Introduction
Cancer is a class of diseases, in which a group of
cells exhibit uncontrolled growth, invasion and sometimes
metastasis (1). Ovarian cancer
remains the most frequent type of cancer among women, which is a
heterogeneous cancer encompassing multiple subgroups with a
complicated molecular basis (2,3).
Improvements in current understanding of the underlying biology of
ovarian cancer may lead to novel therapeutic strategies. Several
models of ovarian carcinogenesis have been suggested. One of the
models involves the malfunction of tumor suppressors and the
occurrence of oncogenes, thereby leading to the hyperproliferation
of epithelial cells and/or inactivation of DNA repair genes.
Another model suggests the activation or deactivation of chromatin
proteins associated with DNA, with resultant effects on different
cellular processes (4,5). In reference to these models, the
field of DNA-targeted drug development has gained increased
attention due to their high sensitivity and specificity.
Furthermore, due to the complicated molecular basis
of ovarian cancer, certain clinical results have evaluated and
encouraged the utility of combination therapies, including the
combination of doxorubicin (Dox) and cisplatin (Cis), which are
beneficial in the treatment of cancer (6–8). An
optimized therapeutic strategy requires a drug delivery system,
which can guide the drug into the targeted cancer cells and improve
therapy. It is known that the overexpression of high-affinity
folate receptors (FRs) on human cancer cells provides advantages in
treating tumor cells without interfering with neighboring normal
tissue (9,10). For this reason, the FR is used as
an attractive target for cancer treatment, further enforcing the
selective active targeting in anticancer therapy (11,12).
In addition, a combination therapy is not complete without the
real-time monitoring of the drug activity. Therefore, developing a
sensitive and accurate platform to evaluate drug activities is
critical in scientific investigations and clinical
applications.
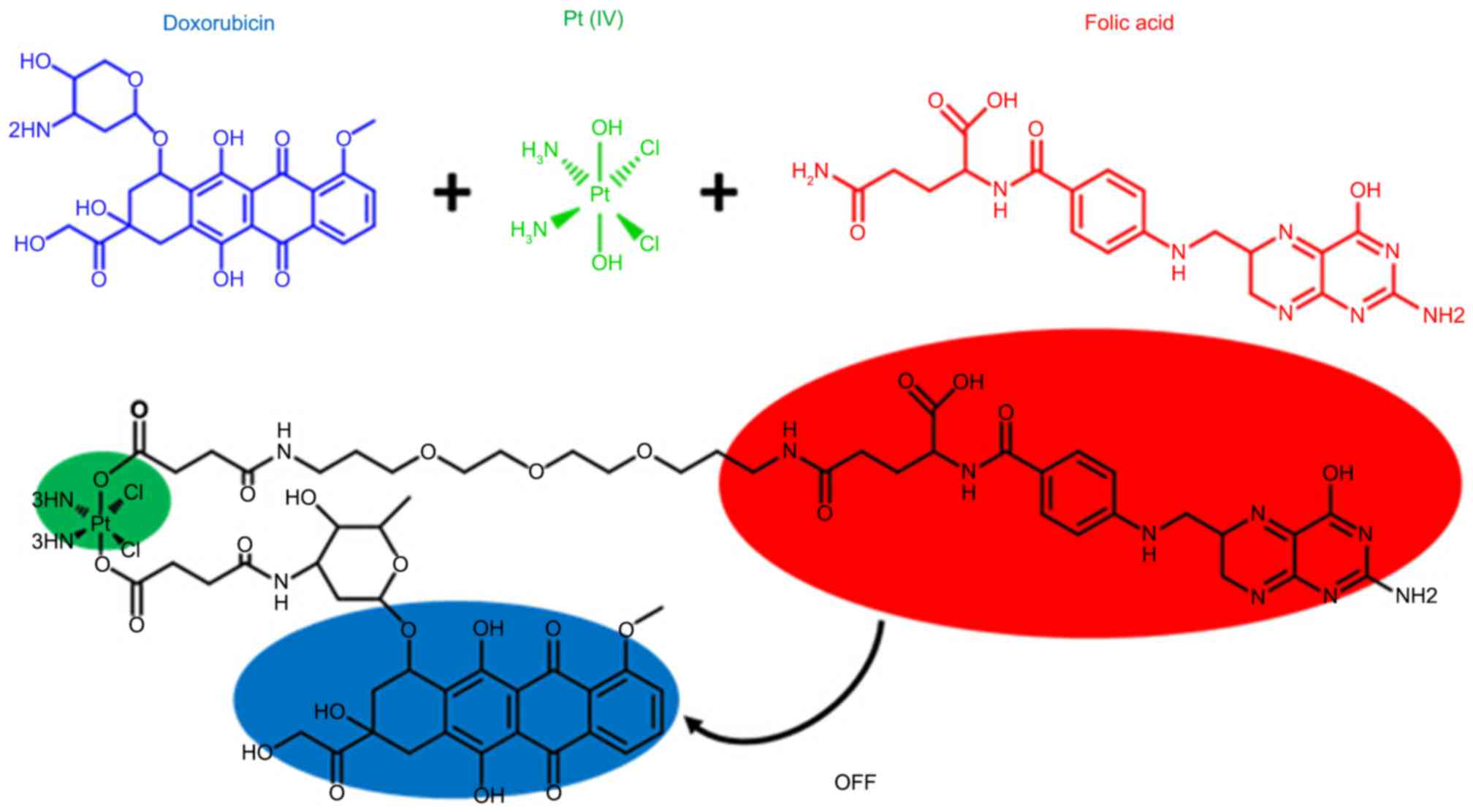
Inspired by the multifunctional chemotherapy, the
present study was performed to design a combination therapy
integrating a targeted delivery platform and an activatable
