Introduction
The proliferation and migration of vascular smooth
muscle cells (VSMCs), and the deposition of extracellular matrix
(ECM) results in intimal hyperplasia (IH) (1–8). The
proliferation and migration of VSMCs is stimulated and regulated by
a series of factors in the injury response (9–16).
Growth factors, metalloproteinases induced by matrix proteins,
white blood cells, endothelial cells, fibroblasts and plasma are
all involved in the proliferation and migration of VSMCs in
response to injury (10,11,17).
Also included are platelet-derived growth factor (PDGF), basic
fibroblast growth factor, acidic fibroblast growth factor,
insulin-like growth factor 1, tumor growth factor (TGF), epidermal
growth factor, connective TGF, and vascular endothelial growth
factor (VEGF) (18–22). Among these, PDGF may be vital in IH
by stimulating the migration of VSMCs. There are a variety of
factors, which affect VSMC proliferation and migration, however,
the underlying mechanism remains to be fully elucidated, and there
remains no effective therapeutic target for coronary artery bypass
vein graft IH.
Peroxiredoxin-1 (Prx-1), as a member of the Prx
family, is a class of antioxidant enzyme. Previous studies on Prx-1
have focussed predominantly on its roles in antioxidant activity,
including ROS scavenging, which protect cells from damage caused by
ROS (23,24). Previously, studies have shown that
Prx1 is expressed at high levels in various types of tumor tissues,
including esophageal squamous cell carcinoma (25), pancreatic cancer (26), astrocytoma (27) and prostate cancer (28), and it has been reported to be
closely associated with tumor growth, proliferation,
differentiation, invasion and migration (25–33).
Although Prx1 has been shown to promote the proliferation of VSMCs
(29), the effects of Prx1 on the
invasion and migration of VSMCs and the underlying mechanisms
remain to be fully elucidated.
The present study was designed to examine the effect
of Prx1 on the proliferation, invasion and migration abilities of
VSMCs, and to reveal the underlying mechanisms. The expression
levels of Prx1 in the rat vein graft IH group were detected using
western blot analysis. Retroviral transfection of the Prx1 plasmid
was performed to construct stable VSMCs overexpressing Prx1. An MTT
assay was used to detect the effect of overexpressed Prx1 on VSMC
proliferation. The effect of overexpressed Prx1 on VSMC viability
was measured using a Transwell assay. A wound-healing assay was
used to detect the effect of overexpressed Prx1 on VSMC migration
ability. The Prx1-overexpressing cells were then transfected with
the TLR4 interference plasmid, and MTT and wound-healing assays
were used to determine the effect of TLR4-silencing on the
Prx1-induced proliferation and migration of the VSMCs.
Materials and methods
Antibodies and reagents
The rabbit-anti-rat Prx1 antibody (cat. no.
ab208919) and goat anti-rabbit IgG-horseradish peroxidase (HRP)
secondary antibody (cat. no. ab6721) were purchased from Abcam
(Cambridge, MA, USA). The rabbit-anti-rat β-actin antibody (cat.
no. sc-130656) was from Santa Cruz Biotechnology, Inc. (Dallas, TX,
USA). The RevertAid™ First Strand cDNA Synthesis kit and DreamTaq™
Green PCR master mix were purchased from Fermentas; Thermo Fisher
Scientific, Inc., Waltham, MA, USA), Lipofectamine™2000 and
TRIzol® reagent were from Invitrogen; Thermo Fisher
Scientific, Inc.). The gel DNA extraction kit, ribonuclease A and
trypsin were from Takara Bio, Inc. (Otsu, Japan). The plasmid
extraction and purification kit was from TianGen Biotech Co., Ltd.
(Beijing, China). The GeneAmp PCR-System 9600 was from Applied
Biosystems; Thermo Fisher Scientific, Inc.). TheUV-1700
spectrophotometer was from Shimadzu Corporation (Kyoto, Japan).
Precooled Matrigel gum was from BD Biosciences (San Jose, CA, USA).
Transwell chambers were purchased from EMD Millipore (Billerica,
MA, USA).
Construction of the rat common carotid
artery grafting model
Healthy, clean, male Sprague-Dawley rats (300–400 g;
10–12 weeks old; n=6) were purchased from the Laboratory Animal
Center of Jilin University (Jilin, China). The rats were treated
according to institutional guidelines of the Laboratory Animal
Center of Jilin University, and the experimental protocol was
approved by the local ethical committee. The rats were housed under
controlled environmental conditions with ambient temperature of
25°C, relative humidity of 65%, and a 12/12-h light-dark cycle.
