Introduction
As a type of dermal cell located in the base of the
hair follicle, dermal papilla cells (DPCs) have the ability to
induce hair follicle regeneration and hair growth (1). Platelet-rich plasma (PRP) is plasma
enriched with a higher proportion of platelets, compared with that
with that which is usually found in whole blood. α-granules in
platelets contain growth factors, indicating that PRP has the
ability to promote cell proliferation and differentiation (2). Previous studies have reported that
PRP may induce mesenchymal stem cell proliferation and chondrogenic
differentiation in vitro (3–5). It
has been reported that PRP is essential in hair follicle
regeneration (6). Thus, it was
hypothesized that PRP may contribute to hair follicle regeneration
via changes in DPC proliferation levels. Hair follicle regeneration
is currently known to have an impact in treating alopecia and
dermal wounds (7,8). The initial generation of a hair
follicle is intimately linked with signal exchange between
mesenchymal and epithelial cells via the formation of hair placodes
(9,10). Alopecia is defined as a loss of
hair from the body or head, and may be induced by nutritional
deficiencies, fungal infection and traumatic damage (11). Increased chronic ulcers, skin
disease, trauma caused by burns and accidental skin defects mean
the restoration of dermal wounds is becoming an important medical
concern (12). Thus, it is
important to investigate the influence of PRP on DPC and develop
therapeutic strategies for hair follicle regeneration.
Previous studies have investigated mechanisms of
hair follicle regeneration. For example, S100 calcium binding
protein A4 and S100 calcium binding protein A6 may be important in
activating stem cells at the beginning of follicle regeneration
(13,14). Wingless-type mouse mammary tumor
virus integration site family member 10b promotes hair follicle
growth and regeneration via activation of the canonical Wnt
signaling pathway and, thus, may be used as therapeutic target in
the treatment of hair follicle-associated diseases (15,16).
Via the Gpr44 receptor, prostaglandin D2 may function in inhibiting
hair follicle regeneration (17).
As a crucial ATPase of the BAF chromatin-remodeling complex,
brahma-related gene 1 may regulate the processes of epidermal
repair and hair regeneration in bulge stem cells (18).
Preliminary studies demonstrated that PRP at a
concentration of 5% should be used to treat human hair DPCs
(HHDPCs) in the present study (data not shown). Using RNA-seq data
of HHDPCs from normal samples and samples treated with 5% PRP, the
present study aimed to identify differentially expressed genes
(DEGs) and predict their possible function using Gene Ontology (GO)
and pathway enrichment analyses. The interactions and associations
between the DEGs were investigated using protein-protein
interaction (PPI) networks. Furthermore, regulatory networks were
constructed to screen key genes and transcription factors
(TFs).
Materials and methods
PRP preparation
Samples of whole blood (10 ml) were taken from the
median cubital vein of each of the 8 male, healthy participants
(mean age, 24.9 years) and mixed with 3.2% sodium citrate
(vol/vol=10:1). PRP was prepared using a two-step centrifugation
method. Firstly, the whole blood was centrifuged at 400 × g
for 10 min at room temperature, allowing separation of blood into
three layers, the topmost platelet-poor plasma layer, an
intermediate PRP layer and the bottommost red blood cell layer.
Subsequently, the upper two layers were centrifuged again at 3,800
× g for 10 min at room temperature. The platelets in PRP
were activated by 0.2 ml 10% CaCl2 and 1,000 U bovine
thrombin. After a 10 min incubation, PRP was centrifuged at 1,500 ×
g for 5 min at room temperature, and the supernatant was
stored at −80°C. All participants provided informed consent, and
the present study was approved by the ethics committee of the
Hangzhou First People's Hospital (Hangzhou, China).
HHDPCs cultivation
At 37°C in a humidified 5% CO2 incubator
(Thermo Fisher Scientific, Inc., Waltham, MA, USA), the HHDPCs
(Shanghai Hu Zheng Industrial Co., Ltd., Shanghai, China) were
cultivated in a medium consisting of 10% fetal bovine serum (FBS;
Gibco; Thermo Fisher Scientific, Inc.), RPMI-1640 medium (Gibco;
Thermo Fisher Scientific, Inc.) and 1% double antibody (Gibco;
Thermo Fisher Scientific, Inc.). HHDPCs were passaged at 80–90%
confluence and pancreatin was used for digestion (Gibco; Thermo
Fisher Scientific, Inc.). Subsequently, the cells were centrifuged
at 300 × g for 5 min at room temperature and the supernatant
was removed. HHDPCs were preserved in a frozen stock solution which
consisted of 10% dimethyl sulfoxide, 40% FBS and 50% RPMI-1640.
