypertensive
effect by natriuresis (4).
However, EETs have natriuretic, vasodilative, anti-inflammatory and
anti-apoptotic properties (5–7).
Therefore, the homeostasis of 20-HETE and EETs is important in the
pathogenesis of hypertension.
In our previous study (8), it was reported that CYP4F2
transgenic mice showed hypertension and hyperglycemia, with
increased intrarenal 20-HETE and decreased intrarenal EETs in the
urine and kidney homogenate. Coincidentally, hypertensive Ren-2
transgenic rats exhibit increased synthesis of 20-HETE and
deficient synthesis of EETs in the kidney, which may contribute to
the development of hypertension (9). Similar disturbances in 20-HETE and
EETs also occur in diabetic nephropathy induced by high glucose
(10). The decrease of EETs may be
caused by decreased generation via the CYP2C and CYP2J families or
by increased degradation via sEH (11). Until now, whether 20-HETE affects
the level of EETs remains to be fully elucidated. The aim of the
present study was to investigate the metabolic mechanism underlying
endogenous epoxygenases regulation by high levels of 20-HETE, and
identify the cause of the reduced EETs in CYP4F2 transgenic
mice.
Materials and methods
Sample collection
Blood samples were obtained from male patients with
metabolic syndrome (MS) as the test group (45–56 years old; mean,
51 years old) and from a normal control group (45–54 years old;
mean, 50 years old). Each group contained 20 samples, which were
age-matched. The sample collection methods were approved by the
Ethics Committee of Shengjing Hospital of China Medical University
(Shenyang, China), and written informed consent was obtained from
all patients prior to commencement of the investigation.
RNA purification and reverse
transcription-polymerase chain reaction (RT-PCR) arrays
RNA was extracted from 1.5 ml whole blood samples
using the QIAamp® RNA Blood Mini kit (Qiagen GmbH,
Hilden, Germany). Reverse transcription was performed with an
RT2 First Strand kit (Qiagen GmbH), which comprised an
effective genomic DNA elimination step. The genomic DNA elimination
reaction conditions were 42°C for 5 min and then on ice for 1 min,
then the reverse transcription reaction conditions were 42°C for 5
min followed by 95°C for 5 min. The cDNA template was then detected
using the Human Oxidative Stress RT2 Profiler™ (Qiagen
GmbH) PCR Array in an ABI 7900HT detection system (Applied
Biosystems; Thermo Fisher Scientific., Waltham, MA, USA). PCR
reactions were performed to evaluate the expression of 84 genes
associated with oxidative stress. The reaction conditions were 95°C
for 10 min followed by 40 cycles of 95°C for 15 sec and 60°C for 1
min. Data analysis was performed using PCR Array Data Analysis
software (Qiagen GmbH).
Animals
CYP4F2 transgenic mice from the FVB/N strain
were generated and characterized as previously described (12). The mice were fed with standard
mouse chow and water ad libitum, and bred in a 12:12 h light-dark
cycle system at 23°C. Experiments were performed on 14-20-week-old
CYP4F2 transgenic mice weighing 24–33 g. Transgenic mice were
matched by sex, weight and age with wild-type control mice, and
each group contained 6 mice. All experiments conformed to the Guide
for the Care and Use of Laboratory Animals (13).
Western blot analysis
The mice were sacrificed by cervical dislocation
when they were 14-20-weeks old. Total protein from the livers and
kidneys of the mice was extracted and the concentration was
determined using the Bradford method. The denatured protein (60 µg)
was separated on a 10% SDS-PAGE gel by electrophoresis and
transferred onto polyvinylidene difluoride membranes at 4°C. The
membranes were subsequently blocked in 5% skim milk in PBS, and
incubated at 4°C overnight with the following primary antibodies:
Rabbit anti-sEH (sc-25797; 1:200; Santa Cruz Biotechnology, Inc.,
Santa Cruz, CA, USA) and mouse polyclonal anti-GAPDH (10494-1-AP;
1:10,000; ProteinTech Group, Inc., Chicago, IL, USA). Subsequently,
the membranes were incubated at room temperature for 1 h with
polyclonal horseradish peroxidase-conjugated goat anti-rabbit IgG
(ZB-2301; 1:4,000; ZSGB-BIO, Beijing, China) as the secondary
antibody. The final detection reaction was performed with an ECL
detection kit (Beyotime Institute of Biotechnology, Jiangsu,
China). The blotted membranes were scanned, and the density of
bands was quantified using Image J Lab Software, version 2.0.1
(Bio-Rad Laboratories, Inc., Hercules, CA, USA).
