Introduction
Endometriosis affects ~10% of women of reproductive
age, and is a complex disease characterized by the presence of
endometrial tissue outside the uterine cavity. This extra tissue
subsequently leads to an ectopic growth, which results in an
inflammatory response, adhesions and fibrosis, followed by chronic
pelvic pain, dyspareunia and infertility (1).
Chemokines are small molecules produced during the
inflammatory process and are responsible for the attraction of
cells, depending on the selective receptors expressed on their
membranes. Endometrial stromal cells (ESCs) exhibit the ability to
invade and proliferate, and regulation of these processes is
controlled by chemokines produced in the endometriotic milieu. In
addition, these help recruit leukocytes to the peritoneal cavity
(2,3). C-X-C motif chemokine ligand 12
(CXCL12) is the single natural ligand of chemokine receptors C-X-C
chemokine receptor type (CXCR) 4 and CXCR7. The refluxed
endometrial cells are attracted to the peritoneal cavity via the
CXCL12/CXCR4 axis (4).
Overexpression of CXCR4 in stromal cells has been observed by
immunohistochemistry (5). The
eutopic endometrial stromal cells from endometriosis patients are
attracted to the peritoneal cavity via the interaction between
CXCR4 expressed on their membrane and CXCL12 produced in the
peritoneal cavity. Furthermore, the activation of the CXCL12/CXCR4
axis is associated with the alternative phenotype of endometriotic
cells (6). Our previous study
indicated that greater levels of CXCL12 secreted from ESCs in the
endometriotic milieu suppress ESC apoptosis by increasing autophagy
via the nuclear factor-κB signaling pathway (7). However, the systemic role of the
CXCL12/CXCR4 axis and the molecular alterations induced by CXCL12
challenge in endometriosis remain to be fully elucidated.
Alterations in microRNA (miRNA) expression occur in
numerous pathological and physiological processes, and miRNAs are
associated with endometriosis. Dysregulation of miRNAs leads to
alterations in cellular differentiation, proliferation and
apoptotic processes involved in the pathogenesis of endometriosis
(8). Thus, elucidating the
biological consequences of dysregulated miRNAs and identifying
their specific targets is necessary to understand the underlying
molecular mechanisms associated with the disease.
Therefore, the present study aimed to fully
elucidate the systemic role of CXCL12 in endometriosis, and
investigate key miRNAs associated with CXCL12 stimulation of ESCs.
Furthermore, the underlying cellular regulatory mechanisms of
CXCL12 in ESCs were investigated by building networks between
miRNAs, genes and gene ontologies (GOs).
Materials and methods
Subjects and sample collection
Female patients (n=20) of reproductive age from the
Department of Obstetrics and Gynecology, Nanjing Drum Tower
Hospital (Nanjing, China) were recruited as the subjects of the
present study (May-June 2015). The present study was approved by
the Research Ethics Committee of Nanjing Drum Tower Hospital and
written informed consent was obtained from all patients prior to
sampling; endometrial biopsies were obtained for research purposes
only. The patients selected for inclusion in the study had not
received any hormonal therapy or medication for 6 months prior to
surgery and had not experienced any pelvic inflammatory-associated
complications. Endometrial tissues were collected from 18 patients
undergoing tubal ligation, and they were used for the control group
and the CXCL12-treated group. Evaluation of endometrial histology
was performed, as was a comparison of the date to the expected day
of the menstrual cycle, to determine its phase. All endometrial
samples used for in vitro experiments were histologically
verified to be in the secretory phase of the menstrual cycle. All
samples (>300 mg) were collected under sterile conditions and
transported to the laboratory on ice in Dulbecco's modified Eagle's
medium (DMEM)/F-12 medium (Gibco; Thermo Fisher Scientific, Inc.,
Waltham, MA, USA).
