Introduction
Matrine is a component of the traditional Chinese
medical herb Sophora flavescens ait. It performs a variety of
medical effects as a κ-opioid receptor and µ-receptor agonist
(1). Since Matrine has a wide
range of clinical effects, including anti-inflammatory, antiviral,
immunoinhibitory, antifibrotic and antidiarrhea effects (2,3), it
has been widely used in treatment of viral hepatitis, hepatic
fibrosis, cardiac arrhythmia and skin diseases, including atopic
dermatitis and eczema in China (4–7).
Therefore, matrine can serve as a potential anticancer drug to
treat various types of human cancer. A previous study reported that
matrine can suppress the proliferation of hepatoma G2 (HepG2)
cells, possibly by inducing apoptosis through the activation of
B-cell lymphoma-2-activated X protein (8). However, its antitumor mechanism in
p53 deficient hepatoma Hep3B cells remains to be elucdated.
In response to various cellular stresses, the tumor
suppressor gene, p53, acts as an important safeguard mechanism by
preventing cells from undergoing uncontrolled proliferation in
response to DNA damage (9,10). The downstream DNA damage response
(DDR) involves a series of events that lead to either cell-cycle
arrest induced by p21 or apoptosis induced by p53 upregulated
modulator of apoptosis (PUMA) (11). Mouse double-minuted (MDM)2, a
target gene of p53 that can form a negative feedback loop with p53,
it is also considered as an antiapoptotic factor that can inhibit
apoptosis of tumor cells (12).
MDM2 has attracted great attention following its identification as
a negative modulator of p53. MDM2 can bind to p53 through its
N-terminus to repress p53 anticancer functions, while its
C-terminus serves as an E3 ubiquitin ligase to mediate p53
degradation, which maintains p53 at a low protein level during
normal homeostasis without stress signals (12–14).
In addition to interacting with and modulating p53, MDM2 may
compete with effectors that are capable of binding with p73
(15–17). With the exception of interacting
with and regulating p53 family members, growing evidence suggests
that MDM2 has numerous p53-independent functions. For example, MDM2
was shown to bind to and ubiquitinate Rb, resulting in Rb
degradation and release of E2F1, which promotes cell cycle
progression (18,19). Since MDM2 is able to bind RNA and
shuttle between the nucleus and the cytoplasm, properties of most
internal ribosome entry site (IRES) trans-acting factors
(ITAFs)/ribonucleoproteins (RNPs), this suggests that the
dephosphorylated cytoplasmic MDM2 may act as an ITAF/RNP to
regulate translation through binding of its C-terminus to specific
RNAs (20). Additionally, previous
studies also demonstrated that MDM2 can physically interact with
the IRES of the 5′-untranslated region of inhibitor of apoptosis
protein (IAP)3, and in turn induce translation of the latter, which
allows for development of resistance to anticancer treatment
(21,22).
As a negative modulator of p53, it may be suggested
that MDM2 is an indirect oncogene, when overexpressed would be
oncogenic by preventing the release of activated p53. This
hypothesis is supported by numerous previous studies (23,24).
For example, in MDM2 overexpression mice, the incidence rate of
tumor formation is significantly increased. In particular. MDM2
overexpression is even observed in cancer types that lack MDM2 gene
amplification, including acute lymphoblast leukemia cells (23,24).
Regardless of any molecular mechanism involved, MDM2 overexpression
is associated with the development of tumors and poor
prognosis.
A previous study reported that matrine at
concentrations of 0.25, 0.5, 1.0 and 2.0 mg/ml inhibits the growth
of HepG2 cells in a dose- and time-dependent manner (8). The present study used identical
concentrations of matrine with minimal modification to investigate
the influence of matrine on MDM2 expression and induction of Hep3B
cell death. It was revealed that matrine markedly suppressed MDM2
transcription and caused significant apoptosis of
MDM2-overexpressing Hep3B cells. In addition, the present study
examined the expression of the p53 family member, p73, as well as
its downstream effectors, p21 and PUMA. Investigating these effects
may assist with elucidating the molecular mechanism by which
matrine induces MDM2 downregulation and apoptosis of the
p53-deficient Hep3B cells.
Materials and methods
Reagents
Matrine was supplied by Xi'an Tianyuan Biologics
Plant (Xi'an, China), with a purity of >99%. Matrine was
dissolved in sterile double distilled water at a stock
concentration of 40 mg/ml, was stored at −20°C in the dark and was
subsequently diluted in Dulbecco's modified Eagle's medium (DMEM)
to obtain the desired concentration. Actinomycin D, benzo(a)pyrene
and etoposide were purchased from Sigma-Aldrich (St. Louis, MO,
USA). DMEM and fetal bovine serum (FBS) were products from Gibco
(Thermo Fisher Scientific, Inc., Waltham, MA, USA). The protein
isolation kit was obtained from KeyGen Biotech. Co., Ltd. (Nanjing,
China). MDM2 CRISPR activation plasmid, and MDM2 (cat. no. BS1223),
PUMA (cat. no. AB10418), p73α (cat. no. 4662), p73α (Try99) (cat.
no. 4665S) and β-actin (cat. no. 4967) antibodies were all obtained
from Santa Cruz Biotechnology, Inc. (Santa Cruz, CA, USA). IAP3
(2F1) antibody (cat. no. ab5746) was purchased from Abcam
(Cambridge, MA, USA). An mRNA extraction kit and SYBR green I mix
for quantitative polymerase chain reaction (qPCR) were supplied by
Qiagen (Hilden, Germany) and Invitrogen (Thermo Fisher Scientific,
Inc.), respectively. Activated caspase-9 (cat. no. BS7070),
activated caspase-3 (cat. no. BS4301) and activated poly ADP-ribose
polymerase (PARP; cat. no. BS7047) antibodies, as well as
IRDye-conjugated secondary antibodies (cat. no. BA29880) were all
purchased from Bioworld Technology, Inc. (St. Louis Park, MN, USA).
The dilution ratio of all primary and secondary antibodies were
1:500 and 1:5,000, respectively. The annexin V-fluorescein
isothiocyanate (FITC)/propidium iodide (PI) apoptosis detection kit
was purchased from Multi-Sciences Biotechnology (Hangzhou,
China).
Cell culture and treatment
Both Hep3B and L0-2 cells were obtained from the
Cell Bank of Type Culture Collection of the Chinese Academy of
Science (Shanghai, China). The two cell lines were grown in DMEM
containing 10% (v/v) FBS and were cultured at 37°C in a humidified
5% CO2 atmosphere.
