Introduction
There are ~1,000,000 cases of breast cancer
diagnosed annually worldwide (1).
Breast cancer is the most frequent and most life-threatening type
of cancer among women (2). Various
genetic and environmental risk factors may be associated with the
incidence of breast cancer, including family history, oral
contraceptive pills, menarche and reproductive age, dietary
factors, exposure to sunlight, and levels of vitamin D in the blood
(1,3).
In addition to its contribution to the regulation of
calcium and phosphate metabolism, and its facilitating of bone
mineralization (4), the active
form of vitamin D (1,25-dihydroxyvitamin D3), termed calcitriol,
performs a wide range of biological activities, including
proapoptotic, prodifferentiative and antiproliferative effects, in
target tissues (3–6) and is able to inhibit the
proliferation of human cancer cells (7–9).
The source of the precursor of vitamin D may be
through dietary intake or it can be synthesized from
7-dehydrocholesterol in the skin as a result of exposure to
ultraviolet B sunlight (4,8,10).
When transferred to the liver, vitamin D3 is hydroxylated in the
five carbon side chain and is then converted to 25-hydroxyvitamin
D3, which is the major form of vitamin D present in the blood.
25-hydroxyvitamin D3 is also hydroxylated in other tissues,
predominantly the kidney, through a mitochondrial enzyme containing
cytochrome P450, 1-α hydroxylase (CYP27B1), in the methyl region,
which is then converted to 1,25-dihydroxyvitamin D3. This form of
vitamin D is bound to vitamin D-binding proteins in the blood
stream and activates or represses the transcription of its target
gene through binding to the vitamin D receptor (VDR) in tissues
(6,11–13).
By binding to the retinoid X receptor, this complex binds to
vitamin D response elements in the promoter region of the target
genes and regulates the transcription of genes, including those
contributing to the regulation of cell growth, differentiation,
apoptosis and inflammation, and consequent antitumor activities
(4,5,8).
Vitamin D decreases the incidence of certain types of cancer via
the inhibition of tumor angiogenesis, mutual adherence stimulation
of cells and intercellular connection by gap junctions (14). It can also affect hypoxia, the cell
cycle and the receptors of hormones, and these effects result in
prevention of the progression and metastasis of cancer (15).
The activity of vitamin D is terminated by the
catalytic enzyme, 24-hydroxylase, encoded by CYP24A1 and
responsible for vitamin D catabolism, which is then metabolized to
calcitroic acid, a bile secretion (1,4,9,10).
The overexpression of CYP24A1 is associated with a poor prognosis
in several types of human cancer, including breast cancer (5). The expression of CYP27B1 can be
considered a central mechanism in the association between active
vitamin D and its antitumor effects (12).
These enzymes are active not only in the kidney, but
also in several other tissues, including breast tissue (1,4,9,16).
Experimental studies have revealed an association
between vitamin D and protection against breast cancer; vitamin D
levels have been found to be inversely correlated with the risk of
breast cancer (10,17). Women with a high dietary intake of
vitamin D have been shown to have a reduced risk of breast cancer
(1,4); the risk of breast cancer was found to
be five times higher in women with 25-hydroxyvitamin D3
concentrations <150 nmol/l, compared with women with blood
concentrations >150 nmol/l (18).
The present study was performed to investigate the
expression of CYP27B1 and CYP24A1 in cancerous and normal breast
tissues, and to understand their role in the incidence of breast
cancer (12). The study was
designed to estimate the capacities of cancerous and normal breast
tissues to produce local 1, 25-dihydroxyvitamin D3, and its
importance in controlling the proliferation and differentiation of
breast tissue. The results of the present study may be used towards
developing preventive or therapeutic measures for breast cancer
using precursors of vitamin D.
