Introduction
Epirubicin (EPI) is one of the most well-known and
widely used anthracyclines, a class of effective genotoxic
anticancer drugs used to treat a variety of human malignancies
(1,2). The major mechanism of EPI is
inhibition of topoisomerase 2α, which induces DNA damage through
intercalating the DNA double helix (3). EPI-containing regimens, including
5-fluorouracil/EPI/cyclophosphamide and EPI/cyclophosphamide, are
widely used as an adjuvant or neoadjuvant in the treatment of
metastatic cancer (3–5). Although these EPI-containing regimens
are highly effective, serious side effects, including
cardiomyopathy and congestive heart failure, limits their long-term
administration (5–7). To circumvent this problem,
strategies, including use of prodrugs and targeted delivery
systems, have been previously investigated in an attempt to
increase the selectivity of EPI for cancer tissues or cells
(6,8,9).
These strategies require further exploration to identify the most
effective method for using EPI, with the fewest side effects.
The transferrin receptor (TfR) is a transmembrane
homodimer, able to bind up to two molecules of transferrin (Tf),
and internalize them by TfR-mediated endocytosis (10). As a 78 kD a monomeric glycoprotein,
Tf is an important chelator primarily involved in serum iron
transportation, essential for cellular proliferation (11). TfR is highly expressed on cells
that have a high proliferation rate, reflecting the high cellular
metabolic requirements for iron (11). Due to their rapid rate of division,
the majority of cancer cells have high rates of iron uptake. In
addition, TfR is generally overexpressed on cancer cells, ~100-fold
more compared with that on normal cells (10,12,13).
Therefore, due to the difference in the expression of TfR between
cancerous and normal cells, Tf has been previously used as a
targeting ligand (10,14,15).
However, it is difficult for Tf to be synthesized as a conjugate
with other anticancer drugs, or constructed in a drug targeting
delivery system. This is due to its high molecular weight and that
it is a competitive inhibitor of endogenous Tf (10–12).
HAIYPRH, a TfR-targeting peptide, has a high
affinity for the TfR with a Kd of ~10 nM
(16). In addition, the binding
sites on HAIYPRH for the Tf are distinct from the binding sites for
the TfR, allowing a co-delivery of HAIYPRH and endogenous Tf to
TfR-expressing cells (12).
Furthermore, the molecular weight of HAIYPRH is ~1 kDa,
significantly lower compared with the molecular weight of Tf. These
advantageous features of HAIYPRH make it a promising targeting
ligand. Oh et al (12)
reported that conjugates of HAIYPRH and artemisinin demonstrated
significantly increased selectivity for cancer cells.
In the present study, HAIYPRH was used as a
targeting ligand to synthesize a HAIYPRH-EPI conjugate. In
addition, the anticancer activity and cellular uptake of the
conjugate were each evaluated. This study may provide an effective
strategy to increase selectivity of EPI for cancer cells, and
reduce the systemic toxicity of EPI.
Materials and methods
Materials
EPI was supplied by Shandong Xinshidai
Pharmaceutical Co., Ltd. (Linyi, China). The HAIYPRH-EPI conjugate
was synthesized by Hangzhou Dangang Biotechnology Co., Ltd.
(Hangzhou, China). Glutamic acid was used as a linker between
HAIYPRH and EPI, and the structure of the conjugate is detailed in
Fig. 1. The HAIYPRH-EPI conjugate
was identified by liquid chromatography-mass spectrometry. Mass
spectrometric detection was performed using a Sciex 2000 QTrap (AB
Sciex, Concord, ON, Canada) equipped with electrospray ionization
(ESI). The mass spectrometer was employed in the positive ion
elctrospray mode with multiple reaction monitoring. The optimal ESI
source conditions were as follows: Nebulizer gas pressure, 40.00
psi; gas flow rate, 10.00 l/min; ion source temperature, 350°C; ion
transitions of m/z 773.8–393.0. Fluorescein
isothiocyanate-conjugated anti-human CD71 (anti-TfR) and the
isotype fluorescein isothiocyanate-conjugated IgG1 were purchased
from eBioscience, Inc. (San Diego, CA, USA). Caspase-3, caspase-8,
and caspase-9 activity assay kits were purchased from Beyotime
Institute of Biotechnology (Haimen, China). Cell culture reagents
and additional reagents were purchased from Gibco (Thermo Fisher
Scientific, Inc., Waltham, MA, USA) or Hyclone (GE Healthcare Life
Sciences, Logan, UT, USA). All chemicals and reagents were of
analytical grade.
Cell lines
U87 and LN229 cells were purchased from Cell Bank of
the Type Culture Collection of Chinese Academy of Sciences
(Shanghai, China). U87 cells were cultured in Dulbecco's modified
Eagle's medium, and LN229 cells were cultured in minimum essential
medium. Each type of medium was supplemented with 10% fetal bovine
serum and 1% penicillin/streptomycin (100 U/ml and 100 mg/ml,
respectively) and the cells were maintained at 37°C in a humidified
incubator containing 5% CO2.
Flow cytometry
Flow cytometric analysis for each glioma cell line
was performed, as previously described (17) with slight modifications. LN229 and
U87 cells were washed twice with phosphate-buffered saline (PBS)
containing 0.1% bovine serum albumin and blocked with normal mouse
serum at 4°C for 60 min prior to staining. A total of
1×106 cells were incubated with fluorescein
isothiocyanate-conjugated anti-human CD71 (anti-TfR) at 4°C for 30
min and were subsequently washed twice with PBS containing 0.1%
bovine serum albumin. The cells were subsequently analyzed using a
flow cytometer (FACSCalibur; BD Biosciences, Franklin Lakes, NJ,
USA). An isotype fluorescein isothiocyanate-conjugated IgG1 was
used as a negative control to define the threshold of the
background staining.