Western blotting
Hep3B and L0-2 cells were collected and lyzed in a
traditional radioimmunoprecipitation buffer (1 M Tris-HCl, 5 M
NaCl, 1% Nonidet P-40, 1% sodium deoxycholate, 0.05% SDS and 1 mM
phenylmethylsulfonyl fluoride) for 15 min. The proteins were
denatured at 96°C for 5 min following mixing with 5 µl SDS-loading
buffer. The proteins were subsequently separated on 12% SDS-PAGE
gels and were transferred onto polyvinylidene difluoride membranes
(EMD Millipore, Billerica, MA, USA). The immunoblotting assay was
performed as described previously (25). The protein band densities were
measured using Quantity One software (Bio-Rad, Hercules, CA, USA).
The data was expressed as the density normalized against that of
β-actin.
Reverse transcription (RT)-qPCR
The total RNA was extracted from cells using the
RNeasy Mini kit (cat. no. 75144; Qiagen), according to the
manufacturer's protocol. cDNA synthesis was performed using 1 µg
total RNA sample mixed with dNTP, reverse transcriptase, random
monomers, oligo-dT as primers, 10X reaction buffer and DNAse I. The
samples were run in a PCR system (T100; Bio-Rad) at the following
temperatures: 42°C for 55 min, 70°C for 10 min and 4°C until
collected. The samples were stored at 4°C. The amplification was
performed using a 7900 Real-Time PCR system (Applied Biosystems;
Thermo Fisher Scientific, Inc.), according to the manufacturer's
protocol for the Quanti-Fast SYBR Green RT-PCR kit (Qiagen). All
specific primers for amplification of specific genes, as well as
the housekeeper gene β-actin, were designed and synthesized by
Qiagen. All samples were run in triplicate.
Pulse-chase and cycloheximide (CHX)
assays
The degradation rate of mRNA was assessed using a
standard actinomycin D analysis. Following the addition of 5 µg/ml
actinomycin D, Hep3B cells were treated with or without matrine.
Subsequently, at different time points, the cells were harvested
for isolation of the total RNA. The mRNA expression of MDM2 was
detected by RT-qPCR.
Protein translation level was evaluated using a
standard protein-synthesis inhibitor CHX assay. Briefly, the cells
were pretreated with 100 µg/ml CHX for 15 min at 4°C to arrest
polyribosome migration. The cells were subsequently treated with or
without matrine, and the cells were harvested and lyzed in a buffer
containing 20 mM Tris-HCl (pH 8.0), 100 mM NaCl, 5 mM MgCl2, 0.5%
Triton X-100 and 500 U/ml RNAsin, as well as a cocktail of protease
inhibitors. Fractionation was performed on a 15–45% (w/v) sucrose
gradient, centrifuged at 24,000 × g for 1 h at 4°C. The fractions
were collected from each gradient tube by upward replacement and
absorption monitored at an optical density of 254 nm, using a
fractionator (Brandel, Gaithersburg, MD, USA). Lastly,
immunoblotting was performed to observe concurrent turnover of
MDM2.
MDM2 plasmid transfection
For transfection, Hep3B cells were seeded into
6-well plates at density of 2×105 cells/well. Following
culturing to 70–80% confluence, the cells were harvested to make a
cell suspension using cell medium. Both Lipofectamine 2000
transfection reagent (Invitrogen; Thermo Fisher Scientific, Inc.)
and MDM2 overexpression plasmid were diluted with the Opti-MEM I
medium (Thermo Fisher Scientific, Inc.) at a final concentration of
5% and 50 nM, respectively. The two mixtures were mixed and
incubated at room temperature for an additional 20 min. Following
incubation, the above transfection mixture was added to new 6-well
plates. The Hep3B cell suspensions (final concentration for MDM2
plasmid, 5 nM) were overlaid onto the transfection mixture.
Following incubation at 37°C for 4 h, the media was removed and the
cells were cultured with fresh cell medium.
3-(4,5-dimethyl-2-thiazolyl)-2,5-diphenyl-2-H-tetrazolium bromide
(MTT) assay
Hep3B and MDM2-overexpressing Hep3B cells were
seeded at a density of 3×103 cells/well in 96-well
plates for 24 h. Different concentrations of matrine were added to
the cells for times indicated. Following treatment, the supernatant
in each well was replaced with 90 µl DMEM (without FBS) and 10 µl
MTT solution (final concentration, 5 mg/ml), and incubated at 37°C
for an additional 4 h. The liquid in each well was carefully
aspirated and 150 µl dimethylsulfoxide was added to dissolve the
formazan product. The optical density was quantified using
Multi-Detection Microplate Readers (Synergy 2; Bio-Tek Instruments,
Inc., Winooski, VT, USA) at a wavelength of 570 nm. The control and
treated group data are expressed as mean ± standard deviation of
three independent experiments.
Flow cytometry
The matrine-induced cell cycle arrest and cell death
were assessed by flow cytometry. For the cell-cycle sample
preparation, the harvested cells were washed twice with
phosphate-buffered saline (PBS) and fixed overnight with 70%
ethanol at 4°C. After washing three times with PBS, the cells were
resuspended with 0.5 ml PBS containing 20 µg/ml PI and 1 µg/ml
RNase A. Following incubation at 4°C for an additional 30 min, the
cells were analyzed using a FACScan (Becton-Dickinson, San Jose,
CA, USA) with WinList software (Verity Software House, Topsham, ME,
USA). For analysis of cell death type, the matrine-treated cells
were harvest and washed twice with PBS. The cells were subsequently
suspended in 400 µl binding buffer containing 10 µl PI and 5 µl
Annexin V-FITC, and were incubated for 20 min at room temperature
in the dark, followed by flow cytometric analysis.
Statistic analysis
The differences between the groups were examined for
statistical significance using the one-way analysis of variance
followed by Dunnett's post-hoc test using SPSS version 19.0
software (SPSS, Inc., Chicago, IL, USA). P<0.05 was considered
to indicate a statistically significant difference.