real-time sensor. In the design, folic acid (FA) acted as a
selective tumor-targeting ligand and a quencher for Dox
fluorescence (13–16). A reducible platinum (Pt) (IV)
prodrug and its palladium (Pd) analogue were introduced as an
activatable ligand with two axial positions linked to FA and Dox.
Once this platform enters the tumor cells through FA targeting, the
prodrug can be activated via reduction by several extracellular or
intracellular reducing agents, including glutathione (GSH),
cysteine and ascorbic acid, to achieve effective antitumor
treatment (17,18), with concomitant breakage of two
axial bonds and the release of free Dox. This system showed a
turn-on effect on fluorescence, thereby realizing the sensitive and
site-specific detection of drugs. This design was based on a
combination therapy, which can achieve optimal effectiveness due to
their synergic roles in the ovarian cancer environment.
The combining of fluorescence imaging, a targeted
delivery platform and combination chemotherapy provides advantages
to treat ovarian cancer cells with improved efficiency and
real-time imaging. Supported by the data of the present study, such
conjugation provides improved efficiency and bioactivity, which can
be used as a multifunctional system for the optimization of ovarian
cancer treatment.
Materials and methods
Chemistry
All chemicals and reagents used in the present study
were of analytical grade. All electrospray ionisation mass
spectrometry (ESI-MS) spectra were recorded on a Mariner System
5304 mass spectrometer [Mariner Systems (UK) Ltd., Wokingham, UK].
Column chromatography was performed using silica gel (200–300 mesh)
eluted with ethyl acetate and petroleum ether. Analytical
reverse-phase high performance liquid chromatography (HPLC)
analysis was performed on a Shimadzu HPLC system (Shimadzu
Corporation, Kyoto, Japan) using the Grace™ Alltech™ Alltima™ C-18
(250×10 mm) column (Thermo Fisher Scientific, Inc., Waltham, MA,
USA) at a flow rate of 3.0 ml/min for preparation, and a C-18
(250×4.6 mm) column at 1.0 ml/min for analysis. The
195Pt NMR spectra were recorded on a Bruker AVANCE-400
spectrometer (Bruker Systems, Inc., Billerica, MA, USA). Elemental
analyses were performed on a CHN-O-Rapid instrument (Heraeus,
Hanau, Germany).