Cytotoxicity evaluation
The cytotoxicity of the conjugate was evaluated in
each glioma cell line using a
3-(4,5-dimethylthiazol)-2,5-diphenyltetrazolium (MTT) assay
(18). Cells in suspension,
containing ~1×104 cells, were seeded into each well of a
96-well plate. These cells were treated with the HAIYPRH-EPI
conjugate (0.01–100 µM) for 24 h at 37°C. To test whether the
cytotoxicity was enhanced by the addition of free Tf, all cells
treated with the HAIYPRH-EPI conjugate were assessed in the
presence or absence of free Tf (25 µM). Free EPI and the
appropriate culture medium were used as controls. After 24 h, the
culture medium was removed, and the cells were incubated with MTT
(5 mg/ml) at 37°C in a humidified incubator containing 5%
CO2 for 4 h. Following the removal of the supernatant,
the formazan crystals were dissolved in 200 µl dimethyl sulfoxide.
The absorbance was recorded using a microplate reader (Model 550;
Bio-Rad Laboratories, Inc., Hercules, CA, USA) at a wavelength of
570 nm. Cell viability was calculated as 100% ×
(absorptiontreatment/absorptioncontrol).
Transmission electron microscopy
(TEM)
LN229 and U87 cells were cultured for 24 h in 6-well
plates. HAIYPRH-EPI (10 µl), EPI (10 µl), and the same volume of
medium were separately added into treatment and control groups, and
the cells were cultured at 37°C under a 5% CO2
atmosphere. After 24 h of treatment, the cells were harvested,
fixed in 2.5% glutaraldehyde and rinsed with PBS. The cells were
subsequently post-fixed in 1% osmium tetroxide, dehydrated through
a series of graded ethanol and acetone, and embedded in epoxy
resin. Ultra-thin sections (60–70 nm) were cut and these sections
were stained with 2% uranyl acetate and lead citrate. Images were
captured using a transmission electron microscope (H-600; Hitachi,
Ltd., Tokyo, Japan).
Analysis of caspase activity
The activities of caspase-3, caspase-8 and caspase-9
in control and drug-treated cells were evaluated using caspase
activity assay kits (Beyotime Institute of Biotechnology). LN229
and U87 cells were treated separately with HAIYPRH-EPI (10 µl), EPI
(10 µl) or the same volume of medium. Following a 24 h treatment
period, LN229 and U87 cells were lysed using lysis buffer (Beyotime
Institute of Biotechnology, Haimen, China). The cell lysates were
centrifuged at 26,487 × g for 10 min at 4°C. The cell lysate