Food and water were provided ad libitum. Experiments were
performed following 1 week of acclimation. All rats were
anesthetized by intraperitoneal injection of chloral hydrate (30
mg/kg). The rats were fixed in the supine position and the cervical
region were shaved and sterilized. A 3 cm long longitudinal
incision was made along the median line of the anterior neck under
an operating microscope to exposure the external jugular vein,
followed by the injection of 1.5 mg/kg heparin saline into the
common cardinal vein. The rats were then divided into two groups,
the experimental group (n=3) and the control group (n=3). In the
experimental group, a 0.5–0.8 cm long segment of the vessel was
removed and the vessel was ligated using nylon 6 oligomer. The
common carotid artery was exposed and a segment of the same length
was cut. Immediately, a segment of the external jugular vein was
anastomosed to the common carotid artery. No transplantation
experiments were performed in the control group. Subsequently, the
incisions in all six rats were closed. At 4 weeks
post-transplantation, the rats were anesthetized by intraperitoneal
injection of chloral hydrate (30 mg/kg). The graft was harvested,
flushed with saline solution, cleaned of adipose tissue and then
was fixed in 4% paraformaldhyde. Subsequently, the graft was
stained with hematoxylin and eosin solution (Sigma-Aldrich; Merck
Millipore, Darmstadt, Germany). The arterial vasculature at same
site and of the same length, were harvested from the normal rats
(n=3), which had not received transplantation, and were cultured in
the same environment as the experimental group. All animals
survived 1 month following transplantation. The rats were
sacrificed by an overdose of pentobarbital sodium
(intraperitoneally, 100 mg/kg body weight; Sigma-Aldrich; Merck
Millipore). The degree of IH was assessed and confirmed by
morphometric analysis.
Cell culture and transfection
experiments in vitro
The CRL-1476 rat VSMC line was purchased from the
Cell Bank of the Chinese Academy of Sciences (Shanghai, China). The
cells were cultured at 37°C in a humidified atmosphere with 5%
CO2, and then seeded into six-well plates until the
VSMCs were adherent and at logarithmic growth phase at 24 h. When
60–80% confluent, the cells were transfected with retrovirus for
the overexpression of Prx1 and either the pGenesil-1 scramble short
hairpin RNA or pGenesil-1-small interfering (si)RNA-TLR4
recombinant plasmid using Lipofectamine™2000 according to the
manufacturer's protocol.
Reverse transcription-quantitative
polymerase chain reaction (RT-qPCR) analysis
The mRNA expression level of Prx1 in the CRL-1476
cells was investigated using RT-qPCR analysis. Total RNA was
extracted from the CRL-1476 cells using TRIzol reagent according to
the manufacturer's protocol. The RNA preparations were quantified
using agarose gel electrophoresis, following which the optical
density (OD)260/OD280 ratio of the RNA was measured on a
spectrophotometer and then stored at −20°C.
cDNA was synthesized by using 1.25 µM oligo dT
(Promega Corporation, Madison, WI, USA), 0.15 µg random primers
(Invitrogen; Thermo Fisher Scientific, Inc.), and 200 U SuperScript
III reverse transcriptase (Invitrogen; Thermo Fisher Scientific,
Inc.) from 2 µg total RNA in a total volume of 20 µl. The PCR
mixture included 2.5 µl Taq buffer (10X), 5 µl MgCl2 (25
mmol/l), 2 µl dNTP (2.5 mmol/l), 0.5 µl upstream primer (20
µmol/l), 0.5 µl downstream primer (20 µmol/l), 2 µl cDNA template,
0.3 µl Taq (5 U/µl), 0.6 µl TaqMan probe (10 µmol/l) and 11.6 µl
double distilled H2O. The sequences of the primers were
as follows: Prx1, forward 5′-CCCGGATGCTTTTGTTCGAGA-3′ and reverse
5′-CATGTGGCAGAATAAGTAGCCAT-3′; TLR4, forward
5′-GCCTTGAATCCAGATGAAAC-3′ and reverse 5′-CTGTGAGGTCGTTGAGGTTAG-3′;
β-actin, forward 5′-CACGATGGAGGGGCCGGACTCATC-3′ and reverse
5′-TAAAGACCTCTATGCCAACACAGT-3′. RT-qPCR analysis was performed at
95°C for 5 min, followed by 40 cycles at 95°C for 40 sec, 55°C for
40 sec and 72°C for 40 sec. Fluorescence was detected following
each cycle and analyzed using the ABI PRISM 7000 sequence detection
system (Applied Biosystems; Thermo Fisher Scientific, Inc.). The
relative mRNA level was calculated using the comparative threshold
cycle (Cq) method (2−ΔΔCq) (34) normalized by β-actin expression. The
PCR products were analyzed using agarose gel and confirmed by
melting curve analyses.
