Introduction
Cardiovascular diseases remain a significant health
and socioeconomic burden in developed countries (1). Surgical bypass with an autologous
vein remains the primary treatment (2), however, a usable vascular graft is
often absent due to limited sources and donor site morbidity. In
addition, current widely used synthetic materials have several
limitations including immunological and thrombotic complications.
Furthermore, these vascular grafts are usually non-degradable and
lack growth potential (3,4).
The development of tissue engineering technology is
promising for potential improvement over currently used synthetic
grafts. A blood vessel made of autologous cells and a biocompatible
scaffold with the potential to remodel, repair and grow would be a
major therapeutic advance (5).
Promising results have been demonstrated with small diameter (<6
mm) tissue engineered blood vessels (TEBVs) under low blood
pressure (6–8), however, few studies have focused on
cases with larger vessels (>6 mm in diameter), which demand an
increased number of seed cells and improved biomechanical
properties.
However, tissue engineering approaches are limited
by the large number of cells that must be obtained for regenerative
medicine. Stem cells are promising cell sources with increased
proliferation and broad differentiation capacity, making them
suitable for the preparation of TEBVs (9–11).
Although several types of stem and progenitor cells have been
investigated for their potential as sources of seed cells in
vascular tissue engineering (12–16),
hair follicles are increasingly used for stem cell research due to
the fact that they are a rich source of easily accessible
multipotent adult stem cells. Hair follicle stem cells (HFSCs) have
been demonstrated to possess osteogenic, adipogenic, chondrogenic,
neurogenic and myogenic lineage differentiation potential (7,17–19).
In a previous study, human HFSCs (hHFSCs) were
successfully induced to differentiate into functional smooth muscle
cells (SMCs) by transforming growth factor-β1 (TGF-β1) and
platelet-derived growth factor BB (PDGF-BB) in combination with
low-serum culture medium (20).
The aim of the present study was to engineer a large vessel (6 mm
in diameter) using induced hHFSCs and polyglycolic acid (PGA) with
8 weeks of in vitro culture. The rapid degradation of the
PGA prevents the accumulation of degraded fragments in vivo.
The culture system used indicated potential to construct large
muscular vessels with differentiated SMCs induced from hHFSCs.
Materials and methods
Isolation and culture of hHFSCs
hHFSCs were obtained from human scalp tissue from
healthy adult patients (average age, 30 years) undergoing cosmetic
plastic surgery, as described previously (20). All protocols for human tissue
handling were approved by the Research Ethical Committee of
Shanghai 9th People's Hospital, and written informed consent was
obtained from the patients. hHFSCs at the second passage were used
in the subsequent study. The hHFSCs were characterised by
determining their CD marker profile (K15, K19 and integrin β1) and
their ability to differentiate into osteogenic, adipogenic and
chondrogenic lineages (data not shown), as reported previously
(21,22).
Induction of SM differentiation
As previously reported (20), hHFSCs reaching subconfluence were
cultured in low-glucose Dulbecco's modified Eagle's medium
(LG-DMEM; Gibco; Thermo Fisher Scientific, Inc., Waltham, MA, USA)
containing 10% foetal bovine serum (FBS; GE Healthcare Life
Sciences, Logan, UT, USA) supplemented with 5 ng/ml recombinant
human TGF-β1 (R&D Systems, Inc., Minneapolis, MN, USA) and 10
ng/ml recombinant human PDGF-BB (R&D Systems, Inc.) with 1%
FBS. DMEM supplemented with 1% FBS was defined as the basal medium
(BM). Human umbilical artery SMCs (hUASMCs) were obtained from
ScienCell Research Laboratories (Carlsbad, CA, USA) and used as the
positive control. The culture media were changed every 2 days. Cell
characterisation and functional evaluation (data not shown) were
performed subseqeuent to 8 days of culture as described previously
(20).
Culture of hHFSC-PGA sheets in
dishes
In the current study, 35 mg unwoven PGA fibres
(Albany International Research Co., Albany, NY, USA) were
constructed into an approximately 35×80×2 mm mesh. The scaffold was
first soaked in 75% ethanol (Sigma-Aldrich; Merck Millipore,
Darmstadt, Germany) for 2 h. Subsequently, it was washed three
times with phosphate-buffered saline and incubated in DMEM for 10
min. The medium was removed, and the scaffold was incubated in an
incubator (Binder GmbH, Tuttlingen, German) at 37°C prior to use.
