Introduction
Cambodia is a tropical country featuring plateaus,
flat plains, hills and highland, the area of which is covered
predominantly in forest. By virtue of its tropical climate,
Cambodia has a diverse range of natural resources, amongst which
plants are primarily used for complementary medicines by the local
population. As many as 515 species amongst 134 families of
medicinal plants have been found in Cambodia (1), allowing Cambodians to couple the use
of medicinal plants with their traditional treatment of diseases.
Cambodian medicinal plants are crucial in folk medicine in
communities, with traditional healers offering assurance for their
curable effects (2). According to
the Ministry of Health in Cambodia, 45% of the Cambodian population
use herbal plants for therapeutic purposes, despite a shortage of
scientific evidence (3).
Liver disease, predominantly attributed to hepatitis
and alcoholism, is considered a primary contributor to morbidity
and mortality rates in humans. The accumulation of deleterious
substances in hepatocytes caused by infection, injury, exposure to
xenobiotics, autoimmunity or genetic disorders may lead to liver
damage in the form of inflammation, scarring, cirrhosis or liver
dysfunction (4,5). Oxidative stress is critical in liver
injury and may be triggered by reactive oxygen species (ROS)
generated by various signal transduction pathways (6). Oxidant-induced liver injury is caused
by toxins, including tert-Butyl hydroperoxide
(t-BHP), which exerts cytotoxic effects through glutathione
(GSH) depletion coupled with intracellular over-influx of
Ca2+ (4,7). Although there is general recognition
that the drugs currently available for treating patients with liver
disease are mandatory, natural products originating from plants
have attracted considerable interest among hepatologists in terms
of their efficacy and safety (8).
Traditional medicinal plants have long been used in
folk medicine for ameliorating liver diseases. A number of these
plants have shown potential in the treatment of liver disease due
to their therapeutic mechanisms. For example, Silybum
marianum and Picrorhiza kurroa have been confirmed to be
clinically efficacious in the treatment of toxic hepatitis, fatty
liver disease, cirrhosis, ischemic injury, radiation toxicity and
viral hepatitis, based upon their antioxidative, antilipid
peroxidative, antifibrotic, anti-inflammatory, immunomodulatory and
liver regenerating effects (9). In
addition, phytochemicals, including glycyrrhizin, matrine and
silymarin, have been shown to exert therapeutic effects against
hepatitis, alcoholic liver disease and liver cirrhosis (10). Phytochemicals protect hepatocytes
(11) via the induction of heme
oxygenase-1 (HO-1), which is catabolized to produce carbon
monoxide, biliverdin and free iron (12). HO-1 is well-known as a valuable
therapeutic candidate as it exerts anti-inflammatory,
anti-apoptotic and antiproliferative effects (13). In addition, HO-1 is understood to
be upregulated in the liver and splanchnic organs during portal
hypertension (14) by the
activation of nuclear factor-E2-related factor-2 (Nrf2) (15). Nrf2 is known to be important in the
regulation of phase II detoxifying enzymes and associated proteins,
including HO-1, GSH, catalase, superoxide dismutase,
glutathione-S-transferase, γ-glutamyl cysteine ligase, NAD(P)H:
quinone oxidoreductase-1, glutathione peroxidase and glutathione
reductase (16). Nrf2-regulated
phase II detoxifying enzymes can be critical in protecting the
liver from oxidative stress imposed by ROS (15,16).
Alcoholic liver disease (ALD) is reported to affect the Cambodian
population (17) due to high
levels of alcohol consumption among men and women (18). Several species of Cambodian
medicinal plants have been used for the treatment of ALD via a
number of traditional approaches. However, the majority of these
plants have received limited scientific investigation, particularly
screening assays of their hepatoprotective activities (19).
Thus, the present study was designed to determine
the hepatoprotective characteristics of 64 Cambodian plants by
examining the cytoprotective effects of the Cambodian plant
extracts against t-BHP-induced hepatotoxicity in HepG2
cells. Several laboratories have used human liver-derived HepG2
cells as a model for investigating hepatocyte proliferation and
function; these cells perform several of the functions of normal
hepatocytes, including the secretion of plasma proteins and
extensive degradation of insulin (20). Therefore, HepG2 cells were selected
for use in the present study. Selective extracts with
hepatoprotective effects were further evaluated to examine their
cytoprotective mechanism associated with the activities of ROS,
HO-1 and Nrf2.
Materials and methods
Chemicals and reagents
Roswell Park Memorial Institute (RPMI) medium, fetal
bovine serum (FBS), trypsin-EDTA solution and
antibiotic-antimycotic solution (containing 10,000 units of
penicillin, 10,000 µg of streptomycin and 25 µg of amphotericin
B/ml) were purchased from Gibco; Thermo Fisher Scientific, Inc.
(Waltham, MA, USA). Tin protoporphyrin IX (SnPP), an inhibitor of
HO activity, was obtained from Porphyrin Products (Logan, UT, USA).
All other relevant chemicals and reagents were obtained from
Sigma-Aldrich; Merck Millipore (Darmstadt, Germany).
Preparation of Cambodian plants
A total of 64 Cambodian plants were imported from
O'reusey Market (Phnom Penh, Cambodia). All plants were identified
by Professor Sun Kaing Cheng (Laboratory of Phytochemistry, Faculty
of Pharmacy, University of Health Sciences (Phnom Penh, Cambodia).