Materials and methods
Collection and preparation of tissue
samples
Tumor and adjacent normal tissue samples from 30
patients with breast cancer who had undergone surgery were
collected and examined from the Tumor Bank, Cancer Institute of
Imam Khomeini Hospital (Tehran, Iran). The present study was
approved by the ethics committee of Isfahan University of Medical
Sciences (Isfahan, Iran). The histopathological results of
diagnostic tests of the patients were also collected. Pathological
examination confirmed that the normal tissue samples had no
malignant signs. The clinical characteristics of the patients are
shown in Table I.
 | Table I.Demographic parameters of the
subjects. |
Table I.
Demographic parameters of the
subjects.
| Parameter | Subjects; n
(%) |
|---|
| Mean age
(years) | 50.8±11.3 |
| Age
range (years) | 32℃79 |
| Tumor stage |
|
| T1 | 7 (23.3) |
| T2 | 20 (66.7) |
| T3 | 3 (10) |
| Tumor grade |
|
| I | 7 (23.3) |
| II | 16 (53.3) |
|
III | 7 (23.3) |
| Tumor size |
|
| <5
cm | 22 (73.3) |
| ≥5
cm | 8 (26.7) |
| Lymphatic
invasion |
|
|
Yes | 17 (56.7) |
| No | 13 (43.3) |
RNA extraction and cDNA synthesis
Total RNA was extracted from the homogenized tumor
and normal tissues using QIAzol solution (Qiagen GmbH, Hilden,
Germany) and an RNeasy Mini kit (Qiagen GmbH), respectively,
according to the manufacturer's protocols. The RNA concentration
was quantified using Nanodrop spectrophotometer, and its quality
was determined by electrophoresis on a 1% agarose gel.
Subsequently, the RNA was reverse transcribed into cDNA using a
QuantiTect Reverse Transcription kit (Qiagen GmbH) and was prepared
for analyzing the gene expression levels of CYP24A1 and
CYP27B1.
Quantitative polymerase chain reaction
(qPCR) analysis
qPCR analysis was performed using the TaqMan
technique with the ABI Step One Plus system (Thermo Fisher
Scientific, Inc., Waltham, MA, USA) and the TaqMan Gene Expression
master mix (Thermo Fisher Scientific, Inc.). Assay-on-Demand
primers and probes (Thermo Fisher Scientific, Inc.; Table II) were used for detecting the
gene expression of CYP24A1 and CYP27B1. The 18S rRNA gene was
included as an internal control. Each sample was examined in a
final volume of 20 µl, containing 2 µl synthesized cDNA, 10 µl
master mix and 1 µl specific mixed primer and probes. The PCR
conditions were as follows: 10 min at 95°C, followed by 50 cycles
at 50°C for 2 min and 95°C for 15 sec. The quantification cycle
(Cq) values were measured in the samples and the expression levels
of the genes were calculated using the 2-ΔΔCq method
(19).
 | Table II.TaqMan probes for analysis of gene
expression. |
Table II.
TaqMan probes for analysis of gene
expression.
| Gene | Sequence | Amplicon length
(bp) |
|---|
|
CYP24A1(FAM-MGB) | Hs00989014 m1 | 108 |
|
CYP27B1(FAM-MGB) | Hs01096142 g1 | 74 |
| 18S rRNA | Hs99999901 s1 | 187 |
Statistical analysis
Following measurement of the Cq values of the
examined genes in the tumor and normal tissue samples and of the
internal control gene (18SrRNA), the differences in gene expression
between the two groups were examined using the IBM SPSS 19 (IBM
SPSS, Armonk, NY, USA) and Microsoft Excel (Microsoft Corporation,
Redmond, WA, USA) software programs. In addition, comparison of the
gene expression levels of CYP24A1 and CYP27B1 with tumor stage,
tumor grade, age range, tumor size and the presence of lymphatic or
non-lymphatic invasion were performed using one-way analysis of
variance and independent t-tests. P<0.05 was considered to
indicate a statistically significant difference. Data are presented
as the mean ± standard deviation.