Cell culture and treatments
ESCs were purified and cultured from endometrial
tissue as previously described (7). The ESCs exhibited 92% purity
(Vimentin positive). ESCs from the same patients were used in
control and treatment groups (healthy ESCs and recombinant human
(rh)CXCL12-treated healthy ESCs). To clarify the miRNAome and
mRNAome of CXCL12-treated ESCs, cells were treated with 100 ng/ml
rhCXCL12 (R&D Systems, Inc., Minneapolis, MN, USA) for 48 h,
whereas the control ESCs were cultured with 1% phosphate-buffered
saline (PBS; Corning Incorporated, Corning, NY, USA) as the vehicle
control. The rhCXCL12 treatment dose and exposure time were
selected according to our previous study (7).
Isolation of total RNA
Total RNA from cultured healthy ESCs and
corresponding rhCXCL12-treated healthy ESCs (n=18) was extracted
using Qiagen miRNeasy Mini kit (Qiagen GmbH, Hilden, Germany). The
quality of the extracted RNA was verified via absorbance
measurements at wavelengths of 230, 260 and 280 nm using a
spectrophotometer (NanoDrop 2000; Thermo Fisher Scientific,
Inc.).
miRNA and mRNA microarray
analysis
Total RNA was processed for miRNA and mRNA
microarray analysis, using Affymetrix miRNA 4.0 and Affymetrix
GeneChip Human 2.0 (Affymetrix, Inc., Santa Clara, CA, USA) as
previously described (9).
Following analysis, significantly differentially expressed miRNAs
and mRNAs were selected according to their P-values (10).
Bioinformatics analysis
A comprehensive GO and miRNA-gene network was built
to enrich the gene dataset. Genes that were differentially
expressed underwent GO analysis (geneontology.org) to verify their primary function.
This identification of the regulatory network of genes allowed
their categorization into different hierarchies, based on their
molecular function (11).
Differential gene expression values and Sanger miRNA database
(Gminix-Cloud Biotechnology Information, GCBI, http://www.gcbi.com.cn/gclib/html/index,
Gminix Informatics Co., Ltd., Shanghai, China) interactions were
used to investigate associations between miRNAs and genes, and to
create an miRNA-gene network. An adjacency matrix of miRNAs and
genes A = [ai, j] was created according to the attributed
associations between genes and miRNA, where ai, j represents the
weight of the association between gene ai and miRNA j.