The herbarium specimens (no. WKP-2013-23-WKP-2013-86) were stored
at the College of Pharmacy, Wonkwang University (Iksan, Korea). Dry
plants (20 g each) were extracted with 70% ethanol (1 liter of
each) for 2 h, and the extracts were concentrated in vacuo
to obtain 70% ethanol extracts. Plant extraction was supported by
the College of Pharmacy, Wonkwang University.
Cell culture and viability assay
Human liver-derived HepG2 cells were obtained from
American Type Culture Collection (Manassas, VA, USA). The cells
were maintained at 5×105 cells/ml in RPMI 1640 medium
supplemented with 10% heat-inactivated FBS, penicillin G (100
U/ml−1), streptomycin (100 mg·ml−1) and
L-glutamine (2 mM), and were incubated at 37°C in a humidified
atmosphere containing 5% CO2 and 95% air. The cells were
pretreated for 3 h with the indicated concentrations of Cambodian
plant extract and stimulated for 24 h with t-BHP. The
control group was treated with equal volume of DMSO as the
extracts. The determination of cell viability was performed using a
3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT)
assay. MTT (2.5 mg·ml−1; 50 µl) was added to each well,
containing a cell suspension of 2×105 cells/ml in
96-well plates, at a final concentration of 0.5 mg·ml−1.
The mixture was incubated for 3–4 h at 37°C, and the liquid was
removed from the wells in turn. Subsequently, DMSO (150 µl) was
added to each well, and the absorbance was read at 540 nm on a UV
Max microplate reader (Bio-Rad Laboratories, Inc., Hercules, CA,
USA). The relative optical density of formazan in the control
group, which contained cells treated with neither glutamate nor the
plant extracts, was taken as 100% viability.
Western blot analysis
The human liver-derived HepG2 cells were harvested
and pelleted by centrifugation at 200 × g for 3 min at 4°C.
The cells were then washed with PBS and lysed with 20 mM Tris-HCl
buffer (pH 7.4) containing a protease inhibitor mixture (0.1 mM
phenylmethanesulfonyl fluoride, 5 mg/ml aprotinin, 5 mg/ml
pepstatin A and 1 mg/ml chymostatin). The protein concentration was
determined using a Lowry protein assay kit (P5626; Sigma-Aldrich;
Merck Millipore). An equal quantity of protein from each sample (30
µg) was resolved using 12% sodium dodecyl sulfate-polyacrylamide
gel electrophoresis and then electrophoretically transferred onto a
Hybond enhanced chemiluminescence (ECL) nitrocellulose membrane
(Bio-Rad Laboratories, Inc.). The membrane was blocked with 5%
skimmed milk and sequentially incubated with primary antibodies
against HO-1 (1:1,000; cat. no. sc-10789), Nrf2 (1:1,000; cat. no.
sc-722), Lamin B (1:1,000; cat. no. sc-6216), actin (1:1,000; cat.
no. sc-1616) all obtained from Santa Cruz Biotechnology, Inc.
(Dallas, TX, USA), a further anti-HO-1 antibody (1:1,000; Merck
Millipore; cat. no. 374090) and horseradish peroxidase-conjugated
secondary antibodies (1:1,000; Santa Cruz Biotechnology, Inc.; cat.
nos. sc-2741 and sc-2004) followed by ECL detection.
Preparation of nuclear and cytosolic
fractions
The cells were homogenized (1:20, w/v) in PER
Mammalian Protein Extraction buffer (Pierce; Thermo Fisher
Scientific, Inc.) containing freshly added protease inhibitor
cocktail I (EMD Millipore, Billerica, MA, USA) and 1 mM
phenylmethylsulfonyl fluoride. The cytosolic fraction of the cell
was prepared by centrifugation at 15,000 × g for 10 min at
4°C. The nuclear and cytoplasmic extracts of the cells were
prepared using NE-PER nuclear and cytoplasmic extraction reagents
(Pierce; Thermo Fisher Scientific, Inc.), respectively.
Reverse transcription-quantitative
polymerase chain reaction (RT-qPCR) analysis
Total RNA was isolated from the cells using TRIzol
(Invitrogen; Thermo Fisher Scientific, Inc.), in accordance with
the manufacturer's protocol, and quantified spectrophotometrically
at 260 nm. The total RNA (1 µg) was reverse-transcribed using the
High Capacity RNA-to-cDNA kit (Applied Biosystems; Thermo Fisher
Scientific, Inc.). The cDNA was then amplified using the SYBR
Premix Ex Taq kit (Takara Bio, Inc., Shiga, Japan) using a
StepOnePlus Real-Time PCR system (Applied Biosystems; Thermo Fisher
Scientific, Inc.). Briefly, each 20 µl of reaction volume contained
10 µl of SYBR-Green PCR master mix, 0.8 µM of each primer, and
diethyl pyrocarbonate-treated water. The primer sequences were
designed using PrimerQuest (Integrated DNA Technologies, Cambridge,
MA, USA; www.idtdna.com/Primerquest/Home/Index). The primer
sequences were as follows: HO-1, forward 5′-CTCTTGGCTGGCTTCCTT-3′
and reverse 5′-GGCTCCTTCCTCCTTTCC-3′ and glyceraldehyde 3-phosphate
dehydrogenase (GAPDH), forward 5′-ACTTTGGTATCGTGGAAGGACT-3′ and
reverse 5′-GTAGAGGCAGGGATGATGTTCT-3. The optimal conditions for PCR
amplification of the cDNA were established according to the
manufacturer's protocol. The data were analyzed using StepOne
software version 2.3 (Applied Biosystems; Thermo Fisher Scientific,
Inc.), and the cycle number at the linear amplification threshold
(Cq) values for the endogenous control gene (GAPDH) and the target
gene were recorded. Relative gene expression, calculated as the
target gene expression normalized to the expression of the
endogenous control gene, was calculated using the comparative Cq
method (2−ΔΔCq) (21).