Results
Determination of mRNA expression
levels of CYP27B1 and CYP24A1
In the present study, the mRNA expression levels of
two key enzymes, CYP27B1 and CYP24A1, were examined in breast tumor
tissues and adjacent nomal tissues using TaqMan RT-qPCR analysis.
The data were normalized using the 18SrRNA gene as an internal
control. The differences in the expression levels of these two
genes in the tumor and normal tissues were examined, and the
results were analyzed.
The results showed that the mRNA expression of
CYP24A1 was significantly upregulated in the tumorous breast tissue
(P<0.01), compared with the normal tissue, with the mRNA
expression of CYP24A1 undetected in certain samples of normal
tissue (Fig. 1A).
The analysis of the mRNA expression levels of
CYP27B1 in the tumor and normal tissue showed downregulation in the
expression of the gene in the tumorous breast tissue (P<0.01),
as shown in Fig 1B.
Determination of the association
between demographic parameters and gene expression levels of
CYP27B1 and CYP24A1
The comparison of gene expression levels of CYP24A1
and CYP27B1 in the tumor tissues showed no statistically
significant difference in the gene expression levels of CYP24A1
between different tumor stages (Fig.
2A), however, there were significant differences between the
gene expression of CYP27B1 and tumor stage, in which the gene
expression of CYP27B1 at stage 1 was higher, compared with the
levels at stage 2 and stage 3 (Fig.
2B).
No statistical differences were found in the gene
expression levels of CYP24A1 and CYP27B1 in different tumor grades
(Fig. 3A and B).
In examining the association between the gene
expression levels of CYP24A1 and CYP27B1 and the age range of the
patients, the only significant difference was found in the gene
expression of CYP24A1 (P<0.05; Fig.
4A), although the gene expression of CYP27B1 increased with
increasing age (Fig. 4B).
No statistically significant differences were found
between the gene expression levels of CYP24A1 and CYP27B1 and tumor
size (Fig. 5A and B).
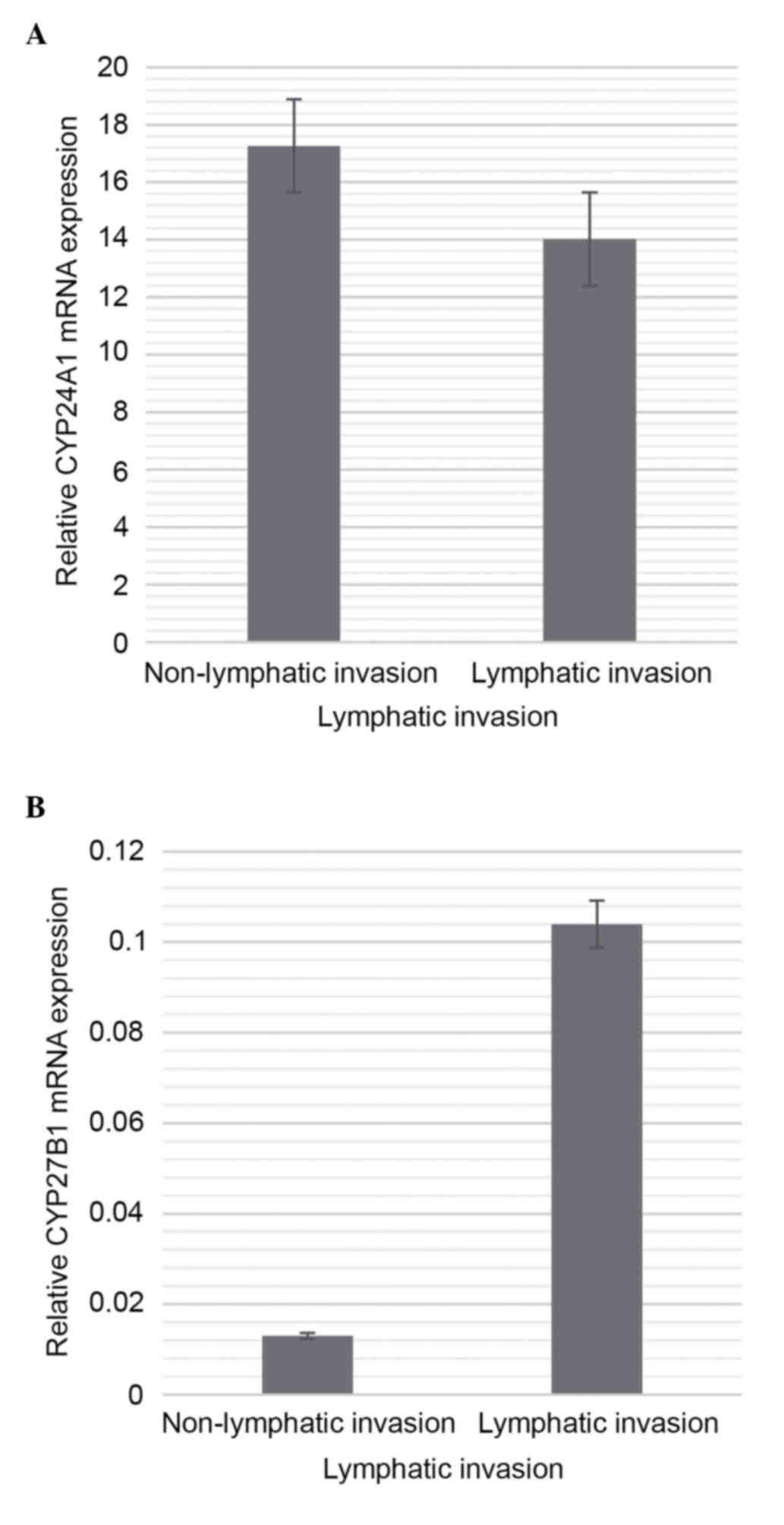
No statistically significant differences between
lymphatic or non-lymphatic invasion and the gene expression levels
of CYP24A1 and CYP27B1 were found (Fig. 6A and B).
Discussion