RNA extraction and RNA-seq library
construction
Following cell counting, HHDPCs were spread on
6-well plates (Applied Biosystems; Thermo Fisher Scientific, Inc.;
2×105 cells/well) and starved for 24 h. HHDPCs in the
treatment group were treated with 5% PRP whilst the HHDPCs in the
control group were treated with RPMI-1640 medium containing 10% FBS
and 1% penicillin-streptomycin solution. Each experiment was
repeated twice. Total RNA from HHDPC treatment samples and normal
HHDPC samples was extracted using TRIzol (Invitrogen; Thermo Fisher
Scientific, Inc.), according to the manufacturer's protocols. The
purity and integrity of total RNA were checked by a
spectrophotometer (Merinton Instrument, Ltd., Beijing, China) and
2% agarose gel electrophoresis. Following this, the RNA-seq library
was prepared using NEBNext® Ultra™ RNA Library Prep Kit
for Illumina® (New England Biolabs, Inc. Ipswich, MA,
USA) according to the manufacturer's protocols. Briefly, mRNAs were
isolated and divided into ~200 nt fragments. Subsequently,
double-stranded cDNAs were synthesized and modified, and DNA
cluster amplification was performed using a Phusion®
Human Specimen Direct Polymerase Chain Reaction kit (Thermo Fisher
Scientific, Inc.). The reaction mixture was subjected to the
following cycling conditions: An initial denaturation step at 94°C
for 30 sec, followed by 11 cycles of denaturation at 98°C for 10
sec, annealing at 65°C for 30 sec and extension at 72°C for 30 sec,
and a final extension step at 72°C for 5 min. Using Illumina HiSeq
2500 v4 100PE (Illumina, Inc., San Diego, CA, USA), high-throughput
sequencing was conducted for the RNA-seq library.
DEGs screening
RNA-seq data were preprocessed by the Next
Generation Sequencing Quality Control Toolkit (19). Any sequences containing >20%
bases with a quality value <20 were filtered out. Using TopHat2
software (ccb.jhu.edu/software/tophat/index.shtml) (20), RNA-seq data was aligned to human
genome hg19, which was downloaded from the University of
California, Santa Cruz website (genome.ucsc.edu) (21). The parameter was set as no-mixed
and other parameters used default settings. Cuffdiff software
(cufflinks.cbcb.umd.edu/) (22) was used to identify the DEGs between
RNA-seq data of normal HHDPCs and treated HHDPCs. The adjusted
P<0.05 and |log2fold change (FC)|>1 served as the
cut-off criteria.
Functional and pathway enrichment
analysis
GO analysis aims to describe subcellular location,
molecular function and the biological processes of gene products
(23). The Kyoto Encyclopedia of
Genes and Genomes (KEGG) pathway database introduces functions of
molecules or genes (24). Using
the GOFunction (25) of
bioconductor (www.bioconductor.org/), as well as annotation files
GO.db (26) and org.Hs.eg.db
(27), GO and KEGG pathway
enrichment analyses were performed for the DEGs. P<0.05 and ≥2
genes enriched in one pathway served as the cut-off criteria.
PPI network construction
The STRING online software (28) was applied to screen interactions of
proteins encoded by DEGs, and the Cytoscape software (www.cytoscape.org/) (29) was used to visualize the PPI
network. A combined score >0.7 was used as the cut-off
criterion.
Regulatory network construction
Using information provided by the ENCODE website
(genome.ucsc.edu/ENCODE/encode.hg17.html) regarding TF
binding sites, (30), in addition
to genetic and long non-coding RNAs (lncRNAs) location information
on the genome, differentially expressed TF-DEG pairs and
differentially expressed TF-differentially expressed lncRNA pairs
were screened. A certain amount of overlapping occurred between TF
binding sites and the cut-off criterion was defined as 1,000 bp
upstream to 500 bp downstream of the transcription start site.