sEH activity assay
The liver and kidney tissues were homogenized in
deionized water with 1 mM PMSF, and cytosolic supernatants were
obtained by centrifugation at 3,500 × g for 10 min at 4°C. The
activity of sEH in the hepatic and renal homogenates was determined
using Epoxy fluor 7 (Cayman Chemical Company, Ann Arbor, MI, USA)
and the sEH inhibitor,
trans-4-[4-(3-adamantan-1-ylureido)-cyclohexyloxy]-benzoic acid
(t-AUCB), as previously described (14). The sEH inhibitor, t-AUCB, was
obtained from Professor Bruce D. Hammock (Department of Entomology
and UCD Cancer Research Center, University of California, Davis,
CA, USA).
RT-quantitative PCR (RT-qPCR)
Total RNA was isolated from the liver and kidney
tissue using TRIzol reagent (Invitrogen; Thermo Fisher Scientific,
Inc.), and reverse transcribed into cDNA (1 µg) using a reverse
transcription reagent kit (Promega Corporation, Madison, WI, USA).
The PCR reactions were performed on the ABI 7,900 system (Applied
Biosystems; Thermo Fisher Scientific, Inc.) in a 20 µl SYBR Green
PCR reaction containing 1X SYBR Green PCR master mix (Applied
Biosystems, Thermo Fisher Scientific, Inc.), 8 ng cDNA, and 10 nM
forward and reverse primers (Qiagen GmbH), which were synthesized
with the sequences listed in Table
I. The reaction conditions were 95°C for 10 min followed by 40
cycles of 95°C for 15 sec, 60°C for 1 min and 72°C for 30 sec.
Dissociation curves were generated to ensure that a single and
specific product was amplified. Quantification cycle values (Cq)
were analyzed using SDS2.4 software (Applied Biosystems; Thermo
Fisher Scientific, Inc.).
 | Table I.Primer sequences for reverse
transcription-quantitative polymerase chain reaction analysis. |
Table I.
Primer sequences for reverse
transcription-quantitative polymerase chain reaction analysis.
| Gene | Sequence | Length (bp) |
|---|
| Cyp2c29 | F:
5′-GGCCTCAAAGCCTACTGTCA-3′ |
|
|
| R:
5′-AACGCCAAAACCTTTAATC-3′ | 130 |
|
| Cyp2c38 | F:
5′-CACTATGGAGACAGAGGTCTA-3′ |
|
|
| R:
5′-CCAAATACAGAGTGAAAACG-3′ | 156 |
| Cyp2j5 | F:
5′-CAACACTGCGATGGGCTTTG-3′ |
|
|
| R:
5′-GACTCTCGGTCAGACAAGCTC-3′ | 121 |
| Human EPHX2 | F:
5′TCAGCAGGATGGTCACTGAG'3 |
|
|
| R:
5′CATGTGCTGGGACATCTGAG'3 | 209 |
|
| Mouse Ephx2 | F:
5′-TGAACACGCCGTTTATGCC-3′ |
|
|
| R:
5′-TCTCATCACTGGCTCGGAAG-3′ | 171 |
|
| Gapdh | F:
5′-TGCACCACCAACTGCTTAGC-3′ |
|
|
| R:
5′-GGCATGGACTGTGGTCATGAG-3′ | 87 |
|
AA hydroxylation assay of hepatic and
renal microsomes
The hepatic and renal microsomes were prepared
according to the method described previously (12). The conversion of AA was assessed in
a reaction mixture of 100 mM potassium phosphate buffer (pH 7.4)
containing 3.3 mM MgCl2, 80 µM AA (Cayman Chemical
Company), 1 mM NADPH (Roche Applied Science, Basel, Switzerland)
and 0.6 µg/µl mouse renal microsomes. Following vortexing and 5 min
preincubation at 37°C, NADPH was added to start the reaction at
37°C for 30 min. The reaction was terminated by acidification with
formic acid to pH 3.5, following which the production was measured
using liquid chromatography-tandem mass spectrometry (LC-MS/MS)
following the addition of 2 ng of 20-HETE-d6 (Cayman Chemical
Company).