It has been revealed that vitamin D has a protective
role in inhibiting the growth of proliferating tumor cells, in
addition to its role in calcium and phosphate homeostasis (20,21).
The two key enzymes, CYP27B1 and CYP24A1 are important in vitamin D
anabolism and catabolism, respectively, and are required to
maintain adequate levels of vitamin D in the blood (13,22).
In the present study, the mRNA expression levels of CYP27B1 and
CYP24A1 were examined in tumorous and normal breast tissues using
TaqMan RT-qPCR analysis.
The results showed that the mRNA expression level of
CYP27B1 in the normal tissue was higher, compared with that in the
tumor tissue, whereas the mRNA expression level of CYP24A1 in the
tumor tissue was significantly higher, compared with that in the
normal tissue.
As mentioned previously, the active form of vitamin
D, calcitriol, performs a wide range of biological activities in
target tissues, including proapoptotic, prodifferentiative and
antiproliferative effects (3–6). It
has been revealed that the progression of cancer is associated with
alterations in the balance of the expression of CYP27B1 and
CYP24A1, in addition to alterations in the expression of VDR and
polymorphisms (1,23).
Anderson et al (5) showed that an increase in the
expression of CYP24A1 is found in several types of human tumor,
including those of the colon, lung and ovary, although not in tumor
tissue of the breast. The results of the present study were not
concordant with these in terms of the breast tissue, however, the
previous study showed the upregulation of CYP24A1 in colon, lung
and ovary tumor tissues (5,17).
The results of a study by Fischer et al (17) showed decreased mRNA expression of
CYP24A1 in breast carcinoma, compared with benign breast tissue
(17), which was also
contradictory to the results of the present study.
The study by Anderson et al (5) on cancer cell lines revealed that the
induction of CYP24A1 may lead to the metabolism and inactivation of
1,25 (OH)2D3 and consequently result in the
insensitivity of the cells to the growth inhibitory effects of the
vitamin. These results conformed to those of a previous study on
prostate cancer, showing that the degree of growth inhibition by
1,25(OH)2D3 is inversely correlated with the
activity of CYP24A1 in cells (5).
