Introduction
The kidneys are crucial for the elimination of
endogenous metabolites and xenobiotics, including drugs and
environmental chemicals. At least 37 xenobiotic transporters have
been identified in mammalian kidneys (1). Transporter proteins provide
organ/tissue defense and are involved in the therapeutic effects
and toxicity of numerous drugs and toxicants (2–4).
Important basolateral uptake transporters include organic anion
transporter (OAT) 1, 2 and 3, organic cation transporter (OCT) 1, 2
and 3 and organic anion transporting polypeptide (OATP4C1).
Important efflux transporters on the apical brush-border membrane
primarily include multidrug resistance protein [P-glycoprotein,
encoded by multidrug resistance protein gene 1b (Mdr1b)], multidrug
resistance-associated protein (MRP) 2 and 4, multidrug and toxin
extrusion protein (MATE) 1 and 2 and breast cancer resistance
protein (BCRP) (2,5,6).
Renal OATs are important in the uptake of common
drugs, toxins and nutrients (3,7). For
example, OAT1 and 3 are critical for renal mercury absorption and
accumulation from inorganic (3,8,9) or
organic mercury (10). Deletion of
OAT1 and 3 protects against nephrotoxicity induced by mercury
chloride (11) and aristolochic
acid I (12). Thus, alterations of
OAT1 and 3 greatly impact chemical-induced nephrotoxicity (13).
In addition, renal OCTs are important in
transporting cationic xeno- and endobiotics across biological
membranes. For example, OCT1 and 2 mediate the renal uptake and
accumulation of platinum compounds (14) and cadmium (15); modification of OCT expression
levels causes altered proximal tubular cell accumulation of
cisplatin and cadmium, resulting in decreased or increased toxicity
(14–16).
Mdr1 and MRP2 are expressed in proximal tubular
epithelial cells and are crucial for protection against
toxicant-induced kidney injury. For example, Mdr1 protects against
paraquat-induced toxicity in human and murine proximal tubular
cells (17), while MRP2 protects
against mercury-induced kidney injury (18,19).
MATE1 and 2 mediate cisplatin-induced nephrotoxicity
via a reduction in cellular efflux (16). MATE and OCT2 affect cisplatin
accumulation and toxicity in coordination (20). The efflux transporter BCRP is
involved in the elimination of mercury from proximal tubular cells
(6).
The expression of renal transporters may be affected
by drugs and toxicants, and by physiological variations. For
example, females express reduced levels of renal OAT1 and 3, and
are therefore less susceptible to mercury-induced kidney injury
(21). Older rats are more
susceptible to mercury nephrotoxicity compared with younger rats
(22), and methylmercury may cross
the placenta to the fetus, resulting in accumulation and toxicity
(19). Thus, understanding the
ontogeny and aging-associated alterations in renal transporter
expression is important for the evaluation of drug or toxicants
effects in sensitive populations. For this reason, the ontogeny of
the expression of renal transporters, including OCTs (23), MATE1 and 2 (24) and OATs (1) has been investigated in mice. However,
in rats the majority of studies have examined the expression of
OATs (25–27).
Recently, the whitepaper of Pediatric Transporter
Working Group presented a systematic review of the ontogeny of
clinically relevant transporters in intestine, liver and kidney
(4). Different developmental
patterns for individual transporters exist; however, these remain
to be fully elucidated, particularly with regard to elderly
populations. The present study aimed to address this. Kidneys were
collected from male Sprague Dawley rats at 11 time points: Fetal
(−2 days), birth (1 day), nursing (7 and 14 days), weaning (21
days), puberty (28 and 35 days), maturation (60 days), adulthood
(180 days) and aging (540 and 850 days). The mRNA expression levels
of six primary renal uptake transporters (OAT1 and 3, OATP4C1, and
OCT1, 2 and 3) and six primary efflux transporters (Mdr1b, MRP2 and
4, MATE1 and 2, and BCRP) were examined, and three selected
transporters (OAT1 and 3, and MDR1) were additionally examined at
the protein levels via western blot analysis. The results obtained
may be of physiological, pharmacological and toxicological
significance.
Materials and methods
Animals
Sprague Dawley rats (weight, 250–300 g; 10 male, 30
female as parents of the experimental mice) were purchased from the
Experimental Animal Center of Third Military Medical College
(Chongqing, China) and acclimatized for one week prior to mating
overnight. Rats were housed in specific pathogen-free-grade animal
facilities under a 12-h light/dark cycle, at 22±2°C and 50%
humidity, and had access to feed and water ad libitum. All
animal procedures experiments were performed in accordance with
Chinese Guidelines of Animal Care and Welfare, and the present
study was approved by the Animal Care and Use Committee of Zunyi
Medical College (Zunyi, China).
Tissue collection
If a vaginal plug was present the next morning this
was designated as day 0 of gestation. Kidneys were collected from
male rats only at developmental days −2, 1, 7, 14, 21, 28, 35 and
60 and ageing days 180, 540 and 850, where day 0 was the day of
birth. Rats were anesthetized with 7% chloral hydrate (5 ml/kg),
sacrificed by cervical dislocation, and kidneys were frozen in
liquid nitrogen and stored at −80°C prior to analysis.
RNA isolation
Kidney tissue (50–100 mg) was homogenized in 1 ml
TRIzol® (Takara Biotechnology Co., Ltd., Dalian, China).
The quality and quantity of RNA were determined by measuring the
absorbance at wavelengths of 260 and 280 nm and calculating the
260/280 ratio, and by gel electrophoresis.
Reverse transcription-quantitative
polymerase chain reaction (RT-qPCR)
Total RNA was reverse transcribed with the High
Capacity Reverse Transcriptase kit (Applied Biosystems; Thermo
Fisher Scientific, Inc.). The iQ™ SYBR® Green Supermix
(Bio-Rad Laboratories, Inc., Hercules, CA, USA) was used for qPCR
analysis. The primers were designed with Primer3 software (version
4.0) and are listed in Table I.
The 15 µl PCR reaction mix contained 3 µl cDNA (10 ng/µl), 7.5 µl
iQTM SYBR Green Supermix (Bio-Rad Laboratories, Inc.), 0.5 µl
primer mix (10 µM each), and 4 µl ddH2O. The thermocycling
conditions were as follows: 5 min denaturation at 95°C; 40 cycles
of annealing and extension at 60°C for 45 sec, and denaturation at
95°C for 10 sec. A dissociation curve was performed following the
40 cycles to verify the quality of primers and amplification.
Relative expression of genes was calculated by the
2−∆∆Cq method (28),
and normalized to the housekeeping gene GAPDH and β-actin of the
same sample, and the relative transcript levels were calculated as
percentage of −2 days.
 | Table I.Primer sequences used in reverse
transcription-quantitative polymerase chain reaction analysis. |
Table I.