Quantification of eicosanoids using
LC-MS/MS
The eicosanoids were measured using the API 3200
Q-trap LC-MS/MS System (Applied Biosystems; Thermo Fisher
Scientific, Inc.). The livers and kidneys were homogenized in
methanol (0.1% formic acid), and added to 2 ng 20-HETEd6 internal
standard (Cayman Chemical Company). Lipids were extracted using
ethyl acetate, dried under nitrogen, resuspended in methanol, and
separated on a reversed-phase Symmetry C18 column (3.5 µm; 2.1×150
mm; Waters Corporation, Milford, MA, USA.) at a flow rate of 0.2
ml/min using solvent A (water, 0.1% formic acid) and solvent B
(acetonitrile:methanol 6:1, 0.1% formic acid) as follows: 0–2 min,
25% B; 2–10 min, 25–75% B; 10–18 min, 75–95% B; 18–30 min, 95% B;
30–30.5 min, 95–25% B; 30.5–40 min, 25% B). The effluent was
ionized using negative ion electrospray and quantified by multiple
reaction monitoring.
Statistical analysis
All data are expressed as the mean ± standard
deviation. Statistical analyses were performed using SPSS 17.0
software (SPSS, Inc., Chicago, IL, USA). Differences were evaluated
using Student's t-test and P<0.05 was considered to indicate a
statistically significant difference. All experiments were
performed in triplicate.
Results
Oxidative stress PCR array assay
As previously reported, the ratio of 20-HETE/EETs is
disturbed in CYP4F2 transgenic mice, associated with
hypertension and hyperglycemia (8,15),
however, the mechanism remains to be elucidated. In order to screen
out the possible pathogenetic factor, 20 blood samples from an MS
group with clinical phenotypes including hypertension, abdominal
obesity, diabetes and dyslipidemia, and a control group were
collected and analyzed using an oxidative stress PCR array. In the
84 oxidative stress genes, which were evaluated, statistically
significant alterations (>5-fold; P<0.05) in the expression
of 27 genes were detected in the patients with MS, 24 of which were
upregulated and three of which were downregulated (data not shown).
Among these genes, the expression of epoxide hydrolase 2,
cytoplasmic (EPHX2), coding for the sEH protein was
6.96-fold higher in the MS group, compared with the control group
(Table II). This result was also
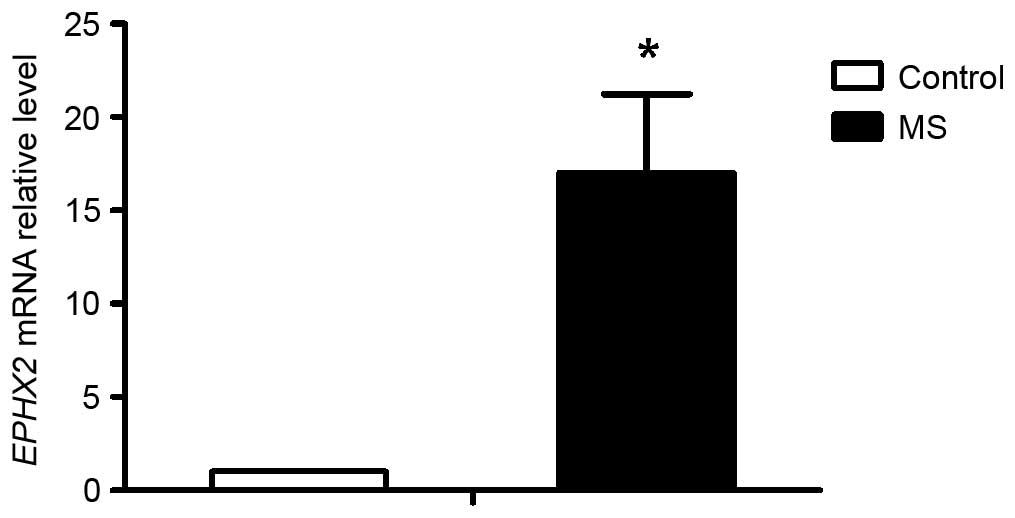
confirmed in the RT-qPCR assay, as shown in Fig. 1, in which the expression of sEH was
17.04-fold higher in the MS group, compared with the control group.
sEH, functions to enzymatically hydrolyze EETs (16), which has been implicated in various
diseases. In the present study, it was hypothesized that sEH may be
important in the disturbed ratio of 20-HETE/EETs in CYP4F2
transgenic mice.
 | Table II.Gene expression of EPHX2 in patients
with metabolic syndrome. |
Table II.