General procedure for FA-prodrug-Dox
derivatives (1e-2e)
(1) Synthesis of M
(NH3) 2Cl2
(O2CCH2CH2CO2H)
[O2CCH2-CH2CONH-polyethylene
glycol (PEG)-FA], Cis-[MCl2(NH3)2]
(1 mmol; QianKun Chemistry Technology Co., Ltd., Shanghai, China)
was suspended in water (5 ml) and a 10-fold excess of
H2O2 was added. The mixture was stirred for 4
h at 50°C. Recrystallization of
Cis-[MCl2(OH)2(NH3)2]
was performed in situ and collected. The mixture was washed
with cold water, ethanol and ether, and dried in a desiccator.
Subsequently, succinic anhydride (4 mmol) was added to a suspension
of
Cis-[MCl2(OH)2(NH3)2]
in dimethylformamide (DMF; 5 ml; QianKun Chemistry Technology Co.,
Ltd.), and the reaction mixture was stirred at 70°C for 4 h. The
resulting solution was dried in vacuo, leaving a dark yellow oil,
which was dissolved in a small volume of acetone (5 ml). The
addition of ether precipitated a solid, which was collected and
dried in vacuo to leave the product as a powder. To a solution of
Cis-[MCl2(NH3)2(O2CCH2CH2CO2H)2
in DMF (10 ml), DMF solution (0.5 ml) containing HATU (1.5 mmol)
was added,. This mixture was stirred for 10 min at room
temperature. To the resulting solution, DMF solution containing PEG
linker (0.8 mmol) and N,N-diisopropylethylamine (DIPEA; 1.2 mmol;
QianKun Chemistry Technology Co., Ltd.) was added. The mixture was
stirred at room temperature for 24 h in the dark. The DMF was then
removed under a vacuum and the resulting compound was purified
using HPLC. FA (0.8 mmol; QianKun Chemistry Technology Co., Ltd.)
was dissolved in 5 ml DMF coupled with 0.5 mmol of
dicyclohexylcarbodiimide (DCC) and 1 mmol of N-hydroxysuccinimide
(NHS). The reaction was stirred for 14 h at room temperature in the
dark to obtain the folate-NHS ester. The resulting folate-NHS was
reacted with M
(NH3)2Cl2O2CCH2CH2CO2H)(O2CCH2-CH2CONH-PEG)
in DMF and purified by reprecipitation. (2) Synthesis of M
(NH3)2Cl2(O2CCH2CH2CONH-Dox)
(O2CCH2-CH2CONH-PEG-FA): The
synthesis of the final Dox-prodrug-FA conjugates was performed
using standard amide coupling reactions. Following the procedure
mentioned above, 1.0 equiv prodrug-PEG-FA was reacted with 1.5
equiv 1-ethyl-3-(3-dimethylaminopropyl) carbodiimide (EDC; QianKun
Chemistry Technology Co., Ltd.) coupled with DIPEA (20 µl) in 2 ml
DMF at a temperature of 70°C. The resultant mixture was evaporated
under pressure and washed with water to remove the remaining EDC,
following which 1.0 equiv Dox (QianKun Chemistry Technology Co.,
Ltd.) was added and the reaction was set at the temperature of 70°C
overnight. The product was purified by reverse-phase HPLC with an
eluting system consisting of water solution (A) and acetonitrile
solution (B) under a linear gradient. The linear gradient stretched
over 20 min from t=0 min at 20% solution B to t=20 min at 80%
solution B.
FA-Cis (NH3,
NH3, Cl, Cl) Pt (IV)-Dox (1)
Yellow powders, yield 65%; Rf=0.25 (PE/EtOAC=2:1);
ESI-MS calculated for
C64H82Cl2N12O25Pt(M+H)+:
1686.4738; found: 1686.4716. 195Pt NMR(DMSO-d6):
δ1298.21 ppm. Calculated for
C64H82Cl2N12O25Pt:
C, 45.55; H, 5.02; N, 9.96; O, 23.70. Found: C, 44.35; H, 5.40; N,
9.83; O, 23.02.