Nuclear magnetic resonance (NMR)
analysis
The 1H-NMR spectra of the ethanolic
extracts of T. crispa and P. weberi were recorded in
(CD3) CO2 and D2O using a JEOL JNM
ECP-400 spectrometer (400 MHz for 1 h), and chemical shifts were
referenced relative to the residual solvent peak. The resultant
spectra of the possible functional group present in the plants were
determined.
Statistical analysis
The data are expressed as the mean ± standard
deviation of at least three independent experiments. To compare
three or more groups, one-way analysis of variance, followed by the
Newman-Keuls post-hoc test, was used. Statistical analysis was
performed using GraphPad Prism software version 3.03 (GraphPad
Software, Inc., San Diego, CA, USA). P<0.05 was considered to
indicate a statistically significant difference.
Results
Effects of Cambodian plant extracts on
t-BHP-induced hepatotoxicity in human liver-derived HepG2
cells
The ethanolic extracts of 64 Cambodian plants on
human liver-derived HepG2 cells were assessed in vitro. As
shown in Table I, 51 plants
exhibited a cytoprotection value of <80%, whereas 19 plants
showed a cytoprotection value of >80%. At a final concentration
of 100 µg/ml, plant extracts of B. flabellifer (root), C.
halicacabum (whole plant), C. trifolia (stem), C.
caryophyllus (bark), C. rotundus (rhizome), F.
benjamina (stem), Q. indica (whole plant), S.
glabra (rhizome) and W. cochinchinensis (stem) resulted
in maintained cell viability (Table
I). At a final concentration of 300 µg/ml, the plant extracts
A. martinii (root), B. bracteata (stem), B.
ceiba (bark), D. lomentaceum (stem), M.
duperreana (bark), M. citrifolia (fruit), P.
humilis (stem), P. weberi (whole plant), P.
emblica (fruit) and T. crispa (stem) resulted in
maintained cell viability (Table
I). The 19 plant extracts with cytoprotective values >80% at
a final concentration of 100 µg/ml were used for further analysis
(Table II). The results revealed
the half maximal effective concentration (EC50) values
for B. flabellifer (66.25 µg/ml), C. halicacabum
(63.18 µg/ml), C. trifolia (59.23 µg/ml), C.
caryophyllus (64.33 µg/ml), C. rotundus (68.75 µg/ml),
F. benjamina (62.98 µg/ml), Q. indica (62.9 µg/ml),
S. glabra (80.41 µg/ml), W. cochinchinensis (72.95
µg/ml), A. martinii (121.1 µg/ml), B. bracteata
(138.9 µg/ml), B. ceiba (148.8 µg/ml), D. lomentaceum
(125.7 µg/ml), M. duperreana (105.2 µg/ml), M.
citrifolia (157.8 µg/ml), P. humilis (151.3 µg/ml),
P. weberi (121.7 µg/ml), P. emblica (144.9 µg/ml) and
T. crispa (144.3 µg/ml), as shown in Table II.
 | Table I.Protective effects of ethanolic
extracts (100 and 300 µg/ml) derived from Cambodian plants on
t-BHP-induced hepatotoxicity in HepG2 cells. |
Table I.
Protective effects of ethanolic
extracts (100 and 300 µg/ml) derived from Cambodian plants on
t-BHP-induced hepatotoxicity in HepG2 cells.