Primer sequences used in reverse
transcription-quantitative polymerase chain reaction analysis.
|
|
| Sequence
(5′-3′) |
|---|
|
|
|
|
|---|
| Gene | GenBank no. | Forward | Reverse |
|---|
| β-actin | NM_007393 |
catccgtaaagacctctatgccaac |
atggagccaccgatccaca |
| OAT1 | NM_017224 |
cttgtacaccggagagc |
aggcatggaggggtagaact |
| OAT3 | NM_031332 |
gttgacatcccagccaagtt |
ctgcatttctgaaggcacaa |
| OCT1 | U17013 |
agcagctcaccaatcaaagc |
gtggagtctgtagtgcctgt |
| OCT2 | NM_031584 |
ttgtctgctcctccatgtgt |
agagccttccctttggtctc |
| OCT3 | NM_031332 |
gtctctctctggcctggttt |
gcacaaagatgagggccaaa |
| OATP4C1 | NM_001002024 |
tcaagctggcaaaacttccc |
ccgcaaagctcgatgtcaat |
| MRP2 | NM_012833 |
tctcttgcgctcacagaaga |
gaaactggaatacgccgcat |
| MRP4 | AY533524 |
accaggatgccgacatctac |
cgtgcaaagtgtggcagata |
| BCRP | NM_181381 |
ccagcctcggtattccatct |
cagccgaagaatctccgttg |
| MATE1 | NM_001014118 |
cctgagtggtatccttggca |
ggcctggtcaatgtttcctg |
| MATE2-K | NM_001191920 |
cacctcccagttcttcctgt |
tcccaatctcgaaggtccac |
| Mdr1b | NM_012623 |
tgtttgactgcagcatcacc |
agctgagtccctttgtctcc |
Protein extraction and
quantification
Kidneys from each group were pooled and ~100 mg was
homogenized in 1 ml radioimmunoprecipitation assay lysis buffer
(Beyotime Institute of Biotechnology, Shanghai, China) containing 1
mM phenylmethanesulfonyl fluoride and proteinase inhibitors. The
lysates were centrifuged at 12,000 × g for 10 min at 4°C, and
supernatants were collected and stored at −80°C prior to analysis.
Protein concentrations were quantified by the Bicinchoninic Acid
assay (Beyotime Institute of Biotechnology).
Western blot analysis
Aliquoted proteins were denatured with loading
buffer (catalog no. P0015; Beyotime Institute of Biotechnology) at
90°C for 10 min, and ~10 µg protein/lane was separated on 10%
SDS-PAGE gels and transferred to polyvinylidene difluoride
membranes. Membranes were blocked with 5% dry non-fat milk in TBS
containing 0.07% Tween-20 (TBST) at room temperature for 2 h,
followed by incubation overnight at 4°C with the following primary
antibodies, diluted 1:1,000 in 1% bovine serum albumin
(Sigma-Aldrich; Merck Millipore, Darmstadt, Germany) in TBST.
Antibodies were Goat anti-OAT1 (cat.no. sc-161977; Santa Cruz
Biotechnology, Inc., Dallas, TX, USA), goat anti-OAT3 (cat. no.
sc-107836; Santa Cruz Biotechnology, Inc.), rabbit anti-MDR1 (cat.
no. ab170904; Abcam, Cambridge, UK) and mouse anti-β-actin (cat.
no. AA128-1; Beyotime Institute of Biotechnology). Following three
washes with TBST, membranes were incubated with horseradish
peroxidase-conjugated anti-rabbit (cat. no. A0208), anti-mouse
(cat. no. A0126), or anti-goat (cat. no. A0181) IgG secondary
antibodies, all obtained from Beyotime Institute of Biotechnology)
and used at a dilution of 1:5,000 for 1 h at room temperature.
Protein-antibody complexes were visualized using an Enhanced
Chemiluminescent reagent (catalog no. P0018; Beyotime Institute of
Biotechnology), and a ChemiDoc XRS system (Bio-Rad Laboratories,
Inc.). Band intensities were semi-quantified by densitometry using
Quantity One® software (version 4.6.2, Bio-Rad
Laboratories, Inc.).
Statistical analysis
Statistical analyses were performed in SPSS software
version 16.0 (SPSS, Inc., Chicago, IL, USA). Data were expressed as
the mean ± standard error. Age-associated differences were analyzed
by one-way analysis of variance, followed by the least significant
difference post hoc test. P<0.05 was considered to
indicate a statistically significant difference.
Results
Basolateral uptake transporter
expression levels in kidneys
On the basolateral membrane of the kidneys, the
primary anion transporters are OAT1 and 3, and OATP4C1; the primary
cation transporters are OCT1, 2 and 3.
OAT1 and 3. OAT1 and OAT3 are involved in the active
uptake of chemicals and toxicants. The mRNA expression levels of
OAT1 and 3 increased significantly with age from −2 to 850 days
(Fig. 1). OAT1 mRNA expression
levels were low in fetal kidneys, increased gradually following
birth and increased markedly on maturation and adulthood (peaking
at 180 days). Compared with OAT1 mRNA expression levels at −2 days,
OAT1 levels at 180 days were 35-fold greater, and were maintained
at that high level until 850 days (Fig. 1).
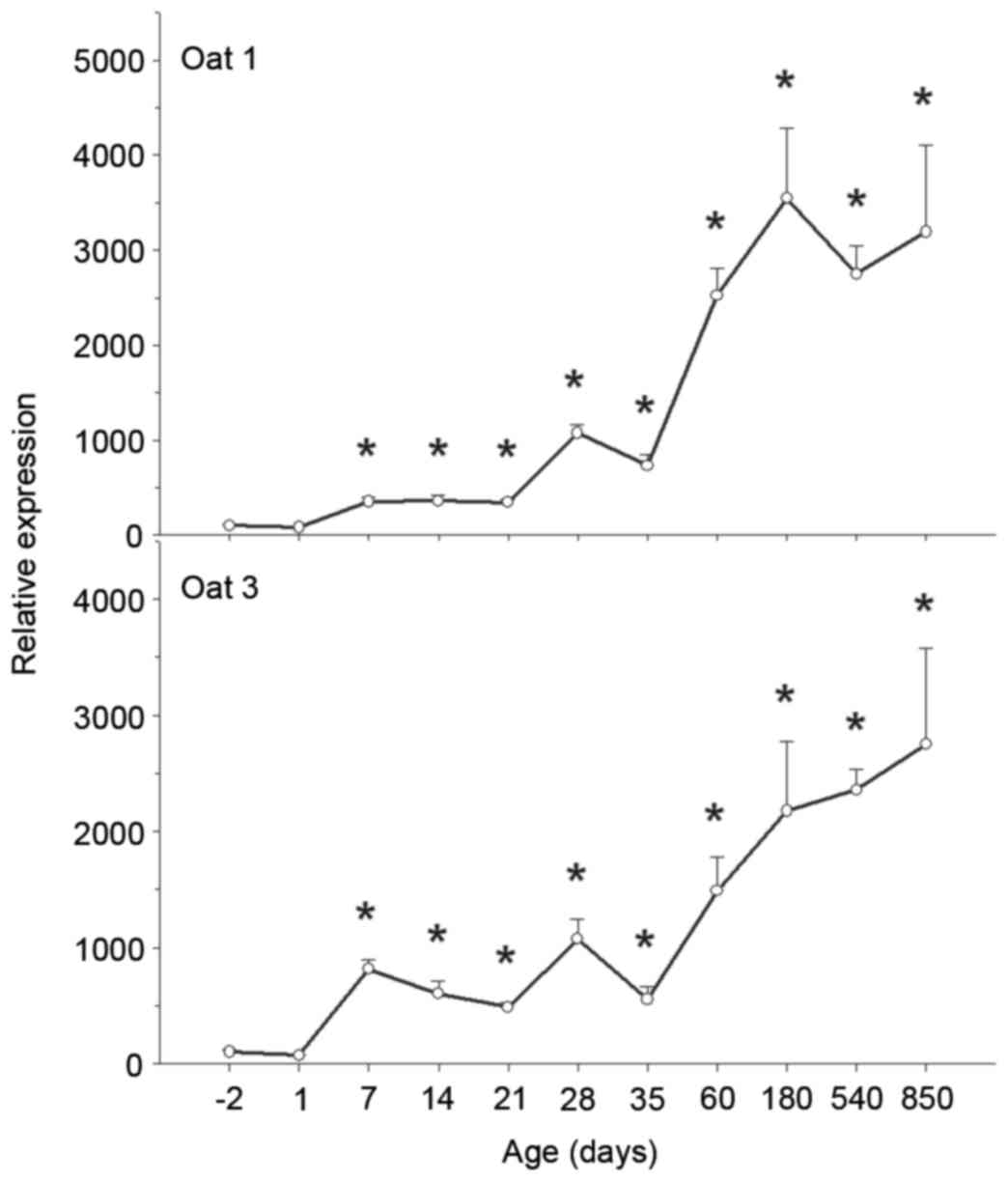 | Figure 1.Ontogeny of OAT1 and 3 mRNA
expression levels in rat kidney. Kidneys were collected at −2, 1,
7, 14, 21, 28, 35, 60, 180, 540 and 850 days, and subjected to
reverse transcription-quantitative polymerase chain reaction
analysis using specific primers. mRNA expression levels of OAT1 and
3 increased over time. Data are expressed as the mean ± standard
error (n=6). *P<0.05 vs. −2 days. OAT, organic anion
transporter. |
OAT3 mRNA expression levels were low in fetal and
newborn kidneys; however, these levels increased rapidly following
birth and continued to increase during adulthood. Compared with
OAT3 mRNA expression levels at birth, OAT3 levels at 180 days were
22-fold greater, and were maintained at that high level and even
increased until 850 days (Fig.