FA-Cis (NH3,
NH3, Cl, Cl) Pd (IV)-Dox (2)
Yellow powders, yield 62%; Rf=0.25 (PE/EtOAC=2:1);
ESI-MS calculated for
C64H82Cl2N12O25Pd,
(M+H)+: 1597.4182; found: 1597.4163. Calculated for
C64H82Cl2N12O25Pd:
C, 48.08; H, 5.30; N, 10.51; O, 25.02; gound: C, 47.86; H, 5.11; N,
10.13, O, 24.92.
Antiproliferation assay
Target tumor cell lines were grown to log phase in
RPMI 1640 medium supplemented with 10% fetal bovine serum (both
Gibco; Thermo Fisher Scientific, Inc.) and 1% antibiotics.
Following diluting to 1×105 cells ml−1 with
complete medium, 100 µl of the obtained cell suspension was added
to each well of 96-well culture plates. The cells were incubated
with FA-prodrug-Dox at 37°C, 5% CO2 for 24 h prior to
the cytotoxicity assessments. The samples at pre-set concentrations
(1 µg/µl) were added to six wells, and Dox and Cis were co-assayed
as positive references. After 48 h exposure at 37°C and 5%
CO2, 40 µl of PBS containing 2.5 mg ml−1 of
3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide was
added to each well. After 4 h, 100 µl per well extraction solution
(10% SDS-5% isobutyl alcohol-0.01 M HCl) was added. Following
incubation overnight at 37°C, the optical density was measured at
570 nm on an ELISA microplate reader. In all experiments, three
replicate wells were measured for each drug concentration. Each
assay was repeated at least three times. The results are summarized
in Table I.
 | Table I.Structure of compounds 1e and 2e, and
inhibition of A2780-associated cell line proliferation. |
Table I.
Structure of compounds 1e and 2e, and
inhibition of A2780-associated cell line proliferation.
|
|
|
| IC50
(µM) |
|---|
|
|
|
|
|
|---|
| Compound | M | X (X1,
X2) | A2780 | A2780/Dox | A2780/Cis |
|---|
| 1e | Pt | NH3 | 0.85±0.10 | 8.64±0.37 | 0.81±0.03 |
| 2e | Pd | NH3 | 0.96±0.08 | 8.98±0.68 | 1.07±0.11 |
| Cis |
|
| 15.60±1.21 | 18.10±1.33 | 40.80±0.83 |
| Dox |
|
| 1.47±0.10 | 20.32±1.13 | 1.39±0.05 |
| Cis-Dox |
|
| 12.40±1.16 | 16.70±1.08 | 41.00±1.35 |
Time course of fluorescence emission
spectra assay in vitro
In the experiments, incubation was performed in the
presence of the reducing agent, GSH. Each FA-prodrug-Dox (1 µM) in
PBS (pH 7.4) was incubated with GSH solution (5 mM), and their
fluorescence emissions (λex=497; λem=594 nm) were measured at
different time points. The change in fluorescence emission
(λex=497; λem=594 nm) was read using a Cary Eclipse fluorometer
(Varian Medical Systems, Inc., Palo Alto, CA, USA).
Fluorescence imaging using confocal
laser scanning microscopy
A2780 cells (Chuanbo Biotech Co., Ltd., Nanjing,
China) at a density of 5×104 cells per well were grown
in a 35-mm diameter plastic-bottom µ-dish (Ibidi GmbH, Martinsried,
Germany) and maintained at 37°C in 5% CO2 for 24 h prior
to treating with FA-Pt (IV)-Dox conjugate 1e (1 µM). At designated
time intervals (3, 6 and 12 h), the medium was removed, and the
cells were washed three times with RPMI 1640 medium and twice with
PBS. Images of the cells were captured using an LSM 510 confocal
laser scanning microscope (Carl Zeiss AG, Oberkochen, Germany) with
the appropriate instrument filter sets.
Flow cytometric analysis
The cells were seeded into 24-well plates at a
density of 15×104 cells per well in 0.5 ml RPMI 1640
medium and incubated in a humidified 5% CO2 atmosphere
for 24 h at 37°C. The cells were then treated with FA-Pt (IV)-Dox
1e (1 µM) and, at designated time intervals (3, 6 and 12 h), the
cells were washed three times with RPMI 1640 medium and twice with
PBS, following which the cells were harvested by trypsin treatment.
