Introduction
The etiology of inflammatory bowel disease (IBD)
remains unclear (1–3). There are three main forms of IBD,
ulcerative colitis (UC), Crohn's disease (CD) and microscopic
colitis (MC), which exhibit differences in prevalence, clinical
course and prognosis (3–6). While the onset of UC and CD occurs
predominantly in individuals <40 years, the onset of MC occurs
in those >60 years (3,7). In addition to the morbidity caused by
IBD, it considerably reduces the quality of life of patients
(3,5,6).
The gastrointestinal endocrine cells are a component
of the local regulatory system of the gut, the neuroendocrine
system (NES) (8), which also
includes the enteric nervous system (8). The NES regulates gastrointestinal
motility, secretion, absorption, cell proliferation, visceral
sensitivity, local immune defense and appetite (8,9).
Abnormalities in several intestinal endocrine cells have been
reported in IBD (10–29). It is believed that the interaction
between the hormones secreted by the gut endocrine cells and the
immune system serve a major role in the pathophysiology of the IBD
(30,31).
The primary aim of the present study was to
establish the presence of abnormalities in the colonic endocrine
cells following dextran sulfate sodium (DSS)-induced colitis in
rats, which closely mimics human UC (32). Furthermore, the existence of a
correlation between any colonic endocrine cell abnormalities and
changes in the densities of various types of immune cells was
determined.
Materials and methods
Rats
A total 24 male Wistar rats (age, 12 weeks; Hannover
GALAS; Taconic Biosciences, Lille Skensved, Denmark) with a mean
body weight of 280 g (range, 231–380 g) were housed in Macrolon III
cages with water and food available ad libitum. They were
fed a standard diet (B & K Universal, Nittedal, Norway)
consisting of cereal products (88.5%), soy protein (6%), animal
protein (2.5%), soy oil (0.5%), and vitamin, mineral and amino-acid
supplements (2.5%). The animals were maintained at a temperature of
21±1°C and a relative humidity of 55±5%, and under a 12/12-h
light/dark cycle.
The animals were left to acclimatize in the animal
house for 7 days prior to the experiment, and were then divided
into 2 groups of 12 animals each: Control and DDS-induced colitis
(DSS group). Animals in the control group were provided with normal
drinking water for 7 days, while those in the DDS-colitis group
were instead provided with distilled water containing 5% DSS
(molecular weight, 40 kDa; prepared daily; TdB Consultancy AB,
Uppsala, Sweden) for 7 days, according to a previously described
protocol (33,34). All of the animals were monitored
twice daily and were weighed once daily. Animals that showed any
signs of pain were given a subcutaneous, 1 ml injection of Temgesic
solution (containing 0.3 g/ml Temgesic; Merck Millipore, Darmstadt,
Germany).
At the end of the 7-day period, the animals were
sacrificed by CO2 inhalation, and the colon was
dissected out via a postmortem laparotomy. Tissue samples were
collected from the lower part of the colon for further,
histopathological and immunohistochemical examinations.
The local ethical committee for the Protection of
Vertebrate Animals used for Experimental and Other Scientific
Purposes approved the study protocols (project no. 20124629).
Histopathology and
immunohistochemistry
The tissue samples were fixed overnight in 4%
buffered paraformaldehyde, embedded in paraffin and then sectioned
at a thickness of 5 mm. The sections were deparaffinized and then
stained with hematoxylin-eosin, or immunostained using the
ultraView Universal DAB Detection kit (version 1.02.0018, Ventana
Medical Systems, Inc., Basel, Switzerland) and the BenchMark Ultra
IHC/ISH staining module (Ventana Medical Systems, Inc.).
For immunostaining, the sections were incubated with
one of the following primary antibodies for 32 min at 37°C:
Monoclonal mouse anti-N-terminal of purified chromogranin A (CgA;
cat. no. M869; Dako, Glostrup, Denmark) diluted 1:1,000, monoclonal
mouse antiserotonin (cat. no. 5HT-209; Dako) diluted 1:1,200,
polyclonal antiporcine peptide YY (PYY; cat. no. PYY 11A; Alpha
Diagnostic International, San Antonio, TX, USA) diluted 1:1,400,
polyclonal rabbit antisynthetic human pancreatic polypeptide (PP;
cat. no. #114; Diagnostic BioSystems, Pleasanton, CA, USA) diluted
1:800, polyclonal rabbit antiporcine oxyntomodulin
‘glicentin/enteroglucagon’ (cat. no. BP508; Acris Antibodies GmbH,
Herford, Germany) diluted 1:400, polyclonal rabbit antisynthetic
human somatostatin (cat. no. A566; Dako) diluted 1:200, monoclonal
mouse antihuman CD45 (cat. no. M0701; Dako) diluted 1:100,
monoclonal mouse antihuman CD5 (cat. no. IS082; Dako) diluted
1:200, monoclonal mouse antihuman CD57 (cat. no. IS647; Dako)
diluted 1:100, monoclonal mouse antihuman CD23 (cat. no. IS781;
Dako) diluted 1:100, monoclonal mouse antihuman CD68 (cat. no.