Differentiated and undifferentiated hHFSCs (6×107) were
each evenly seeded onto the PGA mesh in 100-mm culture dishes
(Falcon; BD Biosciences, San Jose, CA, USA). To accomplish the
complete adhesion of the hHFSCs to the fibres, the cell-scaffold
constructs were then maintained in the incubator at 37°C with 95%
humidity and 5% CO2 for approximately 4 h. Thereafter,
sufficient induced culture medium or BM was added to the two dishes
to cover the constructs. The cell-PGA sheets were incubated at 37°C
for another 5 days prior to use.
Induced culture in dishes
Subsequent to culture in dishes for 5 days, the
cell-PGA sheets were wrapped around the silicone tubes and fixed by
biodegradable sutures (Ethicon, Inc., Somerville, NJ, USA) for
another 8 weeks of culture. The culture media was changed twice a
week. The cell-PGA constructs cultured in BM were used as the
controls.
Histological analysis
Subsequent to 8 weeks of culture, the engineered
vessel walls were harvested, fixed in 10% formalin (Sigma-Aldrich;
Merck Millipore) and embedded in paraffin (Sigma-Aldrich; Merck
Millipore). Following this, they were sequentially cut into
sections of 4 mm thickness. The sections were then tested with
haematoxylin and eosin or Masson's trichrome and Gömöri staining
(all stains were from Sigma-Aldrich; Merck Millipore).
Hydroxyproline assay
For hydroxyproline assessment, the vessel wall was
dried and weighed. The total hydroxyproline content of each vessel
was determined by a colorimetric assay described by Reddy and
Ewemeka (23). In the current
study, a Sigma-MAK008, Hydroxyproline Assay Kit (Sigma-Aldrich;
Merck Millipore) and a Genesys 20 Spectrophotometer (Z376027;
Sigma-Aldrich; Merck Millipore) were used. Normal human saphenous