|
|
|
|
| Protection (%) |
|---|
|
|
|
|
|
|
|---|
| No. | Plant family | Plant species | Plant region | 100 µg/ml | 300 µg/ml |
|---|
| 1 | Anacardiaceae | Anacardium
occidentale L. | Bark | 11.5 | 45.1 |
| 2 |
| Mangifera
duperreana pierre | Bark | 45.1 | 85.1 |
| 3 | Annonaceae | Cananga
latifolia finet & gagnep. | Stem | −15.1 | −85.1 |
| 4 |
| Dasymaschalon
lomentaceum finet & gagnep. | Stem | 48.8 | 87.8 |
| 5 | Apocynaceae | Calotropis
procera (aiton) R. Br. | Stem | −33.9 | −19.8 |
| 6 |
| Streptocaulon
juventas (Lour.) merr. | Root | −26.7 | −33.1 |
| 7 |
| Willughbeia
cochinchinensis (Pierre) K. Schum. | Stem | 88.7 | 17.5 |
| 8 | Araceae | Alocasia
macrorrhiza schott. | Stem | −3.3 | −17.4 |
| 9 |
| Amorphophallus
harmandii engl. & gehrm. | Tuber | 1.9 | 19.3 |
| 10 | Arecaceae | Borassus
flabellifer L. | Root | 83.1 | 12.4 |
| 11 |
| Borassus
flabellifer L. | Male flower | 48.4 | −30.1 |
| 12 | Asparagaceae | Dracaena
angustifolia (medik.) roxb. | Stem | 9.6 | 51.7 |
| 13 |
| Peliosanthes
weberi (L. modr.) N. tanaka | Whole plant | 42.5 | 87.6 |
| 14 | Asteraceae | Blumea
balsamifera (L.) DC. | Stem | 26.9 | 47.2 |
| 15 | Capparaceae | Crateva
adansonii DC. | Bark | 0.7 | 1.9 |
| 16 | Caricaceae | Carica
papaya L. | Root | 3.9 | 15.6 |
| 17 | Combretaceae | Quisqualis
indica L. | Whole plant | 83.9 | 20.6 |
| 18 | Costaceae | Costus
speciosus (J. koenig) Sm. | Rhizome | −15.8 | −24.1 |
| 19 | Cyperaceae | Cyperus
rotundus L. | Rhizome | 89.6 | 14.4 |
| 20 | Dilleniaceae | Tetracera
indica (christm. & panz) merr. | Stem | 1.4 | 21.2 |
| 21 |
Dipterocarpaceae | Shorea
siamensis miq. | Flowers | 55.8 | 69.3 |
| 22 | Ebenaceae | Diospyros
ehretioides wall. ex G. don | Root | 46.8 | −10.3 |
| 23 |
| Diospyros
rhodocalyx kurz | Fruit | 0.7 | 3.5 |
| 24 | Euphorbiaceae | Croton
crassifolius geiseler | Rhizome | 10.1 | −31.2 |
| 25 | Fabaceae | Bauhinia
bracteata (benth.) baker | Stem | 36.0 | 88.1 |
| 26 |
| Cassia alata
L. | Stem | 30.4 | 30.0 |
| 27 |
| Dalbergia
hancei benth. | Stem | 74.8 | 48.7 |
| 28 | Lamiaceae | Gmelina
asiatica L. | Stem | 4.1 | 10.8 |
| 29 |
| Cinnamomum
cambodianum lecomte | Bark | 57.0 | 54.5 |
| 30 |
| Cinnamomum
caryophyllus (lour.) S. moore | Bark | 87.4 | 5.3 |
| 31 | Lecythidaceae | Careya
arborea roxb. | Bark | −76.1 | −16.7 |
| 32 | Loganiaceae | Strychnos
nux-vomica L. | Seed | 67.8 | 75.8 |
| 33 | Malvaceae | Abutilon indicum
(L.) sweet | Stem | 10.2 | 23.0 |
| 34 |
| Bombax ceiba
L. | Bark | 31.8 | 81.6 |
| 35 | Meliaceae | Walsura
villosa wall. ex wight & arn. | Bark | 42.5 | −34.3 |
| 36 | Menispermaceae | Fibraurea
tinctoria lour. | Stem | 37.9 | −80.8 |
| 37 |
| Tinospora
crispa (L.) hook. f. & thomson | Stem | 36.2 | 88.2 |
| 38 | Moraceae | Ficus
benjamina L. | Stem | 87.7 | 21.9 |
| 39 |
| Morus alba
L. | Bark | 10.4 | 30.1 |
| 40 | Pandanaceae | Pandanus
humilis lour. | Stem | 40.9 | 85.3 |
| 41 | Phyllanthaceae | Flueggea
virosa (roxb. ex willd.) baill. | Wood | 14.2 | 50.3 |
| 42 |
| Phyllanthus
emblica L. | Fruit | 34.5 | 85.5 |
| 43 | Piperaceae | Piper
retrofractum vahl | Fruit | −8.5 | −12.9 |
| 44 | Poaceae | Chrysopogon
aciculatus (retz.) trin. | Whole plant | 50.3 | 48.6 |
| 45 |
| Coix
lacryma-jobi L. | Whole plant | 65.2 | 2.0 |
| 46 |
| Cynodon
dactylon (L.) pers. | Aerial part | 70.4 | 24.0 |
| 47 |
| Imperata
cylindrica (L.) raeusch. | Rhizome | 12.2 | 27.4 |
| 48 |
| Oryza
rufipogon griff. | Whole plant | 20.3 | −15.3 |
| 49 | Rubiaceae | Anthocephalus
chinensis (lam.) rich. ex walp. | Wood | 27.1 | 45.5 |
| 50 |
| Gardenia
obtusifolia roxb. ex hook. f. | Rhizome | −25.1 | −22.8 |
| 51 |
| Morinda
citrifolia L. | Fruit | 30.7 | 84.3 |
| 52 | Rutaceae | Aegle
marmelos (L.) corrêa | Fruit | 0.8 | 23.6 |
| 53 |
| Feroniella
lucida swingle | Bark | −52.5 | −43.2 |
| 54 | Sapindaceae | Cardiospermum
halicacabum L. | Whole plant | 82.5 | 15.9 |
| 55 | Simaroubaceae | Brucea
javanica (L.) merr. | Whole plant | −65.9 | −42.0 |
| 56 |
| Smilax
glabra roxb. | Rhizome | 84.4 | 15.8 |
| 57 | Solanaceae | Physalis
angulata L. | Whole plant | −4.2 | −3.7 |
| 58 | Urticaceae | Pouzolzia
zeylanica (L.) benn. & R. Br. | Whole plant | 17.0 | 3.9 |
| 59 | Vitaceae | Ampelocissus
martinii planch. | Root | 45.9 | 85.9 |
| 60 |
| Cayratia
trifolia (L.) domin | Stem | 88.5 | 10.9 |
| 61 |
| Cissus
modeccoides planch. | Stem | 59.8 | 25.4 |
| 62 |
| Leea rubra
blume ex spreng. | Stem | 10.4 | 30.2 |
| 63 | Zingiberaceae | Alpinia
conchigera griff. | Rhizome | −44.8 | −42.0 |
| 64 |
| Amomum
krervanh pierre ex gagnep. | Fruit | 10.7 | 8.8 |
 | Table II.Protective effects of ethanolic
extracts from Cambodian plants on t-BHP-induced
hepatotoxicity in HepG2 cells. |
Table II.