Western blot analysis
Protein was extracted from the cells and tissue
samples from the vein grafts in each group for western blot
analysis. Protein was isolated from cells using
radioimmunoprecipitation assay buffer (Sigma-Aldrich; Merck
Millipore) according to the manufacturer's instructions. Following
centrifugation at 12,000 × g for 30 min at −4°C, the supernatant
was collected. The protein concentration was measured by the
bicinchoninic acid protein assay (Sigma-Aldrich; Merck Millipore).
Equal quantities of protein were separated on 12% SDS-PAGE gels,
followed by transfer onto nitrocellulose membranes. The membranes
were blocked with 5% skim milk in phosphate-buffered saline (PBS)
with Tween 20 for 1 h and probed with the rabbit anti-rat primary
antibodies (1:800) overnight at 4°C. Following three washes with
Tris-buffered saline-Tween 20, the membranes were incubated with
goat anti-rabbit IgG-HRP secondary antibody (1:1,000) for 1 h at
room temperature. The blots were visualized by enhanced
chemiluminescence (Santa Cruz Biotechnology, Inc.). The intensities
of the bands were quantified using the NIH ImageJ software package
(http://rsb.info.nih.gov/ij/). The
expression level of β-actin served as an internal control for
protein loading.
Transwell assay
VSMCs in logarithmic growth phase were collected and
digested, and then resuspended with serum-free medium, adjusting
the cell concentration to 1×105/100 µl. Precooled
Matrigel gum (BD Biosciences) was diluted with serum-free
Dulbecco's modified Eagle's medium (Invitrogen; Thermo Fisher
Scientific, Inc.), and added to evenly coat the bottom film of
Transwell chambers (50 µl/well), followed by incubation for 30 min
at room temperature. 100 µl cell suspension was added in each upper
chamber of the Transwell chamber, 600 µl medium containing 10%
fetal bovine serum (Gibco; Thermo Fisher Scientific, Inc.) was
added in the lower chambers to avoid the generation of bubbles. The
paved 24-well cell Transwell chamber (8 µm) cell culture plate was
placed in a 37°C incubator of saturated humidity and 5%
CO2, and cultured for 24 h. Following removal of the
chamber, the medium was aspirated, washed twice using PBS, and a
cotton swab was used to remove the cells on the upper chamber
surface. Anhydrous methanol fixing was performed for 10 min,
followed by 30 min staining with 0.05% crystal violet dye at room
temperature. Following washing twice with PBS, the number of cells
in three randomly selected visual fields per well were counted
under a microscope, and the average calculated. Three replicate
wells were used and the experiment was repeated three times.
Wound healing assay
The migratory ability of the cells was examined
using a wound healing assay. Briefly, the VSMCs were seeded into
six-well plates at a density of 1×105 cells per well,
and were cultured for 48 h to grow to full confluence in the
plates. Subsequently, the cell layer were wounded using 200 µl
pipette tips and washed with PBS to remove detached cells. The
cells were then cultured with 5% CO2 at 37°C for 48 h.
Photomicrographs of the initial wounds and final wounds were
captured using an inverted microscope. The initial and final wound
sizes were measured using AxioVision Rel.4.7 software (Zeiss GmbH,
Jena, Germany).
MTT assay
Cell proliferation was determined using an MTT
assay. The VSMCs were inoculated onto a 24-well plate at a density
of 1×105 cells per well 24 h post-transfection. The
cells were cultured at 37°C in a humidified atmosphere of 5%
CO2 for 24 h. Subsequently, 20 ml MTT dye was added (5
mg/ml), and incubation was continued for 4 h. Dimethyl sulfoxide
(100 µl) was added and agitated for 5 min to mix thoroughly, and
the absorbance was detected using ELISA at 595 nm.
Statistical analysis
Data are shown as the mean ± standard deviation of
triplicate determinations, calculated using SPSS software (version
10.0; SPSS, Inc., Chicago, IL, USA). Student's t-test was used for
comparison between two groups. P<0.05 was considered to indicate
a statistically significant difference.