The harvested cells were suspended in PBS and centrifuged at 120 ×
g for 5 min at room temperature. The supernatants were
discarded and the cell pellets were washed with PBS to reduce the
background fluorescence from the medium. Following two cycles of
washing and centrifugation, the cells were resuspended with 200 µl
cell fixing solution. One-color flow cytometry was performed using
a FACS Calibur flow cytometer (BD Biosciences, Franklin Lakes, NJ,
USA). In order to assess the fluorescence enhancement of the
compounds upon incubation with A2780 cells, the results were
analyzed using FlowJo 7.6.1 software (TreeStar, Inc., Ashland, OR,
USA).
Statistical analysis
All biological experiments were repeated 3–5 times
with similar outcomes. Where applicable, data are reported as the
mean ± standard deviation.
Results and Discussion
Chemistry
Six FA-prodrug-Dox derivatives were synthesized. The
general reaction pathway for the synthesis is outlined in Fig. 1.
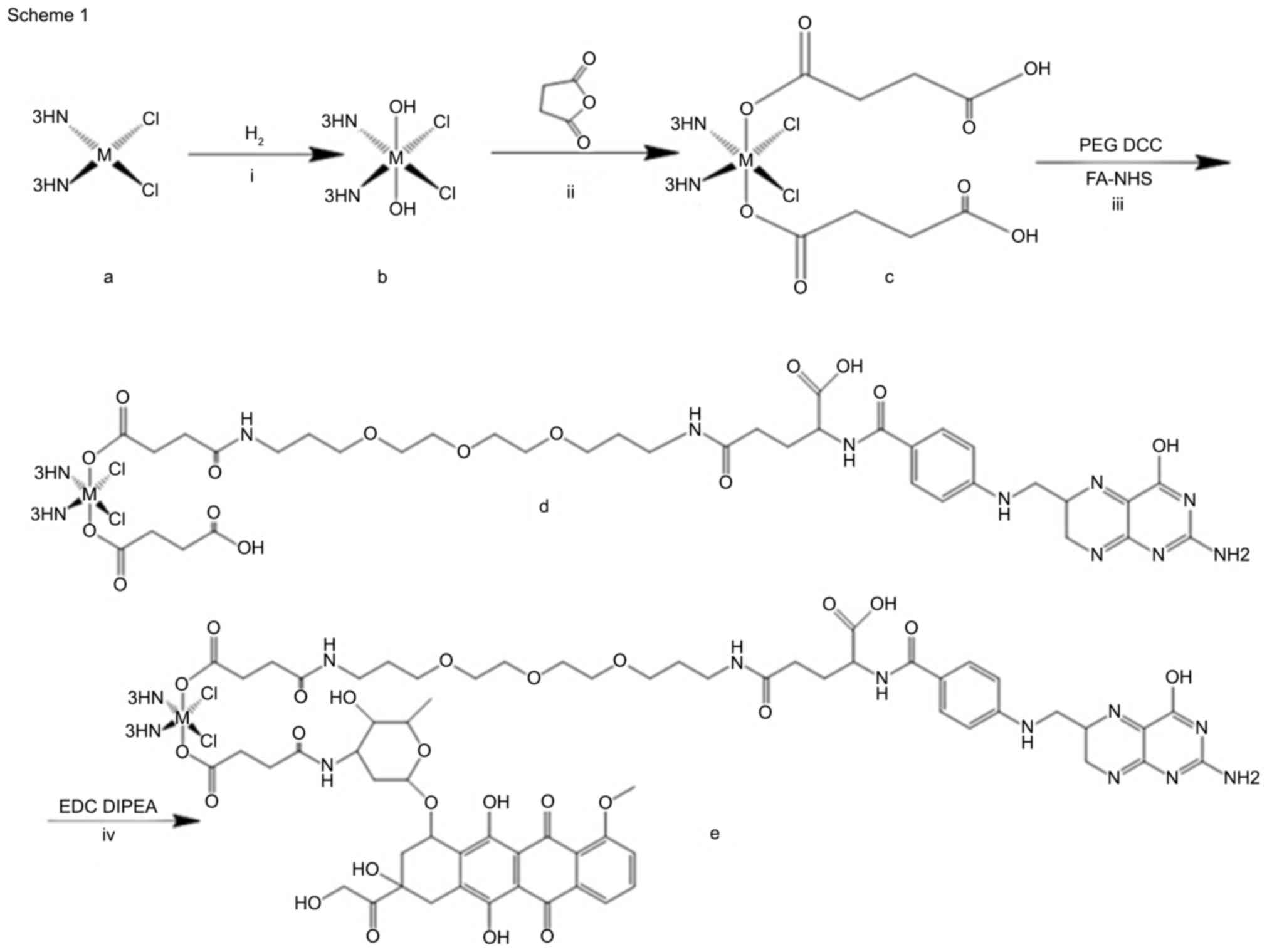 | Figure 1.General synthesis of compounds 1e,
and 2e. Reagents and conditions were as follows: (i)
H2O2, 50°C, 4 h; (ii) succinic anhydride,
70°C, 4 h; (iii) PEG, DCC, room temperature, Folate-NHS, 24 h. (iv)
EDC, DIPEA, 70°C, overnight. Dcc, dicyclohexylcarbodiimide; NHS,
N-hydroxysuccinimide; EDC, 1-ethyl-3-(3-dimethylaminopropyl)
carbodiimide; PEG, polyethylene glycol; DIPEA,
N,N-diisopropylethylamine. |
Synthesis of
M(NH3)2Cl2(O2CCH2CH2CO2H)
(O2CCH2-CH2CONH-PEG-FA)
Cis-[MCl2(NH3)2] (1
mmol) was suspended in water (5 ml), to which a 10-fold excess of
H2O2 was added. The mixture was stirred for 4
h at 50°C. Recrystallization of
Cis-[MCl2(OH)2(NH3)2]