The regulatory status of miRNAs and genes was
evaluated via GCBI. (http://www.gcbi.com.cn/gclib/html/index, Gminix
Informatics Co., Ltd.). The evaluation criteria included the
degrees of miRNA; the number of genes regulated by that particular
miRNA, and the degree of each gene; and the number of miRNAs that
regulated that particular gene. Those that exhibited the greatest
degree values were identified as the most important in the analysis
of the network (12).
Statistical analysis
GO analysis for all miRNA and the regulated-genes
were obtained, based on the GO and Kyoto Encyclopedia of Genes and
Genomes (www.genome.jp/kegg/) databases.
P-values and the false discovery rate were calculated using
Fisher's and χ2 tests (SPSS 19.0; IBM SPSS, Armonk, NY,
USA), and 39 GOs were identified, with P<0.001 considered to
indicate a statistically significant difference.
Results
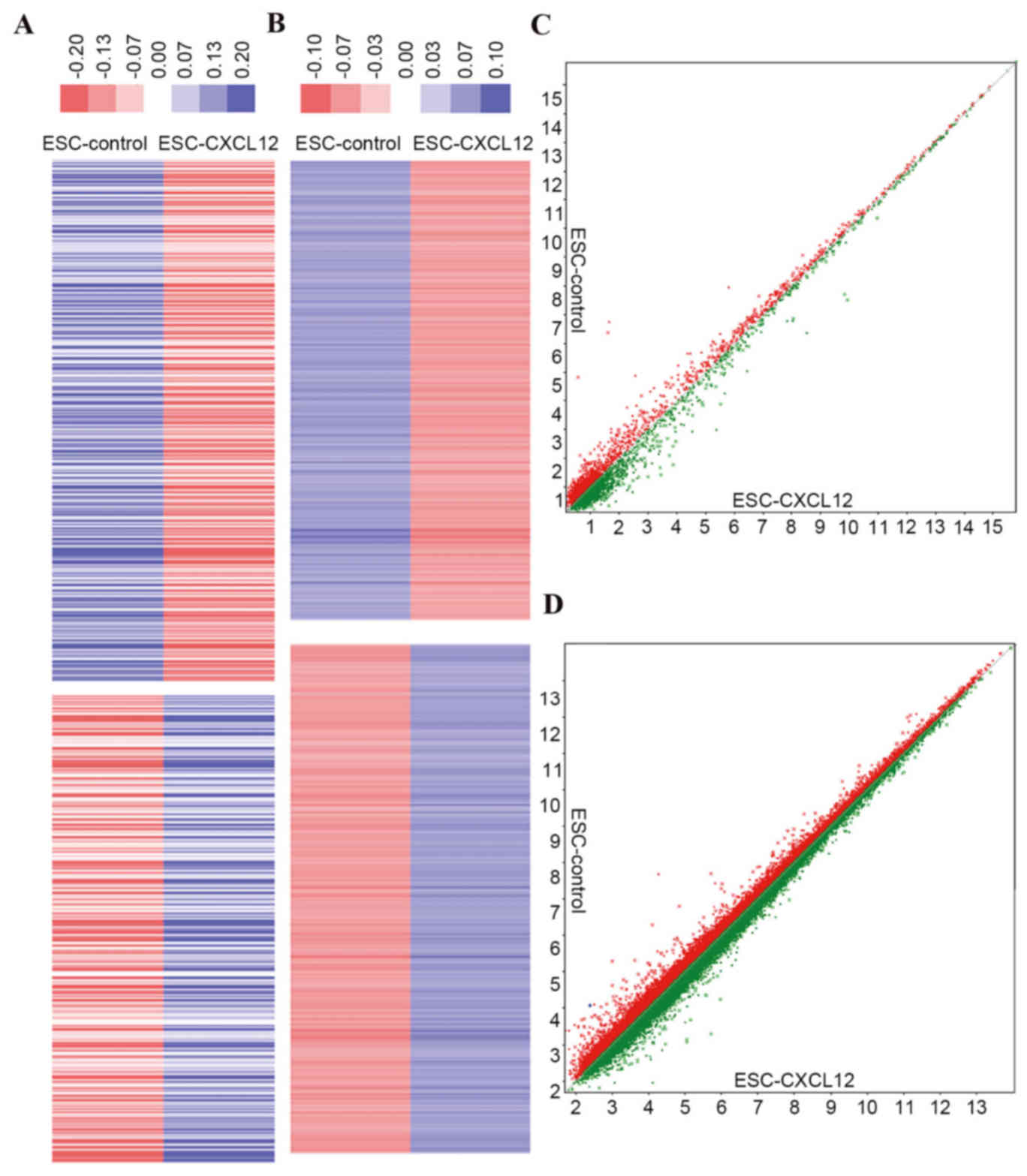
Microarray data
miRNAs and mRNAs induced by rhCXCL12 treatment in
ESCs were measured by miRNA and gene chips. A total of 35 miRNAs
and 1,671 mRNAs (Fig. 1) were
differentially expressed in CXCL12-treated ESCs compared with
controls.
GO analysis
The primary functions of the genes that were
differentially expressed were analyzed via GO. Potential target
genes of the 35 miRNAs were used to search for intersections of
these miRNAs with 1,671 differentially expressed mRNAs. A total of
63 intersection genes were identified. The 39 mRNAs that exhibited
a negative association with the above 22 miRNAs were analyzed and
are presented in Table I. GOs for
all of the above intersection genes were obtained, based on the GO
and Kyoto Encyclopedia of Genes and Genomes (www.genome.jp/kegg/) databases. P-values and the false
discovery rate were calculated using Fisher's and χ2
tests, and 39 GOs were identified according to the standard of
P<0.001. The present study identified three classes of GOs
(associated with immune cell chemoattractants, inflammatory and
immune responses, and pathological processes of endometriotic
lesions in endometriosis) strictly associated with CXCL12 challenge
(Fig. 2), indicating that these
GOs may be important in regulating the role of CXCL12 in
endometriosis.
 | Table I.Differentially expressed miRNA and
predicted targets. |
Table I.