supernatant was mixed with buffer containing Ac-DEVD-pNA (for
caspase-3), Ac-IETD-pNA (for caspase-8), and Ac-LEHD-pNA (for
caspase-9). The release of pNA was quantified by determining the
absorbance using a microplate reader (Model 550; Bio-Rad
Laboratories, Inc.) at 405 nm. Caspase activities were expressed as
a relative percentage of the control value.
Evaluation of cellular uptake
The cellular uptake of EPI was visualized by
fluorescence microscopy. LN229 and U87 cells (1×105
cells/well) were seeded separately into 24-well plates. Following
incubation for 24 h, the culture media containing free EPI or
HAIYPRH-EPI (10 µM) with or without free Tf (25 µM) was added.
After a further 2-h incubation, the treated cells were thoroughly
washed three times with PBS to remove the excessive drug that was
not taken up by the cells. The fluorescence intensity (FI) of the
cells was detected using fluorescence microscopy (Eclipse TE2000-U;
Nikon Corporation, Tokyo, Japan).
The extent of cellular uptake was further determined
using a flow cytometer (FACSCalibur; BD Biosciences, Franklin
Lakes, NJ, USA). LN229 and U87 cells harvested during the
logarithmic growth phase were seeded into 24-well plates at a
density of 1×106 cells/well. Following incubation for 24
h, the culture media containing free EPI or HAIYPRH-EPI (10 µM) was
added. Following a further 2-h incubation, the treated cells were
thoroughly washed three times with PBS.
Statistical analysis
The results were expressed as the mean ± standard
deviation and were obtained from at least three independent
experiments. The statistical analysis of the data was performed
using a one-way analysis of variance or Student's t-test. P<0.05
was considered to indicate a statistically significant
difference.
Results
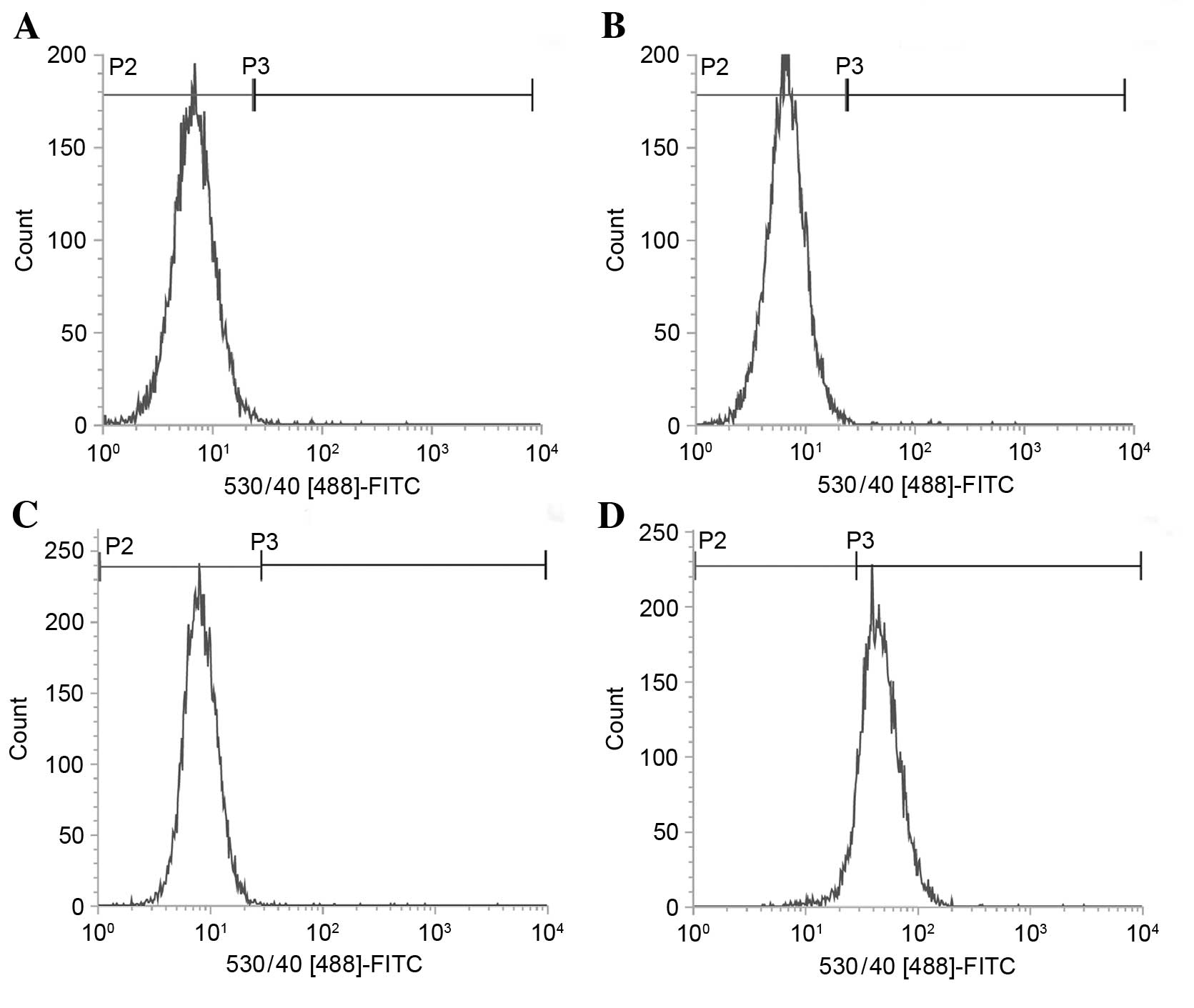
Flow cytometry analyses for TfR
surface expression
To identify the difference in the surface expression
of TfR between LN229 and U87 cells, flow cytometry was used. The FI
reflected the expression of surface TfR. As demonstrated in
Fig. 2, the U87 cell line did not
express detectable surface TfR and the FI levels of surface TfR did
not demonstrate significant differences when compared with their
negative control (Fig. 2A). By
contrast, in the LN229 cell line surface TfR was detected and the