Results
MDM2 expression in matrine-treated
Hep3B and L0-2 cells
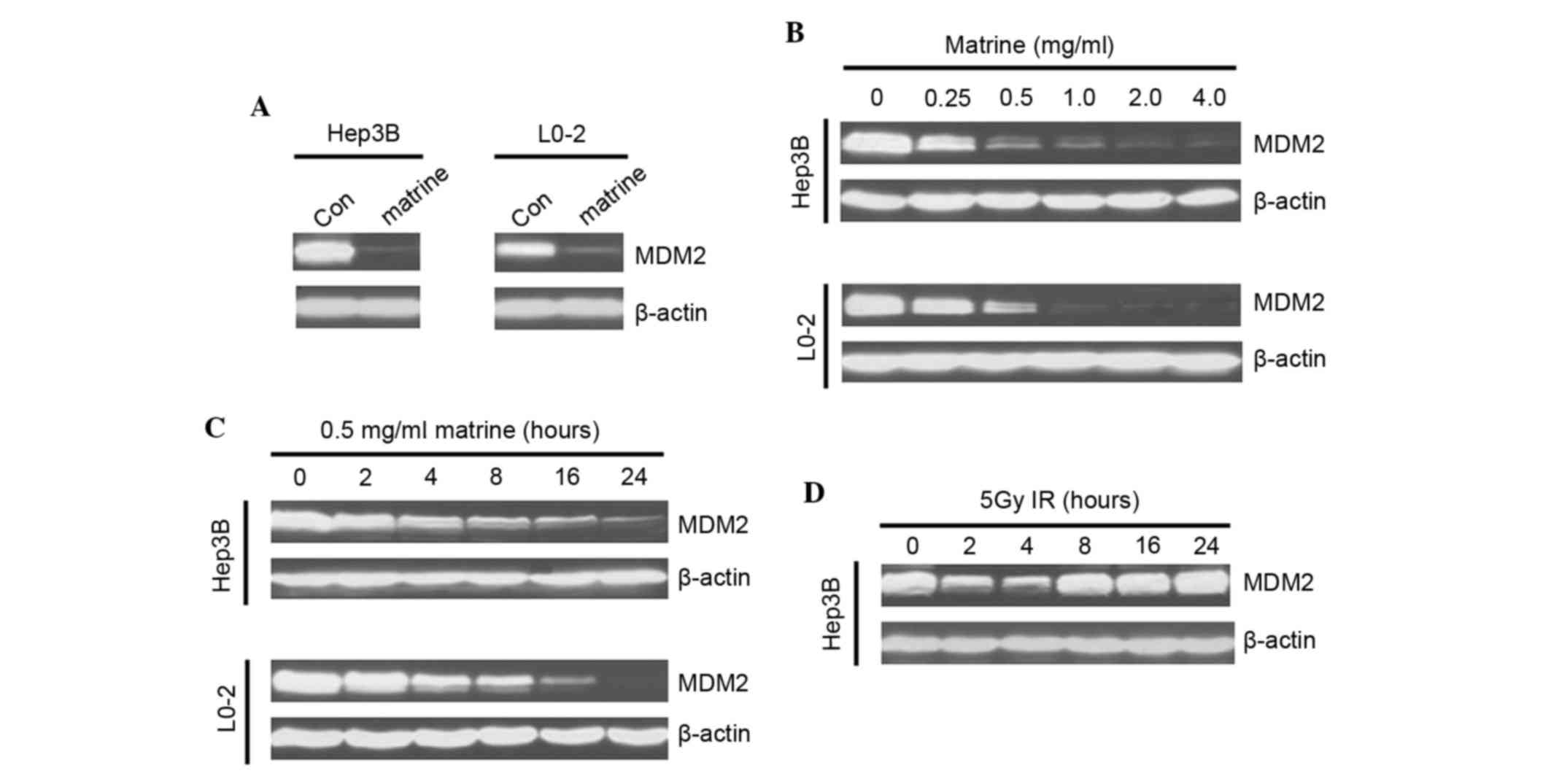
To assess whether MDM2 expression levels were
influenced by matrine, western blotting analysis was performed. It
was revealed that matrine notably downregulated MDM2 in the two
studied cell lines (Fig. 1A).
Matrine suppressed the protein expression of MDM2 in a
dose-dependent manner, even at low concentrations (Fig. 1B). The matrine-induced MDM2
inhibition occurred at ~4 h post-treatment and was followed by a
steady-state downregulation (Fig.
1C), with a style distinct from irradiation-treament that
instead induced a rapid and transient (1–2 h) decrease of MDM2
expression, followed by a clear upregulation (Fig. 1D).
Matrine-induced decrease of the mRNA
synthesis of MDM2
The present study next investigated the mechanism by
which matrine suppress the expression of MDM2. As shown in Fig. 2A, the mRNA expression of MDM2 was
clearly inhibited by matrine. To investigate whether the
matrine-induced MDM2 mRNA decrease is associated with mRNA
stability, pulse-chase and RT-qPCR reactions were performed. As
shown in Fig. 2B, matrine caused
no affect on the mRNA stability of MDM2. MDM2 protein stabilization
was further assessed using a standard CHX pulse-chase assay. As
shown in Fig. 2C, compared with
the control groups, the half-life of MDM2 was not affected by
matrine, suggesting that matrine decreases MDM2 via the inhibition
of mRNA synthesis. Enhanced expression of p73 by matrine is a
result of matrine-mediated inhibition of MDM2, since MDM2 can
compete with p73 for binding to its upstream effectors (17).