Protective effects of ethanolic
extracts from Cambodian plants on t-BHP-induced
hepatotoxicity in HepG2 cells.
| No. | Plant species | Plant region | EC50
(µg/ml) |
|---|
| 1 | Ampelocissus
martinii planch. | Root | 121.1 |
| 2 | Bauhinia
bracteata (benth.) baker | Stem | 138.9 |
| 3 | Bombax ceiba
L. | Bark | 148.8 |
| 4 | Borassus
flabellifer L. | Root | 66.2 |
| 5 | Cardiospermum
halicacabum L. | Whole plant | 63.2 |
| 6 | Cayratia
trifolia (L.) domin | Stem | 59.2 |
| 7 | Cinnamomum
caryophyllus (Lour.) S. moore | Bark | 64.3 |
| 8 | Cyperus
rotundus L. | Rhizome | 68.8 |
| 9 | Dasymaschalon
lomentaceum finet & gagnep. | Stem | 125.7 |
| 10 | Ficus
benjamina L. | Stem | 63.0 |
| 11 | Mangifera
duperreana pierre | Bark | 105.2 |
| 12 | Morinda
citrifolia L. | Fruit | 157.8 |
| 13 | Pandanus
humilis lour. | Stem | 151.3 |
| 14 | Peliosanthes
weberi (L. rodr.) N. tanaka | Whole plant | 121.7 |
| 15 | Phyllanthus
emblica L. | Fruit | 144.9 |
| 16 | Quisqualis
indica L. | Whole plant | 62.9 |
| 17 | Smilax
glabra roxb. | Rhizome | 80.4 |
| 18 | Tinospora
crispa (L.) hook. f. & thomson | Stem | 144.3 |
| 19 | Willughbeia
cochinchinensis (pierre) K. Schum. | Stem | 73.0 |
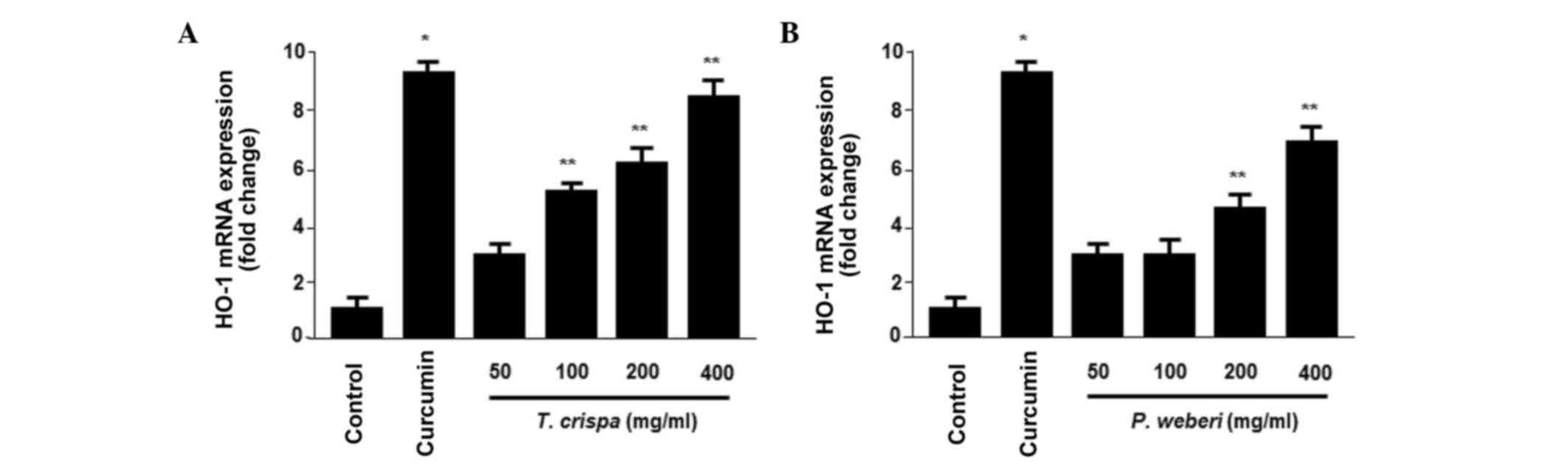
Effects of Cambodian plant extracts on
the mRNA and protein expression levels of HO-1 in human
liver-derived HepG2 cells
HO-1 has been reported to be upregulated in
hepatocytes (14). Therefore, the
present study performed further screening of the 19 plant extracts
for the protein expression of HO-1 in the HepG2 cells (Fig. 1). Whether treatment of the HepG2
cells with the selected extracts affected the mRNA expression of
HO-1 was subsequently examined. The protein and mRNA expression
levels were determined following treatment for 12 h. Amongst the 19
plant extracts, the T. crispa and P. weberi extracts
significantly increased the protein (Fig. 1E and J) and mRNA (Fig. 2A and B) levels of HO-1 in a
dose-dependent manner, with maximal values observed at 400 µg/ml.