FI levels were higher when compared with the corresponding negative
control (Fig. 2B).
Cytotoxicity of HAIYPRH-EPI
The cytotoxicity of the HAIYPRH-EPI conjugate and
free EPI was determined to evaluate the in vitro anticancer
effects of each condition. As presented in Fig. 3, the HAIYPRH-EPI conjugate
exhibited higher cytotoxicity compared with free EPI for the LN229
cell line (P<0.05; Fig. 3A). By
contrast, the conjugate exhibited lower cytotoxicity in the U87
cell line compared with free EPI (P<0.05; Fig. 3B). In addition, when Tf was added,
the cytotoxicity of HAIYPRH-EPI for the LN229 cells was increased
(P<0.05; Fig. 3A). However, Tf
caused no effect on the cytotoxicity of HAIYPRH-EPI for U87 cells
(P>0.05; Fig. 3B) or the
cytotoxicity of EPI for the two cell lines.
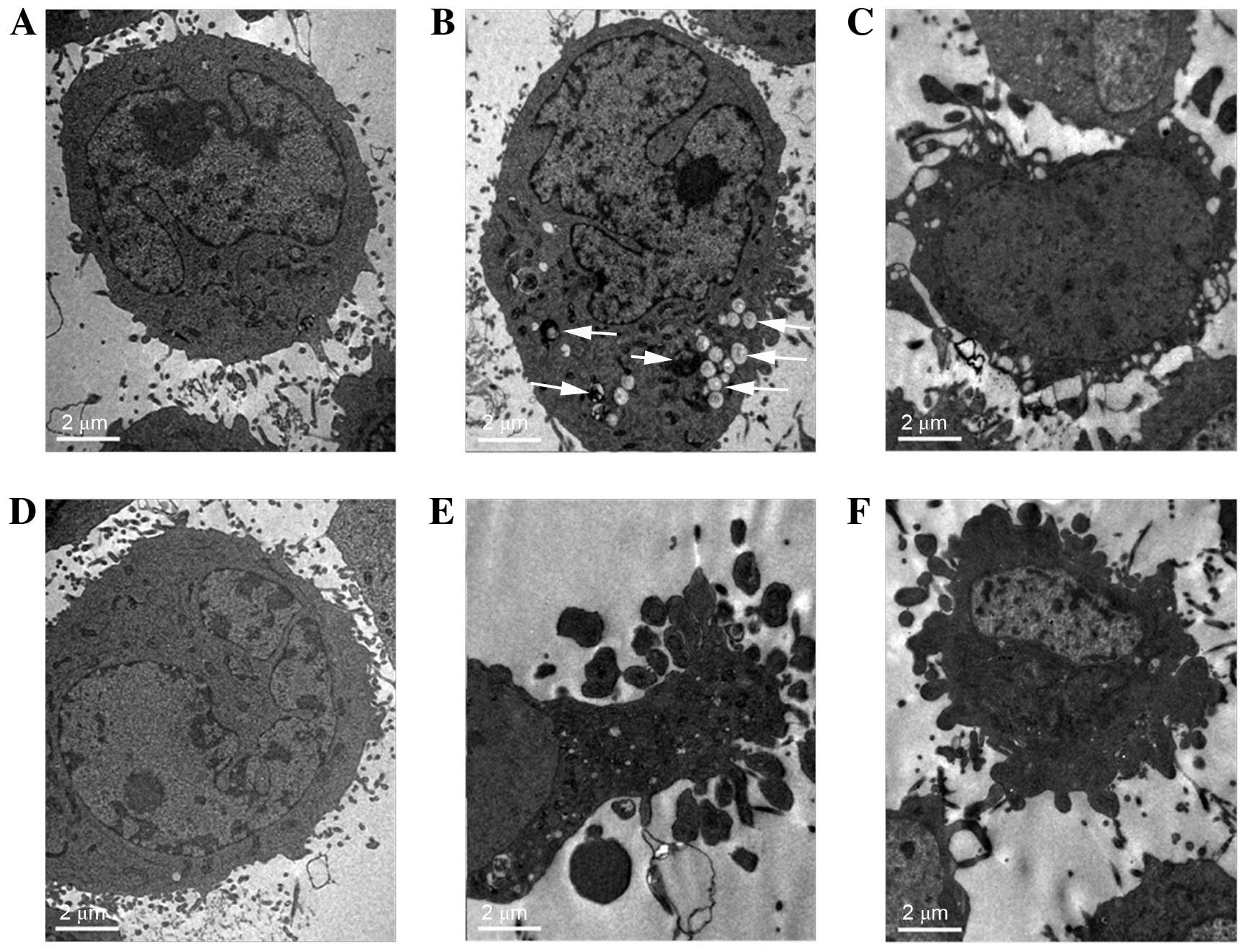
TEM analysis of cell morphology
TEM is the most common method used for morphological
observation by clearly differentiating cellular ultrastructure. In
the present study, changes in the nuclei and organelles were
observed using TEM. As demonstrated in Fig. 4, untreated U87 cells exhibited
intact cell membranes with dense cellular contents. Numerous
organelles, including the mitochondria and rough endoplasmic
reticulum, were easily observed. The nuclei were large and the
nuclear membrane was unbroken. Compared with the control, treatment
of the U87 cells with HAIYPRH-EPI (10 µM), induced mild apoptosis,
and a few myelin figures and fatty drops (white arrows) were
observed. The U87 cells treated with only EPI (10 µM) exhibited
severe apoptosis accompanied with clear apoptotic bodies. By
contrast, both groups of LN229 cells treated with EPI (10 µM) or
HAIYPRH-EPI (10 µM) exhibited severe apoptosis.
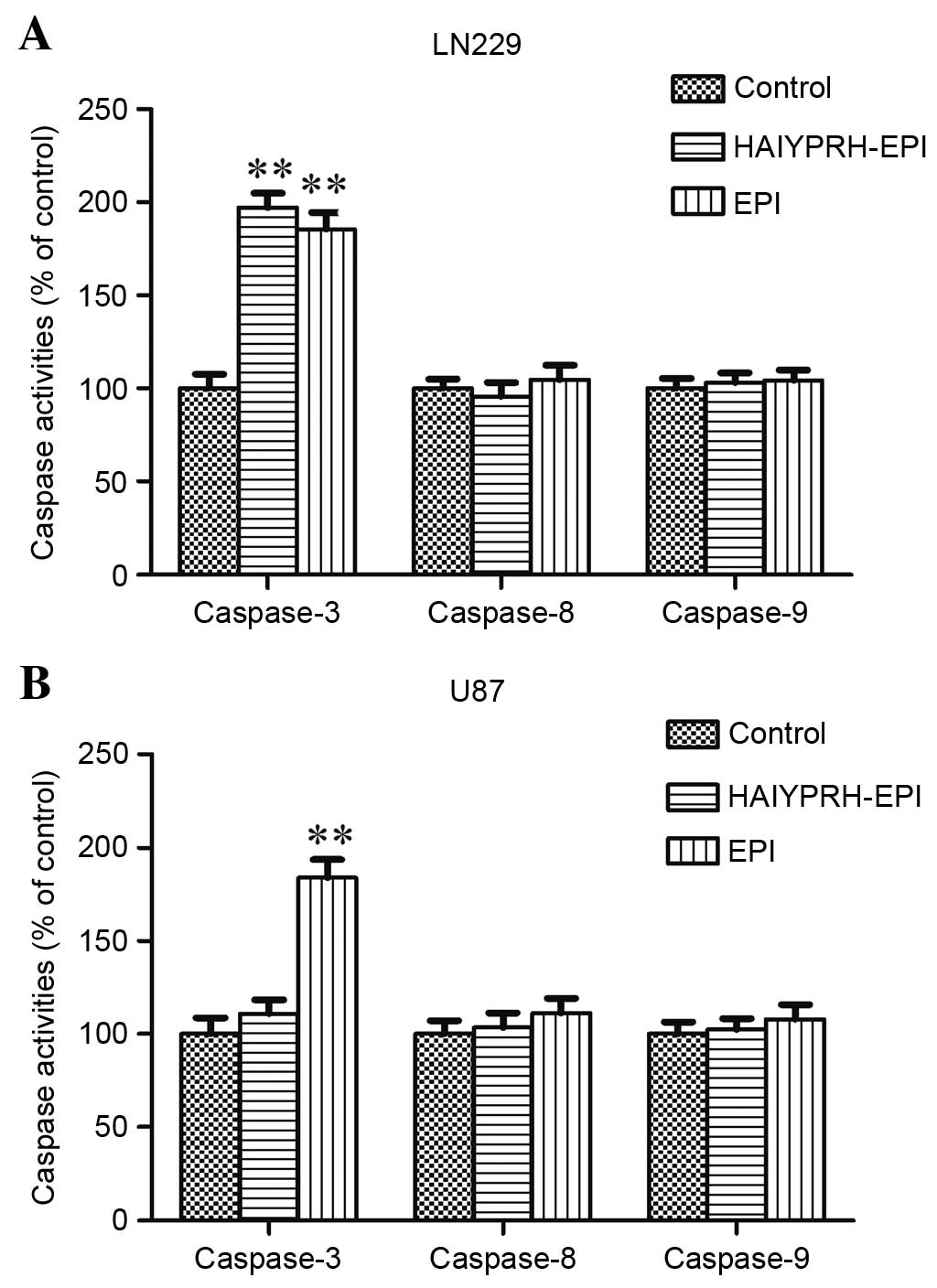
Analysis of caspase activity
Caspases are significant effector molecules involved
in the execution of apoptosis (19). When the LN229 cells were treated
with EPI or HAIYPRH-EPI for 24 h, the activity of caspase-3
increased in both groups (Fig. 5A,
P<0.01); however, the activities of caspase-8 or caspase-9 were
not affected by EPI or HAIYPRH-EPI (Fig. 5A, P>0.05). When the U87 cells
were treated with EPI or HAIYPRH-EPI for 24 h, the activities of
caspase-3 increased only in the EPI group (Fig. 5B, P<0.01), and the activities of
caspase-8 or caspase-9 remained unaffected by EPI or HAIYPRH-EPI
(Fig. 5A, P>0.05).