1).
The protein expression levels of OAT1 (Fig. 2) and 3 (Fig. 3) followed similar patterns to the
mRNA expression levels. OAT1 protein was undetectable at day 1,
reached a peak on maturation, and remained high on aging (P<0.05
vs. −2 days; Fig. 2). OAT3 protein
was undetectable at −2 and 1 days; however, protein expression
levels subsequently increased throughout weaning, maturation,
adulthood and aging (P<0.05 vs. −2 days; Fig. 3).
 | Figure 2.Protein expression levels of OAT1 in
rat kidney. Kidneys were collected at −2, 7, 14, 21, 28, 60, 180,
540 and 850 days, and subjected to western blot analysis using
specific antibodies. Protein expression levels of OAT1 increased
over time, peaking at 60 days. Data are expressed as the mean ±
standard error of 3–4 replicates of pooled samples. *P<0.05 vs.
−2 days. OAT, organic anion transporter. |
OCT1, 2 and 3, and OATP4C1
OCTs are responsible for excretion of cationic
substances into urine. Tissue OCT expression is important for the
disposition and excretion of xenobiotics. On the basolateral
membrane of the kidneys, OCT1, 2 and 3 are the primary cation
transporters, whereas OATP4C1 is the primary anion transporter. The
mRNA expression levels of OCT1, 2 and 3, and OATP4C1 increased
significantly with age from −2 to 850 days (Fig. 4).
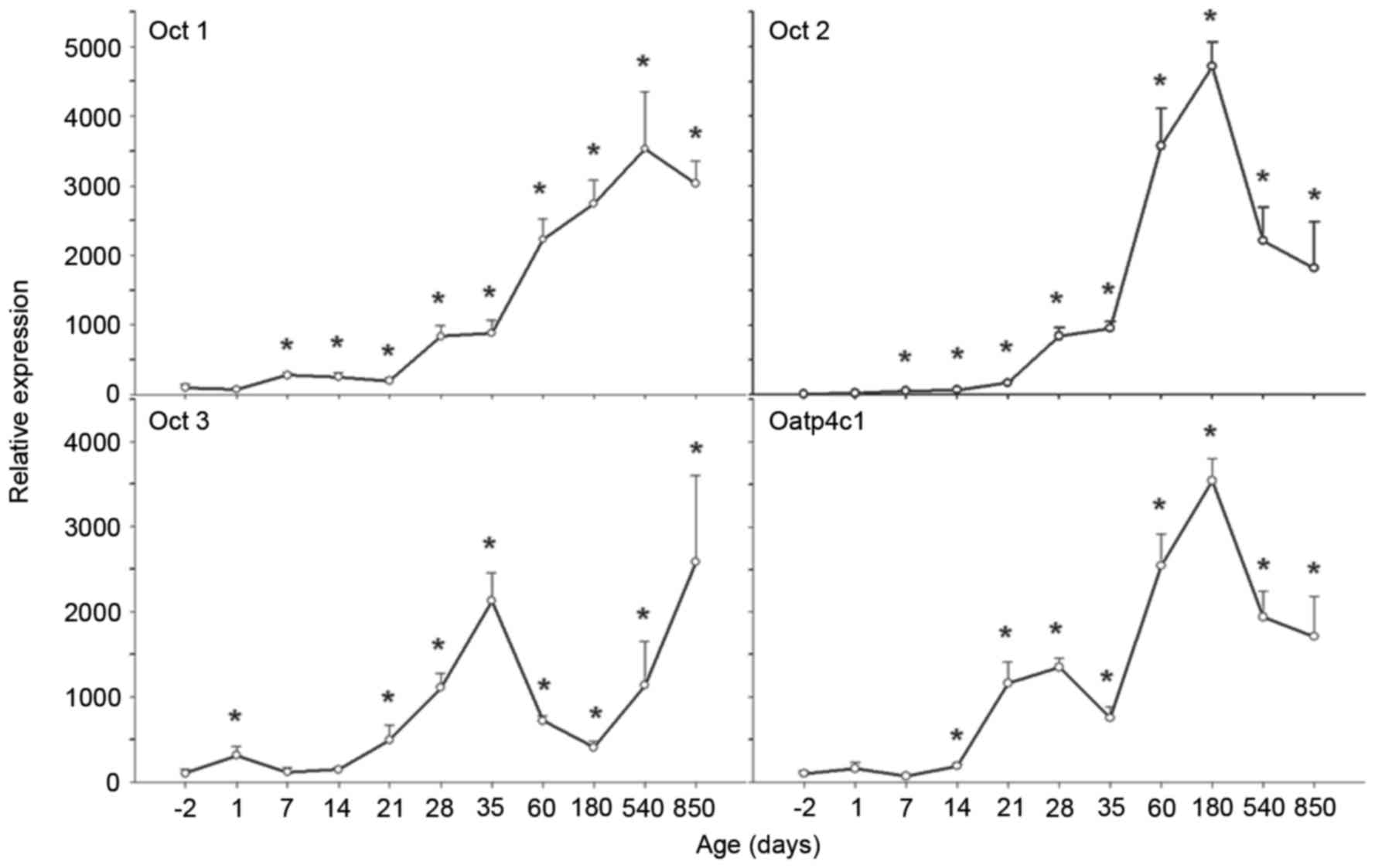 | Figure 4.Ontogeny of OCT1, 2 and 3, and
OATP4C1 mRNA expression levels in rat kidney. Kidneys were
collected at −2, 1, 7, 14, 21, 28, 35, 60, 180, 540 and 850 days,
and subjected to reverse transcription-quantitative polymerase
chain reaction analysis using specific primers. mRNA expression
levels of OCT1 increased over time, levels of OCT2 and OATP4C1
increased over time and subsequently decreased on aging. OCT3 mRNA
expression levels exhibited a biphasic pattern. Data are expressed
as the mean ± standard error (n=6). *P<0.05 vs. −2 days. OCT,
organic cation transporter; OATP, organic anion-transporting
polypeptide. |
OCT1 mRNA expression levels were low in fetal
kidneys, increased gradually following birth, and increased
markedly on weaning, maturation and adulthood (peaking at 540
days). Compared with OCT1 mRNA expression levels at −2 days, OCT1
levels at 420 days were 35-fold greater, and were maintained at
that high level until 850 days (P<0.05 vs. −2 days; Fig. 4).