vein with a diameter of 4 mm served as the control. The veins were
obtained from human adult patients undergoing cardiovascular
surgery with autologous vein graft. The residual saphenous veins
were contributed for future experimental studies, written informed
consent was obtained from the patients. All protocols for human
tissue handling were approved by the Research Ethical Committee of
Shanghai 9th People's Hospital.
Statistical analysis
Each experiment was repeated a minimum of three
times. The results were expressed as the mean ± standard deviation.
Significant differences were measured using Student's t-test.
P<0.05 was considered to indicate a statistically significant
differences. All of the statistical analyses were performed using
SPSS software, version 16 (SPSS, Inc., Chicago, IL, USA).
Results
Culture of hHFSC-PGA constructs in
dishes
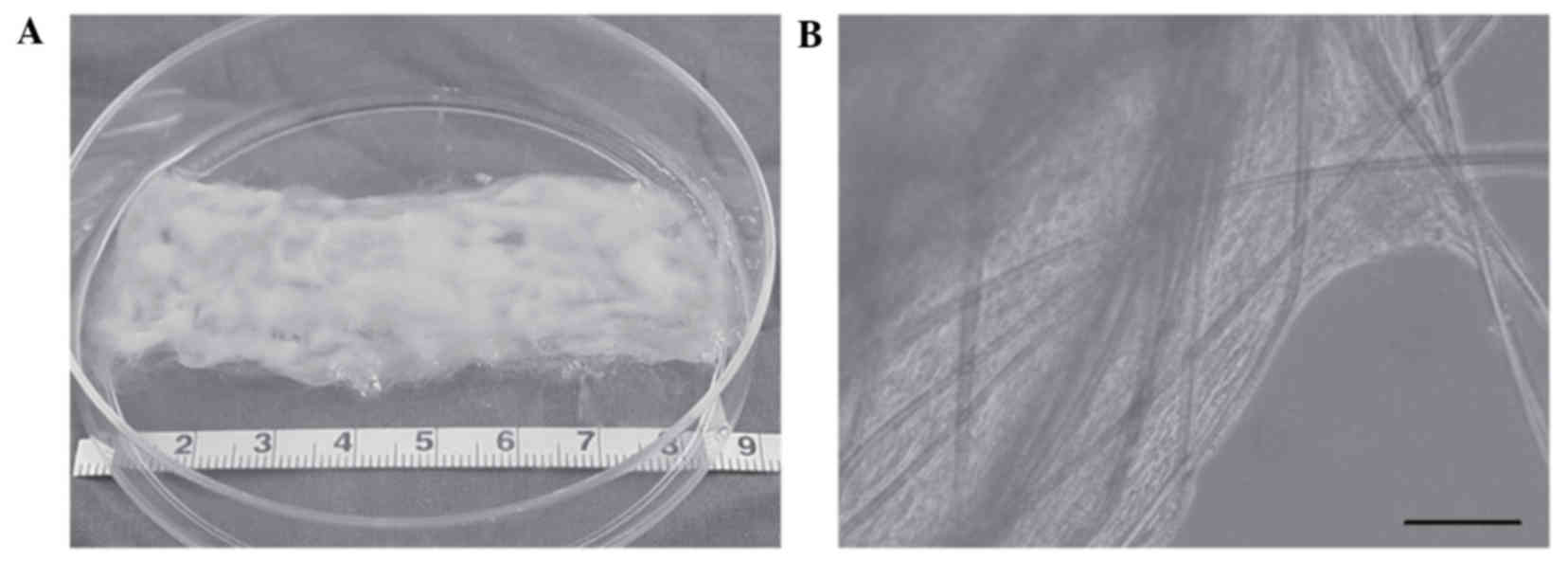
Subsequent to 24 h of culture, the cells began to
spread and extended along the length of the fibres. Following an
additional 5 days of culture in the dishes, a cell-PGA sheet had
formed (Fig. 1A). Micrographs
indicated that abundant hHFSCs had adhered to the PGA fibres, with
secreted extracellular matrix (ECM) filling the spaces between the
fibres (Fig. 1B).
Induced culture in dishes
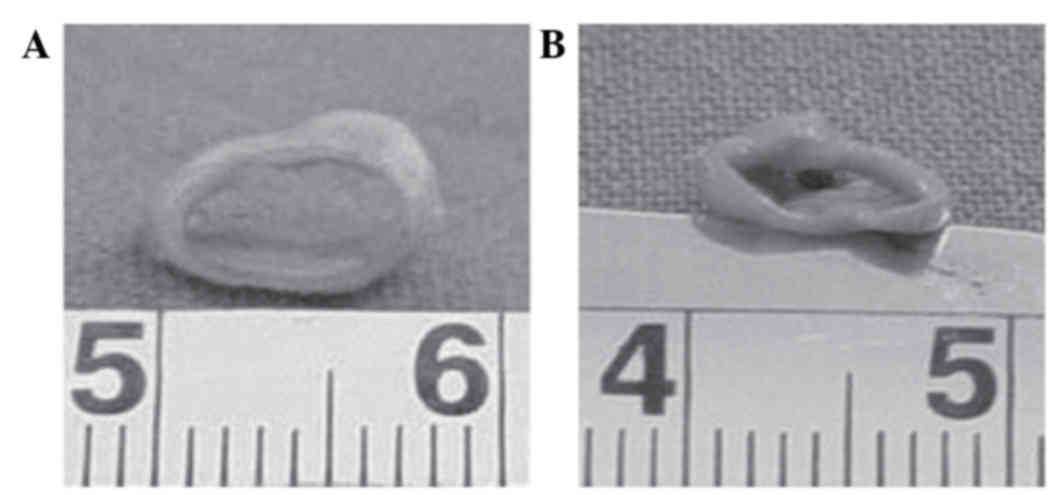
The hHFSC-PGA constructs were incubated in culture
dishes for 8 weeks subsequent to being wrapped around silicone
tubes (Fig. 2). In the induced
group, the constructs demonstrated a glossy and tubular structure
with a round lumen 6 mm in diameter (Fig. 3A). In contrast, the vessel walls in
the static culture group exhibited a collapsed lumen and rough
surface (Fig. 3B).