Results
DEGs analysis
Compared with normal HHDPC samples, there were 365
upregulated and 142 downregulated genes screened in the treated
HHDPC samples. Furthermore, 178 differentially expressed (including
131 upregulated and 47 downregulated) lncRNAs were identified in
the treated HHDPC samples.
Functional and pathway enrichment
analysis
The enriched KEGG pathways for upregulated genes
were listed in Table I, including
cell cycle (P<0.0001), progesterone-mediated oocyte maturation
(P<0.0001) and DNA replication (P=0.000614). The enriched KEGG
pathways for downregulated genes included p53 signaling pathway
(P<0.0001), mitogen-activated protein kinase signaling pathway
(P=0.00709) and cell cycle (P=0.020687; Table II). In addition, no GO functions
were enriched for the DEGs.
 | Table I.Pathways enriched for upregulated
genes. |
Table I.
Pathways enriched for upregulated
genes.
| Category | Term | Description | Gene number | Gene symbol | P-value |
|---|
| KEGG | 4110 | Cell cycle | 25 | BUB1,
BUB1B | <0.0001 |
| KEGG | 4914 |
Progesterone-mediated oocyte
maturation | 12 | CCNA2,
CCNB1 |
8.13×10−8 |
| KEGG | 4114 | Oocyte meiosis | 13 | AURKA,
BUB1 |
2.12×10−7 |
| KEGG | 5130 | Pathogenic
Escherichia coli infection | 8 | ACTB,
ACTG1 |
1.10×10−5 |
| KEGG | 4115 | p53 signaling
pathway | 8 | CCNB1,
CCNB2 |
4.72×10−5 |
| KEGG | 4145 | Phagosome | 11 | ACTB,
ACTG1 | 0.000182 |
| KEGG | 4540 | Gap junction | 8 | CDK1,
PDGFRB | 0.000346 |
| KEGG | 3030 | DNA
replication | 5 | FEN1,
MCM3 | 0.000614 |
| KEGG | 4974 | Protein digestion
and absorption | 7 | COL18A1,
COL2A1 | 0.000971 |
| KEGG | 4510 | Focal adhesion | 11 | ACTB,
ACTG1 | 0.001748 |
 | Table II.Pathways enriched for downregulated
genes. |
Table II.
Pathways enriched for downregulated
genes.
| Category | Term | Description | Gene number | Gene symbol | P-value |
|---|
| KEGG | 4115 | p53 signaling
pathway | 6 | BBC3,
CCND2 | <0.0001 |
| KEGG | 250 | Alanine, aspartate
and glutamate metabolism | 3 | ASNS, GLS2,
GOT1 | 0.002425 |
| KEGG | 5219 | Bladder cancer | 3 | CDKN1A, MYC,
VEGFA | 0.005288 |
| KEGG | 4010 | MAPK signaling
pathway | 7 | DDIT3,
DUSP2 | 0.00709 |
| KEGG | 330 | Arginine and
proline metabolism | 3 | GLS2, GOT1,
SAT1 | 0.010639 |
| KEGG | 910 | Nitrogen
metabolism | 2 | ASNS,
GLS2 | 0.016056 |
| KEGG | 4964 | Proximal tubule
bicarbonate reclamation | 2 | GLS2,
PCK2 | 0.016056 |
| KEGG | 4110 | Cell cycle | 4 | CCND2,
CDKN1A | 0.020687 |
| KEGG | 4612 | Antigen processing
and presentation | 3 | CIITA, HLA-DQA1,
HLA-DQA2 | 0.026471 |
| KEGG | 5310 | Asthma | 2 | HLA-DQA1,
HLA-DQA2 | 0.026583 |
PPI network analysis
The PPI network for upregulated genes had 192 nodes
and 1,017 interactions (Fig. 1).
In this network, cyclin-dependent kinase 1 (CDK1, degree=76),
polo-like kinase 1 (PLK1, degree=65), cell division cycle 20
(CDC20, degree=50), cyclin B1 (CCNB1, degree=49), aurora kinase B
(AURKB, degree=47) and cyclin-dependent kinase 2 (CDK2, degree=46)
demonstrated higher degrees. In addition, these genes interacted
with each other for example, CDK1-PLK1, CDC20-CCNB1,
AURKB-CDK2 and CDK1-AURKB in the PPI network.