Similarly to OCT1, OCT2 mRNA expression levels were
low in fetal kidneys, increased gradually following birth, and
increased markedly on weaning, maturation and adulthood (peaking at
180 days). Compared with OCT2 mRNA expression levels at −2 days,
OCT2 levels at 180 days were 470-fold greater; however, these
levels decreased on aging (P<0.05 vs. −2 d; Fig. 4).
The ontogeny of OCT3 differed from OCT1 and 2. OCT3
mRNA expression levels were low in fetal and newborn kidneys,
increased rapidly following birth and reached a first peak at 35
days. Compared with OCT3 mRNA expression levels at birth, OCT3
levels at 35 days were 20-fold greater. Subsequently, OCT3 mRNA
expression levels declined, and reached a trough at 180 days.
Following this, however, OCT3 mRNA expression levels increased
again and reached a second peak at 850 days. Compared with OCT3
mRNA expression levels at birth, OCT3 levels at 850 days were
25-fold greater (P<0.05 vs. −2 days; Fig. 4).
OATP4C1 mRNA expression levels were low in fetal
kidneys, increased gradually following birth, and increased
markedly after 14 days, peaking at 180 days. Compared with OATP4C1
mRNA expression levels at −2 days, OATP4C1 levels at 180 days were
35-fold greater. Subsequently, OATP4C1 mRNA expression levels
decreased (P<0.05 vs. −2 days; Fig.
4).
Apical efflux transporter expression levels in
kidneys. The brush-border efflux transporters BCRP, MDR1 MRP2 and
4, and MATE1 and 2-K contribute to the secretion of chemicals from
the kidneys into the urine.
BCRP and Mdr1b
BCRP and MDR1 transport an extensive range of
endogenous and exogenous lipophilic substrates, including lipids,
steroids, peptides and xenobiotics. The mRNA expression levels of
BCRP and Mdr1b increased significantly with age from −2 to 850 days
(Fig. 5).
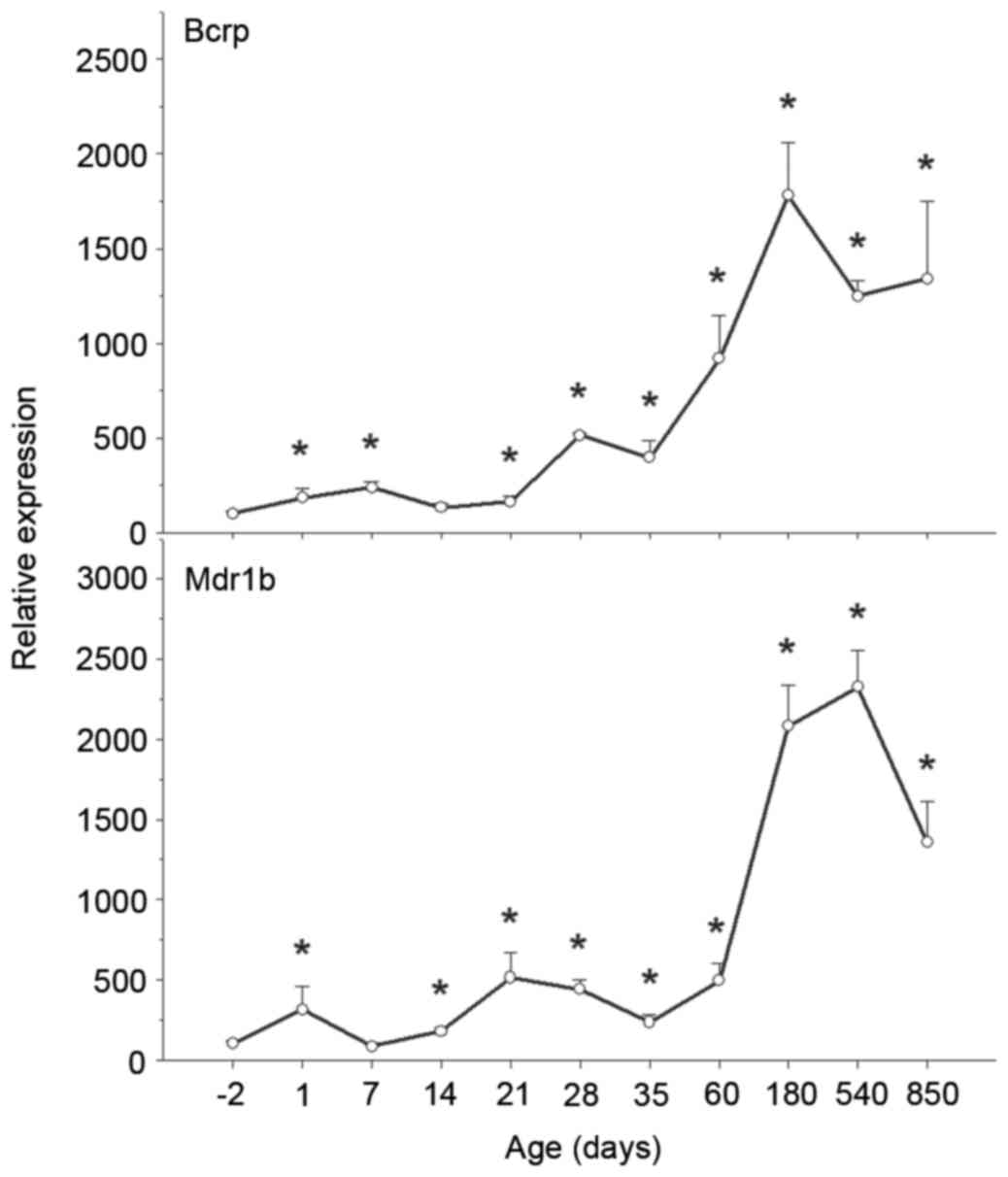 | Figure 5.Ontogeny of BCRP and Mdr1b mRNA
expression levels in rat kidney. Kidneys were collected at −2, 1,
7, 14, 21, 28, 35, 60, 180, 540 and 850 days, and subjected to
reverse transcription-quantitative polymerase chain reaction
analysis using specific primers. mRNA expression levels of BCRP
increased over time, whereas levels of Mdr1b1 increased over time
and subsequently decreased on aging. Data are expressed as the mean
± standard error (n=6). *P<0.05 vs. −2 days. BCRP, breast cancer
resistance protein; Mdr1b, multidrug resistance protein gene
1b. |
BCRP mRNA expression levels were low in fetal and
newborn kidneys, and increased rapidly following birth, reaching a
peak at 180 days and subsequently declining. Compared with BCRP
mRNA expression levels at birth, BCRP levels at 180 days were
18-fold greater (P<0.05 vs. −2 days; Fig. 5).