Histological observation
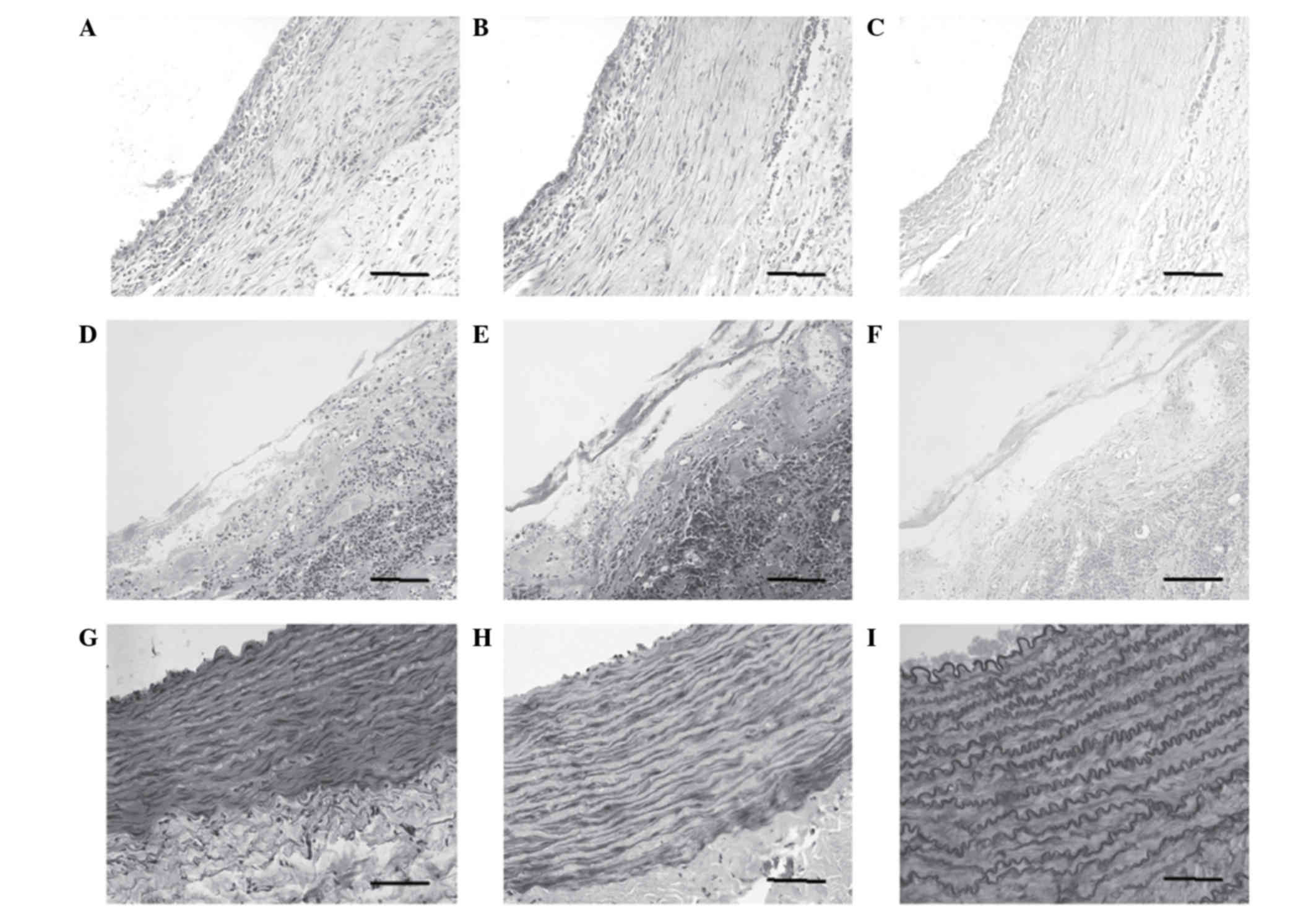
Subsequent to 8 weeks of further induced culture
in vitro, several smooth muscle-like cells and few
collagenous fibres were observed by histological examination
(Fig. 4A and B). The PGA fibres
had degraded completely, and few elastic fibres were observed at
this time (Fig. 4C). In contrast,
disorganised cells, randomly collagenous fibres and small elastic
fibres were observed in the undifferentiated group (Fig. 4D-F). The above results were further
confirmed by immunohistochemical staining for smooth muscle α-actin
and calponin (data not shown), using the hUASMCs as a positive
control (Fig. 4G-I).