M0814; Dako) diluted 1:100 and monoclonal mouse antihuman mast-cell
tryptase (cat. no. M7052; Dako) diluted 1:100. CD45 is considered a
common leukocyte antigen and is expressed exclusively on cells of
the hematopoietic system and their progenitors. CD5 is expressed on
B and T lymphocytes, CD57 is expressed by subsets of natural killer
cells and CD8+ lymphocytes, and by a small proportion of
CD4+/CD45R0+ T lymphocytes, CD23 is expressed
on B lymphocytes, CD68 labels human monocytes, macrophages, and
myeloid cells, and mast-cell tryptase is expressed predominantly in
mast cells (35).
Quantification of endocrine and immune
cells
The endocrine and immune cells were quantified by
counting each cell type in 10 randomly chosen microscopic fields.
Measurements were performed on a computer linked to a microscope
(BX43; Olympus Corporation, Tokyo, Japan) that was equipped with a
digital camera (DP26; Olympus Corporation), and using cellSens
imaging software (version 1.7; Olympus Corporation). The number of
endocrine cells in the epithelial lining of the intestinal lumen
and immune cells in the lamina propria of each field were counted
on a computer screen, and the area of the epithelial cells was
determined by manual drawing using the computer mouse. A 40X
objective was used, for which each frame (field) on the monitor