was performed in situ, collected and washed with cold water,
ethanol and ether, and dried in a desiccator. Succinic anhydride (4
mmol) was added to a suspension of
Cis-[MCl2(OH)2(NH3)2]
in DMF (5 ml), and the reaction mixture was stirred at 70°C for 4
h. The resulting solution was precipitated by ether and collected.
To a solution of
Cis-[MCl2(NH3)2(O2CCH2CH2CO2H)2]
in DMF (10 ml), DMF solution (0.5 ml) containing HATU (1.5 mmol)
was added. This mixture was stirred for 10 min at room temperature.
To the resulting solution, DMF solution containing PEG linker (0.8
mmol) and DIPEA (20 µl) was added. The mixture was stirred at room
temperature for 24 h in the dark. The DMF was then removed under a
vacuum and the resulting compound was purified by column
chromatography. FA (0.8 mmol) was dissolved in 5 ml DMF coupled
with 0.5 mmol of DCC and 1 mmol of NHS. The mixture was stirred for
14 h at room temperature in the dark to produce folate-NHS ester.
The resulting folate-NHS was reacted with M
(NH3)2Cl2(O2CCH2CH2CO2H)
(O2CCH2-CH2CONH-PEG) in DMF and
purified by reprecipitation.
Synthesis of
M(NH3)2Cl2(O2CCH2CH2CONH-Dox)
(O2CCH2-CH2CONH-PEG-FA)
The synthesis of the final Dox-prodrug-FA conjugates
was performed using standard amide coupling reactions. Following
the procedure mentioned above, 1.0 equiv prodrug-PEG-FA (0.5 mmol)
was reacted with 1.5 equiv EDC coupled with DIPEA (20 µl) in 2 ml
DMF at the temperature of 70°C, the resulting mixture was
evaporated under pressure and washed with water to remove the
remaining EDC. Subsequently, 1.0 equiv Dox was added and incubated
at a temperature of 70°C overnight. The general formula of the
final purified compound was Dox-[Pt (IV)/Pd (IV)]-FA.