Hydroxyproline assay
The hydroxyproline content was significantly higher
(P<0.05) in the induced group than in the control group at the
same time points (Fig. 5). In
addition, the hydroxyproline concentration in the induced group
reached approximately 65% of that in the hUASMCs.
Discussion
hHFSCs have been previously successfully isolated
from patients, expanded, differentiated and used to construct
autologous tissue (22,24–26).
This method eliminates the need for immunosuppressants and
mitigates the risk of teratoma formation associated with embryonic
stem cells and induced pluripotent stem cells (27). In a previous study, hHFSCs were
induced to differentiate into functional SMCs by TGF-β1 and PDGF-BB
in combination with low-serum culture medium (20). In the current study, a large
diameter vessel wall was engineered using the aforementioned
differentiated hHFSCs and PGA unwoven fibre mesh in vitro.
Subsequent to culture, the newly formed tissues exhibited SM-like
characteristics, including morphological performance, the
expression of SM cell-specific markers (SM α-actin and calponin)
and appropriate hydroxyproline content. These results demonstrated
that hHFSCs may be utilised as a potential cell source for the
tissue engineering of SMs, particularly that of the large diameter
aorta.
Tissue engineering predominantly focuses on the
incorporation of isolated cells with supporting scaffolds (28). An optimal scaffold degrades
proportionally with tissue regeneration to be gradually replaced by
newly formed functional tissue, and it supports cellular adhesion
and collagenous matrix deposition (29). In vascular tissue engineering, the
scaffold should reflect the biomechanical properties of blood
vessels and serve as a platform for cell attachment and
proliferation (30). It should be
non-thrombogenic, non-immunogenic, biocompatible, haemocompatible,
biodegradable and elastic (31,32).
Furthermore, it should also control the extent and the strength of
cell adhesion, proliferation, differentiation and maturation to
achieve the desired phenotype and proper function (33). PGA is one of the most extensively
used polymer scaffold materials in the engineering of numerous
types of tissues, including blood vessels. It is a polyester that
undergoes rapid degradation via hydrolysis of ester bonds, leaving
behind glycolic acid and is further catabolised into water and
carbon dioxide (34). The
mechanical properties and degradation profile of PGA make it an
attractive candidate for vascular tissue engineering (35). Numerous studies have demonstrated
the successful use of PGA scaffolds for constructing vascular
grafts (7,31,32,34,36).
In the current study, cells in the experimental group were
demonstrated to possess good proliferative ability and ECM
secretion on PGA.
In the histological examination, it was identified
that the content and distribution of SMCs and elastin in the
experimental group were not as dense as those in normal blood
vessels. Normal blood vessels have been demonstrated to develop
under the influence of the mechanical force of blood, which is an
important physiological component of the environment experienced by
cells: It promotes the circumferential orientation of the cells in
addition to the deposition of the extracellular matrix, and it
likely contributes to the survival of implanted substitutes
(36). It is suggested that
dynamic culturing is important in vascular tissue engineering.
In vitro investigations have demonstrated that low shear
stress induces SMC proliferation (37–40)
and promotes collagen alignment (41) and that cyclic stretching induces
clear alterations in the SMC phenotype, function and gene
expression (42,43). SMCs use multiple sensing mechanisms
to perceive the mechanical stimulus generated from pulsatile
stretching and transduce it into intracellular signals. This
results in the modulation of gene expression and cellular functions
including proliferation, apoptosis, migration and remodelling
(44–49). Therefore, a bioreactor, which is
currently is under development, may be used in future studies to
mimic the physiological environment of the arterial vessel
wall.
In conclusion, a large vessel (6 mm in diameter) was
constructed using PGA seeded with hHFSCs in vitro. Induced
culture constructs exhibited improved performance histologically
and in hydroxyproline content when compared with constructs of the
undifferentiated group. Further research focused on dynamic
culturing of the constructs and further seeding of endothelial
cells on the surface of the lumen to engineer composite vascular
conduits is required, in order to progress in this area of
bio-engineering.