Gene expression of EPHX2 in patients
with metabolic syndrome.
| Gene | Description | Fold change |
|---|
| EPHX2 | Epoxide hydrolase 2,
cytoplasmic | 6.96a |
Expression and activity of sEH in
CYP4F2 transgenic mice
In order to confirm the association between sEH and
the disturbed 20-HETE/EETs ratio in CYP4F2 transgenic mice,
the present study measured the expression of sEH. As shown in
Fig. 2A and B, no difference was
found in the hepatic expression of sEH between the transgenic and
wild-type mice at either the mRNA or protein levels. However, the
renal expression of sEH in the transgenic mice was ~30% higher,
compared with that in the wild-type mice. To further examine the
alteration of sEH in the transgenic mice, the activity of sEH was
measured using its specific substrate, epoxy fluor 7. As shown in
Fig. 2C, the transgenic mice had
higher activity of sEH, compared with the wild-type mice, by
~1.6-fold and 1.4-fold in the liver and kidney, respectively. Taken
together, these results suggested that 20-HETE activated sEH in the
liver and kidney.
Eicosanoid quantification using
LC-MS/MS analysis
Activated sEH can metabolize EETs into the less
bioactive DiHETEs (3). Therefore,
the present study measured EETs (14, 15-EET, 11, 12-EET and 8,
9-EET) and DiHETEs (14, 15-DiHETE, 11, 12-DiHETE and 8, 9-DiHETE)
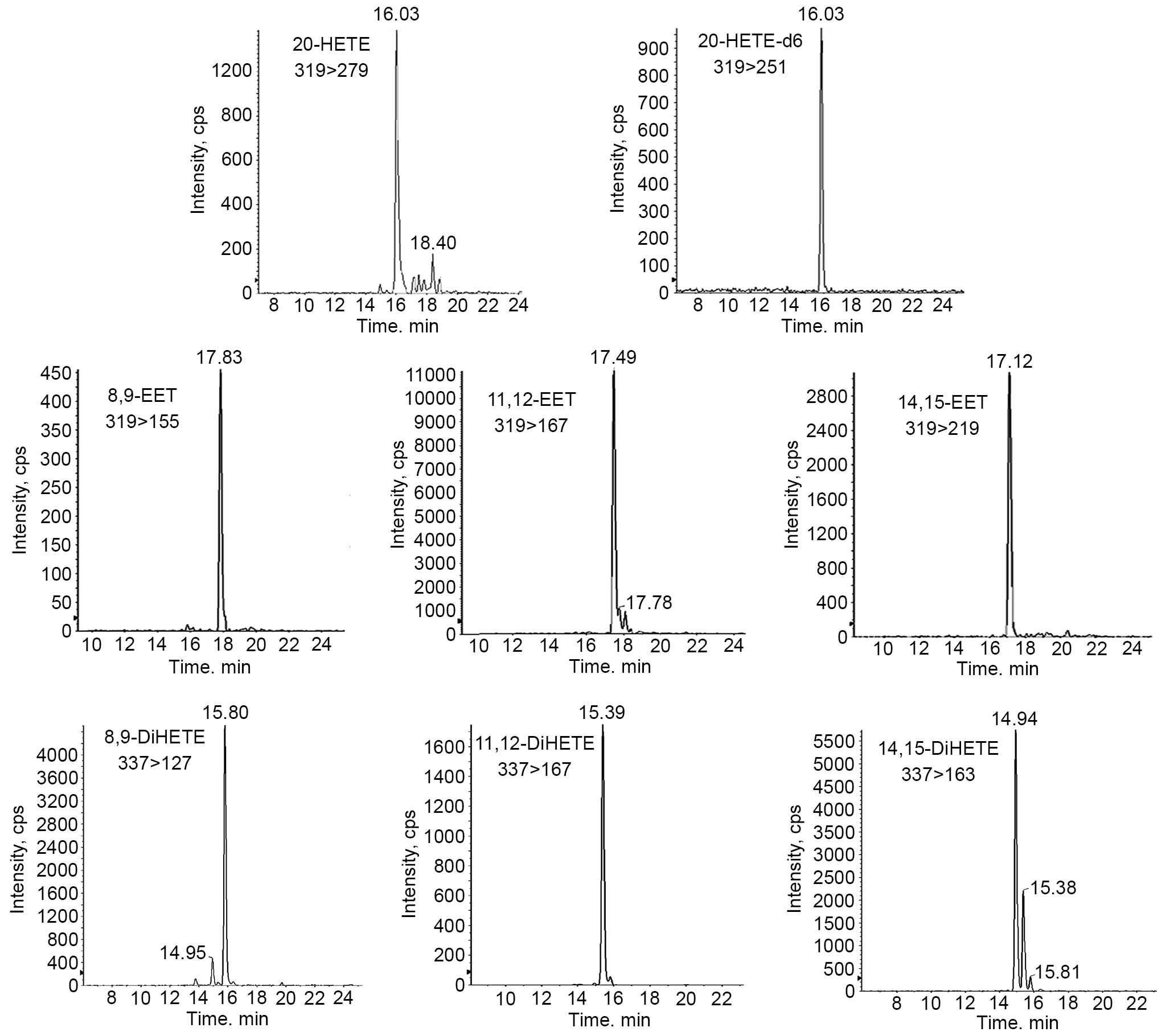
in the hepatic and renal homogenates using LC-MSMS (Fig. 3). The CYP4F2 transgenic mice
had elevated levels of 20-HETE, compared with the levels in the
controls in the liver (0.55±0.1, vs. 0.17±0.05 ng/mg pro) and the
kidney (0.70±0.13, vs. 0.37±0.14 ng/mg pro), as previously reported
(15). The total DiHETE levels
were markedly higher in the liver (7.92±1.71, vs. 2.74±0.6 ng/mg