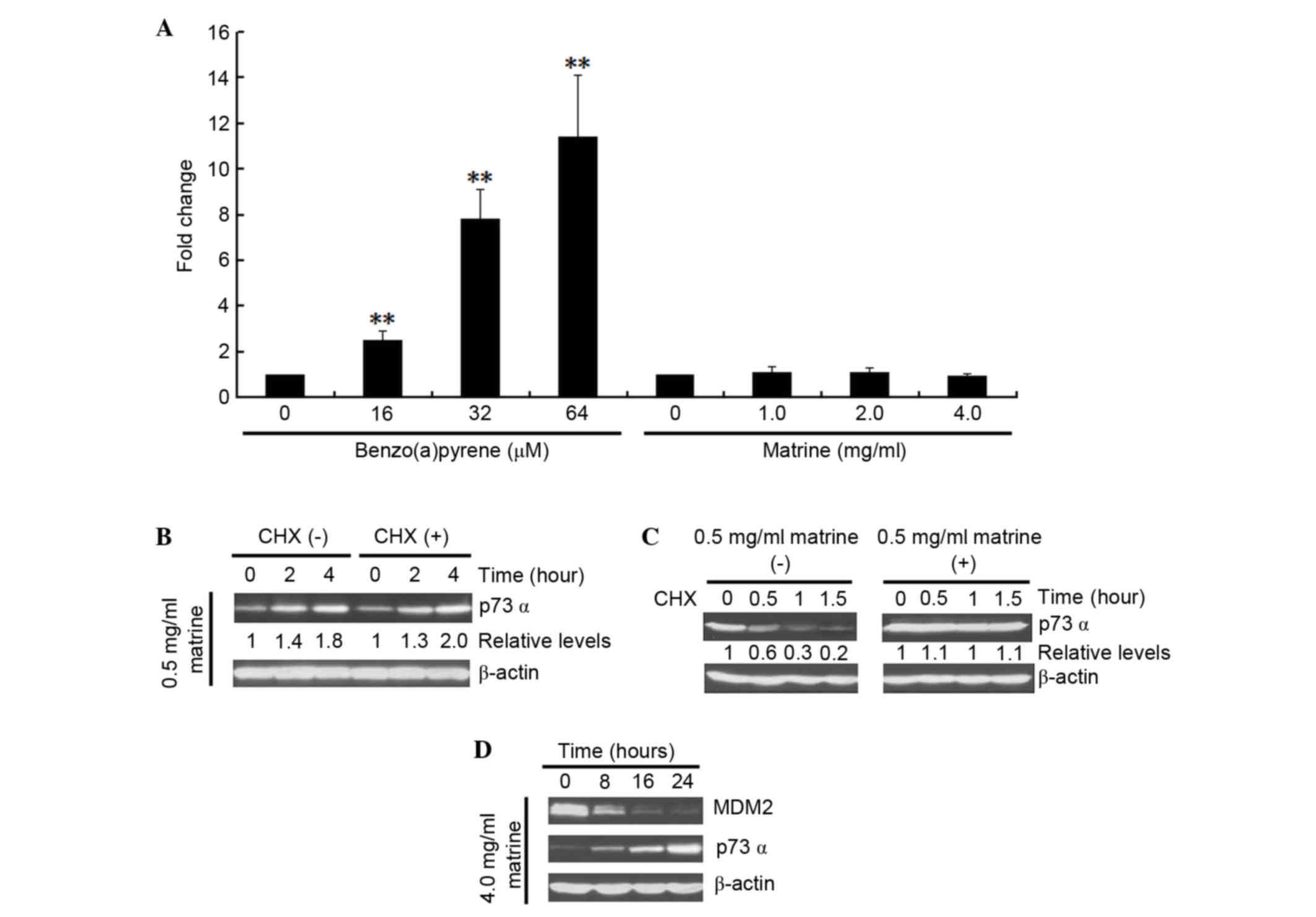
The present study investigated whether the
matrine-induced MDM2 decrease can affect the expression of p73α.
Firstly, a possible direct influence of matrine on p73 mRNA level
was investigated by comparing it with the known effect of
benzo(a)pyrene on the mRNA expression of p73 (26). As shown in Fig. 3A, benzo(a)pyrene increased p73
transcription in the cells. By contrast, matrine failed to either
increase or decrease the mRNA expression of p73. Whether matrine
can affect p73 translation was next determined. As shown in
Fig. 3B, no difference in the
expression of p73 was detected in the presence or absence of the
protein synthesis, as assessed by CHX treatment. Lastly, the
turnover of p73 protein was measured in matrine-treated cells, as
shown in Fig. 3C. Compared with
the control groups, matrine notably increased the half-life of p73.
These results suggested that the observed matrine-upregulated
expression of p73 in Hep3B cells occurs only at the
post-translational level. Additionally, the expression of p73 after
matrine treatment in Hep3B cells was assessed, as shown in Fig. 3D. Matrine increased the expression
of p73 following the downregulation of MDM2.
p73 cell-cycle arrest function is not
activated in matrine-treated Hep3B cells
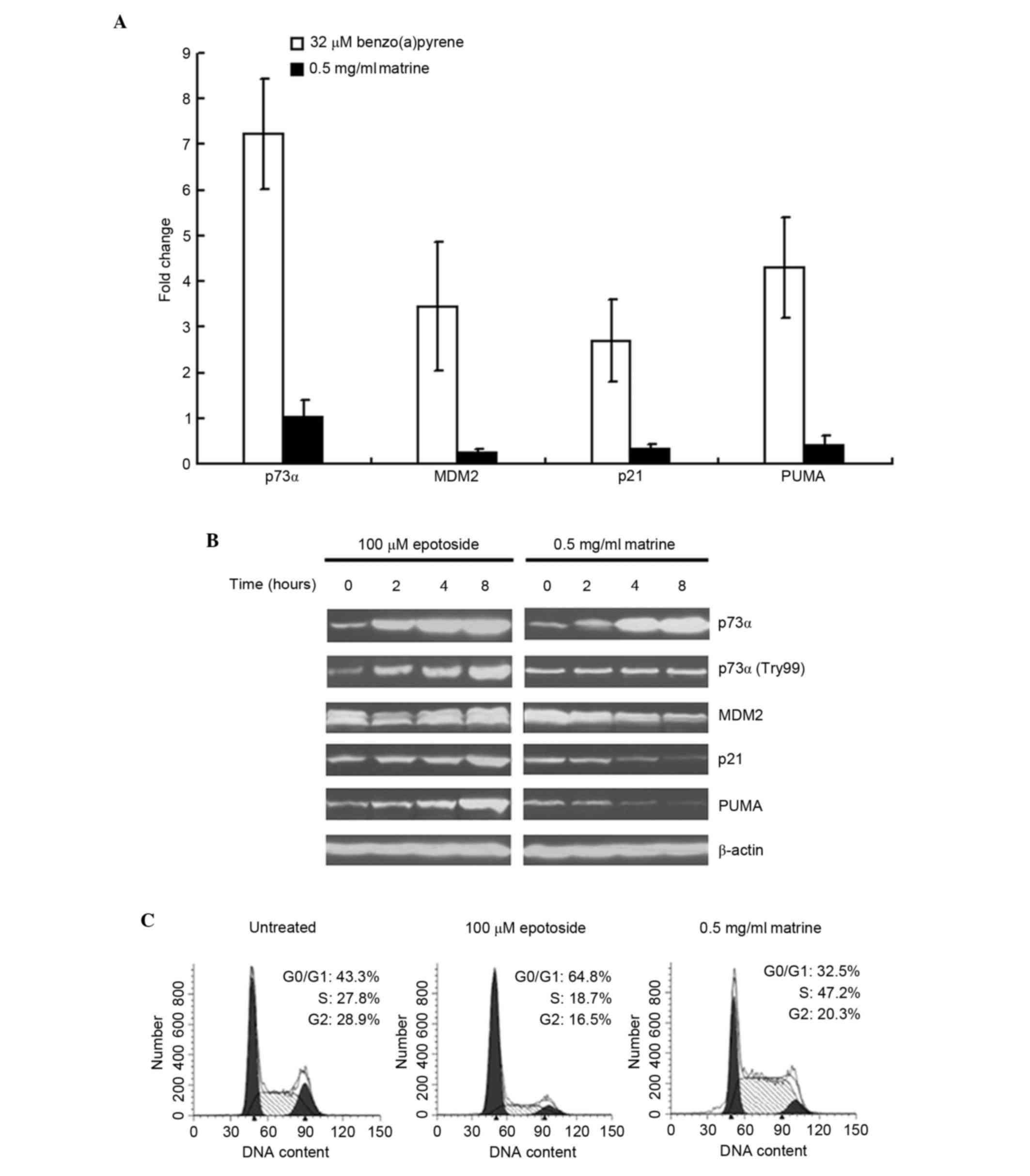
p21 and PUMA are two downstream targets of p73.