represented a tissue area of 0.035 mm2. The data are
presented as density measurements (i.e., the number of endocrine
cells/mm2 epithelium, and the number of immune cells per
field). Immunostained sections were coded and mixed, and
measurements were made by the same person (Professor Magdy
El-Salhy), who was blind to the identity of the sections.
Statistical analysis
Differences between the control and DSS groups were
tested using the Mann-Whitney nonparametric test. The existence of
a correlation between abnormalities/alterations in the densities of
endocrine cells and immune cells was determined using the
nonparametric Spearman's correlation test. The data are presented
as the mean ± standard error, and P<0.05 was considered to
indicate a statistically significant difference.
Results
The histopathological examinations of the colonic
tissues produced normal results in the control group, whereas the
DSS group had severe-to-moderate inflammation with disturbed
mucosal architecture, crypt abscesses, edema, bleeding and
infiltration of immune cells into the mucosa and submucosa.
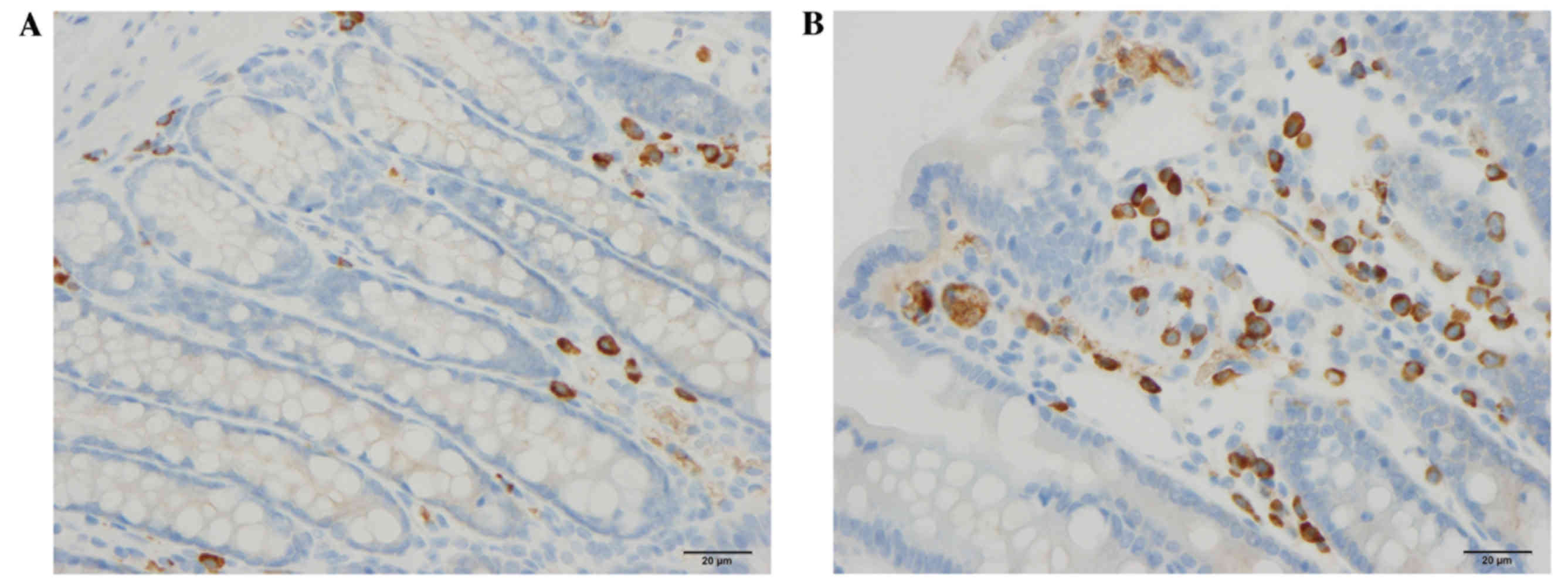
Endocrine cells
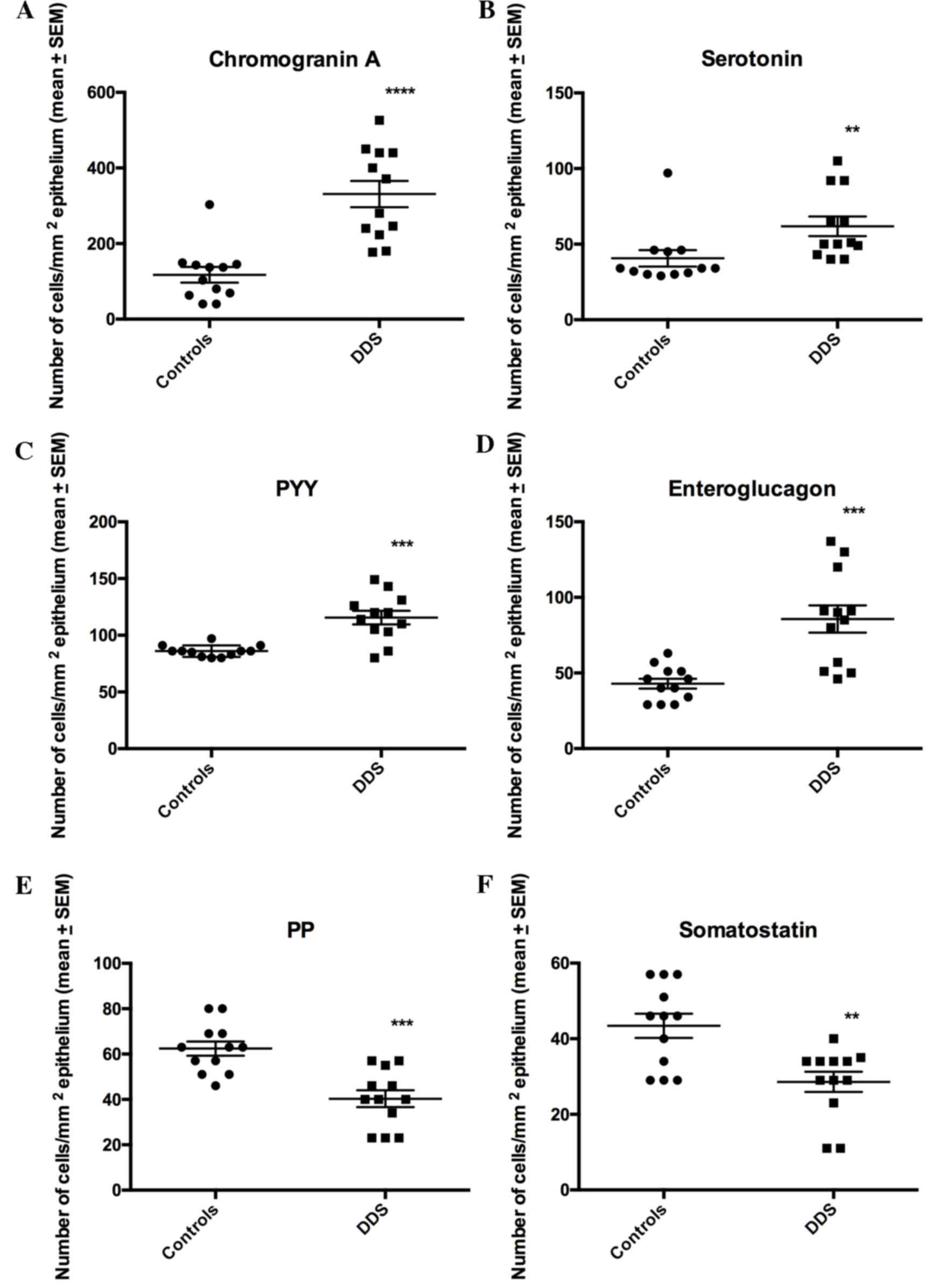
The densities of CgA, serotonin, PYY and
enteroglucagon cells were all significantly higher in the DDS group
(333.1±34.7, 61.8±6.5, 115.6±5.9 and 85.7±9.0 cells/mm2