The PPI network for downregulated genes had 38 nodes
and 58 interactions (Fig. 2). In
this network, c-myc (MYC, degree=12), activating
transcription factor 3 (degree=9), DNA-damage-inducible transcript
3 (degree=8) and early growth response 1 (EGR1, degree=8)
indicated higher degrees.
Regulatory network analysis
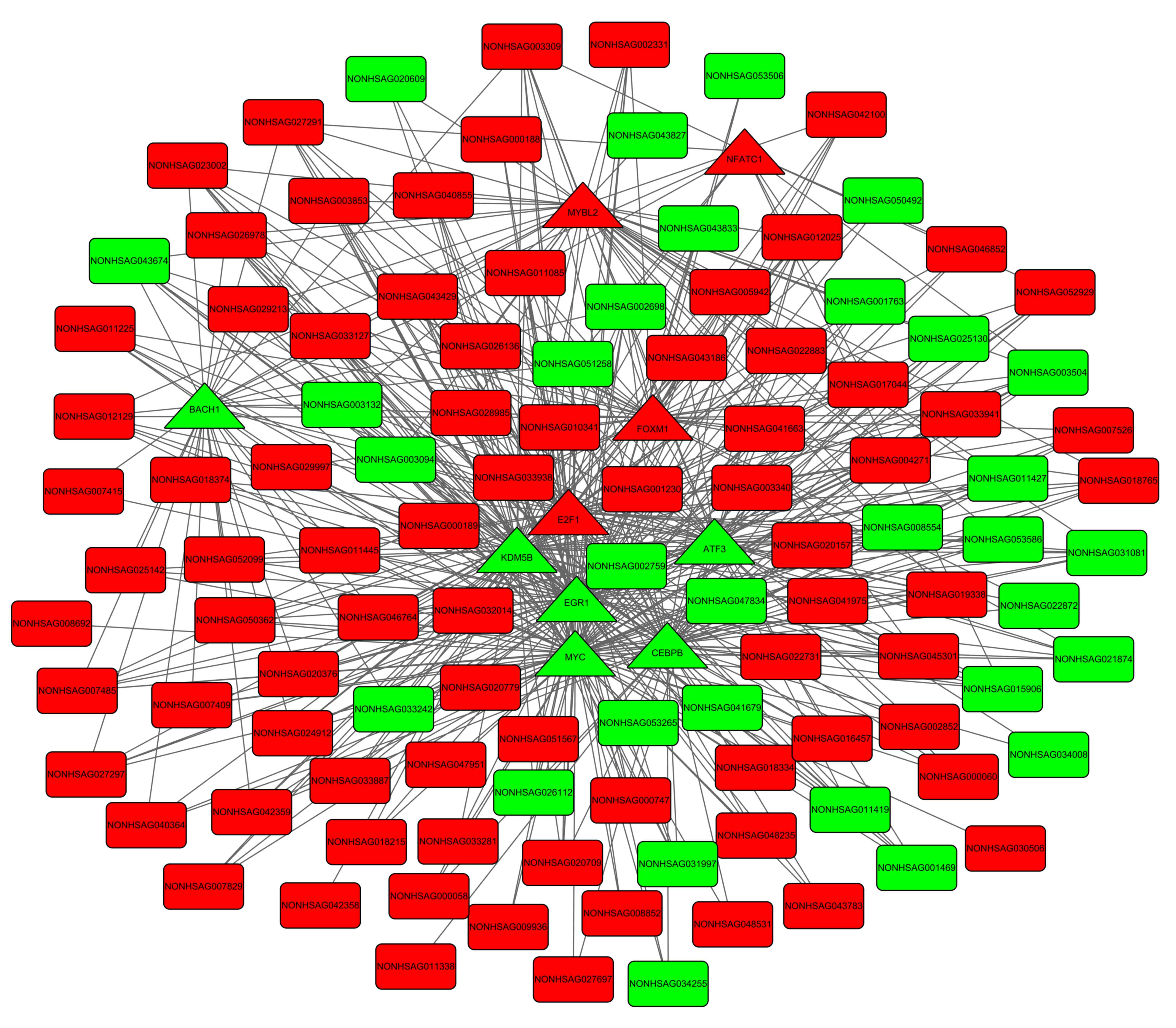
A total of 453 TF-DEG pairs and 530 TF-lncRNA pairs
were identified. The TF-DEG and TF-lncRNA regulatory networks are
presented in Figs 3 and 4. Notably, the target genes of TFs
CCAAT/enhancer binding protein-β (C/EBP-β), E2F
transcription factor (E2F) 1, EGR1 and MYC
were enriched in pathways associated with the cell cycle. In the
TF-DEG regulatory network, these TFs targeted CDK1,
PLK1, CCNB1 and AURKB (for example,
MYC→PLK1, MYC→CCNB1,
MYC→AURKB, CEBPB→AURKB,
E2F1→CDK1 and E2F1→CCNB1).