pro) and kidney (1.32±0.24, vs. 0.64±0.13 ng/mg pro) of the
transgenic mice, compared with the wild-type control mice. Of note,
the levels of EETs were significantly higher in the liver
(4.25±0.77, vs. 2.98±0.13 ng/mg pro), but lower in the kidney
(6.64±0.21, vs. 7.43±0.42 ng/mg pro), compared with the wild-type
mice (Table III). These results
demonstrated that 20-HETE showed various moderating effects on the
generation of EETs in the liver and kidney, whereas EET degradation
was enhanced by 20-HETE-activited sEH in the two tissues.
 | Table III.Eicosanoid quantification using
liquid chromatography-tandem mass spectrometry. |
Table III.
Eicosanoid quantification using
liquid chromatography-tandem mass spectrometry.
| Eicosanoid | WT | TG |
|---|
| Hepatic homogenate
(ng/mg pro) |
|
|
|
20-HETE | 0.17±0.05 |
0.55±0.10a |
|
EETs | 2.98±0.13 |
4.25±0.77a |
|
DiHETEs | 2.74±0.60 |
7.92±1.71a |
| Renal homogenate
(ng/mg pro) |
|
|
|
20-HETE | 0.37±0.14 |
0.70±0.13a |
|
EETs | 7.43±0.42 |
6.64±0.21a |
|
DiHETEs | 0.64±0.13 |
1.32±0.24a |
Detection of endogenous epoxygenases,
and the expression of Cyp2c29, Cyp2c38 and Cyp2j5 using RT-qPCR
analysis
Cyp2c29, Cyp2c38 and Cyp2j5 are
major epoxygenases in the liver and kidney, which can catalyze AA
into EETs. To demonstrate whether the endogenous epoxygenase was
affected by the high production of 20-HETE in the transgenic mice,
the present study measured the mRNA levels of Cyp2c29,
Cyp2c38 and Cyp2j5 in the liver and kidney using
RT-qPCR analysis. As shown in Fig.
4A, the mRNA levels of Cyp2c29 and Cyp2j5 in the
transgenic mice were marginally higher in the liver, compared with
those in the wild-type mice (by ~20 and 30%, respectively).