Similarly to BCRP, Mdr1b mRNA expression levels were
low in fetal kidneys, increased gradually following birth and
increased markedly on maturation, peaking at 540 days. Compared
with Mdr1b mRNA expression levels at −2 days, Mdr1b levels at 540
days were 23-fold greater; however, these levels decreased at 850
days (Fig. 5). The protein
expression levels of MDR1 followed a similar pattern: Undetectable
at 1 day, and increasing on maturation, prior to decreasing at 850
days (P<0.05 vs. −2 days; Fig.
6).
MRP2 and 4
In renal proximal tubules, MRP2 and 4 actively
transport numerous organic anions into urine, including drugs and
metabolic waste. The mRNA expression levels of MRP2 and 4 increased
significantly with age from −2 to 850 days (Fig. 7).
 | Figure 7.Ontogeny of MRP2 and 4 mRNA
expression levels in rat kidney. Kidneys were collected at −2, 1,
7, 14, 21, 28, 35, 60, 180, 540 and 850 days, and subjected to
reverse transcription-quantitative polymerase chain reaction
analysis using specific primers. mRNA expression levels of MRP2 and
4 increased over time. Data are expressed as the mean ± standard
error (n=6). *P<0.05 vs. −2 days. MRP, multidrug
resistance-associated protein. |
MRP2 mRNA expression levels were reduced 50% in
neonatal kidneys (14 days) compared with fetal kidneys, and
subsequently increased markedly following weaning, continuing to
increase throughout and peaking at 850 days. Compared with MRP2
mRNA expression levels at −2 days, MRP2 levels at 850 days were
3-fold greater (P<0.05 vs. −2 days; Fig. 7).
MRP4 mRNA expression levels were low in fetal
kidneys and increased gradually following birth and through
weaning, peaking at 28 days. Compared with the MRP4 mRNA expression
levels at −2 days, MRP4 levels at 28 days were 6-fold greater
(P<0.05 vs. −2 days; Fig.
7).
MATE1 and 2-K
The MATE transporters mediate cellular efflux of a
variety of organic cations, including numerous drugs. The mRNA
expression levels of MATE1 and 2-K increased significantly with age
from −2 to 850 days (Fig. 8).
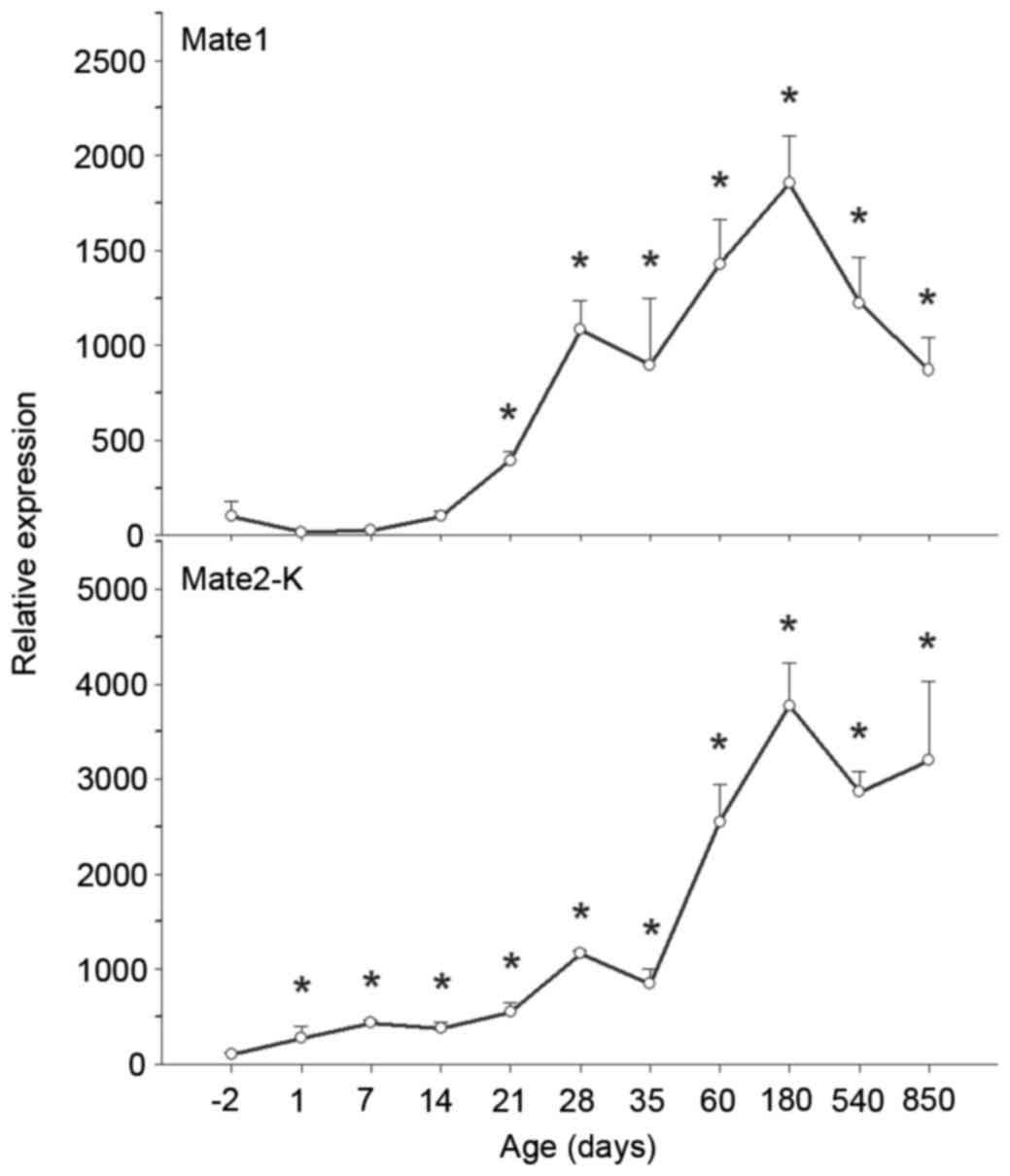 | Figure 8.Ontogeny of MATE1 and 2-K mRNA
expression levels in rat kidney. Kidneys were collected at −2, 1,
7, 14, 21, 28, 35, 60, 180, 540 and 850 days, and subjected to
reverse transcription-quantitative polymerase chain reaction
analysis using specific primers. mRNA expression levels of MATE2-K
increased over time, whereas levels of MATE1 increased over time
and subsequently decreased on aging. Data are expressed as the mean
± standard error (n=6). *P<0.05 vs. −2 days. MATE, multidrug and
toxin extrusion protein. |
MATE1 mRNA expression levels were low in fetal
kidneys, increased gradually following birth, and increased
markedly following weaning, peaking at 180 days. Compared with
MATE1 mRNA expression levels at −2 days, MATE1 levels at 180 days
were 18-fold greater. MATE1 mRNA expression levels subsequently
decreased (P<0.05 vs. −2 days; Fig.
8).
MATE2-K mRNA expression levels were low in fetal
kidneys, increased gradually following birth, and increased
markedly following maturation, peaking at 180 days. Compared with
MATE2-K mRNA expression levels at −2 days, MATE2-K levels at 180
days were 37-fold greater, and were maintained at that high level
on aging (P<0.05 vs. −2 days; Fig.