Differentially expressed miRNA and
predicted targets.
| miRNA | Up/downregulated | Fold change | Gene ID | Up/downregulated |
|---|
| hsa-miR-150-5p | Down | 0.028877827 | CCL5 | Up |
| hsa-miR-4306 | Down | 0.374462719 | ELAVL3 | Up |
|
|
|
| SLA2 |
|
| hsa-miR-4286 | Down | 0.410782037 | ZFP36L1 | Up |
| hsa-miR-4539 | Down | 0.416821327 | ELAVL3 | Up |
|
|
|
| HSF5 |
|
|
|
|
| RAB39B |
|
| hsa-miR-6507-5p | Down | 0.424400129 | RGS21 | Up |
|
|
|
| ZFP36L1 |
|
| hsa-miR-29b-2-5p | Down | 0.440211274 | ANKS4B | Up |
| hsa-miR-18b-5p | Down | 0.440222564 | HSF5 | Up |
| hsa-miR-1290 | Down | 0.44241555 | ELAVL3 | Up |
|
|
|
| EN2 |
|
| hsa-miR-502-5p | Down | 0.447388476 | ELAVL3 | Up |
|
|
|
| LIMD2 |
|
|
hsa-miR-550a-3-5p | Down | 0.488144777 | GP1BA | Up |
|
|
|
| HOXB13 |
|
| hsa-miR-1260a | Down | 0.495246952 | ELAVL3 | Up |
|
|
|
| HOXB13 |
|
|
|
|
| LIMD2 |
|
|
|
|
| SLA2 |
|
| hsa-miR-6886-3p | Down | 0.498411064 | DPCR1 | Up |
| hsa-miR-466 | Up | 2.127169505 | SORL1 | Down |
| hsa-miR-4708-5p | Up | 2.223631924 | IFNG | Down |
| hsa-miR-424-5p | Up |
|
|
| 2.348038396 | DSEL | Down |
|
|
|
| PDK4 |
|
|
|
|
| SH3BGRL2 |
|
| hsa-miR-8084 | Up | 2.398208347 | LRCH2 | Down |
| hsa-miR-498 | Up | 3.346560277 | RNASEH2B | Down |
|
|
|
| SH3BGRL2 |
|
| hsa-miR-4476 | Up | 4.050657207 | FAM155A | Down |
| hsa-miR-4428 | Up | 4.247547261 | SORL1 | Down |
|
hsa-miR-4668-5p | Up | 4.362360508 | SCD | Down |
| hsa-miR-1184 | Up | 5.207536333 | DSEL | Down |
|
|
|
| SH3BGRL2 |
|
|
hsa-miR-3613-3p | Up | 5.322665855 | DSEL | Down |
|
|
|
| LRCH2 |
|
|
|
|
| SH3BGRL2 |
|
|
|
|
| VNN2 |
|
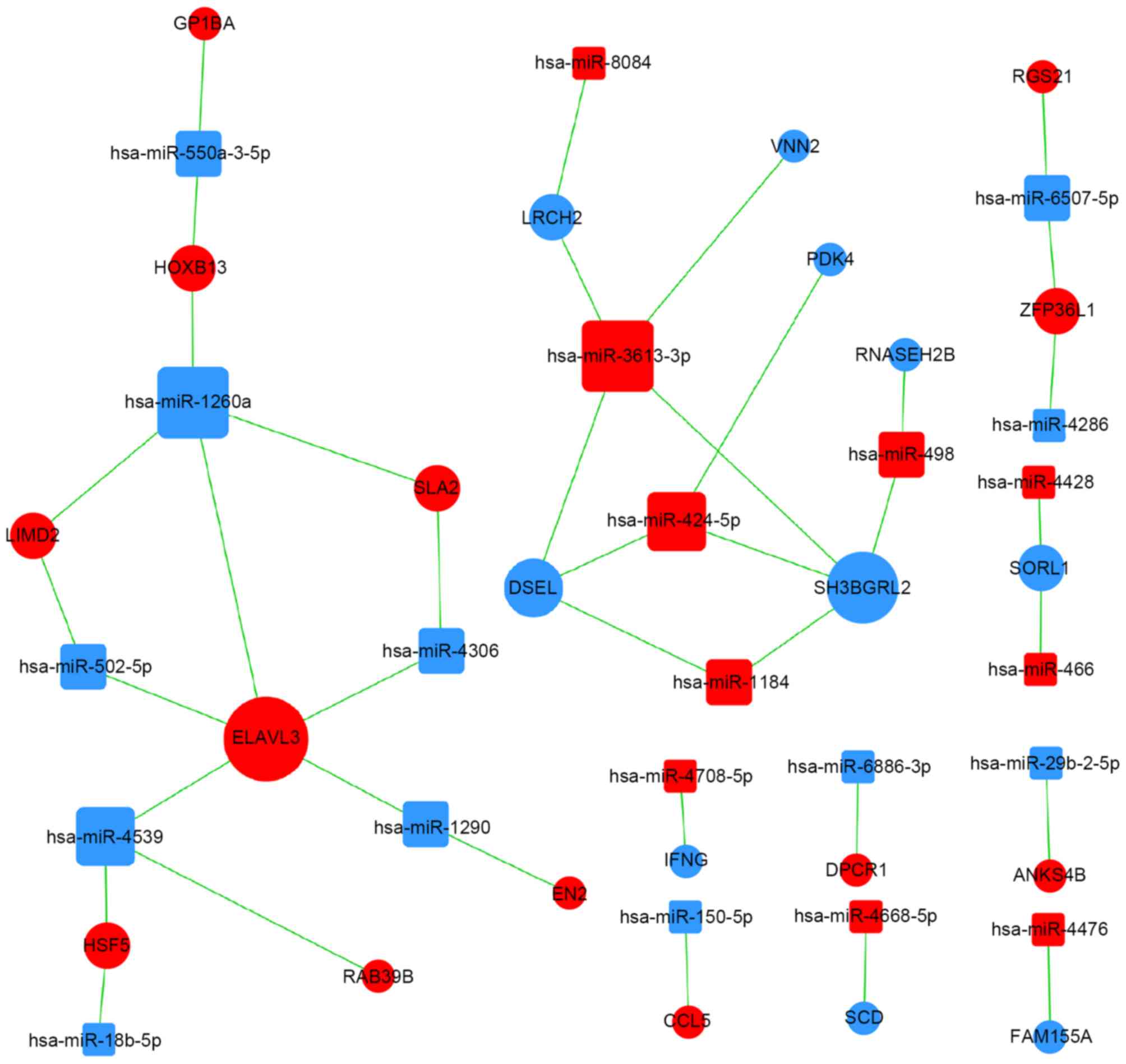
miRNA regulatory networks
Interactions between miRNAs and genes in the Sanger
miRNA database led to the subsequent creation of an miRNA-gene