However, in the kidney, the mRNA levels of Cyp2c29,
Cyp2c38 and Cyp2j5 were decreased in the transgenic
mice (by ~50, 50 and 30%, respectively; Fig. 4B). These results demonstrated that
the enhanced 20-HETE in the transgenic mice increased the
expression of endogenous epoxygenases in the liver and suppressed
the expression of endogenous epoxygenases in the kidney.
Detection of endogenous epoxygenase
activity using LC-MS/MS analysis
The lack of antibodies for the three isoforms
presents a challenge in further detecting the protein expression
levels. Therefore, the present study measured endogenous
epoxygenase activity in the liver and kidney using LC-MS/MS
analysis. Following the incubation of hepatic and nephric
microsomes with AA, it was demonstrated that 20-HETE induced the
total level of EETs in the liver by 1.2-fold, and caused a 0.8-fold
decrease in the kidney, compared with the wild-type controls (shown
in Fig. 5A and B).
Discussion
As previously reported, the disturbed ratio of renal
20-HETE/EETs is found in CYP4F2 transgenic mice, which is
considered to be the molecular basis of hypertension (8). The decrease in EETs may be induced by
overdegradation by sEH or by reduced generation by the endogenous
epoxygenases. In the present study, it was demonstrated that
20-HETE activated sEH in the liver and kidney. In addition, 20-HETE
showed an opposing action in regulating the endogenous
epoxygenases, decreasing endogenous EET synthesis in the kidney and
increasing endogenous EET synthesis in the liver.
20-HETE and EETs have been suggested to have
vasoactive properties. As they have opposite vascular effects,
compared with vasoconstrictors or vasodilators, their proportion
determines the vascular resistance and further affects the blood
pressure (17). In order to reveal
the effect of 20-HETE on AA epoxygenation in the liver and kidney,
the present study detected the levels of 20-HETE, EETs and DiHETEs
in the hepatic and renal homogenates, respectively. It was found
that the overexpression of CYP4F2 in the transgenic mice was
associated with higher levels of 20-HETE in the liver and kidney,
decreased EETs in the kidney and increased EETs in the liver. EETs,
as the major substrate of sEH, can be rapidly metabolized to
DiHETEs (11). On the subsequent
measurement of the activity of sEH, the present study demonstrated
for the first time, to the best of our knowledge, that the 20-HETE
increased the protein activity of sEH in the liver and kidney of
the hypertensive CYP4F2 transgenic mice. Consistently,
previous studies have shown that the protein expression of sEH is
upregulated in certain hypertensive models, including
two-kidney-one-clip and angiotensin-II infusion hypertension
(18–20), however, the mechanism remains to be
elucidated. It has been reported that proinflammatory cytokines
elevate the protein level of sEH (13). As 20-HETE can induce the expression
of cellular adhesion molecules and cytokines (21), the present study hypothesized that
20-HETE-induced inflammation may be the reason for the increased
protein level and activity of sEH. By contrast, EETs have important
biological effects on anti-hypertension and anti-inflammation. The
inhibition of sEH can enhance the beneficial properties of EETs,
and has been regarded as a possible treatment for cardiovascular
diseases and hyperglycemia (16,22).
The present study demonstrated that the increased
DiHETEs in the CYP4F2 transgenic mice was due to the
enhanced degradation of EETs via sEH. Although the activation of
sEH explains the higher level of DiHETEs in the transgenic mice,
the altered levels of EETs was not caused solely by excessive
degradation. It has been reported that Cyp4a14-knockout mice
present with a hypertensive phenotype, which is caused by the high
levels of Cyp4a12 and 20-HETE in the kidney (23). Our previous study (8) found that the transgenic mice with
high expression levels of CYP4F2 showed significantly suppressed
levels of all homologous Cyp4a mRNAs, which suggested an
interaction exists between the different isoforms of 20-HETE
synthesis. Therefore, the present aimed to investigate whether an
interaction exists between 20-HETE and endogenously expressed EETs.