Therefore, the present study examined the expression levels of p21
and PUMA in matrine-treated Hep3B cells. As shown in Fig. 4A, benzo(a)pyrene clearly increased
the expression levels of p21 and PUMA, as well as the mRNA
expression of MDM2. By contrast, matrine failed to increase or
decrease the expression levels of the above genes. Additionally, as
shown in Fig. 4B, the
matrine-induced p73 protein increase was not to the same level as
that of epotoside-induced. The data in Fig. 4B also demonstrated that epotoside
increased the protein expression levels of p21 and PUMA, whereas
matrine decreased the expression levels. In addition, matrine
failed to induce accumulation of the p73 activated form
(p73α-Try99), compared with epotoside, which increased p73α-Try99
levels. The cell-cycle analysis also confirmed that p21 was not
functional in the matrine-treated cells. As shown in Fig. 4C, matrine failed to induce G1
arrest. By contrast, epotoside clearly induced cell accumulation in
G1 phase, whereas matrine decreased cell numbers in G1, S and
G2/M.
Matrine inhibited the expression of
IAP3 in MDM2-overexpressing Hep3B cells
IAP3 is a translational target of MDM2, which
exhibits reduced expression level following MDM2 silencing
(21). The present study assessed
the expression levels of IAP3. As shown in Fig. 5A, matrine markedly decreased the
expression levels of MDM2 and IAP3, whereas both genes were
increased in the epotoside-treated cells. To investigate whether
MDM2 inhibition affected the expression of IAP3 in matrine-treated
Hep3B cells, an MDM2-overexpressing Hep3B cells cell line was
treated with matrine. As shown in Fig.
5B, a more marked decrease of IAP3 was induced in the
matrine-treated MDM2-overexpressing Hep3B cells compared with in
the matrine-treated negative plasmid-transfected Hep3B cells.
Additionally, the IAP3 protein stability was also evaluated in the
matrine-treated Hep3B cells. As shown in Fig. 5C, compared with the control group,
matrine caused no effect on IAP3 protein stability. These results
suggested that matrine can strongly suppress the expression of IAP3
in MDM2-overexpressing Hep3B cells at both the mRNA and protein
expression levels.
Matrine sensitized Hep3B cells to
epotoside-induced apoptosis
The present study assessed the effect of matrine on
Hep3B and MDM2-overexpressing Hep3B cell viabilities using an MTT
assay. As shown in Fig. 6A,
matrine exhibited cytotoxic activity in each of the cell lines.
Matrine exhibited a more marked cytotoxic effect on
MDM2-overexpressing Hep3B cells that express very high level of
MDM2. To investigate the mechanism by which matrine induces
MDM2-overexpressing Hep3B cell death, the activation of several
apoptotic effectors was assessed. As shown in Fig. 6B, cleavage of caspases-3 and −9,
and PARP in MDM2-overexpressing Hep3B cells was observed 4 h
post-treatment. Whether matrine has synergistic effects on
epotoside-induced apoptosis was determined by treating the Hep3B
cells with both. When administered alone, a 24-h treatment induced
27.4and 34.2% apoptosis for etoposide and matrine treatment,
respectively. When the same doses were administered to cells in
combination, as shown in Fig. 6C,
compared with the control and either alone treated groups, the
apoptosis ratio was significantly increased in the combination
group (>70%; P<0.01). These results suggested that matrine
sensitized Hep3B cells to epotoside-induced apoptosis.