Results
Expression of Prx1 in grafted vessels
of the IH rat model
The expression levels of Prx1 in the rat carotid
artery bypass graft model were detected using western blot
analysis. Additionally, the mRNA expression levels of Prx1 in each
group were detected using RT-qPCR analysis. Each group was analyzed
in duplicate and in three separate trials. The results showed that
the protein (Fig. 1A) and mRNA
(Fig. 1B) expression levels of
Prx1 in the IH groups were significantly higher, compared with
those in the control groups.
Overexpression of Prx1 promotes VSMC
invasion and migration
A retroviral vector was transfected into VSMCs, and
the transfection efficiency was detected using western blot and
RT-qPCR analyses. The results showed that the expression of Prx1 in
the retroviral cells was significantly higher, compared with that
in the control group. Date analysis showed that the relative mRNA
expression levels of Prx1 in the IH groups were increased by almost
50%, compared with those in the control groups (data not shown),
which indicated that the VSMCs overexpressing Prx1 had been
constructed successfully.
At 48 h post-VSMC transfection with the Prx1 gene, a
Transwell assay was used to detect the effect of the overexpression
of Prx1 on the invasion capability of the VSMCs. The results
suggested that, compared with the control group, the number of
cells able to pass through Transwell chamber was significantly
increased from 40±5/visual field to 150±17/visual field (Fig. 2A), in the Prx1-overexpressed group,
indicating that the overexpression of Prx1 promoted the invasive
capability of the VSMCs. A wound healing assay was used to detect
the effect of the overexpression of Prx1 on VSMC migration. The
results showed that the VSMC migration ability in the
Prx1-overexpressed group was significantly higher, compared with
that in the control group, with the degree of wound healing
increased from 55 to 100% (Fig.
2B), which suggested that the overexpression of Prx1 enhanced
the invasion and migration capacities of the VSMCs.
Overexpression of Prx1 promotes VSMC
proliferation
Cell viability was determined using an MTT assay.
The results showed that number of surviving cells in the
Prx1-overexpressing group were 5.625±0.1×105,
8.9±0.737×105 and 10.635±0.065×105 on days 1,
2 and 3, respectively, which were significantly higher, compared
with the number of surviving cells in the control group, which were
3.0±0.025×105, 4.1±0.035×105 and
5.06±0.023×105 on days 1, 2 and 3, respectively
(Fig. 3). These results
demonstrated that the overexpression of Prx1 promoted cell
proliferation of the VSMCs.
TLR4 silencing inhibits the
Prx1-induced proliferation of VSMCs
To verify the transfection efficiency of TLR4 siRNA
in the VSMCs, RT-qPCR analysis was performed, and the mRNA
expression levels of TLR4 in the control group, scramble group and
siRNA group were detected. The results showed that the mRNA
expression of TLR4 was effectively inhibited in the TLR4
siRNA-transfected group, compared with those in the control and
scramble groups (Fig. 4A). An MTT
assay was then used to determine the proliferation of the
Prx1-overexpressed VSMCs following TLR4 gene silencing. The results
showed that the number of surviving cells in the TLR4 siRNA
Prx1-overexpressed group on days 1–3 were
3.824±0.43×105, 5.395±0.6×105 and
6.456±0.28×105, respectively, which were significantly
lower, compared with the numbers in the Prx1-overexpressed group on
these days (5.825±0.12, 9.1±0.5375 and 10.735±0.075×105,
respectively; Fig. 4B). This
suggested that the silencing of TLR4 inhibited the cell
proliferation induced by Prx1.