The mRNA levels of the three endogenously expressed EETs,
Cyp2c29, Cyp2c38 and Cyp2j5, which show high
levels of expression in the liver and kidney (24,25)
were examined. The data demonstrated that the transgene,
CYP4F2, had markedly reduced expression levels of
Cyp2c29, Cyp2c38 and Cyp2j5 in the kidney, but
not in the liver, at the mRNA level (Fig. 4A and B). The lack of antibodies of
the three isoforms presents a challenge for the further
investigations. To investigate the activity of the three endogenous
epoxygenases, hepatic and nephric microsomes were separated and
incubated with AA in vitro. As shown in Fig. 5, total EET production was markedly
lower in the kidney and marginally higher in the liver of
transgenic mice, compared with the wild-type controls. The
discrepancy in regulatory effects of 20-HETE in different tissues
has also been reported in previous studies; 20-HETE acts as a
potent constrictor of renal and dilator of bovine coronary arteries
(26). In the kidney, 20-HETE has
been found to decrease cortical blood flow and increase medullary
blood flow (27).
In conclusion, the results of the present study
demonstrated that CYP4F2 transgenic mice, with high levels of
20-HETE, increased the degradation of endogenous EETs by activating
sEH in the liver and kidney. 20-HETE suppressed endogenous
epoxygenases in the kidney, but not in liver. The mechanism
underlying the 20-HETE-induced differential regulation of
endogenous epoxygenase activity in the liver and kidney requires
further investigation. The effects of EETs in cardiovascular
physiology are vasodilatory, anti-inflammatory and anti-apoptotic,
which are opposite to that of the function of 20-HETE (4–7).
Therefore, it was inferred that the amplified disturbed ratio of
20-HETE/EETs in CYP4F2 transgenic mice may further lead to
hypertension.
Acknowledgements
The authors would like to thank Professor Bruce D.
Hammock (Department of Entomology and UCD Cancer Research Center,
University of California) for providing the sEH inhibiter. This
study was supported by the National Natural Science Foundation of
China (grant no. 81270343) and the Ministry of Education of China
(grant no. 20122104110020).
References
|
1
|
Brash AR: Arachidonic acid as a bioactive
molecule. J Clin Invest. 107:1339–1345. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Roman RJ: P-450 metabolites of arachidonic
acid in the control of cardiovascular function. Physiol Rev.
82:131–185. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Shen HC: Soluble epoxide hydrolase
inhibitors: A patent review. Expert Opin Ther Pat. 20:941–956.
2010. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Miyata N and Roman RJ: Role of
20-hydroxyeicosatetraenoic acid (20-HETE) in vascular system. J
Smooth Muscle Res. 41:175–193. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Campbell WB: New role for
epoxyeicosatrienoic acids as anti-inflammatory mediators. Trends
Pharmacol Sci. 21:125–127. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Chen JK, Capdevila J and Harris RC:
Cytochrome p450 epoxygenase metabolism of arachidonic acid inhibits
apoptosis. Mol Cell Biol. 21:6322–6331. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Zhang Y, Oltman CL, Lu T, Lee HC,
Dellsperger KC and VanRollins M: EET homologs potently dilate
coronary microvessels and activate BK(Ca) channels. Am J Physiol
Heart Circ Physiol. 280:H2430–H2440. 2001.PubMed/NCBI
|
|
8
|
Liu X, Wu J, Liu H, Lai G and Zhao Y:
Disturbed ratio of renal 20-HETE/EETs is involved in
androgen-induced hypertension in cytochrome P450 4F2 transgenic
mice. Gene. 505:352–359. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Certíková Chábová V, Kramer HJ, Vanecková
I, Thumová M, Skaroupková P, Tesar V, Falck JR, Imig JD and
Cervenka L: The roles of intrarenal 20-hydroxyeicosatetraenoic and
epoxyeicosatrienoic acids in the regulation of renal function in
hypertensive Ren-2 transgenic rats. Kidney Blood Press Res.
30:335–346. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Eid S, Maalouf R, Jaffa AA, Nassif J,
Hamdy A, Rashid A, Ziyadeh FN and Eid AA: 20-HETE and EETs in
diabetic nephropathy: A novel mechanistic pathway. PLoS One.
8:e700292013. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Yu Z, Xu F, Huse LM, Morisseau C, Draper
AJ, Newman JW, Parker C, Graham L, Engler MM, Hammock BD, et al:
Soluble epoxide hydrolase regulates hydrolysis of vasoactive
epoxyeicosatrienoic acids. Circ Res. 87:992–998. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Liu X, Zhao Y, Wang L, Yang X, Zheng Z,
Zhang Y, Chen F and Liu H: Overexpression of cytochrome P450 4F2 in
mice increases 20-hydroxyeicosatetraenoic acid production and
arterial blood pressure. Kidney Int. 75:1288–1296. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
National Research Council (US) Committee
for the Update of the Guide for the Care and Use of Laboratory
Animals, . Guide for the Care and Use of Laboratory Animals. 8th.