Acknowledgements
The present study was supported by the National
Natural Science Foundation of China (grant no. 81000842). The
authors would additionally like to thank Mr Demin Ying, Mrs Lijuan
Zong and Mr Bing Zhong for their technical assistance.
References
|
1
|
Kohn JC, Lampi MC and Reinhart-King CA:
Age-related vascular stiffening: Causes and consequences. Front
Genet. 6:1122015.PubMed/NCBI
|
|
2
|
Lee AY, Mahler N, Best C, Lee YU and
Breuer CK: Regenerative implants for cardiovascular tissue
engineering. Transl Res. 163:321–341. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Kurobe H, Maxfield MW, Breuer CK and
Shinoka T: Concise review: Tissue-engineered vascular grafts for
cardiac surgery: Past, present, and future. Stem Cells Transl Med.
1:566–571. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Nemeno-Guanzon JG, Lee S, Berg JR, Jo YH,
Yeo JE, Nam BM, Koh YG and Lee JI: Trends in tissue engineering for
blood vessels. J Biomed Biotechnol. 2012:9563452012.PubMed/NCBI
|
|
5
|
Teebken OE and Haverich A: Tissue
engineering of small diameter vascular grafts. Eur J Vasc Endovasc
Surg. 23:475–485. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Wang Y, Hu J, Jiao J, Liu Z, Zhou Z, Zhao
C, Chang LJ, Chen YE, Ma PX and Yang B: Engineering vascular tissue
with functional smooth muscle cells derived from human iPS cells
and nanofibrous scaffolds. Biomaterials. 35:8960–8969. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Wang C, Cen L, Yin S, Liu Q, Liu W, Cao Y
and Cui L: A small diameter elastic blood vessel wall prepared
under pulsatile conditions from polyglycolic acid mesh and smooth
muscle cells differentiated from adipose-derived stem cells.
Biomaterials. 31:621–630. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Wilhelmi M, Jockenhoevel S and Mela P:
Bioartificial fabrication of regenerating blood vessel substitutes:
Requirements and current strategies. Biomed Tech (Berl).
59:185–195. 2014.PubMed/NCBI
|
|
9
|
Nerem RM and Seliktar D: Vascular tissue
engineering. Annu Rev Biomed Eng. 3:225–243. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Naito Y, Shinoka T, Duncan D, Hibino N,
Solomon D, Cleary M, Rathore A, Fein C, Church S and Breuer C:
Vascular tissue engineering: Towards the next generation vascular
grafts. Adv Drug Deliv Rev. 63:312–323. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Khait L and Birla RK: Bypassing the
patient: Comparison of biocompatible models for the future of
vascular tissue engineering. Cell Transplant. 21:269–283. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Zhu GC, Gu YQ, Geng X, Feng ZG, Zhang SW,
Ye L and Wang ZG: Experimental study on the construction of small
three-dimensional tissue engineered grafts of electrospun
poly-ε-caprolactone. J Mater Sci Mater Med. 26:1122015. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Koobatian MT, Liang MS, Swartz DD and
Andreadis ST: Differential effects of culture senescence and
mechanical stimulation on the proliferation and leiomyogenic
differentiation of MSC from different sources: Implications for
engineering vascular grafts. Tissue Eng Part A. 21:1364–1375. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
14
|
GN, Tan A, Gundogan B, Farhatnia Y, Nayyer
L, Mahdibeiraghdar S, Rajadas J, De Coppi P, Davies AH and
Seifalian AM: Tissue engineering vascular grafts a fortiori:
Looking back and going forward. Expert Opin Biol Ther. 15:231–244.