This can also be estimated using tissue samples, and future
investigations aim to examine cell lines.
CYP24A1 is a major target gene of VDR following the
binding of vitamin D to the receptor (10). The product of this gene, which is
the major enzyme in the process of vitamin D catabolism, has been
identified as an important regulator of the biological effect of
the signaling system of vitamin D (3,24).
Ren et al (25) showed that
the increased expression of CYP24A1 in breast tumors occurs in
response to the increased local production of
1,25(OH)2D3 in the tissue. Further
investigations are required in order to understand the increased
expression of VDR or increased levels of vitamin D in the induction
of the expression of CYP24A1.
As in the present study, the increased mRNA
expression of CYP24A1 has been detected in several types of cancer,
including lung, colon, ovary, cervical and esophageal carcinoma,
which suggests the involvement of CYP24A1 in tumorigenesis
(10,12).
Regarding the significant increase in the mRNA
expression of CYP24A1 in malignant cells, several studies have
concluded that CYP24A1 can be considered as a potential oncogene in
cancer (12,17,26–28).
The markedly higher expression levels of CYP24A1 in the tumor
tissues examined in the present study support this.
Regarding the mRNA expression of CYP27B1, the
present study showed that the expression of CYP27B1 was
downregulated in the tumor tissue, compared with that in the normal
tissue. The present study found that the tissue synthesis of
1,25(OH)2D3 from the blood supplied a local
reservoir of vitamin D in the normal breast tissue.
In confirmation of the results of the present study,
the higher expression of CYP27B1 in the normal breast tissue
suggested that the paracrine production of
1,25(OH)2D3 may be important for maintaining
the safe function of normal breast cells (1). In tumor breast cells, the ability of
active vitamin D synthesis is altered and, therefore, VDR-mediated
vitamin D function decreases (29).
Previous studies on breast cancer cell lines have
revealed that the mRNA expression of CYP27B1 decreases to a certain
extent (7,13), which agrees with the present study.
However the present study investigated tissue samples. The fact
that the activity of CYP27B1 in breast tumor tissue is lower,
compared with that in normal breast tissue, and the activity of
CYP24A1 is higher, compared with normal breast tissue may indicate
potentially malignant deterioration in tumor tissue (29,30).
In the study by Segersten et al (7), the mRNA expression of CYP27B1 in the
malignant breast tissue was lower, compared with that in the normal
tissue (7). However, Friedrich
et al (16) found higher
expression levels of CYP27B1 in breast cancer tissue, compared with
benign tissue, which may be due to the heterogeneity of the breast
cancer (16).
In comparing the mRNA expression of CYP24A1 with
tumor stage, tumor grade, age range, tumor size, and lymphatic or
non-lymphatic status, the only significant association found in the
present study was between the mRNA expression of CYP24A1 and the
age range of the patients. A significant association was also found
between the mRNA expression of CYP27B1 and tumor stage. No other
significant associations were observed.
In terms of the link between the mRNA expression of
CYP24A1 and age range, the results of the present study showed that
gene expression levels of CYP24A1 in patients aged ≥53 years were
higher, compared with those in patients aged 43–52 years. This
finding indicated higher gene expression levels of CYP24A1 at
increased patient age and, as a result, the increase in active
vitamin D catabolism during tumorigenesis.
In terms of the association between the gene
expression of CYP27B1 and tumor stage, the expression at stage 1
was significantly higher, compared with that at stage 2. This was
confirmed by the results of the comparison of the gene expression
of CYP27B1 in tumor and normal tissues in the present study. The
results showed that malignant tissues at a higher stage showed
reduced gene expression of CYP27B1, causing a decrease in active
vitamin D synthesis.