epithelium, respectively) than in the control group (117.4±20.7,
40.7±5.5, 86.0±1.5 and 42.9±3.3 cells/mm2 epithelium;
P<0.0001, P=0.0006, P=0.002 and P=0.0003, respectively; Figs. 1–3). Conversely, the densities of PP and
somatostatin cells were significantly higher in the control group
(62.4±3.1 and 43.4±3.2 cells/mm2 epithelium,
respectively) than in the DSS group (40.3±3.7 and 28.6±2.7
cells/mm2 epithelium, respectively; P=0.0002 and 0.007,
respectively; Figs. 1 and 4).
Immune cells
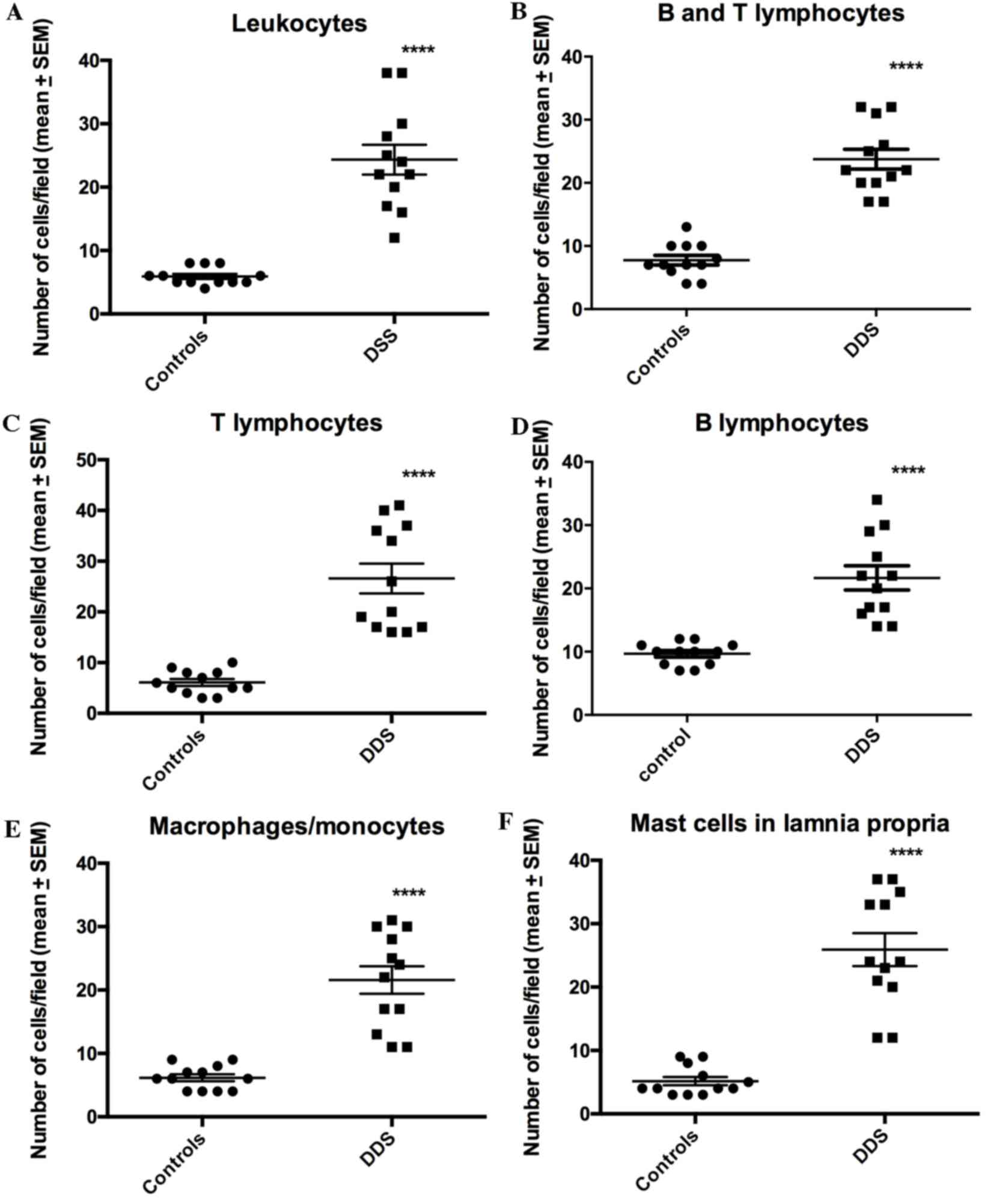
The densities of all of the immune cell types were
significantly higher in the DDS group than in the control group
(Figs. 5–8): Leukocytes, 5.9±0.4 vs. 23.3±2.2
cells/field (P<0.0001); B/T lymphocytes, 7.8±0.8 vs. 23.8±1.6
cells/field (P<0.0001); T lymphocytes, 6.8±0.7 vs. 26.6±2.9
cells/field (P<0.0001); and B lymphocytes, 9.8±0.6 vs. 22.1±2.3
cells/field (P<0.0001).
Correlation between endocrine and
immune cells
The Spearman correlation coefficients and P-values
between different endocrine cell types and various immune cells are
summarized in Table I. The
abnormalities in CgA, serotonin, PYY, and enteroglucagon cells were
identified to be positively correlated with the alterations of all
types of immune cells, while a negative correlation was observed
for PP and somatostatin cells.