2015. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Sundaram S, One J, Siewert J, Teodosescu
S, Zhao L, Dimitrievska S, Qian H, Huang AH and Niklason L:
Tissue-engineered vascular grafts created from human induced
pluripotent stem cells. Stem Cells Transl Med. 3:1535–1543. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Rammal H, Harmouch C, Lataillade JJ,
Laurent-Maquin D, Labrude P, Menu P and Kerdjoudj H: Stem cells: A
promising source for vascular regenerative medicine. Stem Cells
Dev. 23:2931–2949. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Heydarkhan-Hagvall S, Schenke-Layland K,
Yang JQ, Heydarkhan S, Xu Y, Zuk PA, MacLellan WR and Beygui RE:
Human adipose stem cells: A potential cell source for
cardiovascular tissue engineering. Cells Tissues Organs.
187:263–274. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Harris LJ, Abdollahi H, Zhang P, McIlhenny
S, Tulenko TN and DiMuzio PJ: Differentiation of adult stem cells
into smooth muscle for vascular tissue engineering. J Surg Res.
168:306–314. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Hsu YC, Pasolli HA and Fuchs E: Dynamics
between stem cells, niche, and progeny in the hair follicle. Cell.
144:92–105. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Xu ZC, Zhang Q and Li H: Human hair
follicle stem cell differentiation into contractile smooth muscle
cells is induced by transforming growth factor-β1 and
platelet-derived growth factor BB. Mol Med Rep. 8:1715–1721.
2013.PubMed/NCBI
|
|
21
|
Jahoda CA, Whitehouse J, Reynolds AJ and
Hole N: Hair follicle dermal cells differentiate into adipogenic
and osteogenic lineages. Exp Dermatol. 12:849–859. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Yu H, Fang D, Kumar SM, Li L, Nguyen TK,
Acs G, Herlyn M and Xu X: Isolation of a novel population of
multipotent adult stem cells from human hair follicles. Am J
Pathol. 168:1879–1888. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Reddy GK and Ewemeka CS: A simplified
method for the analysis of hydroxyproline in biological tissues.
Clin Biochem. 29:225–229. 1996. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Naito Y, Rocco K, Kurobe H, Maxfield M,
Breuer C and Shinoka T: Tissue engineering in the vasculature. Anat
Rec (Hoboken). 297:83–97. 2014. View
Article : Google Scholar : PubMed/NCBI
|
|
25
|
Drewa T, Joachimiak R, Kaznica A, Sarafian
V and Pokrywczynska M: Hair stem cells for bladder regeneration in
rats: Preliminary results. Transplant Proc. 41:4345–4351. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Lin H, Liu F, Zhang C, Zhang Z, Kong Z,
Zhang X and Hoffman RM: Characterization of nerve conduits seeded
with neurons and Schwann cells derived from hair follicle neural
crest stem cells. Tissue Eng Part A. 17:1691–1698. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Jiang Y, Jahagirdar BN, Reinhardt RL,
Schwartz RE, Keene CD, Ortiz-Gonzalez XR, Reyes M, Lenvik T, Lund
T, Blackstad M, et al: Pluripotency of mesenchymal stem cells
derived from adult marrow. Nature. 418:41–49. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
DiMuzio P and Tulenko T: Tissue
engineering applications to vascular bypass graft development: The
use of adipose-derived stem cells. J Vasc Surg. 45:(Suppl A).
A99–A103. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Collins MN and Birkinshaw C: Hyaluronic
acid based scaffolds for tissue engineering-a review. Carbohydr
Polym. 92:1262–1279. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Lee SJ, Liu J, Oh SH, Soker S, Atala A and
Yoo JJ: Development of a composite vascular scaffolding system that
withstands physiological vascular conditions. Biomaterials.
29:2891–2898. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Pankajakshan D and Agrawal DK: Scaffolds
in tissue engineering of blood vessels. Can J Physiol Pharmacol.
88:855–873. 2010. View
Article : Google Scholar : PubMed/NCBI
|
|
32
|
Gui L, Zhao L, Spencer RW, Burghouwt A,
Taylor MS, Shalaby SW and Niklason LE: Development of novel
biodegradable polymer scaffolds for vascular tissue engineering.
Tissue Eng Part A. 17:1191–1200. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Bačáková L, Novotná K and Pařízek M:
Polysaccharides as cell carriers for tissue engineering: The use of
cellulose in vascular wall reconstruction. Physiol Res. 63:(Suppl
1). S29–S47. 2014.PubMed/NCBI
|
|
34
|
Thottappillil N and Nair PD: Scaffolds in
vascular regeneration: Current status. Vasc Health Risk Manag.