Epigenetic mechanisms can also silence genes; for
example, CYP27B1 is inhibited through alterations in epigenetic
methylation (4,31). Investigations performed by McCarthy
et al (3) showed that the
aberrant methylation of gene promoters is an important mechanism in
the progression of breast and colon cancer. Hypermethylation of the
CYP27B1 promoter has been reported in >40% of cases of breast
cancer, therefore, in breast cancer development, epigenetic
alterations can alter gene expression (23), which may be effective in the
treatment of cancer using chemopreventive drugs (3).
In addition, women who are exposed to more
ultraviolet radiation may be able to neutralize the lower activity
of CYP27B1 through supplying more vitamin D (30).
However, polymorphisms in CYP27B1 cause diversity in
the regulation of the gene expression (12), a consideration for future
investigations.
To compare the mRNA and protein expression levels of
protein in CYP27B1 and CYP24A1, further investigations are required
in order to examine the expression of CYP27B1 and CYP24A1 using
western blot analysis. Studies have shown splice variants in
CYP24A1, CYP27B1 and VDR, which may deactivate the enzymes and
reduce protein levels (17,24).
In addition, assessment of the expression levels of CYP27B1 and
CYP24A1 in cancerous and benign breast lesions using
immunohistochemistry has shown a reduction in the expression of
CYP27B1 and an increase in the expression of CYP24A1 in carcinoma
(29). Whether the changes in
enzymatic expression can be justified by the serum level of vitamin
D, and whether the changes in enzymatic activity are caused by
splice variants require consideration in future investigations.
The present study showed that the capacity of local
production of active vitamin D in the breast depends on the
presence of 25-hydroxyvitamin D, as the substrate of CYP27B1, and
the rate of 1,25(OH)2D3 catabolism, which is
performed by CYP24A1.
The results of the present study showed that the
breast cells did not completely depend on reserving vitamin D from
systemic sources, and they had the capacity for the local
production of 1,25(OH)2D3. However, the local
production of vitamin D alters as the tissue becomes cancerous, and
the alterations may be involved in the tumorigenesis of the breast
cancer (16). Therefore, it can be
argued that the disorders in the regulatory system of vitamin D
anabolism and catabolism, and the disturbance in the association
between VDR, CYP27B1 and CYP24A1 may be involved in the progression
of cancer.
Future investigations may enable the detection of
vitamin D analogues with selective anticancer activity, but without
systemic side effects or with mild side effects (16). Understanding the alterations in the
expression of enzymes effective in the signaling system of vitamin
D in tumorigenesis may provide a reasonable basis for using the
elements of vitamin D to prevent or treat breast cancer (12,16,24,28).
The results of the present study confirmed the
importance of the components of vitamin D anabolism-catabolism in
breast cancer, and suggested that evaluating the mRNA expression
levels of CYP24A1 and CYP27B1 may be useful for estimating the risk
of the progression of breast cancer. Therefore, it can be concluded
that breast cells have an enzymatic system producing
1,25(OH)2D3, which acts as an
autocrine/paracrine growth inhibitor. Increasing the local levels
of 1,25(OH)2D3 in the tissue through the use
of vitamin D analogues, and performing further investigations on
benign and malignant breast cell models of are important in the
development of attitudes towards their clinical uses for preventing
and treating breast cancer, and for elucidating the role of altered
expression in the signaling pathway of vitamin D.
Acknowledgements
The present study was supported by Isfahan
University of Medical Sciences (grant no. 391438).
References
|
1
|
Colston KW, Lowe LC, Mansi JL and Campbell
MJ: Vitamin D status and breast cancer risk. Anticancer Res.
26:2573–2580. 2006.PubMed/NCBI
|
|
2
|
Harirchi I, Kolahdoozan S, Karbakhsh M,
Chegini N, Mohseni SM, Montazeri A, Momtahen AJ, Kashefi A and
Ebrahimi M: Twenty years of breast cancer in Iran: Downstaging
without a formal screening program. Ann Oncol. 22:93–97. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
3
|
McCarthy K, Laban C, Bustin SA, Ogunkolade
W, Khalaf S, Carpenter R and Jenkins PJ: Expression of
25-hydroxyvitamin D-1-alpha-hydroxylase and Vitamin D receptor mRNA
in normal and malignant breast tissue. Anticancer Res. 29:155–157.