Discussion
The present study screened 178 differentially
expressed lncRNA, 365 upregulated genes and 142 downregulated genes
in treated HHDPC samples compared with normal HHDPC samples. Only
selected DEGs and lncRNAs have been presented in the present study.
DEGs enriched in pathways have been listed in Tables I and II. Pathway enrichment indicated that
these upregulated and downregulated genes were enriched in
proliferation-associated pathways, including those involved in the
cell cycle and DNA replication, indicating that PRP may affect cell
proliferation of HHDPCs. The DEGs and lncRNAs that were key nodes
in the networks have been mentioned. In the PPI networks,
upregulated CDK1 (degree=76), PLK1 (degree=65),
CDC20 (degree=50), CCNB1 (degree=49), AURKB
(degree= 47), CDK2 (degree=46) and downregulated MYC
(degree=12) had higher degrees. Furthermore, CEBPB,
E2F1, EGR1 and MYC, which may be key TFs for
their target genes, were enriched in pathways associated with the
cell cycle.
CDK1 activity is continuously required to
ensure continuing end resection and to maintain a double-strand
break-induced checkpoint. Furthermore, CDK1 is involved in
later stages of homologous recombination (31). It has been demonstrated that
CDK1 is the only crucial cell cycle CDK and is able to drive
cell division alone (32). As an
important moderator of cell division (33), PLK1 is essential for mitotic
entry at the appropriate time (34). PLK1 appears to be required
for centrosome-mediated microtubule activities and spindle
assembly. In addition, siRNAs targeted against PLK1 may be
potential anti-proliferative agents that inhibit neoplastic cells
(35,36). This suggests that CDK1 and
PLK1 may be associated with cell proliferation.
CDC20 can activate anaphase-promoting complex
(APC/C) in metaphase of the cell cycle, and its C terminus contains
a WD40 repeat domain that regulates protein-protein interactions
(37,38). Suppression of CCNB1, which
is a cyclin controlling mitotic entry, induces a marked arrest of
G2/M phase in HCT116 and SW480 cells, preventing the
expression of CDK1 and cell division cycle 25C (39,40).
AURKB functions in chromosome bi-orientation, spindle assembly and
cytokinesis, and may be degraded by APC/C bound to cadherin 1 in
G1 and in late mitosis (41). In animal cells, CDK2/cyclin E can
trigger initiation of centrosome duplication by targeting substrate
nucleophosmin/B23 (42–44). Thus, the expression levels of
CDC20, CCNB1, AURKB and CDK2 may be
associated with cell proliferation. In the PPI network for
upregulated DEGs, it was observed that numerous DEGs demonstrated a
certain level of interaction with each other, for example,
CDK1-PLK1, CDC20-CCNB1, AURKB-CDK2 and
CDK1-AURKB. Therefore, it may be other genes, including
PLK1 and CCNB, which had interaction with
CDC20, CCNB1, AURKB and CDK2, may also
function in cell proliferation.
The proto-oncogene MYC encodes transcription
factor c-myc which is associated with regulation of cell
proliferation and differentiation (45). Conditional activation or
constitutive expression of wild-type CEBPB may promote
differentiation and suppress proliferation of 32D-BCR/ABL cells,
which is contrary to a DNA binding-deficient CEBPB mutant
(46). It is reported that
E2F1, E2F2 and E2F3 function as
transcriptional activators in the process of the G1/S
transition (47). As a member of
the Egr transcription factor family, EGR1 is involved in
cell growth, proliferation and stress responses in various types of
tissues (48). This may suggest
the expression levels of CEBPB, E2F1, EGR1 and
MYC were associated with cell proliferation. In the TF-DEG
regulatory network, it was observed that TFs could target
CDK1, PLK1, CCNB1 and AURKB, for
example MYC→PLK1, MYC→CCNB1,
MYC→AURKB, CEBPB→AURKB,
E2F1→CDK1 and E2F1→CCNB1, suggesting
that TFs may be involved in cell proliferation via regulation of
CDK1, PLK1, CCNB1 and AURKB.