network where significant miRNAs and genes were identified from the
intersections between potential target genes of miRNAs and 1,671
differentially expressed mRNAs. A miRNA-GO network was constructed
according to interactions between significant GOs and genes and
associations between miRNAs and genes (Fig. 3).
Discussion
Local, peritoneal inflammation is characteristic of
endometriosis, and previous studies have suggested that various
components of the immune system, including chemokines, enhance the
implantation of endometrial cells and the progression of the
disease (3,13). The CXCL12-CXCR4 axis has been
demonstrated to exhibit immune functions, including lymphocyte
chemotaxis and non-immune functions, including roles in tissue
repair, angiogenesis and the invasion and migration involved in
endometriosis (5,14). Our previous study indicated that
this chemokine is involved in the regulation of ESC autophagy
(7). This suggested that the
CXCL12-CXCR4 axis may have a broad role in the regulation of
endometriosis.
Endometriotic tissue exhibits various molecular
differences compared with healthy endometrial tissue, including
variations in gene expression, production of proteins,
responsiveness to steroids and cytokines, immune components,
adhesion molecules, and proteolytic enzymes (15,16).
The occurrence and development of endometriosis may be based on
these aberrations in molecular processes, and may be caused by as
yet unknown triggers, including miRNA, which initiate these
molecular alterations. Certain studies have investigated the role
of miRNA expression dysregulation, histone modification and DNA
methylation in endometriotic cells (17). Aberrant gene expression associated
with epigenetic mechanisms may induce differentiation and the
subsequent appearance of an endometriotic phenotype in healthy
endometrial cells (15,16).