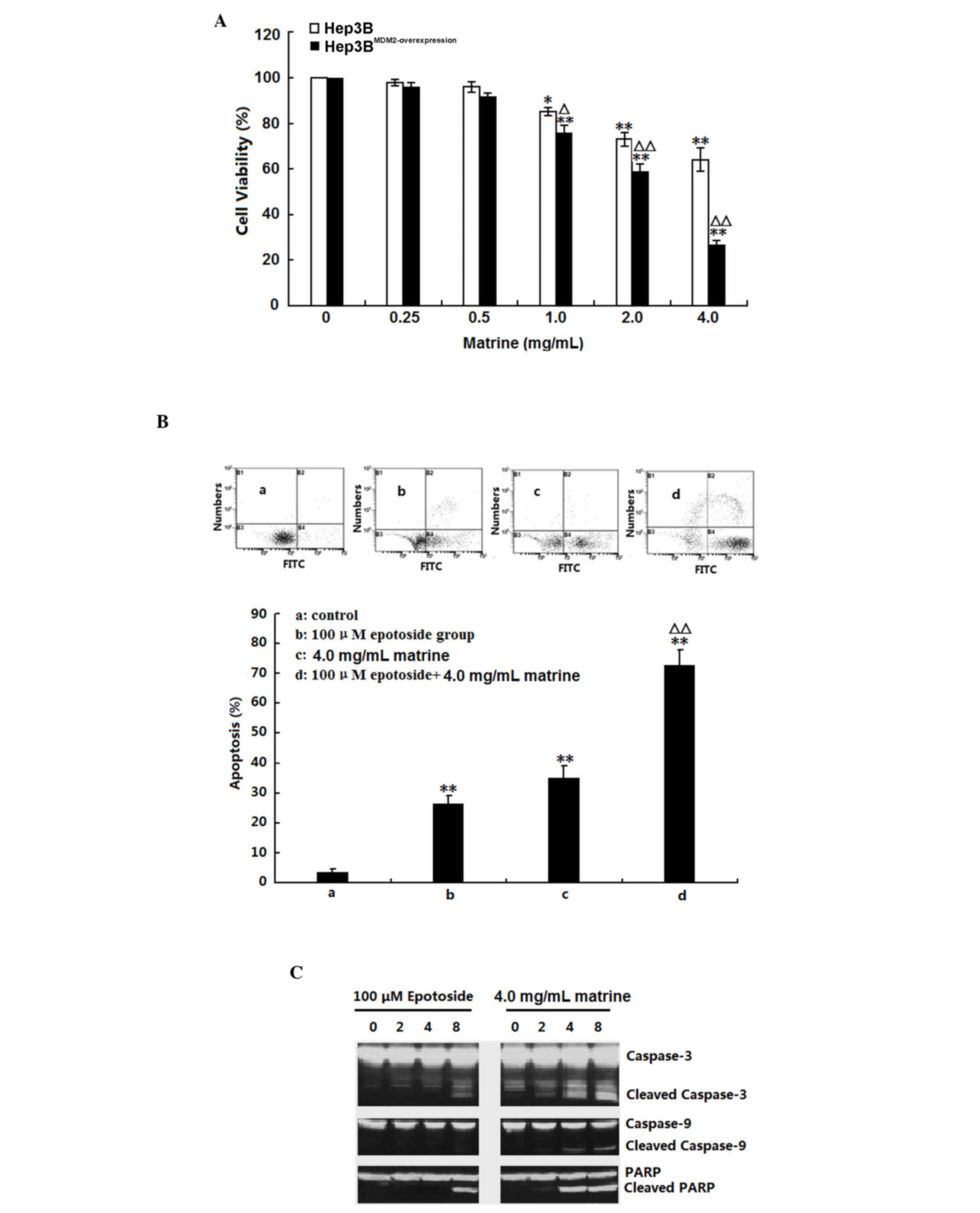 | Figure 6.Apoptotic effect of matrine on Hep3B
and MDM2-overexpressing Hep3B cells. (A) Hep3B and
MDM2-overexpressing Hep3B cells were treated with 0.25–4.0 mg/ml
matrine for 24 h. The cell viability was assessed using a
3-(4,5-dimethyl-2-thiazolyl)-2,5-diphenyl-2-H-tetrazolium bromide
assay. The data are presented as the mean ± standard deviation
(n=3; *P<0.05 and **P<0.01 compared with the corresponding
control group; ∆P<0.05 and ∆∆P<0.01 compared with
the untransfected Hep3B cells). (B) Hep3B cells were treated with
100 µM epotoside or 4.0 mg/ml matrine, alone or in combination, for
24 h. Cell apoptosis was analyzed by dual-parameter flow cytometry
utilizing annexin V-FITC and propidium iodide, and the results of
three independent experiments were pooled and average values are
presented. Group a, control; group b, 100 µM epotoside; group c,
4.0 mg/ml matrine; group d, 100 µM epotoside + 4.0 mg/ml matrine.
The data are presented as the mean ± standard deviation (n=3;
**P<0.01 compared with the control group; ∆∆P<0.01
compared with the epotoside group). (C) Hep3B cells were treated
with 100 µM epotoside or 4.0 mg/ml matrine. The protein expression
levels of cleaved and total Caspase-3, −9 and PARP were assessed by
western blotting. FITC, fluorescein isothiocyanate; PARP, poly ADP
ribose polymerase. |
Discussion
The p53 tumor suppressor gene serves a crucial roles
in maintaining the integrity of the genome and the defense against
tumor metastasis and is mutated or deleted in ~50% of human cancer
types (12). However, numerous
tumors retain wild-type p53, suggesting that the above tumor cells
contain abnormalities of other genes, including overexpression of
the p53 negative regulator, MDM2. MDM2 overexpression has been
reported in ~1/3 human cancer cells that contain wild-type p53 gene
(27). Therefore, MDM2 can be
considered as a proto-oncogene. The present study used a
p53-deficient cell model, Hep3B cells, to investigate the
anticancer activity of a known Chinese herbal medicine, matrine. It
was found that matrine notably decreased the mRNA expression of
MDM2, which resulted in an increase of p73. However, the primary
function of p73 was not activated, which was reflected by the
failure to induce p21 and PUMA. Notably, the matrine-induced
downregulation also resulted in decreased expression of the
apoptotic inhibitor, IAP3.
IAPs form a family of caspase inhibitors that
inhibit the downstream portion of the apoptosis pathway and inhibit
cell death in response to multiple stimuli (28). There are currently eight known
human IAP family members, with IAP3 being the best characterized
member (29). The antiapoptotic
roles of IAP3 have been attributed to its ability to directly bind
to and inhibit the activated forms of caspase-3, −7 and −9, which
are important executors of the intrinsic apoptosis pathway
(30). Additionally,
overexpression of IAP3 has been observed in a variety of human
cancer types (31,32). In the present study, it was
revealed that IAP3 was notably inhibited by matrine at both the
transcriptional and translational levels. Since the mRNA expression
of IAP3 can be regulated by MDM2 (33), the present study further examined
whether matrine-induced IAP3 inhibition was associated with a
decrease in MDM2 using an MDM2-overexpression Hep3B cell model. It
was revealed that more inhibition of IAP3 was observed in the
MDM2-overexpressing Hep3B cells compared with in the wild-type
Hep3B cells. Consistent with previous studies which reported that
knockdown of endogenous MDM2 with siRNA resulted in decreased IAP3
activity (22). The present study
also demonstrated that downregulation of MDM2 in the
MDM2-overexpressing Hep3B cells resulted in potent cell
apoptosis.