Synthesis of
Pt(NH3)2Cl2(O2CCH2CH2CONH-Dox)
(O2CCH2CH2CO2H;
Cis-Dox)
To a solution of Cis-[PtCl2
(NH3)2(O2CCH2CH2CO2H)2]
(10 mg) in DMF (5 ml), 1.5 equiv EDC coupled with DIPEA (10 µl) was
added at the temperature of 70°C for 6 h. The solution was removed
and washed with water to remove the remaining EDC, following which
1.0 equiv Dox was added and incubated at a temperature of 70°C
overnight. The obtained compound was used as a control to further
confirm the FA-targeting effect of the designed compounds (Fig. 2).
Biological activity
To assess the anticancer activities of the
synthesized compounds, the present study evaluated the
antiproliferative activities against A2780, A2780/Dox and A2780/Cis
cell lines. As shown in Table I,
all the compounds showed marked activity against ovarian cancer
cells, suggesting that the FA-prodrug-Dox derivatives exhibited
improved efficacy, compared with the single drugs of Dox and Cis.
In addition, the target compounds exhibited a more potent effect,
compared with the additive effect of the two cytotoxins (Dox-Cis),
the superiority of the designed compounds were predominantly
attributed to the function of FA-targeting. For the compounds, it
was observed that compound 1e showed the most potent biological
activity, with half maximal inhibitory concentration
(IC50) values of 0.85±0.10, 8.64±0.37 and 0.81±0.03 µM
against the A2780, A2780/Dox and A2780/Cis cell lines,
respectively.
In addition, the activity of the assessed compounds
was correlated with the core metallic atom variation and ligand
modifications (Fig. 3). It was
revealed that Pd had a similar antiproliferative activity to Pt,
suggesting that Pd derivatives qualified as a novel substitute for
Pt as an anticancer drug.
In line with the improved anticancer potency, the
present study further investigated the process of drug release in
cancer cells with fluorescent imaging. The fluorescence activation
was first assessed by treating cells with the FA-prodrug-Dox in
reducing conditions and in the presence of GSH. In these
experiments, compound 1e (1 µM) in PBS (pH 7.4) was incubated with
GSH solution (5 mM), as the intracellular GSH concentration is
between 1 and 10 mM. The fluorescence emissions (λex=497; λem=594
nm) were measured at different time points. As shown in Fig. 4A, the fluorescence emission of
FA-Pt (IV)-Dox increased 2-fold as a result of reduction of the
axial bond, reaching a plateau at 1 h. In addition, treatment with
FA-Pt (IV)-Dox (1 µM in PBS; pH 7.4) in the absence of GSH (PBS pH
7.4) showed no effect on the fluorescence emission of Dox,
suggesting that the observed increase in the fluorescence of Dox
was due to GSH-mediated cleavage of the axial bond of the prodrug.
The present study further investigated the drug release of FA-Pd
(IV)-Dox (2e), which showed a similar trend of fluorescent
enhancement with FA-Pt (IV)-Dox (1e), as shown in Fig. 4B, indicating there was no
significant difference in the release efficiency between the Pt
(IV) and Pd (IV) complexes (Fig.
5).
In conclusion, a series of FA-prodrug-Dox
derivatives were synthesized in the present study and were
evaluated for multifunctional anticancer therapy. These compounds
exhibited potent antitumor activities against ovarian cell lines.
Among them, compound 1e demonstrated the most potent activity, with
IC50 values of 0.85±0.10, 8.64±0.37 and 0.81±0.03 µM
against A2780, A2780/Dox and A2780/Cis cell lines, respectively.
The fluorescence imaging of live cell lines also provided an easy
and reliable method for monitoring of the site-specific drug
activities through turn-on systems induced by drug release. The
results of the present study may provide assistance in the
treatment of ovarian cancer cells with improved efficiency and
real-time imaging, which can be used as a multifunctional system
for the optimization of anticancer drugs.
References
|
1
|
Mareel M and Leroy A: Clinical, cellular,
and molecular aspects of cancer invasion. Physiol Rev. 83:337–376.