Our previous study demonstrated a greater secretion
of CXCL12 in eutopic and ectopic ESCs derived from patients with
endometriosis (7). The present
study treated healthy ESCs with CXCL12 to mimic the conditions in
ectopic ESCs. To further elucidate the role of the CXCL12/CXCR4
axis in endometriosis, the present study detected the miRNA and
miRNAome profiles in CXCL12-stimulated ESCs. The 22 miRNAs most
closely associated with the CXCL12/CXCR4 axis in ESCs were
reported. The present study aimed to provide the most complete
miRNAome profiles, and the most detailed microRNA-gene regulatory
networks described to date for the role of CXCL12 in endometriosis.
The results of the present study provide preliminary data to
advance the understanding of the pathogenesis of endometriosis.
Microarray data analysis revealed a total of 35
miRNAs and 1,671 mRNAs significantly expressed in CXCL12-treated
ESCs. Bioinformatics analysis indicated that 63 mRNAs had a
negative correlation with 21 differentially expressed miRNAs, and
39 significant GOs for all of the above intersection genes were
obtained. GOs regarding CXCL12-challenge in ESCs were associated
with: i) Chemoattractants for immune cells, including macrophages,
neutrophils, natural killer (NK) cells, T cells and dendritic
cells, and chemokine biosynthetic regulation; ii) inflammatory and
immune responses, including regulation of chronic inflammatory
responses, T cell activation, innate immune responses, antigen
processing and presentation, adaptive immune responses, and
interleukin production; iii) pathological processes of
endometriotic lesions in endometriosis, including cell-cell
adhesion, mesenchymal to epithelial transition, blood coagulation,
fibrinolysis, cell proliferation, cell cycle arrest, cell-cell
adhesion, Janus kinase/signal transducers and activators of
transcription (STAT) signaling, and chemokine-mediated
signaling.
The upregulation of CXCL12 in reflux eutopic
endometrium recruits immune cells, including macrophages, NK cells,
T cells and dendritic cells into the peritoneal cavity. These
immune cells do not remove endometrial fragments in the pelvic
cavity; rather an inflammatory microenvironment foundation is
created, facilitating the implantation, neo-angiogenesis and
proliferation of ectopic endometrial tissue (18). Conditions for the progression of
the disease may include increased levels of activated peritoneal
macrophages, reduced NK cell activity, an abnormal T lymphocyte
response and a significantly increased number of regulatory T cells
(19).
CXCL12 in ESCs may directly mediate the pathological
process and appearance of lesions in endometriosis, via effects on
autophagy, cell-cell adhesion, mesenchymal to epithelial
transition, blood coagulation, fibrinolysis and cell growth. The
role of CXCL12 in ESC autophagy, which is indicated by GO analysis,
is consistent with the findings of our previous study (7). The GO analysis indicated the
potential role of CXCL12 in epithelial mesenchymal transition
(EMT). Inflammation is regarded as an inducer of EMT in cancer
progression, and EMT is considered to link inflammation and cancer
(20). Epithelial cells are
converted to mesenchymal cells during embryogenesis, tissue
remodeling and wound healing, via EMT. The process is characterized
by alterations in molecular pathways and networks; the loss of
E-cadherin expression is an essential step driving this program in
the initiation of human cancers (21). During arsenite-induced malignant
transformation of skin cancer cells, STAT3 regulates EMT and
malignant transformation (22).
Although EMT occurs in endometriosis, the underlying mechanism
remains to be fully elucidated. Further studies are required to
identify the exact underlying mechanism of CXCL12-induced EMT in
endometriosis.
miRNAs are small endogenous, single-stranded,
non-coding RNA molecules that regulate the translation of specific
targeted protein-coding genes (23). miRNAs regulate the expression of
50–60% of human genes without altering DNA sequences, via various
epigenetic mechanisms. Numerous genes may be targeted by a single
miRNA molecule and various cellular functions are induced via
partial or full base-pairing with the 3′-untranslated region of the
target mRNA (24).