The Hep3B cell apoptosis was predominantly
attributed to matrine-induced IAP3 inhibition, which was further
confirmed by the finding of no induction of p73 function in the
cells. In the present study, although the matrine-induced MDM2
inhibition resulted in an increased expression of p73, the
expression of p21, a p27 downstream target, was inhibited rather
than activated in the matrine-treated Hep3B cells. Another
important p73 transcriptional target for execution of
mitochondria-dependent apoptosis, PUMA, was also not increased and
rather decreased in the matrine-treated Hep3B cells. Although the
present study did not investigate the reason why biological
functions of p21 and PUMA were not activated by the
matrine-increased p73, it was hypothesized that, similar to MDM2,
the mRNA synthesis of p21 and PUMA may be inhibited by matrine,
even though the increased p73 can bind to its promoters. However,
the molecular mechanism underlying the above remains to be
elucidated. The most important observations of the present study
were that matrine notably increased the sensitivity of the
p53-deficient Hep3B cells to epotoside-induced apoptosis. It is
well known that epotoside and numerous other anticancer alkylating
drugs kill cancer cells through induction of DNA damage, the latter
in turn increases immediate accumulation and activation of p53
family members through ATM-Chk2 and ATR-Chk1 pathways, which are
activated by DNA double-strand breaks and single-stranded DNA,
respectively (11,34). The activated p73, similar to p53,
can bind to the promoters of p21 and PUMA, which in turn execute
cell cycle arrest and mintochondria-dependent cell apoptosis
(35). The activated p73 also
triggers mRNA expression of its negative effector, MDM2. This
p73-induced increase of MDM2 expression begins to compete with p73
for binding to the p300/CBP N-terminus and suppresses its
biological functions (17). In
addition to interacting with and inactivating p53, there is
evidence to suggest that MDM2 can also interact with other
molecules, including specific protein and RNA, which may serve a
p53-independent role in oncogenesis (e.g. induction of IAP3)
(33). Aside from regulating its
downstream effectors, MDM2 itself can be modulated by various
upstream signals. For example, cell growth factor-induced
activation of phosphatidylinositol 3-kinase-Akt can phosphorylate
MDM2-serine 166 and 186 in the cytoplasm, which in turn triggers
translocation of MDM2 from the cytoplasm into the nucleus (36). For anther case, cellular stress and
DNA damage can induce dephosphorylation of the central acidic
domain of MDM2, which may result in inhibition of the nuclear
translocation for MDM2, or accumulation in the cytoplasm (37). The present results suggested a
functional role for MDM2 in tumor development, which extends thef
current understanding of resistance to chemical-therapy in
cancer.
In conclusion, the present study indicated that
matrine causes cytotoxicity and induces apoptosis in p53-deficient
Hep3B cells. The major underlying mechanism is the inhibition of
the MDM2-IAP3 pathway. Therefore, it is hypothesized that matrine
may be an interesting candidate drug in the development of
therapies against p53-defective cancer cells.
Glossary
Abbreviations
Abbreviations:
|
MDM2
|
mouse double-minute 2
|
|
DMSO
|
dimethylsulfoxide
|
|
CHX
|
cycloheximide
|
|
MPTP
|
mitochondrial permeability transition
pore
|
|
PUMA
|
p53 upregulated modulator of
apoptosis
|
|
IAPs
|
inhibitors of apoptosis protien
|
|
DDR
|
DNA damage response
|
|
DMEM
|
Dulbecco's modified Eagle's medium
|
|
MTT
|
3-(4,5-dimethyl-2-thiazolyl)-2,5-diphenyl-2-H-tetrazolium
bromide
|
|
PI3K
|
phosphatidylinositol 3-kinase
|
References
|
1
|
Hu ZL, Zhang JP, Qian DH, Lin W, Xie WF,
Zhang XR and Chen WZ: Effects of matrine on mouse splenocyte
proliferation and release of interleukin-1 and −6 from peritoneal
macrophages in vitro. Zhongguo Yao Li Xue Bao. 17:259–261.
1996.PubMed/NCBI
|
|
2
|
Cheng H, Xia B, Zhang L, Zhou F, Zhang YX,
Ye M, Hu ZG, Li J, Li J, Wang ZL, et al: Matrine improves
2,4,6-trinitrobenzene sulfonic acid-induced colitis in mice.
Pharmacol Res. 53:202–208. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Liu J, Zhu M, Shi R and Yang M: Radix
Sophorae flavescentis for chronic hepatitis B: A systematic review
of randomized trials. Am J Chin Med. 31:337–354. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Tan C, Qian X, Jia R, Wu M and Liang Z:
Matrine induction of reactive oxygen species activates p38 leading
to caspase-dependent cell apoptosis in non-small cell lung cancer
cells. Oncol Rep. 30:2529–2535. 2013.PubMed/NCBI
|
|
5
|
Liu XS, Jiang J, Jiao XY, Wu YE and Lin
JH: Matrine-induced apoptosis in leukemia U937 cells: Involvement
of caspases activation and MAPK-independent pathways. Planta Med.
72:501–506. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Jiang H, Hou C, Zhang S, Xie H, Zhou W,
Jin Q, Cheng X, Qian R and Zhang X: Matrine upregulates the cell
cycle protein E2F-1 and triggers apoptosis via the mitochondrial
pathway in K562 cells. Eur J Pharmacol. 559:98–108. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Hu MJ, Zeng H, Wu YL, Zhang YP, Zhang S,
Qiao MM and Fu H: Synergistic effects of matrine and 5-fluorouracil
on tumor growth of the implanted gastric cancer in nude mice. Chin
J Dig Dis. 6:68–71. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Zhang JQ, Li YM, Liu T, He WT, Chen YT,
Chen XH, Li X, Zhou WC, Yi JF and Ren ZJ: Antitumor effect of
matrine in human hepatoma G2 cells by inducing apoptosis and
autophagy. World J Gastroenterol. 16:4281–4290. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Tanigawa S, Fujii M and Hou DX:
Stabilization of p53 is involved in quercetin-induced cell cycle
arrest and apoptosis in HepG2 cells. Biosci Biotechnol Biochem.
72:797–804. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Schavolt KL and Pietenpol JA: p53 and
Delta Np63 alpha differentially bind and regulate target genes
involved in cell cycle arrest, DNA repair and apoptosis. Oncogene.
26:6125–6132. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Reinhardt HC, Aslanian AS, Lees JA and
Yaffe MB: p53-deficient cells rely on ATM- and ATR-mediated
checkpoint signaling through the p38MAPK/MK2 pathway for survival
after DNA damage. Cancer Cell. 11:175–189. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Michael D and Oren M: The p53 and Mdm2
families in cancer. Curr Opin Genet Dev. 12:53–59. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Chène P: Inhibiting the p53-MDM2
interaction: An important target for cancer therapy. Nat Rev
Cancer. 3:102–109. 2003. View
Article : Google Scholar : PubMed/NCBI
|
|
14
|
Shiraishi T and Nielsen PE:
Down-regulation of MDM2 and activation of p53 in human cancer cells
by antisense 9-aminoacridine-PNA (peptide nucleic acid) conjugates.