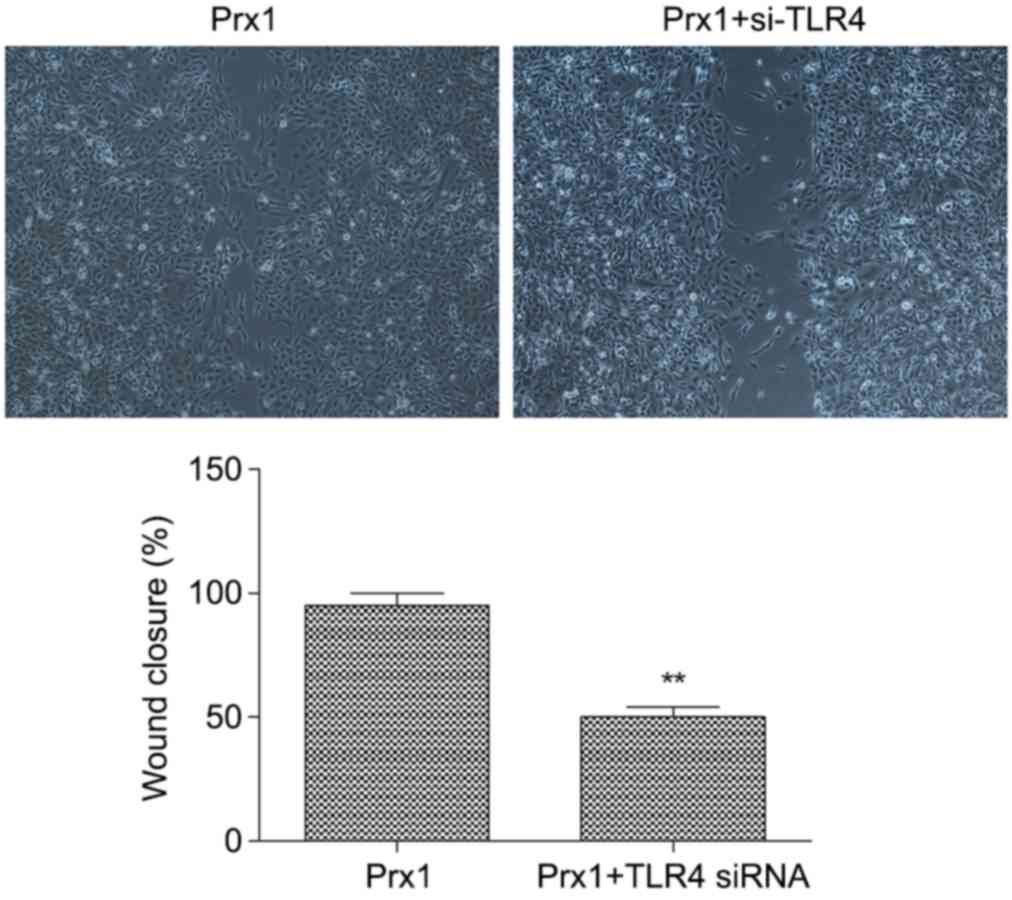
Silencing of TLR4 inhibits the
Prx1-induced migration of VSMCs
The effect of TLR4 gene silencing on cell migration
in the VSMC was determined using a wound healing assay. The results
showed that, in the TLR4 interference Prx1-overexpressed group, the
migration ability of the cells was significantly attenuated,
compared with that in the Prx1-overexpressed group without TLR4
interference. The healing rate was decreased from 95 to 50%
(Fig. 5) suggesting that silencing
of TLR4 inhibited the cell migration induced by Prx1.
Discussion
Previous studies have demonstrated that Prx1 is
expressed at high levels in esophageal squamous cell carcinoma
(25), pancreatic cancer (26), astrocytomas (27) and prostate cancer (28), which is closely associated with
tumor growth, cell proliferation, differentiation, invasion and
migration. Gong et al (25)
found that the downregulation Prx1 inhibits cell proliferation and
invasion of esophageal squamous cell carcinoma, and promotes
apoptosis. Taniuchi et al (26) revealed that Prx1 promotes
pancreatic cancer invasiveness via the p38/mitogen-activated
protein kinase pathway. Prx1 is important in invasion and
malignancy in the development of astrocytoma, offering potential as
a tumor marker and therapeutic target for astrocytoma (27). As a ligand of TLR4, Prx1 stimulates
the inflammatory reaction, and promotes the proliferation,
metastasis and differentiation of endothelial cells in prostate
cancer, whereas suppressing the expression of Prx1 can induce the
expression of vascular endothelial growth factor and reduce
angiogenesis (28,29). Prx1 has also been reported to
promote the development of malignant lung cancer (30), and reducing the expression of Prx1
can induce the apoptosis of lung cancer cells (31). A study by Sun et al
(32) showed that Prx1 is closely
associated with tumor size, vascular invasion, edmondson grade,
α-fetoprotein and lymph node metastasis in hepatocellular
carcinoma. The overexpression of Prx1 also promotes the
epithelial-mesenchymal transition and migration if non-small cell
lung cancer cells induced by TGF-β1 (33). In the presence of TLR4, Prx1
promotes the expression of inflammatory cytokines, IL-6 and tumor
necrosis factor-α, and induces the maturation of dendritic cells
(29). Du et al (35) found that Prx1 is expressed at high
levels in thyroid cancer, and the knockdown of Prx1 promotes
apoptosis in thyroid cancer.
There are limited reports on the effect of Prx1 on
VSMCs, and the effect of Prx1 on cell proliferation, invasion and
migration in VSMCs remains to be fully elucidated. Jones et
al (36) reported that
denatured type I collagens prompt the expression of Prx1 and Prx2,
and the overexpression of Prx1 promotes the growth of VSMCs.