In conclusion, the present study conducted a
comprehensive bioinformatics analysis of genes that may be
associated with cell proliferation. A total of 178 differentially
expressed lncRNA were identified, and additionally, 365 upregulated
and 142 downregulated genes were screened. It was indicated that
CDK1, PLK1, CDC20, CCNB1, AURKB,
CDK2, CEBPB, E2F1, EGR1 and MYC
may be associated with cell proliferation of HHDPCs. Thus, PRP may
be important in HHDPCs. Further investigation is required in order
to elucidate the functional mechanisms of these genes in HHDPCs. In
addition, the proliferative ability of HHDPCs treated with PRP will
be investigated in future work.
Acknowledgements
The present study was supported by the Science and
Technology Projects Fund of Hangzhou City (grant no.
20130633B04).
References
|
1
|
Jahoda C, Horne K and Oliver R: Induction
of hair growth by implantation of cultured dermal papilla cells.
Nature. 311(1984): 560–562. 1984.PubMed/NCBI
|
|
2
|
Eppley BL, Woodell JE and Higgins J:
Platelet quantification and growth factor analysis from
platelet-rich plasma: Implications for wound healing. Plast
Reconstr Surg. 114:1502–1508. 2004.PubMed/NCBI
|
|
3
|
Kocaoemer A, Kern S, Klueter H and Bieback
K: Human AB serum and thrombin-activated platelet-rich plasma are
suitable alternatives to fetal calf serum for the expansion of
mesenchymal stem cells from adipose tissue. Stem Cells.
25:1270–1278. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Vogel JP, Szalay K, Geiger F, Kramer M,
Richter W and Kasten P: Platelet-rich plasma improves expansion of
human mesenchymal stem cells and retains differentiation capacity
and in vivo bone formation in calcium phosphate ceramics.
Platelets. 17:462–469. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Mishra A, Tummala P, King A, Lee B, Kraus
M, Tse V and Jacobs CR: Buffered platelet-rich plasma enhances
mesenchymal stem cell proliferation and chondrogenic
differentiation. Tissue Engineering Part C Methods. 15:431–435.
2009. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Reese RJ: Autologous platelet rich plasma
(PRP): What do we know? Important concepts relevant to hair
restoration surgery. Hair Transplant Forum Int. 2010:14–17.
2010.
|
|
7
|
Ito M, Yang Z, Andl T, Cui C, Kim N,
Millar SE and Cotsarelis G: Wnt-dependent de novo hair follicle
regeneration in adult mouse skin after wounding. Nature.
447:316–320. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Gilhar A and Kalish RS: Alopecia areata: A
tissue specific autoimmune disease of the hair follicle. Autoimmun
Rev. 5:64–69. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Barsh G: Of ancient tales and hairless
tails. Nat Genet. 22:315–316. 1999. View
Article : Google Scholar : PubMed/NCBI
|
|
10
|
Oro AE and Scott MP: Splitting hairs:
Dissecting roles of signaling systems in epidermal development.
Cell. 95:575–578. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Kulick D, Hark L and Deen D: The bariatric
surgery patient: A growing role for registered dietitians. J Am
Diet Assoc. 110:593–599. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Oshima H, Inoue H, Matsuzaki K, Tanabe M
and Kumagai N: Permanent restoration of human skin treated with
cultured epithelium grafting-wound healing by stem cell based
tissue engineering. Hum Cell. 15:118–128. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Ito M and Kizawa K: Expression of
calcium-binding S100 proteins A4 and A6 in regions of the
epithelial sac associated with the onset of hair follicle
regeneration. J Investig Dermatol. 116:956–963. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Kizawa K, Toyoda M, Ito M and Morohashi M:
Aberrantly differentiated cells in benign pilomatrixoma reflect the
normal hair follicle: Immunohistochemical analysis of Ca-binding
S100A2, S100A3 and S100A6 proteins. Br J Dermatol. 152:314–320.