 | Table I.Summary of the Spearman correlation
coefficient (r) and P values between different endocrine
cell types and various immune cells. |
Table I.
Summary of the Spearman correlation
coefficient (r) and P values between different endocrine
cell types and various immune cells.
|
| Immune cell
type |
|---|
|
|
|
|---|
| Endocrine cell
type | Leukocytes | B/T
lymphocytes | T lymphocytes | B lymphocytes |
Macrophages/monocytes | Mast cells |
|---|
| Chromogranin A | r=0.8 | r=0.7 | r=0.6 | r=0.7 | r=0.8 | r=0.7 |
|
| P=0.006 | P=0.009 | P=0.03 | P=0.008 | P=0.0009 | P=0.008 |
| Serotonin | r=0.7 | r=0.7 | r=0.8 | r=0.8 | r=0.8 | r=0.7 |
|
| P=0.007 | P=0.004 | P=0.004 | P=0.006 | P=0.004 | P=0.008 |
| Peptide YY | r=0.6 | r=0.6 | r=0.7 | r=0.6 | r=0.7 | r=0.7 |
|
| P=0.03 | P=0.04 | P=0.02 | P=0.03 | P=0.02 | P=0.02 |
| Enteroglucagon | r=0.6 | r=0.8 | r=0.8 | r=0.7 | r=0.7 | r=0.9 |
|
| P=0.04 | P=0.006 | P=0.006 | P=0.02 | P=0.02 | P=0.0005 |
| Pancreatic
peptide | r=−0.7 | r=−0.7 | r=−0.8 | r=0.7 | r=−0.7 | r=0.7 |
|
| P=0.006 | P=0.007 | P=0.001 | P=0.01 | P=0.006 | P=0.006 |
| Somatostatin | r=−0.7 | r=−0.6 | r=−0.6 | r=−0.8 | r=−0.5 | r=−0.8 |
|
| P=0.01 | P=0.02 | P=0.02 | P=0.0009 | P=0.006 | P=0.0007 |
Discussion
Animal models of IBD are either those with
chemically induced colitis or mutant (knockout) mice (7,32,36–39).