National Academies Press; Washington (DC), USA: 2011, PubMed/NCBI
|
|
14
|
Liu Y, Dang H, Li D, Pang W, Hammock BD
and Zhu Y: Inhibition of soluble epoxide hydrolase attenuates
high-fat-diet-induced hepatic steatosis by reduced systemic
inflammatory status in mice. PLoS One. 7:e391652012. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Lai G, Wu J, Liu X and Zhao Y: 20-HETE
induces hyperglycemia through the cAMP/PKA-PhK-GP pathway. Mol
Endocrinol. 26:1907–1916. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Imig JD and Hammock BD: Soluble epoxide
hydrolase as a therapeutic target for cardiovascular diseases. Nat
Rev Drug Discov. 8:794–805. 2009. View
Article : Google Scholar : PubMed/NCBI
|
|
17
|
Singh H and Schwartzman ML: Renal vascular
cytochrome P450-derived eicosanoids in androgen-induced
hypertension. Pharmacol Rep. 60:29–37. 2008.PubMed/NCBI
|
|
18
|
Imig JD, Zhao X, Capdevila JH, Morisseau C
and Hammock BD: Soluble epoxide hydrolase inhibition lowers
arterial blood pressure in angiotensin II hypertension.
Hypertension. 39:690–694. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Kopkan L, Husková Z, Sporková A, Varcabová
Š, Honetschlägerová Z, Hwang SH, Tsai HJ, Hammock BD, Imig JD,
Kramer HJ, et al: Soluble epoxide hydrolase inhibition exhibits
antihypertensive actions independently of nitric oxide in mice with
renovascular hypertension. Kidney Blood Press Res. 35:595–607.
2012. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Zhao X, Yamamoto T, Newman JW, Kim IH,
Watanabe T, Hammock BD, Stewart J, Pollock JS, Pollock DM and Imig
JD: Soluble epoxide hydrolase inhibition protects the kidney from
hypertension-induced damage. J Am Soc Nephrol. 15:1244–1253.
2004.PubMed/NCBI
|
|
21
|
Ishizuka T, Cheng J, Singh H, Vitto MD,
Manthati VL, Falck JR and Laniado-Schwartzman M:
20-Hydroxyeicosatetraenoic acid stimulates nuclear factor-kappaB
activation and the production of inflammatory cytokines in human
endothelial cells. J Pharmacol Exp Ther. 324:103–110. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Luo P, Chang HH, Zhou Y, Zhang S, Hwang
SH, Morisseau C, Wang CY, Inscho EW, Hammock BD and Wang MH:
Inhibition or deletion of soluble epoxide hydrolase prevents
hyperglycemia, promotes insulin secretion, and reduces islet
apoptosis. J Pharmacol Exp Ther. 334:430–438. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Fidelis P, Wilson L, Thomas K, Villalobos
M and Oyekan AO: Renal function and vasomotor activity in mice
lacking the Cyp4a14 gene. Exp Biol Med (Maywood). 235:1365–1374.
2010. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Athirakul K, Bradbury JA, Graves JP,
DeGraff LM, Ma J, Zhao Y, Couse JF, Quigley R, Harder DR, Zhao X,
et al: Increased blood pressure in mice lacking cytochrome P450
2J5. FASEB J. 22:4096–4108. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Luo G, Zeldin DC, Blaisdell JA, Hodgson E
and Goldstein JA: Cloning and expression of murine CYP2Cs and their
ability to metabolize arachidonic acid. Arch Biochem Biophys.
357:45–57. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Pratt PF, Falck JR, Reddy KM, Kurian JB
and Campbell WB: 20-HETE relaxes bovine coronary arteries through
the release of prostacyclin. Hypertension. 31:237–241. 1998.
View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Oyekan AO: Differential effects of
20-hydroxyeicosatetraenoic acid on intrarenal blood flow in the
rat. J Pharmacol Exp Ther. 313:1289–1295. 2005. View Article : Google Scholar : PubMed/NCBI
|