11:79–91. 2015.PubMed/NCBI
|
|
35
|
Dong Y, Yong T, Liao S, Chan CK, Stevens
MM and Ramakrishna S: Distinctive degradation behaviors of
electrospun polyglycolide, poly (dl-lactide-co-glycolide), and poly
(l-Lactide-co-epsilon-caprolactone) nanofibers cultured
with/without porcine smooth muscle cells. Tissue Eng Part A.
16:283–298. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Xu ZC, Zhang Q and Li H: An elastic large
muscular vessel wall engineered with bone marrow-derived cells
under pulsatile stimulation in a bioreactor. Mol Med Rep.
12:6005–6012. 2015.PubMed/NCBI
|
|
37
|
Sterpetti AV, Cucina A, Santoro L,
Cardillo B and Cavallaro A: Modulation of arterial smooth muscle
cell growth by haemodynamic forces. Eur J Vasc Surg. 6:16–20. 1992.
View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Sho M, Sho E, Singh TM, Komatsu M, Sugita
A, Xu C, Nanjo H, Zarins CK and Masuda H: Subnormalshear
stress-induced intimal thickening requires medial smooth muscle
cell proliferation and migration. Exp Mol Pathol. 72:150–160. 2002.
View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Palumbo R, Gaetano C, Antonini A, Pompilio
G, Bracco E, Rönnstrand L, Heldin CH and Capogrossi MC: Different
effects of high and low shear stress on platelet-derived growth
factor isoform release by endothelial cells: Consequences for
smooth muscle cell migration. Arterioscler Thromb Vasc Biol.
22:405–411. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Qi YX, Qu MJ, Long DK, Liu B, Yao QP,
Chien S and Jiang ZL: Rho-GDP dissociation inhibitor alpha
downregulated by low shear stress promotes vascular smooth muscle
cell migration and apoptosis: A proteomic analysis. Cardiovasc Res.
80:114–122. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Ng CP, Hinz B and Swartz MA: Interstitial
fluid flow induces myofibroblast differentiation and collagen
alignment in vitro. J Cell Sci. 118:4731–4739. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Halka AT, Turner NJ, Carter A, Ghosh J,
Murphy MO, Kirton JP, Kielty CM and Walker MG: The effects of
stretch on vascular smooth muscle cell phenotype in vitro.
Cardiovasc Pathol. 17:98–102. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Jakkaraju S, Zhe X and Schuger L: Role of
stretch in activation of smooth muscle cell lineage. Trends
Cardiovasc Med. 13:330–335. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Haga JH, Li YS and Chien S: Molecular
basis of the effects of mechanical stretch on vascular smooth
muscle cells. J Biomech. 40:947–960. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Standley PR, Cammarata A, Nolan BP,
Purgason CT and Stanley MA: Cyclic stretch induces vascular smooth
muscle cell alignment via NO signaling. Am J Physiol Heart Circ
Physiol. 283:H1907–H1914. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Zhu JH, Chen CL, Flavahan S, Harr J, Su B
and Flavahan NA: Cyclic stretch stimulates vascular smooth muscle
cell alignment by redox-dependent activation of Notch3. Am J
Physiol Heart Circ Physiol. 300:H1770–H1780. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
47
|
Chapman GB, Durante W, Hellums JD and
Schafer AI: Physiological cyclic stretch causes cell cycle arrest
in cultured vascular smooth muscle cells. Am J Physiol Heart Circ
Physiol. 278:H748–H754. 2000.PubMed/NCBI
|
|
48
|
Iwasaki H, Yoshimoto T, Sugiyama T and
Hirata Y: Activation of cell adhesion kinase beta by mechanical
stretch in vascular smooth muscle cells. Endocrinology.
144:2304–2310. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
49
|
Qiu J, Zheng Y, Hu J, Liao D, Gregersen H,
Deng X, Fan Y and Wang G: Biomechanical regulation of vascular
smooth muscle cell functions: From in vitro to in vivo
understanding. J R Soc Interface. 11:201308522013. View Article : Google Scholar : PubMed/NCBI
|