A wide range of physiological and pathological
processes have been revealed to involve miRNAs, including the cell
cycle (in embryogenesis, development, differentiation and
proliferation), metabolism, cell-cell communication, cell survival,
apoptosis, immune responses and oncogenesis (25). Microarray studies have indicated
the presence of a group of specific miRNAs that are differentially
expressed between healthy endometrium without endometriosis,
eutopic endometrial tissue with endometriosis and endometriotic
lesions, suggesting the importance of miRNAs in the pathology of
endometriosis (26,27). Identification of key miRNAs via
bioinformatics analysis provides information regarding an initial
group of expressed miRNAs that are altered in CXCL12-treated ESCs
and, therefore, may target genes that regulate this process. The
results of the present study provide preliminary data to advance
the full elucidation of the role of CXCL12 in endometriosis, and
its underlying epigenetic mechanisms.
In conclusion, the miRNA profiles in
CXCL12-overexpressed ESCs were identified in the present study
using miRNA microarray analysis. The findings suggested that
aberrant miRNA expression may be the epigenetic mechanism
underlying the actions of CXCL12 in endometriosis, and this may
provide novel and promising candidates to act as a diagnostic
biomarkers and therapeutic targets of endometriosis. The present
study, to the best of our knowledge, provides the most complete
miRNAome profile for the evaluation of miRNAs in of
CXCL12-stimulated ESCs, and the most detailed miRNA-gene regulatory
network, targeting genes and GOs for investigation of the
underlying miRNA mechanisms of CXCL12 in ESCs. The data from the
present study will be validated in our further studies regarding
endometriosis.
Acknowledgements
The present study was supported by the National
Natural Science Foundation of China (grant no. 81601354), the
National Science Foundation of Jiangsu Province, China (grant no.
BK20160128), and the Fundamental Research Funds for the Central
Universities (grant no. 021414380180) (all to J. M.), the National
Natural Science Foundation of China (grant no. 81471513 and
91542108), the Development Fund of Shanghai Talents (grant no.
201557) and the Shanghai Rising-Star Program (grant no.
16QA1400800) (all to M-Q. L.).
References
|
1
|
Bulun SE: Endometriosis. N Engl J Med.
360:268–279. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Li MQ, Wang Y, Chang KK, Meng YH, Liu LB,
Mei J, Wang Y, Wang XQ, Jin LP and Li DJ:
CD4+Foxp3+ regulatory T cell differentiation
mediated by endometrial stromal cell-derived TECK promotes the
growth and invasion of endometriotic lesion. Cell Death Dis.
5:e14362014. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Borrelli GM, Carvalho KI, Kallas EG,
Mechsner S, Baracat EC and Abrão MS: Chemokines in the pathogenesis
of endometriosis and infertility. J Reprod Immunol. 981–9.
(2013)2013.PubMed/NCBI
|
|
4
|
Balkwill F: Cancer and the chemokine
network. Nat Rev Cancer. 4:540–550. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Ruiz A, Salvo VA, Ruiz LA, Báez P, García
M and Flores I: Basal and steroid hormone-regulated expression of
CXCR4 in humanendometrium and endometriosis. Reprod Sci.
17:894–903. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Leconte M, Chouzenoux S, Nicco C, Chéreau
C, Arkwright S, Santulli P, Weill B, Chapron C, Dousset B and
Batteux F: Role of the CXCL12-CXCR4 axis in the development of deep
rectal endometriosis. J Reprod Immunol. 103:45–52. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Mei J, Zhu XY, Jin LP, Duan ZL, Li DJ and
Li MQ: Estrogen promotes the survival of human secretory phase
endometrial stromal cells via CXCL12/CXCR4 upregulation-mediated
autophagy inhibition. Hum Reprod. 30:1677–1689. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Burney RO, Hamilton AE, Aghajanova L, Vo
KC, Nezhat CN, Lessey BA and Giudice LC: MicroRNA expression
profiling of eutopic secretory endometrium in women with versus
without endometriosis. Mol Hum Reprod. 15:625–631. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Bao Y, Gao Y, Jin Y, Cong W, Pan X and Cui
X: MicroRNA expression profiles and networks in mouse lung infected
with H1N1 influenza virus. Mol Genet Genomics. 290:1885–1897. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Clarke R, Ressom HW, Wang A, Xuan J, Liu
MC, Gehan EA and Wang Y: The properties of high-dimensional data
spaces: Implications for exploring gene and protein expression
data. Nat Rev Cancer. 8:37–49. 2008. View
Article : Google Scholar : PubMed/NCBI
|
|
11
|
Gene Ontology Consortium, . The gene
ontology (GO) project in 2006. Nucleic Acids Res. 34:(Database
issue). D322–D326. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Joung JG, Hwang KB, Nam JW, Kim SJ and
Zhang BT: Discovery of microRNA-mRNA modules via population-based
probabilistic learning. Bioinformatics. 23:1141–1147. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Ramey JW and Archer DF: Peritoneal fluid:
Its relevance to the development of endometriosis. Fertil Steril.