Denatured collagen protein can also promote the expression of
tenascin-C, resulting in the proliferation of VSMCs, and the
expression of Prx1 can activate the promoter, tenascin-1, with a
20-fold increase in activity. Jin et al (37) found that lipoma-containing lipoma
preferred partner and the cytoskeleton-associated protein, palladin
enhance the migration and proliferation of cells, which can be
induced by angiotensin II, focal adhesion kinase and Prx1, further
contributing to the migration of VSMCs (38). Investigations by Pi et al
(38) showed that apocynin
inhibited the generation of intracellular ROS, and then
significantly inhibited the expression of TLR4 and pro-inflammatory
cytokines, which inhibited the proliferation and migration of
VSMCs. Knocking down TLR4 inhibited the formation, proliferation
and migration of endometrial epithelial cells in the damaged
carotid, although the inhibitory effect was not significant. These
results showed that ROS offers potential for use as a therapeutic
target in TLR4-associated cardiovascular inflammation and disease,
as it is vital in the TLR4-mediated pro-inflammatory response and
cell proliferation of the VSMCs.
In the present study, it was demonstrated that Prx1
was overexpressed in the vein grafts. The overexpression of Prx1
significantly promoted the proliferation, invasion and migration of
the VSMCs. Compared, with the control group, the numbers of living
cells in the Prx1-overexpressed group in the first 3 days increased
0.875-, 1.17- and 1.10-fold, respectively (Fig. 3). The results of the Transwell
assay showed that the number of penetrating cells in the
Prx1-overexpressed group increased from 40/visual field to
150/visual field (Fig. 2A),
indicating the promoting effects of Prx1 on VSMC invasiveness. In
the Prx1-overexpressed groups, the migration ability was
significantly higher, compared with that in the control group
(P<0.05; Fig. 2B). In further
experiments, TLR4 siRNA vectors and recombinant plasmids of Prx1
were co-transfected into the VSMCs. The results demonstrated that
Prx1 significantly inhibited the proliferation and migration of the
VSMCs. Compared, with the Prx1-overexpressed group, the number of
viable cells in the first 3 days decreased by 34, 41 and 40%,
respectively, in the co-transfected groups (Fig. 4), indicating the significant
inhibitory effect of TLR4 on Prx1-induced proliferation in VSMCs.
The wound-healing assay demonstrated that the migration ability of
the TLR4 siRNA and Prx1 co-transfected cells were attenuated
significantly, compared with that in the Prx1-overexpressed group
(P<0.05).
In conclusion, Prx1 was overexpressed in the vein
graft IH group, and the overexpression of Prx1 significantly
promoted the proliferation, invasion and migration ability of the
VSMCs. Knocking down TLR4 significantly attenuated the Prx1-induced
proliferation, invasion and migration of the VSMCs, which indicated
that Prx1 promoted the proliferation and invasion of the VSMCs,
which was TLR4-dependent. These results indicate the possibility of
TLR4 as a novel target for the diagnosis, treatment and prognosis
in IH.
Acknowledgements
This study was supported by the National Natural
Science Foundation of China (grant no. 81170182).
References
|
1
|
Muto A, Model L, Ziegler K, Eghbalieh SD
and Dardik A: Mechanisms of vein graft adaptation to the arterial
circulation: Insights into the neointimal algorithm and management
strategies. Circ J. 74:1501–1512. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Dilley RJ, McGeachie JK and Tennant M: The
role of cell proliferation and migration in the development of a
neo-intimal layer in veins grafted into arteries, in rats. Cell
Tissue Res. 269:281–287. 1992. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Guo H, Makarova N, Cheng YES, Ji RR, Zhang
C, Farrar P and Tigyi G: The early- and late stages in phenotypic
modulation of vascular smooth muscle cells: Differential roles for
lysophosphatidic acid. Biochim Biophys Acta. 1781:571–581. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Wolff RA, Malinowski RL, Heaton NS,
Hullett DA and Hoch JR: Transforming growth factor-beta1 antisense
treatment of rat vein grafts reduces the accumulation of collagen
and increases the accumulation of h-caldesmon. J Vasc Surg.
43:1028–1036. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Jeremy JY, Nystrom ML, Barradas MA and
Mikhailidis DP: Eicosanoids and septicaemia. Prostaglandins Leukot
Essent Fatty Acids. 50:287–297. 1994. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Sasaki Y, Suehiro S, Becker AE, Kinoshita
H and Ueda M: Role of endothelial cell denudation and smooth muscle
cell dedifferentiation in neointimal formation of human vein grafts
after coronary artery bypass grafting: Therapeutic implications.