2009.PubMed/NCBI
|
|
4
|
Haussler MR, Whitfield GK, Kaneko I,
Haussler CA, Hsieh D, Hsieh JC and Jurutka PW: Molecular mechanisms
of vitamin D action. Calcif Tissue Int. 92:77–98. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Anderson MG, Nakane M, Ruan X, Kroeger PE
and Wu-Wong JR: Expression of VDR and CYP24A1 mRNA in human tumors.
Cancer Chemother Pharmacol. 57:234–240. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Ordonez-Moran P, Larriba MJ, Pendas-Franco
N, Aguilera O, Gonzalez-Sancho JM and Munoz A: Vitamin D and
cancer: An update of in vitro and in vivo data. Front Biosci.
10:2723–2749. 2005. View
Article : Google Scholar : PubMed/NCBI
|
|
7
|
Segersten U, Holm PK, Björklund P, Hessman
O, Nordgren H, Binderup L, Akerström G and Hellman P:
25-Hydroxyvitamin D3 1alpha-hydroxylase expression in breast cancer
and use of non-1alpha-hydroxylated vitamin D analogue. Breast
Cancer Res. 7:R980–R986. 2005. View
Article : Google Scholar : PubMed/NCBI
|
|
8
|
Wang J and Jiang YF: Natural compounds as
anticancer agents: Experimental evidence. World J Exp Med. 2:45–57.
2012. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Luo W, Hershberger PA, Trump DL and
Johnson CS: 24-Hydroxylase in cancer: Impact on vitamin D-based
anticancer therapeutics. J Steroid Biochem Mol Biol. 136:252–257.
2013. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Horváth HC, Lakatos P, Kósa JP, Bácsi K,
Borka K, Bises G, Nittke T, Hershberger PA, Speer G and Kállay E:
The candidate oncogene CYP24A1: A potential biomarker for
colorectal tumorigenesis. J Histochem Cytochem. 58:277–285. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Chun RF, Liu PT, Modlin RL, Adams JS and
Hewison M: Impact of vitamin D on immune function: Lessons learned
from genome-wide analysis. Front Physiol. 5:1512014.PubMed/NCBI
|
|
12
|
Townsend K, Banwell CM, Guy M, Colston KW,
Mansi JL, Stewart PM, Campbell MJ and Hewison M: Autocrine
metabolism of vitamin D in normal and malignant breast tissue. Clin
Cancer Res. 11:3579–3586. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Anderson PH, O'Loughlin PD, May BK and
Morris HA: Quantification of mRNA for the vitamin D metabolizing
enzymes CYP27B1 and CYP24 and vitamin D receptor in kidney using
real-time reverse transcriptase-polymerase chain reaction. J Mol
Endocrinol. 31:123–132. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Garland CF, Garland FC, Gorham ED, Lipkin
M, Newmark H, Mohr SB and Holick MF: The role of vitamin D in
cancer prevention. Am J Public Health. 96:252–261. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Aung TT, Chandana SR, D'Silva KJ and
Dimitrov NV: The role of vitamin D in breast cancer. Oncol Rev.
3:19–25. 2009. View Article : Google Scholar
|
|
16
|
Friedrich M, Diesing D, Cordes T, Fischer
D, Becker S, Chen TC, Flanagan JN, Tangpricha V, Gherson I, Holick
MF and Reichrath J: Analysis of 25-hydroxyvitamin
D3-1alpha-hydroxylase in normal and malignant breast tissue.