The HO-1 inducer, curcumin, was used at a concentration of 20 µM as
a positive control, which also increased the expression of
HO-1.
Effects of the upregulation of HO-1 on
t-BHP-induced hepatotoxicity and the inhibition of ROS generation
by T. crispa or P. weberi extracts
HO-1 is considered to possess cytoprotective effects
against oxidative stress-induced cell damage, notably in HepG2
cells (22). Thus, the present
study investigated whether the upregulation of HO-1 triggered by
T. crispa or P. weberi extracts mediated these
cytoprotective effects. The HepG2 cells were co-treated with 400
µg/ml of T. crispa or P. weberi extracts for 12 h in
the absence or presence of SnPP, an inhibitor of HO activity. These
inhibitors significantly suppressed T. crispa/P.
weberi extract-mediated protection (Fig. 3A and B). The T. crispa/P.
weberi extract-induced upregulation of HO-1 was also required
for the suppression of t-BHP-induced ROS generation (Fig. 3C and D).
Effects of T. crispa and P. weberi
extracts on the nuclear translocation of Nrf2
The nuclear translocation of activated Nrf2 is a key
upstream regulator for the expression of HO-1 (23). Therefore, the present study
examined whether treatment of cells with T. crispa or P.
weberi extracts induced the translocation of Nrf2 into the
nucleus. The results of the western blot analysis revealed the
presence of Nrf2 proteins in the nuclear compartment of HepG2
cells. The cells were incubated with 400 µg/ml T. crispa or
P. weberi extracts for 0.5, 1 and 1.5 h. As shown in
Fig. 4A and B, the nuclear
fractions of the T. crispa or P. weberi
extract-treated cells exhibited a gradual increase in the levels of
Nrf2, and the levels of Nrf2 simultaneously decreased in the
cytoplasmic fractions.
Discussion
Previous studies have shown that several medicinal
plants, including Curcuma longa and Glycyrrhiza
glabra exert hepatoprotective effects by quenching ROS, which
is a primary cause of oxidative damage in hepatocytes (24). HO-1, a heme-degrading enzyme, is
involved in anti-inflammatory, anti-apoptotic and antiproliferative
effects under various conditions (13). The induction of HO-1 is also likely
to be a key therapeutic target in hepato-oxidative damage (25). HepG2 cells have been shown to
express HO-1 (26). Several lines
of evidence suggest that t-BHP, often used as a model in
biological investigations (27),
induces apoptotic cell death in HepG2 cells through the depletion
of GSH and increase in intracellular Ca2+ concentrations
(28). Therefore, t-BHP was
used as a cell-death-inducing agent or negative control in the
present study. In identifying the hepatoprotective effects of the
extracts in hepatocytes, curcumin has been extensively selected as
a positive control to identify cytoprotective actions through the
upregulation of HO-1 (29,30). Therefore, curcumin was used as a
positive control for the screening assay in the present study.
Based upon the results from the preliminary
screening for hepatoprotective effects (Table I), 19 active plant extracts were
confirmed by performing an additional screen (Table II). HO-1 screening was then
performed using western blot analysis (Fig. 1). The induction of cytoprotective
enzymes is key to the cytoprotective mechanism. In the present
study, it was shown that the ethanolic extracts of T. crispa
and P. weberi induced the protein and mRNA expression levels
of HO-1 and in HepG2 cells in a concentration-dependent manner
(Figs. 1 and 2). In addition, pre-incubation of cells
with the T. crispa or P. weberi extracts resulted in
enhanced resistance to t-BHP-induced oxidative damage; this
effect was attributable to the expression of HO-1 as the inhibition
of HO enzyme activity by SnPP significantly reduced T.
crispa/P. weberi extract-induced cytoprotection
(Fig. 3). The induction of the
expression of HO-1 was also required to suppress
t-BHP-induced ROS generation. Several reports have indicated
that secondary metabolites of plants can activate Nrf2 by binding
to Kelch-like ECH-associated protein 1, leading to the upregulation
of certain cytoprotective proteins, including HO-1 (15,31).
This suggestion is in accordance with the findings of the present
study showing that T. crispa or P. weberi extracts
significantly increased the levels of Nrf2 and efficiently promoted
the translocation of Nrf2 into the nuclei of HepG2 cells (Fig. 4). T. crispa, known as ‘akar
seruntum’ or ‘akar patawali’ to the Malays, belongs to the family
Menispermaceae and is native to China and Southeast Asia, including
Malaysia. Extracts of T. crispa has been used in folk
medicine as a therapeutic agent for the treatment of fever,
jaundice, hyperglycemia, hypertension, wounds, intestinal worms and
skin infections. In addition, several studies have revealed that
T. crispa possesses anti-inflammatory, antioxidant,
antiallergic, hepatoprotective, antithrombotic, antiviral and
anticarcinogenic activities (32,33).