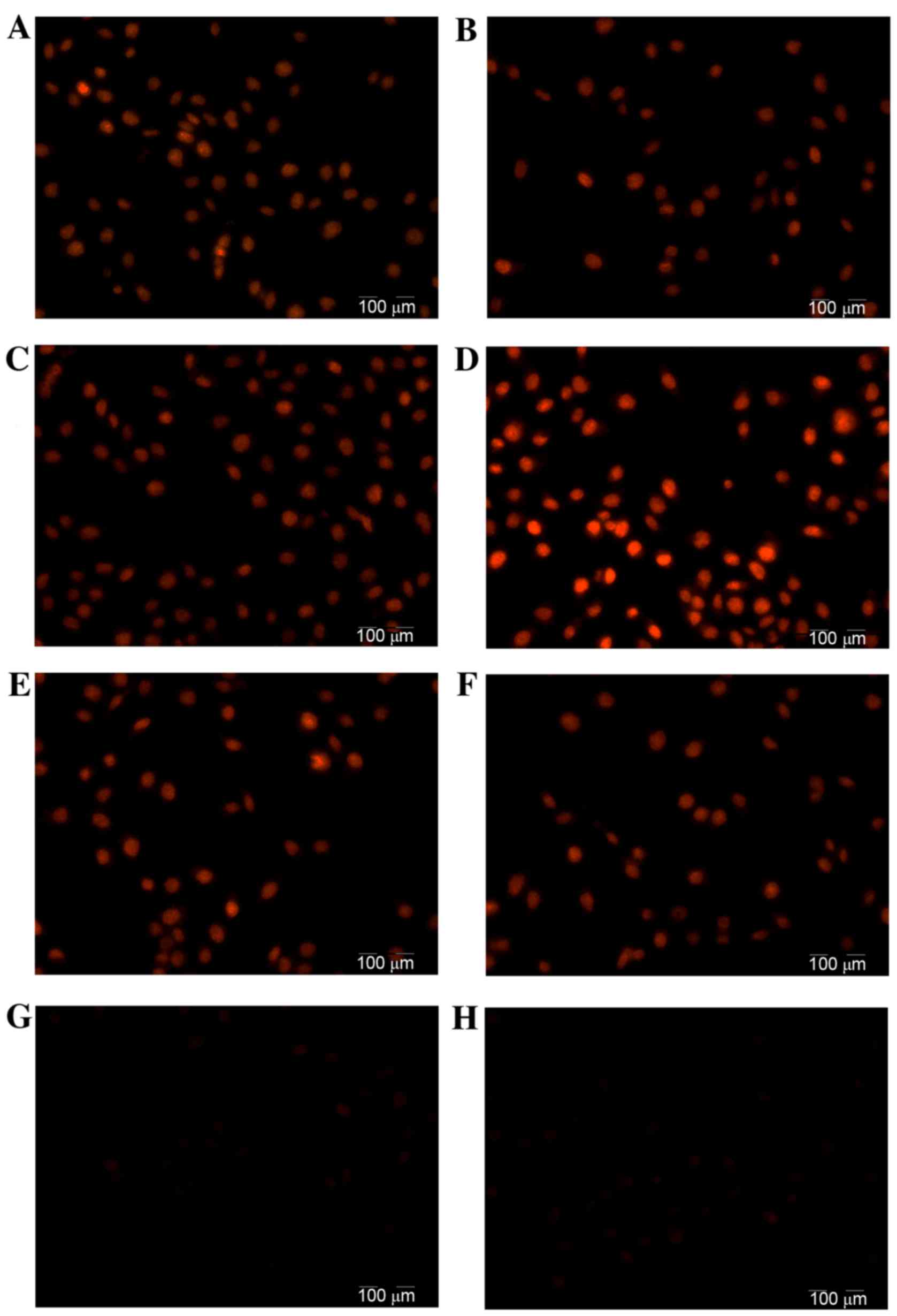
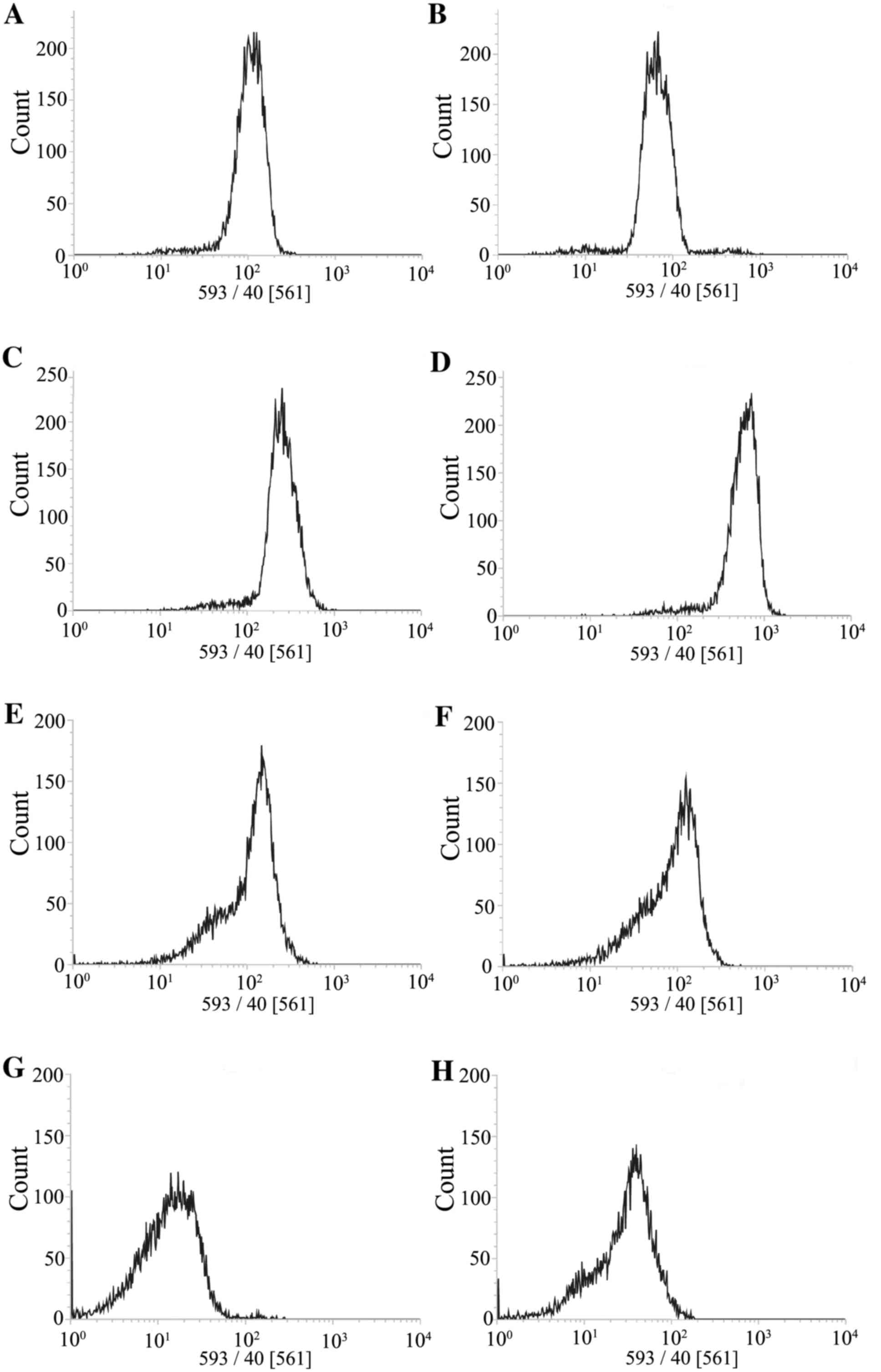
Evaluation of cellular uptake
Since EPI itself harbors florescence, the uptake of
free EPI and HAIYPRH-EPI by the LN229 and U87 cells was
investigated using the methods of fluorescence microscopy and flow
cytometry. As demonstrated in Fig.
6, when incubated with HAIYPRH-EPI, the LN229 cells emitted a
higher fluorescence intensity compared with the U87 cells,
suggesting that the HAIYPRH-EPI cellular uptake of LN229 cells was
higher compared with that of U87 cells. Furthermore, the addition
of Tf significantly increased the intracellular uptake of
HAIYPRH-EPI in LN229 cells. By contrast, when incubated with free
EPI, no obvious difference was observed between the fluorescence of
LN229 and U87 cells, and the addition of Tf caused no significant
change in cellular uptake of EPI inLN229 or U87 cells, when
compared with EPI alone. As demonstrated in Fig. 7, similar results were observed by
flow cytometry. Flow cytometry also confirmed that the LN229 cells
exhibited more uptake of HAIYPRH-EPI compared with the U87 cells,
and that LN299 exhibited enhanced uptake of HAIYPRH-EPI with the
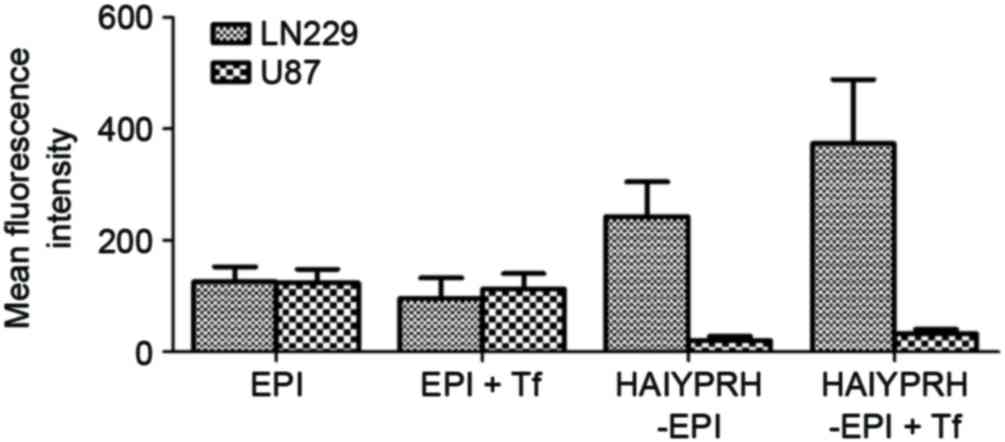
addition of Tf. The mean fluorescence intensity highlighted these
findings (Fig. 8).
Discussion
Although it has been previously demonstrated that
TfR is overexpressed in numerous solid tumor types, the level of
TfR varies among different tumor cell lines (11,20,21).
Wirth et al (21) detected
TfR expression in 41 human tumor cell lines using microarrays. This
previous study identified that TfR expression in these cell lines
was completely different compared with one another. Furthermore,
for glioma cell lines, TfR expression in LN229 cells was positive,
but negative in U87 cells. Notably, another study reported that
both the LN229 and U87 cell lines overexpressed TfR, as determined
by immunofluorescence and western blot analysis (19). Total TfR in cells includes surface
TfR and endocellular TfR. The previous study reported that U87 cell
lines overexpressed TfR, which was the total TfR, not surface TfR
(19). Considering that surface
TfR, not endocellular TfR, executes the delivery of Tf or drugs
across cell membrane and the controversy surrounding the expression
of TfR in LN229 and U87 cells (19,21),
the difference in surface TfR expression between LN229 and U87
cells was examined in the present study. The results demonstrated
that surface TfR expression in LN229 cells was positive, but the
U87 levels had no difference compared with the negative control
(Fig. 2), which was similar to the
results reported by Wirth et al (21).