Heart. 83:69–75. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Yamashita A, Hanna AK, Hirata S, Dardik A
and Sumpio BE: Antisense basic fibroblast growth factor alters the
time course of mitogen-activated protein kinase in arterialized
vein graft remodeling. J Vasc Surg. 37:866–873. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Jia G, Cheng G, Gangahar DM and Agrawal
DK: Involvement of connexin 43 in angiotensin II-induced migration
and proliferation of saphenous vein smooth muscle cells via the
MAPK-AP-1 signaling pathway. J Mol Cell Cardiol. 44:882–890. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Libby P, Schwartz D, Brogi E, Tanaka H and
Clinton SK: A cascade model for restenosis. A special case of
atherosclerosis progression. Circulation. 86:(6 Suppl).
III47–III52. 1992.PubMed/NCBI
|
|
10
|
Lyon CA, Johnson JL, Williams H,
Sala-Newby GB and George SJ: Soluble N-cadherin overexpression
reduces features of atherosclerotic plaque instability.
Arterioscler Thromb Vasc Biol. 29:195–201. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Bhardwaj S, Roy H and Ylä-Herttuala S:
Gene therapy to prevent occlusion of venous bypass grafts. Expert
Rev Cardiovasc Ther. 6:641–652. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Ekstrand J, Razuvaev A, Folkersen L, Roy J
and Hedin U: Tissue factor pathway inhibitor-2 is induced by fluid
shear stress in vascular smooth muscle cells and affects cell
proliferation and survival. J Vasc Surg. 52:167–175. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Chan J, Prado-Lourenco L, Khachigian LM,
Bennett MR, Di Bartolo BA and Kavurma MM: TRAIL promotes VSMC
proliferation and neointima formation in a FGF-2-, Sp1
phosphorylation-, and NFkappaB-dependent manner. Circ Res.
106:1061–1071. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Earley S and Brayden JE: Transient
receptor potential channels and vascular function. Clin Sci.
119:19–36. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Jia G, Mitra AK, Gangahar DM and Agrawal
DK: Regulation of cell cycle entry by PTEN in smooth muscle cell
proliferation of human coronary artery bypass conduits. J Cell Mol
Med. 13:547–554. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Mitra AK, Jia G, Gangahar DM and Agrawal
DK: Temporal PTEN inactivation causes proliferation of saphenous
vein smooth muscle cells ofhuman CABG conduits. J Cell Mol Med.
13:177–187. 2009.PubMed/NCBI
|
|
17
|
Dong LH, Wen JK, Liu G, McNutt MA, Miao
SB, Gao R, Zheng B, Zhang H and Han M: Blockade of the
Ras-extracellular signal-regulated kinase 1/2 pathway is involved
in smooth muscle 22 alpha-mediated suppression of vascular smooth
muscle cell proliferation and neointima hyperplasia. Arterioscler
Thromb Vasc Biol. 30:683–691. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Maile LA, Allen LB, Hanzaker CF, Gollahon
KA, Dunbar P and Clemmons DR: Glucose regulation of thrombospondin
and its role in the modulation of smooth muscle cell proliferation.
Exp Diabetes Res. 2010(pii): 6170522010.PubMed/NCBI
|
|
19
|
Chiu JJ, Usami S and Chien S: Vascular
endothelial responses to altered shear stress: Pathologic
implications for atherosclerosis. Ann Med. 41:19–28. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Panchatcharam M, Miriyala S, Yang F,
Leitges M, Chrzanowska-Wodnicka M, Quilliam LA, Anaya P, Morris AJ
and Smyth SS: Enhanced proliferation and migration of vascular
smooth muscle cells in response to vascular injury under
hyperglycemic conditions is controlled by beta3 integrin signaling.