In the present study, the ethanolic extract from the aerial parts
of T. crispa exerted cytoprotective effects on
t-BHP-induced hepatotoxicity in HepG2 cells (EC50
144.3 µg/ml; Table II). The
cytoprotective activity may be attributed to the presence of
alkaloids, diterpenoid lactones, sesquiterpenoids, phenolics and
aliphatic compounds, which are generally found in T. crispa
(34), which is partially
indicated in Table II. P
weberi, widely distributed in Thailand, Lao, Malaysia and
Southeast Asia, belongs to the family Liliaceae (35–37),
which has antioxidant, antihypertensive, anticholinergic,
antiasthmatic and antifungal actions (38,39).
In the present study, the leaf extract of P. weberi showed
hepatoprotective activity against t-BHP-induced cytotoxicity
in HepG2 (EC50 121.7 µg/ml. The cytoprotective activity
of P. weberi may be due to the presence of steroidal
alkaloids (39). The
1H-NMR analyses of the ethanolic extracts of T.
crispa and P. weberi revealed the presence of aliphatic
or aromatic compounds (40) (data
not shown), which may be responsible for the hepatoprotective
properties of these plant extracts. In the 17 other extracts, a
different mechanism may occur. Nrf2 induces genes encoding numerous
phase II detoxifying enzymes, including HO-1, glutathione,
catalase, superoxide dismutase, glutathione-S-transferase,
γ-glutamyl cysteine ligase, NADPH quinone oxidoreductase-1,
glutathione peroxidase and glutathione reductase (15,16).
Further extensive investigations are likely to provide evidence for
the other representative mechanisms of hepatoprotection in plant
extracts.
In conclusion, among 64 plants extracts from
Cambodian medicinal plants, 19 plant extracts showed promising
hepatoprotective activities. Of these, the extracts of T.
crispa (aerial region) and P. weberi (whole plant)
exerted potent cytoprotective effects against t-BHP-induced
hepatotoxicity in human derived-HepG2 cells, possibly through the
Nrf2-mediated expression of HO-1. Taken together, these results
suggested that the ethalolic extracts of T. crispa and P.
weberi may possess therapeutic applications in liver disease
characterized by oxidative stress.
Acknowledgements
This study was supported by the Basic Science
Research Program through the National Research Foundation of Korea
funded by the Ministry of Education, Science and Technology (grant
nos. 2012R1A1A2042984 and 2008-0062484).
References
|
1
|
Kham L: Medicinal Plants of Cambodia:
Habitat, Chemical Constituents and Ethnobotanical Uses. 1st.
Bendigo Scientific Press; Golden Square: pp. 1–3. 2004
|
|
2
|
Richman MJ, Nawabi S, Patty L and Ziment
I: Traditional Cambodian medicine. J Complement Integr Med. 7:1–14.
2010.
|
|
3
|
World Health Organization, . Health
service delivery profile-Cambodia. Compiled in collaboration
between WHO and Ministry of Health; Cambodia: http://www.wpro.who.int/countries/khm/enAccessed
April 24, 2014.
|
|
4
|
Ling CQ, Chiu JH, Oh B and Cho WC: Natural
products for liver diseases: Basic, clinical and translational
research. Evid Based Complement Alternat Med.
2012:7943432012.PubMed/NCBI
|
|
5
|
El-Serag HB: Current concepts:
Hepatocellular carcinoma. N Engl J Med. 365:1118–1127. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Czaja MJ: Cell signaling in oxidative
stress-induced liver injury. Semin Liver Dis. 27:378–389. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Buc-Calderon P, Latour I and Roberfroid M:
Biochemical changes in isolated hepatocytes exposed to tert-Butyl
hydroperoxide. Implications for its cytotoxicity. Cell Biol
Toxicol. 7:129–143. 1991. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Muriel P and Rivera-Espinoza Y: Beneficial
drugs for liver diseases. J Appl Toxicol. 28:93–103. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Luper S: A review of plants used in the
treatment of liver disease: Part 1. Altern Med Rev. 3:410–421.
1998.PubMed/NCBI
|
|
10
|
Zhang A, Sun H and Wang X: Recent advances
in natural products from plants for treatment of liver diseases.
Eur J Med Chem. 63:570–577. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Schuppan D, Jia JD, Brinkhaus B and Hahn
EG: Herbal products for liver diseases: A therapeutic challenge for
the new millennium. Hepatology. 30:1099–1104. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Pae HO, Kim EC and Chung HT: Integrative
survival response evoked by heme oxygenase-1 and heme metabolites.
J Clin Biochem Nutr. 42:197–203. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Otterbein LE, Soares MP, Yamashita K and
Bach FH: Heme oxygenase-1: Unleashing the protective properties of
heme. Trends Immunol. 24:449–455. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Fernandez M and Bonkovsky HL: Increased
heme oxygenase-1 gene expression in liver cells and splanchnic
organs from portal hypertensive rats. Hepatology. 29:1672–1679.
1999. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Shin SM, Yang JH and Ki SH: Role of the
Nrf2-ARE pathway in liver diseases. Oxid Med Cell Longev.
2013:7632572013.PubMed/NCBI
|
|
16
|
Copple IM, Goldring CE, Kitteringham NR
and Park BK: The Nrf2-Keap1 defence pathway: Role in protection
against drug-induced toxicity. Toxicology. 246:24–33. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Li YM: Alcoholism and alcoholic liver
disease: Focusing on epidemiological investigation in Asia.