2005. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Li YH, Zhang K, Yang K, Ye JX, Xing YZ,
Guo HY, Deng F, Lian XH and Yang T: Adenovirus-mediated Wnt10b
overexpression induces hair follicle regeneration. J Investig
Dermatol. 133:42–48. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Li YH, Zhang K, Ye JX, Lian XH and Yang T:
Wnt10b promotes growth of hair follicles via a canonical Wnt
signalling pathway. Clin Exp Dermatol. 36:534–540. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Nelson AM, Loy DE, Lawson JA, Katseff AS,
FitzGerald GA and Garza LA: Prostaglandin D2 inhibits wound-induced
hair follicle neogenesis through the receptor, Gpr44. J Investig
Dermatol. 133:881–889. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Xiong Y, Li W, Shang C, Chen RM, Han P,
Yang J, Stankunas K, Wu B, Pan M, Zhou B, et al: Brg1 governs a
positive feedback circuit in the hair follicle for tissue
regeneration and repair. Dev Cell. 25:169–181. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Patel RK and Jain M: NGS QC Toolkit: A
toolkit for quality control of next generation sequencing data.
PLoS One. 7:e306192012. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Kim D, Pertea G, Trapnell C, Pimentel H,
Kelley R and Salzberg SL: TopHat2: Accurate alignment of
transcriptomes in the presence of insertions, deletions and gene
fusions. Genome Biol. 14:R362013. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Karolchik D, Baertsch R, Diekhans M, Furey
TS, Hinrichs A, Lu YT, Roskin KM, Schwartz M, Sugnet CW, Thomas DJ,
et al: The UCSC genome browser database. Nucleic Acids Res.
31:51–54. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Trapnell C, Roberts A, Goff L, Pertea G,
Kim D, Kelley DR, Pimentel H, Salzberg SL, Rinn JL and Pachter L:
Differential gene and transcript expression analysis of RNA-seq
experiments with TopHat and Cufflinks. Nat Protoc. 7:562–578. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Tweedie S, Ashburner M, Falls K, Leyland
P, McQuilton P, Marygold S, Millburn G, Osumi-Sutherland D,
Schroeder A, Seal R, et al: FlyBase: Enhancing Drosophila gene
ontology annotations. Nucleic Acids Res. 37:D555–D559. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Kanehisa M and Goto S: KEGG: Kyoto
encyclopedia of genes and genomes. Nucleic Acids Res. 28:27–30.
2000. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Wang J, Zhou X, Zhu J, Gu Y, Zhao W, Zou J
and Guo Z: GO-function: Deriving biologically relevant functions
from statistically significant functions. Brief Bioinform.
13:216–227. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Carlson M, Falcon S, Pages H and Li N: A
set of annotation maps describing the entire Gene Ontology. Version
2.4.5. 2007.
|
|
27
|
Carlson M, Falcon S, Pages H and Li N:
org. Hs. eg. db: Genome wide annotation for Human. Version 3.4.0.
2013.
|
|
28
|
Franceschini A, Szklarczyk D, Frankild S,
Kuhn M, Simonovic M, Roth A, Lin J, Minguez P, Bork P, von Mering C
and Jensen LJ: STRING v9. 1: protein-protein interaction networks,
with increased coverage and integration. Nucleic Acids Res.
41:D808–D815. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Saito R, Smoot ME, Ono K, Ruscheinski J,
Wang PL, Lotia S, Pico AR, Bader GD and Ideker T: A travel guide to
Cytoscape plugins. Nat Methods. 9:1069–1076. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Thomas DJ, Rosenbloom KR, Clawson H,
Hinrichs AS, Trumbower H, Raney BJ, Karolchik D, Barber GP, Harte
RA, Hillman-Jackson J, et al: The ENCODE project at UC santa cruz.
Nucleic Acids Res. 35:D663–D667. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Ira G, Pellicioli A, Balijja A, Wang X,
Fiorani S, Carotenuto W, Liberi G, Bressan D, Wan L, Hollingsworth
NM, et al: DNA end resection, homologous recombination and DNA
damage checkpoint activation require CDK1. Nature. 431:1011–1017.