8).
Discussion
The present study demonstrated the ontogeny and
age-associated variations in 12 primary kidney transporters in
rats. These transporters include the transporters responsible for
renal uptake of xenobiotics (OAT1 and 3, OCT1, 2 and 3, and
OATP4C1) and transporters associated with kidney efflux and
excretion of xenobiotics (MDR1, MRP2, MRP4, BCRP and MATE1 and
2-K). Typically, the mRNA expression levels of these transporters
were low in fetal kidneys, increased gradually following birth, and
increased markedly on maturation and adulthood, maintaining high
expression levels on aging. However, the mRNA expression levels of
certain transporters, OCT2, OATP4C1, Mdr1b and MATE1 were decreased
on aging. The patterns of mRNA and protein expression levels for
the three transporters that underwent western blot analysis were
similar. The profile of the ontogeny and age-associated expression
of these transporters may provide useful information for the
disposition of drugs and toxicants in the kidney, similar to our
previous study on liver (29). The
present study systematically profiled the ontogeny of 12 primary
kidney transporters, and to the best of our knowledge, is among the
first to profile age-associated variations in transporter
expression.
The OAT family has been extensively studied due to
its role in the transport of drugs including cisplatin,
aristolochic acid and tanshinol (12,30),
toxicants including mercury and indoxyl sulfate (13), and nutrients (3,7). OAT
expression is markedly altered during renal failure (31). The importance of OAT1 and 3 in
HgCl2 and MeHg-induced renal injury has been documented
(8,10,11,32).
In addition, our recent studies revealed the alterations of OAT1
and 3 in HgCl2-induced acute and subacute renal injury,
but not following exposure to HgS (33,34).
The pharmacological modulation of the expression and/or function of
OAT1 and 3 may be a potential therapeutic strategy for reducing the
nephrotoxicity of HgCl2 (9). The expression of OAT1 and 3 is
additionally influenced by gender (21), and the increased expression of OAT1
and 3 in males has been revealed to be regulated by the
transcription factor B-cell lymphoma 6 (35). In the kidney, OAT1 transcripts
appeared at mid-gestation, alongside proximal tubule
differentiation, and increased as nephrons matured (36). The present study demonstrated the
ontogeny of OAT expression in the kidney, including during the
aging process.
OCTs are important for the disposition and excretion
of xenobiotics, including platinum compounds (14,20),
cadmium (15,37), metformin (38) and mercury (34). OCT2 and MATEs coordinate to
eliminate cationic drugs, including cisplatin, from the kidney
(14,16). OCT1 and 2 expression has been
demonstrated to be altered during renal failure (31), and OCT mRNA expression levels in
kidneys is influenced by age (23). In the present study, OCT1 and 2
revealed a typical ontogeny pattern, whereas OCT3 exhibited a
biphasic pattern with peaks at 35 and 850 days, which is in
agreement with a previous study (25). The age-associated variations in OCT
expression may affect the ability of the kidney to process heavy
metals and toxicants.
OATP4C1 was identified as a novel uptake transporter
primarily expressed at the basolateral membrane of rat kidney
proximal tubules. It was hypothesized to act as a vectorial
transport partner of an apically-expressed efflux transporter, to
enable the translocation of substrates, including uremic toxins
(39,40), digoxin and estrone 3-sulfate
(41), and sitagliptin, a
therapeutic agent for type 2 diabetes (42), into urine. Numerous factors,
including age-associated variation, may influence the expression of
OATP4C1, thereby altering drug disposition, efficacy and toxicity
(1). Our recent study revealed the
ontogeny and age-associated alterations of OATP expression in the
liver (29), and in the present
study, the ontogeny of OATP4C1, the primary OATP in the kidney, was
characterized.
The transporters BCRP and MDR1 belong to the
multidrug resistance protein family. BCRP has recently been
identified as an additional potential transporter in the
elimination of mercury from proximal tubular cells (6), and aristolochic acid I is an
additional substrate that is excreted by BCRP (43). The organ- and age-specific
expression patterns of these transporters have been demonstrated in
adult organs (44). The ontogeny
of BCRP in the kidney resembles that of the liver with greater
expression in adults (5), and
increased expression of BCRP in male rat kidneys was revealed
(45). In the present study, the
expression levels of BCRP increased with age, and were maintained
at high levels until 850 days. Kidney MDR1 is similar to BCRP
during development (44). Mdr1a
and Mdr1b in kidney exhibit increased expression in females due to
their inhibition by androgens (46), and Mdr1a is important for removing
paraquat from kidneys and protecting against its subsequent
toxicity (17). MDR1 expression is
low at birth, and gradually increases to mature levels at ~30 days
of age (46). In the present
study, MDR1 shared a similar development pattern with BCRP;
however, MDR1 expression levels were decreased during aging,
implying decreased renal excretion function in elderly.
MRP2 and 4 are localized in proximal tubular
epithelial cells and actively transport numerous organic anions
into urine (47), including
HgCl2 (18,19,48)
and the immunosuppressant mycophenolic acid (49). In our recent studies, HgS and
HgS-containing traditional medicines differed from HgCl2
and MeHg as they were unable to increase renal expression of MRP2
and 4 (33,34). The renal toxicity of Hg(2+) differs
in young adult and aged Wistar rats (22), and this may be partially due to the
potential aging-associated expression of MRPs. In kidneys, MRP1 and
5 were expressed at adult levels at birth, whereas MRP2, 3, 4 and 6
expression typically increased with time (50); however, little is known about their
expression during the aging process. The present study profiled the
ontogeny and aging-associated expression of MRP2 and 4 to address
this issue.
In the kidney, MATEs coordinate with OCTs to
eliminate organic cations. More than 40 therapeutic agents and
various endogenous compounds are known to be substrates or
inhibitors of MATEs, including cisplatin, metformin and lamivudine
(51,52). The inhibitory potencies of
ondansetron on MATE1 and 2-K caused increases in tubular cell
accumulation of metformin and cisplatin, with increased renal
toxicity (16,20). In rats treated repeatedly with
HgCl2, MATE2-K expression was increased in an attempt to
eliminate cellular Hg (34). The
mRNA expression levels of MATE1 in the kidneys of males and females
were similar, with levels increasing gradually from prenatal day −2
to 45 days of age. A gender difference appeared at day 30 (24); however, little is known regarding
expression during the aging process. In the present study, MATE1
and 2-K exhibited similar ontogeny patterns, and the levels of mRNA
expression remained high throughout adulthood, although MATE1
levels decreased during aging.
In conclusion, the results of the present study
characterized the ontogeny and age-associated alterations in six
primary renal uptake transporters (OAT1 and 3, OCT1, 2 and 3, and
OATP4C1) and six primary renal efflux transporters (MDR1, BCRP,
MRP2 and 4, and MATE1 and 2-K) at the mRNA level, and at the
protein level of selected transporters (OAT1 and 3, and MDR1). The
results confirmed the ontogeny pattern of certain transporters
described in the literature, and is among the first to demonstrate
the pattern of their expression during development in fetal (−2
days), neonatal (1, 7, 14, 21 and 28 days), mature (35 and 60 days)
and older (180, 540 and 850 days) rat kidneys. These data may
further understanding of age-dependent variations of drug-drug
interactions, and drug efficacy and toxicity.
Acknowledgements
The present study was supported by the Chinese
National Science Foundation (grant nos. 81160415 and 81460632).