Hepatobiliary Pancreat Dis Int. 4:170–172. 2005.PubMed/NCBI
|
|
18
|
Banta JE, Addison A, Job JS, Yel D, Kheam
T and Singh PN: Patterns of alcohol and tobacco use in Cambodia.
Asia Pac J Public Health. 25:(Suppl 5). S33–S44. 2013. View Article : Google Scholar
|
|
19
|
Kraisintu K: The status of medicinal and
aromatic plants in Cambodia, Laos, the Philippines, Thailand and
VietnamLongo G: Medicinal Plants and Their Utilization. ICS-UNIDO;
Trieste: pp. 3–9. 2003
|
|
20
|
Hoshi H and Kan M: Growth factor assays
for normal human hepatocytes and hepatoma cells. J Tissue Cult
Meth. 10:83–92. 1986. View Article : Google Scholar
|
|
21
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(−Delta Delta C(T)) Method. Methods. 25:402–408. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Ghattas MH, Chuang LT, Kappas A and
Abraham NG: Protective effect of HO-1 against oxidative stress in
human hepatoma cell line (HepG2) is independent of telomerase
enzyme activity. Int J Biochem Cell Biol. 34:1619–1628. 2002.
View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Gozzelino R, Jeney V and Soares MP:
Mechanisms of cell protection by heme oxygenase-1. Annu Rev
Pharmacol Toxicol. 50:323–354. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Luper S: A review of plants used in the
treatment of liver disease: Part two. Altern Med Rev. 4:178–188.
1999.PubMed/NCBI
|
|
25
|
Bauer M and Bauer I: Heme oxygenase-1:
Redox regulation and role in the hepatic response to oxidative
stress. Antioxid Redox Signal. 4:749–758. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Gong P, Cederbaum AI and Nieto N: Heme
oxygenase-1 protects HepG2 cells against cytochrome P450
2E1-dependent toxicity. Free Radic Biol Med. 36:307–318. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Van der Zee J, Barr DP and Mason RP: ESR
spin trapping investigation of radical formation from the reaction
between hematin and tert-butyl hydroperoxide. Free Radic Biol Med.
20:199–206. 1996. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Kim JA, Kang YS, Kim YO, Lee SH and Lee
YS: Role of Ca2+ influx in the tert-butyl
hydroperoxide-induced apoptosis of HepG2 human hepatoblastoma
cells. Exp Mol Med. 30:137–144. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Cerný D, Lekić N, Váňová K, Muchová L,
Hořínek A, Kmoníčková E, Zídek Z, Kameníková L and Farghali H:
Hepatoprotective effect of curcumin in
lipopolysaccharide/D-galactosamine model of liver injury in rats:
Relationship to HO-1/CO antioxidant system. Fitoterapia.
82:786–791. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
McNally SJ, Harrison EM, Ross JA, Garden
OJ and Wigmore SJ: Curcumin induces heme oxygenase 1 through
generation of reactive oxygen species, p38 activation and
phosphatase inhibition. Int J Mol Med. 19:165–172. 2007.PubMed/NCBI
|
|
31
|
Itoh K, Chiba T, Takahashi S, Ishii T,
Igarashi K, Katoha Y, Oyake T, Hayashi N, Satoh K, Hatayama I, et
al: An Nrf2/small Maf heterodimer mediates the induction of phase
II detoxifying enzyme genes through antioxidant response elements.
Biochem Biophys Res Commun. 236:313–322. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Kadir FA, Othman F, Abdulla MA, Hussan F
and Hassandarvish P: Effect of Tinospora crispa on
thioacetamide-induced liver cirrhosis in rats. Indian J Pharmacol.
43:64–68. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Higashino H, Suzuki A, Tanaka Y and
Pootakham K: Inhibitory effects of Siamese Tinospora crispa
extracts on the carrageenin-induced foot pad edema in rats (the 1st
report). Folia Pharmacologica Japonica. 100:339–344. 1992.(In
Japanese). View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Hipol RLB, Cariaga MFNM and Hipol RM:
Anti-inflammatory activities of the aqueous extract of the stem of
Tinospora crispa (Family Menispermaceae). J Nat Stud. 11:88–95.
2012.
|
|
35
|
Chaikla P, Suwanthada C and Trisonthi C:
Morphological, anatomic and karyotypic characteristics of
Peliosanthes teta Andrew. Afr J Agr Res. 6:6698–6705. 2011.
|
|
36
|
Eswani N, Kudus KA, Nazre M and Noor AGA:
Medicinal plant diversity and vegetation analysis of logged over
hill forest of Tekai Tembeling forest reserve, Jerantut, Pahang. J
Agr Sci. 2:189–210. 2010.
|
|
37
|
Lundh ECS: Plant use in ante- and
postpartum health care in Lao PDR (unpublished PhD thesis). Uppsala
University; Uppsala: 2007
|
|
38
|
Gupta VK and Sharma SK: Plants as natural
antioxidants. Nat Prod Rad. 5:326–334. 2006.
|
|
39
|
Li HJ, Jiang Y and Li P: Chemistry,
bioactivity and geographical diversity of steroidal alkaloids from
the Liliaceae family. Nat Prod Rep. 23:735–752. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Silverstein RM, Webster FX and Kiemle DJ:
Spectrometric identification of organic compounds. 7th. Hoboken:
Wiley & Sons; pp. 141–142. 2005
|