Nucleic Acids Res. 32:4893–4902. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Ongkeko WM, Wang XQ, Siu WY, Lau AW,
Yamashita K, Harris AL, Cox LS and Poon RY: MDM2 and MDMX bind and
stabilize the p53-related protein p73. Curr Biol. 9:829–832. 1999.
View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Dobbelstein M, Wienzek S, König C and Roth
J: Inactivation of the p53-homologue p73 by the mdm2-oncoprotein.
Oncogene. 18:2101–2106. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Zeng X, Chen L, Jost CA, Maya R, Keller D,
Wang X, Kaelin WG Jr, Oren M, Chen J and Lu H: MDM2 suppresses p73
function without promoting p73 degradation. Mol Cell Biol.
19:3257–3266. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Kowalik TF, DeGregori J, Leone G, Jakoi L
and Nevins JR: E2F1-specific induction of apoptosis and p53
accumulation, which is blocked by Mdm2. Cell Growth Differ.
9:113–118. 1998.PubMed/NCBI
|
|
19
|
Froment P, Dupont J and Christophe-Marine
J: Mdm2 exerts pro-apoptotic activities by antagonizing
insulin-like growth factor-I-mediated survival. Cell Cycle.
7:3098–3103. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Pereboom TC, van Weele LJ, Bondt A and
MacInnes AW: A zebrafish model of dyskeratosis congenita reveals
hematopoietic stem cell formation failure resulting from ribosomal
protein-mediated p53 stabilization. Blood. 118:5458–5465. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Huang X, Wu Z, Mei Y and Wu M: XIAP
inhibits autophagy via XIAP-Mdm2-p53 signalling. Embo J.
32:2204–2216. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Zheng M, Yang J, Xu X, Sebolt JT, Wang S
and Sun Y: Efficacy of MDM2 inhibitor MI-219 against lung cancer
cells alone or in combination with MDM2 knockdown, a XIAP inhibitor
or etoposide. Anticancer Res. 30:3321–3331. 2010.PubMed/NCBI
|
|
23
|
Deb SP, Singh S and Deb S: MDM2
overexpression, activation of signaling networks, and cell
proliferation. Subcell Biochem. 85:215–234. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Jones SN, Hancock AR, Vogel H, Donehower
LA and Bradley A: Overexpression of Mdm2 in mice reveals a
p53-independent role for Mdm2 in tumorigenesis. Proc Natl Acad Sci
USA. 95:15608–15612. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Jiang Y, Zhang XY, Sun L, Zhang GL,
Duerksen-Hughes P, Zhu XQ and Yang J: Methyl methanesulfonate
induces apoptosis in p53-deficient H1299 and Hep3B cells through a
caspase 2- and mitochondria-associated pathway. Environ Toxicol
Pharmacol. 34:694–704. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Jiang Y, Rao K, Yang G, Chen X, Wang Q,
Liu A, Zheng H and Yuan J: Benzo(a)pyrene induces p73 mRNA
expression and necrosis in human lung adenocarcinoma H1299 cells.
Environ Toxicol. 27:202–210. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Yu Q, Li Y, Mu K, Li Z, Meng Q, Wu X, Wang
Y and Li L: Amplification of Mdmx and overexpression of MDM2
contribute to mammary carcinogenesis by substituting for p53
mutations. Diagn Pathol. 9:712014. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Chow KU, Nowak D, Boehrer S, Ruthardt M,
Knau A, Hoelzer D, Mitrou PS and Weidmann E: Synergistic effects of
chemotherapeutic drugs in lymphoma cells are associated with
down-regulation of inhibitor of apoptosis proteins (IAPs),
prostate-apoptosis-response-gene 4 (Par-4), death-associated
protein (Daxx) and with enforced caspase activation. Biochem
Pharmacol. 66:711–724. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Lederman M, Meir T, Zeschnigk M, Pe'er J
and Chowers I: Inhibitor of apoptosis proteins gene expression and
its correlation with prognostic factors in primary and metastatic
uveal melanoma. Curr Eye Res. 33:876–884. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Eckelman BP, Salvesen GS and Scott FL:
Human inhibitor of apoptosis proteins: Why XIAP is the black sheep
of the family. EMBO Rep. 7:988–994. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Wang R, Li B, Wang X, Lin F, Gao P, Cheng
SY and Zhang HZ: Inhibiting XIAP expression by RNAi to inhibit
proliferation and enhance radiosensitivity in laryngeal cancer cell
line. Auris Nasus Larynx. 36:332–339. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Qiao L, Li GH, Dai Y, Wang J, Li Z, Zou B,
Gu Q, Ma J, Pang R, Lan HY and Wong BC: Gene expression profile in
colon cancer cells with respect to XIAP expression status. Int J
Colorectal Dis. 24:245–260. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Gu L, Zhu N, Zhang H, Durden DL, Feng Y
and Zhou M: Regulation of XIAP translation and induction by MDM2
following irradiation. Cancer Cell. 15:363–375. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Nur-E-Kamal A, Li TK, Zhang A, Qi H, Hars
ES and Liu LF: Single-stranded DNA induces ataxia telangiectasia
mutant (ATM)/p53-dependent DNA damage and apoptotic signals. J Biol
Chem. 278:12475–12481. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Sasaki Y, Morimoto I, Ishida S, Yamashita
T, Imai K and Tokino T: Adenovirus-mediated transfer of the p53
family genes, p73 and p51/p63 induces cell cycle arrest and
apoptosis in colorectal cancer cell lines: Potential application to
gene therapy of colorectal cancer. Gene Ther. 8:1401–1408. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Blattner C, Hay T, Meek DW and Lane DP:
Hypophosphorylation of Mdm2 augments p53 stability. Mol Cell Biol.
22:6170–6182. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Okamoto K, Li H, Jensen MR, Zhang T, Taya
Y, Thorgeirsson SS and Prives C: Cyclin G recruits PP2A to
dephosphorylate Mdm2. Mol Cell. 9:761–771. 2002. View Article : Google Scholar : PubMed/NCBI
|