Anticancer Res. 26:2615–2620. 2006.PubMed/NCBI
|
|
17
|
Fischer D, Becker S, Cordes T, Bücker B,
Diedrich K, Friedrich M, Salehin D and Thill M: Vitamin
D-24-hydroxylase in benign and malignant breast tissue and cell
lines. Anticancer Res. 29:3641–3645. 2009.PubMed/NCBI
|
|
18
|
Lowe LC, Guy M, Mansi JL, Peckitt C, Bliss
J, Wilson RG and Colston KW: Plasma 25-hydroxy vitamin D
concentrations, vitamin D receptor genotype and breast cancer risk
in a UK Caucasian population. Eur J Cancer. 41:1164–1169. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(−Delta Delta C(T)) Method. Methods. 25:402–408. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Evans SR, Nolla J, Hanfelt J, Shabahang M,
Nauta RJ and Shchepotin IB: Vitamin D receptor expression as a
predictive marker of biological behavior in human colorectal
cancer. Clin Cancer Res. 4:1591–1595. 1998.PubMed/NCBI
|
|
21
|
Fischer D, Thomé M, Becker S, Cordes T,
Diedrich K, Friedrich M and Thill M: 25-Hydroxyvitamin D3
1alpha-hydroxylase splice variants in benign and malignant ovarian
cell lines and tissue. Anticancer Res. 29:3627–3633.
2009.PubMed/NCBI
|
|
22
|
Fleet JC, DeSmet M, Johnson R and Li Y:
Vitamin D and cancer: A review of molecular mechanisms. Biochem J.
441:61–76. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Welsh JE: Vitamin D metabolism in mammary
gland and breast cancer. Mol Cell Endocrinol. 347:55–60. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Thill M, Hoellen F, Becker S, Dittimer C,
Fischer D, Kümmel S, Salehin D, Friedrich M, Köster F, Diedrich K
and Cordes T: Expression of prostaglandin- and vitamin
D-metabolising enzymes in benign and malignant breast cells.
Anticancer Res. 32:367–372. 2012.PubMed/NCBI
|
|
25
|
Ren S, Nguyen L, Wu S, Encinas C, Adams JS
and Hewison M: Alternative splicing of vitamin D-24-hydroxylase: A
novel mechanism for the regulation of extrarenal
1,25-dihydroxyvitamin D synthesis. J Biol Chem. 280:20604–20611.
2005. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Mimori K, Tanaka Y, Yoshinaga K, Masuda T,
Yamashita K, Okamoto M, Inoue H and Mori M: Clinical significance
of the overexpression of the candidate oncogene CYP24 in esophageal
cancer. Ann Oncol. 15:236–241. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Tanner MM, Grenman S, Koul A, Johannsson
O, Meltzer P, Pejovic T, Borg A and Isola JJ: Frequent
amplification of chromosomal region 20q12-q13 in ovarian cancer.
Clin Cancer Res. 6:1833–1839. 2000.PubMed/NCBI
|
|
28
|
Albertson DG, Ylstra B, Segraves R,
Collins C, Dairkee SH, Kowbel D, Kuo WL, Gray JW and Pinkel D:
Quantitative mapping of amplicon structure by array CGH identifies
CYP24 as a candidate oncogene. Nat Genet. 25:144–146. 2000.
View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Lopes N, Sousa B, Martins D, Gomes M,
Vieira D, Veronese LA, Milanezi F, Paredes J, Costa JL and Schmitt
F: Alterations in Vitamin D signalling and metabolic pathways in
breast cancer progression: A study of VDR, CYP27B1 and CYP24A1
expression in benign and malignant breast lesions. BMC Cancer.
10:4832010. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Grant WB: The likely role of vitamin D
from solar ultraviolet-B irradiance in increasing cancer survival.
Anticancer Res. 26:2605–2614. 2006.PubMed/NCBI
|
|
31
|
Bacchetta J, Sea JL, Chun RF, Lisse TS,
Wesseling-Perry K, Gales B, Adams JS, Salusky IB and Hewison M:
Fibroblast growth factor 23 inhibits extrarenal synthesis of
1,25-dihydroxyvitamin D in human monocytes. J Bone Miner Res.
28:46–55. 2013. View Article : Google Scholar : PubMed/NCBI
|