Glossary
Abbreviations
Abbreviations:
|
BCRP
|
breast cancer resistance protein
|
|
MATE
|
multidrug and toxin extrusion
protein
|
|
Mdr1b
|
multidrug resistance protein 1b
|
|
MRP
|
multidrug resistance-associated
protein
|
|
OAT
|
organic anion transporter
|
|
OATP
|
organic anion-transporting
polypeptide
|
|
OCT
|
organic cation transporter
|
|
RT-qPCR
|
reverse transcription-quantitative
polymerase chain reaction
|
References
|
1
|
Cheng X and Klaassen CD: Tissue
distribution, ontogeny, and hormonal regulation of xenobiotic
transporters in mouse kidneys. Drug Metab Dispos. 37:2178–2185.
2009. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Klaassen CD and Aleksunes LM: Xenobiotic,
bile acid, and cholesterol transporters: Function and regulation.
Pharmacol Rev. 62:1–96. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Sweeney DE, Vallon V, Rieg T, Wu W,
Gallegos TF and Nigam SK: Functional maturation of drug
transporters in the developing, neonatal, and postnatal kidney. Mol
Pharmacol. 80:147–154. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Brouwer KL, Aleksunes LM, Brandys B,
Giacoia GP, Knipp G, Lukacova V, Meibohm B, Nigam SK, Rieder M and
De Wildt SN: Pediatric Transporter Working Group: Human ontogeny of
drug transporters: Review and recommendations of the pediatric
transporter working group. Clin Pharmacol Ther. 98:266–287. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
5
|
de Zwart L, Scholten M, Monbaliu JG,
Annaert PP, Van Houdt JM, Van den Wyngaert I, De Schaepdrijver LM,
Bailey GP, Coogan TP, Coussement WC and Mannens GS: The ontogeny of
drug metabolizing enzymes and transporters in the rat. Reprod
Toxicol. 26:220–230. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Bridges CC, Zalups RK and Joshee L:
Toxicological significance of renal Bcrp: Another potential
transporter in the elimination of mercuric ions from proximal
tubular cells. Toxicol Appl Pharmacol. 285:110–117. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Nigam SK, Bush KT, Martovetsky G, Ahn SY,
Liu HC, Richard E, Bhatnagar V and Wu W: The organic anion
transporter (OAT) family: A systems biology perspective. Physiol
Rev. 95:83–123. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Lash LH, Hueni SE, Putt DA and Zalups RK:
Role of organic anion and amino acid carriers in transport of
inorganic mercury in rat renal basolateral membrane vesicles:
Influence of compensatory renal growth. Toxicol Sci. 88:630–644.
2005. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Di Giusto G, Anzai N, Ruiz ML, Endou H and
Torres AM: Expression and function of Oat1 and Oat3 in rat kidney
exposed to mercuric chloride. Arch Toxicol. 83:887–897. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Zalups RK and Ahmad S: Handling of
cysteine S-conjugates of methylmercury in MDCK cells expressing
human OAT1. Kidney Int. 68:1684–1699. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Torres AM, Dnyanmote AV, Bush KT, Wu W and
Nigam SK: Deletion of multispecific organic anion transporter
Oat1/Slc22a6 protects against mercury-induced kidney injury. J Biol
Chem. 286:26391–26395. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Xue X, Gong LK, Maeda K, Luan Y, Qi XM,
Sugiyama Y and Ren J: Critical role of organic anion transporters 1
and 3 in kidney accumulation and toxicity of aristolochic acid I.
Mol Pharm. 8:2183–2192. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Saito H: Pathophysiological regulation of
renal SLC22A organic ion transporters in acute kidney injury:
Pharmacological and toxicological implications. Pharmacol Ther.
125:79–91. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Kim HJ, Park DJ, Kim JH, Jeong EY, Jung
MH, Kim TH, Yang JI, Lee GW, Chung HJ and Chang SH: Glutamine
protects against cisplatin-induced nephrotoxicity by decreasing
cisplatin accumulation. J Pharmacol Sci. 127:117–126. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Soodvilai S, Nantavishit J, Muanprasat C
and Chatsudthipong V: Renal organic cation transporters mediated
cadmium-induced nephrotoxicity. Toxicol Lett. 204:38–42. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Li Q, Guo D, Dong Z, Zhang W, Zhang L,
Huang SM, Polli JE and Shu Y: Ondansetron can enhance
cisplatin-induced nephrotoxicity via inhibition of multiple toxin
and extrusion proteins (MATEs). Toxicol Appl Pharmacol.
273:100–109. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Wen X, Gibson CJ, Yang I, Buckley B,
Goedken MJ, Richardson JR and Aleksunes LM: MDR1 transporter
protects against paraquat-induced toxicity in human and mouse
proximal tubule cells. Toxicol Sci. 141:475–483. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Zalups RK, Joshee L and Bridges CC: Novel
Hg2+-induced nephropathy in rats and mice lacking Mrp2: Evidence of
axial heterogeneity in the handling of Hg2+ along the proximal
tubule. Toxicol Sci. 142:250–260. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Bridges CC, Joshee L and Zalups RK:
Placental and fetal disposition of mercuric ions in rats exposed to
methylmercury: Role of Mrp2. Reprod Toxicol. 34:628–634. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Yonezawa A and Inui K: Organic cation
transporter OCT/SLC22A and H(+)/organic cation antiporter
MATE/SLC47A are key molecules for nephrotoxicity of platinum
agents. Biochem Pharmacol. 81:563–568. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Hazelhoff MH, Bulacio RP and Torres AM:
Gender related differences in kidney injury induced by mercury. Int
J Mol Sci. 13:10523–10536. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Bridges CC, Joshee L and Zalups RK: Aging
and the disposition and toxicity of mercury in rats. Exp Gerontol.
53:31–39. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Alnouti Y, Petrick JS and Klaassen CD:
Tissue distribution and ontogeny of organic cation transporters in
mice. Drug Metab Dispos. 34:477–482. 2006.PubMed/NCBI
|
|
24
|
Lickteig AJ, Cheng X, Augustine LM,
Klaassen CD and Cherrington NJ: Tissue distribution, ontogeny and
induction of the transporters Multidrug and toxin extrusion (MATE)
1 and MATE2 mRNA expression levels in mice. Life Sci. 83:59–64.
2008. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Slitt AL, Cherrington NJ, Hartley DP,
Leazer TM and Klaassen CD: Tissue distribution and renal
developmental changes in rat organic cation transporter mRNA
levels. Drug Metab Dispos. 30:212–219. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Buist SC, Cherrington NJ, Choudhuri S,
Hartley DP and Klaassen CD: Gender-specific and developmental
influences on the expression of rat organic anion transporters. J
Pharmacol Exp Ther. 301:145–151. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Nakajima N, Sekine T, Cha SH, Tojo A,
Hosoyamada M, Kanai Y, Yan K, Awa S and Endou H: Developmental
changes in multispecific organic anion transporter 1 expression in
the rat kidney. Kidney Int. 57:1608–1616. 2000.PubMed/NCBI
|
|
28
|
Schmittgen TD and Livak KJ: Analyzing
real-time PCR data by the comparative C(T) method. Nat Protoc.
3:1101–1108. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Hou WY, Xu SF, Zhu QN, Lu YF, Cheng XG and
Liu J: Age- and sex-related differences of organic
anion-transporting polypeptide gene expression in livers of rats.