60:1–14. 1993.PubMed/NCBI
|
|
14
|
Ahn SH, Edwards AK, Singh SS, Young SL,
Lessey BA and Tayade C: IL-17A Contributes to the pathogenesis of
endometriosis by triggering proinflammatory cytokines and
angiogenic growth factors. J Immunol. 195:2591–2600. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Nasu K, Nishida M, Kawano Y, Tsuno A, Abe
W, Yuge A, Takai N and Narahara H: Aberrant expression of
apoptosis-related molecules in endometriosis: A possible mechanism
underlying the pathogenesis of endometriosis. Reprod Sci.
18:206–218. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Guo SW: Epigenetics of endometriosis. Mol
Hum Reprod. 15:587–607. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Abe W, Nasu K, Nakada C, Kawano Y,
Moriyama M and Narahara H: miR-196b targets c-myc and Bcl-2
expression, inhibits proliferation and induces apoptosis in
endometriotic stromal cells. Hum Reprod. 28:750–761. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Barrier BF: Immunology of endometriosis.
Clin Obstet Gynecol. 53:397–402. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Berbic M, Hey-Cunningham AJ, Ng C,
Tokushige N, Ganewatta S, Markham R, Russell P and Fraser IS: The
role of Foxp3+ regulatory T-cells in endometriosis: A potential
controlling mechanism for a complex, chronic immunological
condition. Hum Reprod. 25:900–907. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Zhou C, Liu J, Tang Y and Liang X:
Inflammation linking EMT and cancer stem cells. Oral Oncol.
48:1068–1075. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Tellez CS, Juri DE, Do K, Bernauer AM,
Thomas CL, Damiani LA, Tessema M, Leng S and Belinsky SA: EMT and
stem cell-like properties associated with miR-205 and miR-200
epigenetic silencing are early manifestations during
carcinogen-induced transformation of human lung epithelial cells.
Cancer Res. 71:3087–3097. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Lu X, Luo F, Liu Y, Zhang A, Li J, Wang B,
Xu W, Shi L, Liu X, Lu L and Liu Q: The IL-6/STAT3 pathway via
miR-21 is involved in the neoplastic and metastatic properties of
arsenite-transformed human keratinocytes. Toxicol Lett.
237:191–199. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Bartel DP: MicroRNAs: Genomics,
biogenesis, mechanism, and function. Cell. 116:281–297. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Engels BM and Hutvagner G: Principles and
effects of microRNA-mediated post-transcriptional gene regulation.
Oncogene. 25:6163–6169. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Teague EM Ohlsson, Print CG and Hull ML:
The role of microRNAs in endometriosis and associated reproductive
conditions. Hum Reprod Update. 16:142–165. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Braza-Boïls A, Marí-Alexandre J, Gilabert
J, Sánchez-Izquierdo D, España F, Estellés A and Gilabert-Estellés
J: MicroRNA expression profile in endometriosis: Its relation to
angiogenesis and fibrinolytic factors. Hum Reprod. 29:978–988.
2014. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Filigheddu N, Gregnanin I, Porporato PE,
Surico D, Perego B, Galli L, Patrignani C, Graziani A and Surico N:
Differential expression of microRNAs between eutopic and ectopic
endometrium in ovarian endometriosis. J Biomed Biotech.
2010:3695492010.
|