2003. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Weigelt B, Peterse JL and Van't Veer LJ:
Breast cancer metastasis: Markers and models. Nat Rev Cancer.
5:591–602. 2005. View
Article : Google Scholar : PubMed/NCBI
|
|
3
|
Oldenburg RA, Meijers-Heijboer H,
Cornelisse CJ and Devilee P: Genetic susceptibility for breast
cancer: How many more genes to be found? Crit Rev Oncol Hematol.
63:125–149. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Nowsheen S, Aziz K, Tran PT, Gorgoulis VG,
Yang ES and Georgakilas AG: Epigenetic inactivation of DNA repair
in breast cancer. Cancer Lett. 342:213–222. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Leber MF and Efferth T: Molecular
principles of cancer invasion and metastasis (review). Int J Oncol.
34:881–895. 2009.PubMed/NCBI
|
|
6
|
Yoo HS and Park TG: Biodegradable
polymeric micelles composed of doxorubicin conjugated PLGA-PEG
block copolymer. J Control Release. 70:63–70. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Low PS and Antony AC: Folate
receptor-targeted drugs for cancer and inflammatory diseases. Adv
Drug Deliv Rev. 56:1055–1058. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Lu Y, Sega E, Leamon CP and Low PS: Folate
receptor-targeted immunotherapy of cancer: Mechanism and
therapeutic potential. Adv Drug Deliv Rev. 56:1161–1176. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Hilgenbrink AR and Low PS: Folate
receptor-mediated drug targeting: From therapeutics to diagnostics.
J Pharm Sci. 94:2135–2146. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Santra S, Kaittanis C, Santiesteban OJ and
Perez JM: Cell-specific, activatable, and theranostic prodrug for
dual-targeted cancer imaging and therapy. J Am Chem Soc.
133:16680–16688. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Sledge GW, Neuberg D, Bernardo P, Ingle
JN, Martino S, Rowinsky EK and Wood WC: Phase III trial of
doxorubicin, paclitaxel, and the combination of doxorubicin and
paclitaxel as front-line chemotherapy for metastatic breast cancer:
An intergroup trial (E1193). J Clin Oncol. 21:588–592. 2003.
View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Heister E, Neves V, Silva SRP, Mcfadden J
and Coley HM: Carbon nanotubes loaded with anticancer drugs: A
platform for multimodal cancer treatmentCarbon Nanotubes for
Biomedical Applications. Klingeler R and Sim RB: Springer-Verlag
GmbH; Berlin: pp. 223–245. 2009
|
|
13
|
Karukstis KK, Thompson EH, Whiles JA and
Rosenfeld RJ: Deciphering the fluorescence signature of daunomycin
and doxorubicin. Biophys Chem. 73:249–263. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Jakupec MA, Galanski M and Keppler BK:
Tumor-inhibiting platinum complexes-state of the art and future
perspectives. Rev Physiol Biochem Pharmacol. 146:1–54. 2003.
View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Hall MD, Mellor HR, Callaghan R and
Hambley TW: Basis for design and development of platinum (IV)
anticancer complex. J Med Chem. 50:3403–3411. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Tyagi AK, Agarwal C, Chan DC and Agarwal
R: Synergistic anti-cancer effects of silibinin with conventional
cytotoxic agents doxorubicin, cisplatin and carboplatin against
human breast carcinoma MCF-7 and MDA-MB468 cells. Oncol Rep.
11:493–499. 2014.
|
|
17
|
Chun R, Kurzman ID, Couto CG, Klausner J,
Henry C and MacEwen EG: Cisplatin and doxorubicin combination
chemotherapy for the treatment of canine osteosarcoma: A pilot
study. J Vet Intern Med. 14:495–498. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Harrington KJ, Syrigos KN, Uster PS,
Zetter A, Lewanski CR, Gullick WJ, Vile RG and Stewart JS: Targeted
radiosensitisation by pegylated liposome-encapsulated 3′,
5′-O-dipalmitoyl 5-iodo-2′-deoxyuridine in a head and neck cancer
xenograft model. Br J Cancer. 91:366–373. 2004.PubMed/NCBI
|