Toxicol Appl Pharmacol. 280:370–377. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Jia W, Du F, Liu X, Jiang R, Xu F, Yang J,
Li L, Wang F, Olaleye OE, Dong J and Li C: Renal tubular secretion
of tanshinol: Molecular mechanisms, impact on its systemic
exposure, and propensity for dose-related nephrotoxicity and for
renal herb-drug interactions. Drug Metab Dispos. 43:669–678. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Komazawa H, Yamaguchi H, Hidaka K, Ogura
J, Kobayashi M and Iseki K: Renal uptake of substrates for organic
anion transporters Oat1 and Oat3 and organic cation transporters
Oct1 and Oct2 is altered in rats with adenine-induced chronic renal
failure. J Pharm Sci. 102:1086–1094. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Preising C, Schneider R, Bucher M, Gekle M
and Sauvant C: Regulation of expression of renal organic anion
transporters OAT1 and OAT3 in a model of ischemia/reperfusion
injury. Cell Physiol Biochem. 37:1–13. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Sui Y, Yang H, Tian XZ, Liu J and Shi JZ:
Effect of Zhusha Anshen pill, cinnabar, HgS, HgCl2 and MeHg on gene
expression of renal transporters in mice. Zhongguo Zhong Yao Za
Zhi. 40:506–510. 2015.(In Chinese). PubMed/NCBI
|
|
34
|
Zhu QN, Lu YF, Shi JZ, Wu Q, Zhang F, Shi
JS and Liu J: Distinct effect of Wansheng Huafeng Dan containing
ardisia crenata on renal transporters, mercury accumulation and
Kim-1 expression from mercuric chloride. Zhongguo Zhong Yao Za Zhi.
39:1892–1896. 2014.(In Chinese). PubMed/NCBI
|
|
35
|
Wegner W, Burckhardt BC, Burckhardt G and
Henjakovic M: Male-dominant activation of rat renal organic anion
transporter 1 (Oat1) and 3 (Oat3) expression by transcription
factor BCL6. PloS One. 7:e355562012. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Pavlova A, Sakurai H, Leclercq B, Beier
DR, Yu AS and Nigam SK: Developmentally regulated expression of
organic ion transporters NKT (OAT1), OCT1, NLT (OAT2) and Roct. Am
J Physiol Renal Physiol. 278:F635–F643. 2000.PubMed/NCBI
|
|
37
|
Ljubojevic M, Breljak D, Herak-Kramberger
CM, Anzai N and Sabolić I: Expression of basolateral organic anion
and cation transporters in experimental cadmium nephrotoxicity in
rat kidney. Arch Toxicol. 90:525–541. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Tzvetkov MV, Vormfelde SV, Balen D,
Meineke I, Schmidt T, Sehrt D, Sabolić I, Koepsell H and
Brockmöller J: The effects of genetic polymorphisms in the organic
cation transporters OCT1, OCT2, and OCT3 on the renal clearance of
metformin. Clin Pharmacol Ther. 86:299–306. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Kuo KL, Zhu H, McNamara PJ and Leggas M:
Localization and functional characterization of the rat Oatp4c1
transporter in an in vitro cell system and rat tissues. PLos One.
7:e396412012. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Masereeuw R, Mutsaers HA, Toyohara T, Abe
T, Jhawar S, Sweet DH and Lowenstein J: The kidney and uremic toxin
removal: Glomerulus or tubule? Semin Nephrol. 34:191–208. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Yamaguchi H, Sugie M, Okada M, Mikkaichi
T, Toyohara T, Abe T, Goto J, Hishinuma T, Shimada M and Mano N:
Transport of estrone 3-sulfate mediated by organic anion
transporter OATP4C1: Estrone 3-sulfate binds to the different
recognition site for digoxin in OATP4C1. Drug Metab Pharmacokinet.
25:314–317. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Chu XY, Bleasby K, Yabut J, Cai X, Chan
GH, Hafey MJ, Xu S, Bergman AJ, Braun MP, Dean DC and Evers R:
Transport of the dipeptidyl peptidase-4 inhibitor sitagliptin by
human organic anion transporter 3, organic anion transporting
polypeptide 4C1 and multidrug resistance P-glycoprotein. J
Pharmacol Exp Ther. 321:673–683. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Ma L, Qin Y, Shen Z, Bi H, Hu H, Huang M,
Zhou H, Yu L, Jiang H and Zeng S: Aristolochic acid I is a
substrate of BCRP but not P-glycoprotein or MRP2. J Ethnopharmacol.
172:430–435. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Konieczna A, Erdösová B, Lichnovská R,
Jandl M, Cizkova K and Ehrmann J: Differential expression of ABC
transporters (MDR1, MRP1, BCRP) in developing human embryos. J Mol
Histol. 42:567–574. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Tanaka Y, Slitt AL, Leazer TM, Maher JM
and Klaassen CD: Tissue distribution and hormonal regulation of the
breast cancer resistance protein (Bcrp/Abcg2) in rats and mice.
Biochem Biophys Res Commun. 326:181–187. 2005.PubMed/NCBI
|
|
46
|
Cui YJ, Cheng X, Weaver YM and Klaassen
CD: Tissue distribution, gender-divergent expression, ontogeny, and
chemical induction of multidrug resistance transporter genes
(Mdr1a, Mdr1b, Mdr2) in mice. Drug Metab Dispos. 37:203–210. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
47
|
Prevoo B, Miller DS, van de Water FM,
Wever KE, Russel FG, Flik G and Masereeuw R: Rapid, nongenomic
stimulation of multidrug resistance protein 2 (Mrp2) activity by
glucocorticoids in renal proximal tubule. J Pharmacol Exp Ther.
338:362–371. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
48
|
Bridges CC, Joshee L, van den Heuvel JJ,
Russel FG and Zalups RK: Glutathione status and the renal
elimination of inorganic mercury in the Mrp2(−/−) mouse. PLoS One.
8:e735592013. View Article : Google Scholar : PubMed/NCBI
|
|
49
|
El-Sheikh AA, Koenderink JB, Wouterse AC,
van den Broek PH, Verweij VG, Masereeuw R and Russel FG: Renal
glucuronidation and multidrug resistance protein 2-/ multidrug
resistance protein 4-mediated efflux of mycophenolic acid:
Interaction with cyclosporine and tacrolimus. Transl Res.
164:46–56. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
50
|
Maher JM, Slitt AL, Cherrington NJ, Cheng
X and Klaassen CD: Tissue distribution and hepatic and renal
ontogeny of the multidrug resistance-associated protein (Mrp)
family in mice. Drug Metab Dispos. 33:947–955. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
51
|
Staud F, Cerveny L, Ahmadimoghaddam D and
Ceckova M: Multidrug and toxin extrusion proteins (MATE/SLC47);
role in pharmacokinetics. Int J Biochem Cell Biol. 45:2007–2011.
2013. View Article : Google Scholar : PubMed/NCBI
|
|
52
|
Muller F, König J, Hoier E, Mandery K and
Fromm MF: Role of organic cation transporter OCT2 and multidrug and
toxin extrusion proteins MATE1 and MATE2-K for transport and drug
interactions of the antiviral lamivudine. Biochem Pharmacol.
86:808–815. 2013. View Article : Google Scholar : PubMed/NCBI
|






