Int J Biochem Cell Biol. 42:965–974. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Isenovic ER, Kedees MH, Haidara MA,
Trpkovic A, Mikhailidis DP and Marche P: Involvement of ERK1/2
kinase in insulin-and thrombin-stimulated vascular smooth muscle
cell proliferation. Angiology. 61:357–364. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Furgeson SB, Simpson PA, Park I, Vanputten
V, Horita H, Kontos CD, Nemenoff RA and Weiser-Evans MC:
Inactivation of the tumour suppressor, PTEN, in smooth muscle
promotes a pro-inflammatory phenotype and enhances neointima
formation. Cardiovasc Res. 86:274–282. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Egler RA, Fernandes E, Rothermund K,
Sereika S, de Souza-Pinto N, Jaruga P, Dizdaroglu M and Prochownik
EV: Regulation of reactive oxygen species, DNA damage, and c-Myc
function by peroxiredoxin 1. Oncogene. 24:8038–8050. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Jamaluddin M, Wiktorowicz JE, Soman KV,
Boldogh I, Forbus JD, Spratt H, Garofalo RP and Brasier AR: Role of
peroxiredoxin 1 and peroxiredoxin 4 in protection of respiratory
syncytial virus-induced cysteinyl oxidation of nuclear cytoskeletal
proteins. J Virol. 84:9533–9545. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Gong F, Hou G, Liu H and Zhang M:
Peroxiredoxin 1 promotes tumorigenesis through regulating the
activity of mTOR/p70S6K pathway in esophageal squamous cell
carcinoma. Med Oncol. 32:4552015.PubMed/NCBI
|
|
26
|
Taniuchi K, Furihata M, Hanazaki K,
Iwasaki S, Tanaka K, Shimizu T, Saito M and Saibara T:
Peroxiredoxin 1 promotes pancreatic cancer cell invasion by
modulating p38 MAPK activity. Pancreas. 44:331–340. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Zhou J, Liu Q, Wang J, Guo X and Song L:
Expressions of peroxiredoxin 1, peroxiredoxin 6 and GFAP in human
brain astrocytoma and their clinical significance. Nan Fang Yi Ke
Da Xue Xue Bao. 32:1255–1259. 2012.(In Chinese). PubMed/NCBI
|
|
28
|
Riddell JR, Bshara W, Moser MT, Spernyak
JA, Foster BA and Gollnick SO: Peroxiredoxin 1 controls prostate
cancer growth through Toll-like receptor 4-dependent regulation of
tumor vasculature. Cancer Res. 71:1637–1646. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Riddell JR, Wang XY, Minderman H and
Gollnick SO: Peroxiredoxin 1 stimulates secretion of
proinflammatory cytokines by binding to TLR4. J Immunol.
184:1022–1030. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Jiang H, Wu L, Mishra M, Chawsheen HA and
Wei Q: Expression of peroxiredoxin 1 and 4 promotes human lung
cancer malignancy. Am J Cancer Res. 4:445–460. 2014.PubMed/NCBI
|
|
31
|
Hwang KE, Park DS, Kim YS, Kim BR, Park
SN, Lee MK, Park SH, Yoon KH, Jeong ET and Kim HR: Prx1 modulates
the chemosensitivity of lung cancer to docetaxel through
suppression of FOXO1-induced apoptosis. Int J Oncol. 43:72–78.
2013.PubMed/NCBI
|
|
32
|
Sun QK, Zhu JY, Wang W, Lv Y, Zhou HC, Yu
JH, Xu GL, Ma JL, Zhong W and Jia WD: Diagnostic and prognostic
significance of peroxiredoxin 1 expression in human hepatocellular
carcinoma. Med Oncol. 31:7862014. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Ha B, Kim EK, Kim JH, Lee HN, Lee KO, Lee
SY and Jang HH: Human peroxiredoxin 1 modulates TGF-β1-induced
epithelial-mesenchymal transition through its peroxidase activity.
Biochem Biophys Res Commun. 421:33–37. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(−Delta Delta C(T)) method. Methods. 25:402–408. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Du ZX, Yan Y, Zhang HY, Liu BQ, Gao YY,
Niu XF, Guan Y, Meng X and Wang HQ: Suppression of MG132-mediated
cell death by peroxiredoxin 1 through influence on ASK1 activation
in human thyroid cancer cells. Endocr Relat Cancer. 17:553–560.
2010. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Jones FS, Meech R, Edelman DB, Oakey RJ
and Jones PL: Prx1 controls vascular smooth muscle cell
proliferation and tenascin-C expression and is upregulated with
Prx2 in pulmonary vascular disease. Circ Res. 89:131–138. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Jin L, Kern MJ, Otey CA, Wamhoff BR and
Somlyo AV: Angiotensin II, focal adhesion kinase, and PRX1 enhance
smooth muscle expression of lipoma preferred partner and its newly
identified binding partner palladin to promote cell migration. Circ
Res. 100:817–825. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Pi Y, Zhang LL, Li BH, Guo L, Cao XJ, Gao
CY and Li JC: Inhibition of reactive oxygen species generation
attenuates TLR4-mediated proinflammatory and proliferative
phenotype of vascular smooth muscle cells. Lab Invest. 93:880–887.
2013. View Article : Google Scholar : PubMed/NCBI
|