The results of the cytotoxicity assay indicated that
the cytotoxicity of HAIYPRH-EPI was correlated with the expression
of surface TfR. Surface TfR was expressed at greater levels on the
surface of LN229 cells when compared with U87 cells, and due to the
high affinity of HAIYPRH, it mediated the endocytosis of
HAIYPRH-EPI. On the contrary, surface TfR was expressed at lower
levels in U87 cells; thus, the active transport of HAIYPRH-EPI by
surface TfR was rare in these cells. By contrast, the cellular
uptake of HAIYPRH-EPI through passive transport was more difficult
to achieve compared with the transport of free EPI, due to the
strong hydrophilicity of HAIYPRH. Therefore, HAIYPRH-EPI exhibited
lower cytotoxicity in U87 cells and higher toxicity in LN229
cells.
To corroborate the cytotoxicity results, TEM was
used to determine cell apoptosis through morphological observation
of the cellular ultrastructure, and similar results were observed.
When cells are exposed to apoptotic stimuli, there are two major
apoptosis signaling pathways: Intrinsic (mitochondria-mediated)
pathway and extrinsic (transmembrane receptor-mediated) pathway,
both of which use caspases, a family of cysteine proteases
(22). Caspases are divided into
two groups: Initiator caspases and effector caspases. When
initiator caspases bind to adaptor molecules, they are activated,
and are able to activate effector caspases. The major initiator
caspase for the intrinsic pathway is caspase-9, whereas the major
initiator caspase for extrinsic pathway is caspase-8. Each pathway
shares the same effector caspase, caspase-3, which is a key
checkpoint enzyme in the canonical apoptotic pathway (22,23).
To elucidate the initiation event of apoptosis, the present study
probed the activation of the two initiator caspases, caspase-8 and
caspase-9, and the effector caspase, caspase-3. The results
demonstrated that, when treated with EPI or HAIYPRH-EPI, the
activity of caspase-3 increased, whilst the activities of caspase-8
and caspase-9 did not. A previous study also reported that EPI (0.5
µg/ml) induced the activation of caspase-3, but not caspase-8 or
caspase-9, in HeLa cells (24).
These results suggested that the pro-apoptotic effect of EPI or
HAIYPRH-EPI was not mediated by caspase-8 or caspase-9, and that
caspase-3 may be activated by additional upstream factors.
Consistent with the cytotoxicity and cell apoptosis
data, the results of the cellular uptake experiments further
confirmed the original hypothesis. HAIYPRH-EPI is a hydrophilic
substance, and its transport across the cell membrane predominantly
depends on active transport mediated by surface TfR, as opposed to
passive transport. The different expression levels of surface TfR
on LN229 and U87 cells led to different cellular uptakes of
HAIYPRH-EPI, further resulting in different levels of cytotoxicity.
Due to the higher expression of surface TfR, more HAIYPRH-EPI can
be transported into LN229 cells. Therefore, HAIYPRH-EPI was
markedly more active in LN229 cells compared with in U87 cells. By
contrast, EPI is a relatively hydrophobic substance. Its main
transport was passive, so the cellular uptake of free EPI was not
different between LN229 and U87 cells in the present study.
The primary function of Tf is serum iron
transportation and it can reversibly bind two atoms of ferric iron
with high affinity (11). Tf
exhibits a conformational change following iron binding, which has
been demonstrated to be significant in its selective recognition by
the TfR (25). Following binding
to the TfR, the Tf-TfR complex activates a cascade that is
suggested to be important in the mediation of its specific
internalization (26). Therefore,
the internalization mediated by TfR is triggered by Tf (12). As mentioned previously, the binding
sites of Tf and TfR are distinct. Therefore, the addition of Tf can
theoretically enhance the uptake of HAIYPRH-EPI in LN229 cells, and
the results examining the cytotoxicity and cellular uptake also
confirmed this hypothesis.
In conclusion, the cytotoxicity of HAIYPRH-EPI was
dependent upon the expression of the surface transferrin receptor.
Considering that the majority of cancer cells have high rates of
iron uptake, and that TfR is generally overexpressed on cancer
cells, the authors speculated that the HAIYPRH-EPI conjugate may
provide an effective strategy for increasing the selectivity of EPI
for cancer cells and reduce the systemic toxicity of EPI. To
confirm the hypothesis, the therapeutic effects and side effects of
HAIYPRH-EPI with a suitable delivery system in an animal model will
be investigated in a future study.
Acknowledgements
The present study was supported by the National
Science and Technology Major Project (no. 2010ZX09401-306-1-1).
References
|
1
|
Hu DG, Rogers A and Mackenzie PI:
Epirubicin upregulates UDP glucuronosyltransferase 2B7 expression
in liver cancer cells via the p53 pathway. Mol Pharmacol.
85:887–897. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Khasraw M, Bell R and Dang C: Epirubicin:
Is it like doxorubicin in breast cancer? A clinical review. Breast.
21:142–149. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Miyoshi Y, Kurosumi M, Kurebayashi J,
Matsuura N, Takahashi M, Tokunaga E, Egawa C, Masuda N, Kim SJ,
Okishiro M, et al: Topoisomerase IIalpha-positive and
BRCA1-negative phenotype: Association with favorable response to
epirubicin-based regimens for human breast cancers. Cancer Lett.
264:44–53. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Okishiro M, Kim SJ, Tsunashima R, Nakayama
T, Shimazu K, Shimomura A, Maruyama N, Tamaki Y and Noguchi S: MDM2
SNP309 and TP53 R72P associated with severe and febrile neutropenia
in breast cancer patients treated with
5-FU/epirubicin/cyclophosphamide. Breast Cancer Res Treat.