2004. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Santamaría D, Barrière C, Cerqueira A,
Hunt S, Tardy C, Newton K, Cáceres JF, Dubus P, Malumbres M and
Barbacid M: Cdk1 is sufficient to drive the mammalian cell cycle.
Nature. 448:811–815. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Petronczki M, Lénárt P and Peters JM: Polo
on the rise-from mitotic entry to cytokinesis with Plk1. Dev Cell.
14:646–659. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Sumara I, Giménez-Abián JF, Gerlich D,
Hirota T, Kraft C, de la Torre C, Ellenberg J and Peters JM: Roles
of polo-like kinase 1 in the assembly of functional mitotic
spindles. Curr Biol. 14:1712–1722. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Spänkuch-Schmitt B, Bereiter-Hahn J,
Kaufmann M and Strebhardt K: Effect of RNA silencing of polo-like
kinase-1 (PLK1) on apoptosis and spindle formation in human cancer
cells. J Natl Cancer Inst. 94:1863–1877. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Gumireddy K, Reddy MR, Cosenza SC,
Boominathan R, Baker SJ, Papathi N, Jiang J, Holland J and Reddy
EP: ON01910, a non-ATP-competitive small molecule inhibitor of
Plk1, is a potent anticancer agent. Cancer Cell. 7:275–286. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Yu H: Cdc20: A WD40 activator for a cell
cycle degradation machine. Mol Cell. 27:3–16. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Kramer ER, Scheuringer N, Podtelejnikov
AV, Mann M and Peters JM: Mitotic regulation of the APC activator
proteins CDC20 and CDH1. Mol Biol Cell. 11:1555–1569. 2000.
View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Fang Y, Yu H, Liang X, Xu J and Cai X:
Chk1-induced CCNB1 overexpression promotes cell proliferation and
tumor growth in human colorectal cancer. Cancer Biol Ther.
15:1268–1279. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Abel D, Abdul-Hamid O, Dijk M and Oudejans
CB: Transcription factor STOX1A promotes mitotic entry by binding
to the CCNB1 promotor. PLoS One. 7:e297692012. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Stewart S and Fang G: Destruction
box-dependent degradation of aurora B is mediated by the
anaphase-promoting complex/cyclosome and Cdh1. Cancer Res.
65:8730–8735. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Okuda M, Horn HF, Tarapore P, Tokuyama Y,
Smulian AG, Chan PK, Knudsen ES, Hofmann IA, Snyder JD, Bove KE and
Fukasawa K: Nucleophosmin/B23 is a target of CDK2/cyclin E in
centrosome duplication. Cell. 103:127–140. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Tarapore P, Okuda M and Fukasawa K: A
mammalian in vitro centriole duplication system: Evidence for
involvement of CDK2/cyclin E and nucleophosmin/B23 in centrosome
duplication. Cell Cycle. 1:75–78. 2002.PubMed/NCBI
|
|
44
|
Tokuyama Y, Horn HF, Kawamura K, Tarapore
P and Fukasawa K: Specific phosphorylation of nucleophosmin on
Thr(199) by cyclin-dependent kinase 2-cyclin E and its role in
centrosome duplication. J Biol Chem. 276:21529–21537. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Wu KJ, Grandori C, Amacker M, Simon-Vermot
N, Polack A, Lingner J and Dalla-Favera R: Direct activation of
TERT transcription by c-MYC. Nat Genet. 21:220–224. 1999.
View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Guerzoni C, Bardini M, Mariani SA,
Ferrari-Amorotti G, Neviani P, Panno ML, Zhang Y, Martinez R,
Perrotti D and Calabretta B: Inducible activation of CEBPB, a gene
negatively regulated by BCR/ABL, inhibits proliferation and
promotes differentiation of BCR/ABL-expressing cells. Blood.
107:4080–4089. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
47
|
Wu L, Timmers C, Maiti B, Saavedra HI,
Sang L, Chong GT, Nuckolls F, Giangrande P, Wright FA, Field SJ, et
al: The E2F1-3 transcription factors are essential for cellular
proliferation. Nature. 414:457–462. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
48
|
Min IM, Pietramaggiori G, Kim FS, Passegué
E, Stevenson KE and Wagers AJ: The transcription factor EGR1
controls both the proliferation and localization of hematopoietic
stem cells. Cell stem cell. 2:380–391. 2008. View Article : Google Scholar : PubMed/NCBI
|