132:947–953. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Aogi K, Saeki T, Nakamura S, Kashiwaba M,
Sato N, Masuda N, Rai Y, Ohno S, Kuroi K, Nishimura R, et al: A
multicenter, phase II study of epirubicin/cyclophosphamide followed
by docetaxel and concurrent trastuzumab as primary systemic therapy
for HER-2 positive advanced breast cancer (the HER2NAT study). Int
J Clin Oncol. 18:598–606. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Nasr M, Nafee N, Saad H and Kazem A:
Improved antitumor activity and reduced cardiotoxicity of
epirubicin using hepatocyte-targeted nanoparticles combined with
tocotrienols against hepatocellular carcinoma in mice. Eur J Pharm
Biopharm. 88:216–225. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Appel JM, Zerahn B, Moller S, Christensen
HM, Søgaard P, Ejlertsen B, Fogh-Andersen N, Jensen BV and Nielsen
DL: Long-term heart function after adjuvant epirubicin chemotherapy
for breast cancer. Acta Oncol. 51:1054–1061. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Ju RJ, Li XT, Shi JF, Li XY, Sun MG, Zeng
F, Zhou J, Liu L, Zhang CX, Zhao WY and Lu WL: Liposomes, modified
with PTD (HIV-1) peptide, containing epirubicin and celecoxib, to
target vasculogenic mimicry channels in invasive breast cancer.
Biomaterials. 35:7610–7621. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Haisma HJ, Boven E, van Muijen M, de Jong
J, van der Vijgh WJ and Pinedo HM: A monoclonal
antibody-beta-glucuronidase conjugate as activator of the prodrug
epirubicin-glucuronide for specific treatment of cancer. Br J
Cancer. 66:474–478. 1992. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Xu J, Sheng Y, Xu F, Yu Y and Chen Y:
Quantitative subcellular study of transferrin receptor-targeted
doxorubicin and its metabolite in human breast cancer cells. Eur J
Drug Metab Pharmacokinet. 39:301–310. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Tortorella S and Karagiannis TC:
Transferrin receptor-mediated endocytosis: A useful target for
cancer therapy. J Membr Biol. 247:291–307. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Oh S, Kim BJ, Singh NP, Lai H and Sasaki
T: Synthesis and anti-cancer activity of covalent conjugates of
artemisinin and a transferrin-receptor targeting peptide. Cancer
Lett. 274:33–39. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Han L, Huang R, Liu S, Huang S and Jiang
C: Peptide-conjugated PAMAM for targeted doxorubicin delivery to
transferrin receptor overexpressed tumors. Mol Pharm. 7:2156–2165.
2010. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Malarvizhi GL, Retnakumari AP, Nair S and
Koyakutty M: Transferrin targeted core-shell nanomedicine for
combinatorial delivery of doxorubicin and sorafenib against
hepatocellular carcinoma. Nanomedicine. 10:1649–1659.
2014.PubMed/NCBI
|
|
15
|
Nam JP, Park SC, Kim TH, Jang JY, Choi C,
Jang MK and Nah JW: Encapsulation of paclitaxel into lauric
acid-O-carboxymethyl chitosan-transferrin micelles for hydrophobic
drug delivery and site-specific targeted delivery. Int J Pharm.
457:124–135. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Lee JH, Engler JA, Collawn JF and Moore
BA: Receptor mediated uptake of peptides that bind the human
transferrin receptor. Eur J Biochem. 268:2004–2012. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Kato J, Kobune M, Ohkubo S, Fujikawa K,
Tanaka M, Takimoto R, Takada K, Takahari D, Kawano Y, Kohgo Y and
Niitsu Y: Iron/IRP-1-dependent regulation of mRNA expression for
transferrin receptor, DMT1 and ferritin during human erythroid
differentiation. Exp Hematol. 35:879–887. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Qian G, Wang Z, Zhao J, Li D, Gao W, Wang
B, Sui D, Qu X and Chen Y: Synthesis and anti-cancer cell activity
of pseudo-ginsenoside Rh2. Steroids. 92:1–6. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Dixit S, Novak T, Miller K, Zhu Y, Kenney
ME and Broome AM: Transferrin receptor-targeted theranostic gold
nanoparticles for photosensitizer delivery in brain tumors.
Nanoscale. 7:1782–1790. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Xu J, Sheng Y, Xu F, Yu Y and Chen Y:
Quantitative subcellular study of transferrin receptor-targeted
doxorubicin and its metabolite in human breast cancer cells. Eur J
Drug Metab Pharmacokinet. 39:301–310. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Wirth GJ, Schandelmaier K, Smith V, Burger
AM and Fiebig HH: Microarrays of 41 human tumor cell lines for the
characterization of new molecular targets: Expression patterns of
cathepsin B and the transferrin receptor. Oncology. 71:86–94. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Lo YL, Ho CT and Tsai FL: Inhibit
multidrug resistance and induce apoptosis by using glycocholic acid
and epirubicin. Eur J Pharm Sci. 35:52–67. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Pakunlu RI, Wang Y, Saad M, Khandare JJ,
Starovoytov V and Minko T: In vitro and in vivo intracellular
liposomal delivery of antisense oligonucleotides and anticancer
drug. J Control Release. 114:153–162. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Lin Y, Jiang D, Li Y, Han X, Yu D, Park JH
and Jin YH: Effect of sun ginseng potentiation on epirubicin and
paclitaxel-induced apoptosis in human cervical cancer cells. J
Ginseng Res. 39:22–28. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Richardson DR and Ponka P: The molecular
mechanisms of the metabolism and transport of iron in normal and
neoplastic cells. Biochim Biophys Acta. 1331:1–40. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Yashunsky V, Shimron S, Lirtsman V, Weiss
AM, Melamed-Book N, Golosovsky M, Davidov D and Aroeti B: Real-time
monitoring of transferrin-induced endocytic vesicle formation by
mid-infrared surface plasmon resonance. Biophys J. 97:1003–1012.
2009. View Article : Google Scholar : PubMed/NCBI
|