Although neither of these models accurately mimic human IBD, they
are useful tools towards understanding the pathophysiological
mechanisms underlying IBD (32).
DSS-induced colitis is a mixed Th1/Th2
cytokine-mediated colitis (40,41)
and is considered be a model for UC with clinical and morphological
features similar to that of human UC (32,42,43).
However, DSS-induced colitis lacks the chronicity seen in human UC
(32).
The present study identified that the densities of
all of the colonic endocrine cell types were affected in rats with
DSS-induced colitis. Furthermore, the abnormalities in the colonic
endocrine cells were closely correlated with the alterations in
several immune-cell types following the induction of colitis. These
observations lend support to the hypothesized role of gut hormones
in immune activation and inflammation (30,31,44).
CgA belongs to the family of granins (45,46),
and is localized to the stomach and small and large intestines
(47–50). It is commonly used as a marker for
gastrointestinal and endocrine tumor cells (51,52).
The increase in CgA-immunoreactive cells observed in the present
study could reflect an increase in the cell density of the total
colonic endocrine cells following the induction of colitis. This
observation is in line with the previously reported increases in
CgA cells in UC and CD (10,23).
However, CgA itself inhibits the vascular leakage caused by tumor
necrosis factor α (53).
Furthermore, CgA-derived peptides reduce the release of interleukin
(IL)-16 and IL-5, hence reducing the number of lymphocytes at
inflammatory sites and thus the proinflammatory action of
lymphocytes and monocytes (54–56).
The increase in the density of CgA cells reported herein was
closely associated with the increase in immune cells. Taking into
consideration the known interaction between CgA and immune cells,
this increase in CgA density is likely a response defense mechanism
against inflammation.
Serotonin is a potent hormone that exerts several
effects at its numerous receptor types. Thus, it stimulates gastric
and intestinal motility, modulates visceral sensitivity, and
stimulates intestinal secretion (8,57).
The present observation of an increased density of colonic
serotonin cells in DSS-induced colitis relative to healthy controls
is in agreement with previously published observations in patients
with UC, CD and MC, and in animal models of colitis (10,12,58–60).
However, additional studies identified that the serotonin cell
density reduced in UC and remained unchanged in CD (61,62).
It has been reported that serotonin serves an important role in
intestinal inflammation (30,54).
Thus, the number of serotonin cells has been reported to be reduced
in mice lacking the T-lymphocyte receptors (54), IL-13 receptors have been localized
on serotonin cells (63), and
serotonin receptors have been observed in lymphocytes, monocytes,
macrophages and dendritic cells (64). In addition, serotonin affects the
proliferation of lymphocytes, protects natural killer cells,
inhibits the apoptosis of immune cells, and promotes the
recruitment of T cells (65–68).
Thus, the fact that the increase in serotonin-cell density in
DSS-induced colitis was to be closely associated with the increased
densities of the immune-cell types was expected.
PYY and oxyntomodulin are colocalized in the same
endocrine cell type (69,70). However, the degree of that
colocalization differs according to the animal species (70). PYY delays gastric emptying, and is
a key mediator of the ileal brake. It also inhibits gastric and
pancreatic secretion, and stimulates the absorption of water and
electrolytes (71). Oxyntomodulin
has an incretin effect, inhibits gastric and pancreatic secretions,
and reduces gastric motility (8).
PYY and oxyntomodulin have been previously observed to exhibit
anorexigenic effects (72), and
the present observation of increased PYY and oxyntomodulin cell
densities is in agreement with previous observations in UC and IL-2
gene knockout mice (10,59). Whereas the increase in the
oxyntomodulin cell density identified is in line with previous
observations in IL-2 knockout mice, it disagrees with observations
in UC, where oxyntomodulin cell density was unchanged (10,59).
The close correlation between the increase in PYY and oxyntomodulin
cell densities with the increase in the densities of the immune
cells identified in the current study indicates an interaction
between the endocrine and immune systems.
PP stimulates gastric acid secretion and the
motility of the stomach and small intestine, and relaxes the
gallbladder (8). Somatostatin
inhibits intestinal contraction, and gut exocrine and
neuroendocrine secretions (8). In
addition, somatostatin inhibits lymphocyte proliferation,
immunoglobulin synthesis and neutrophil elastase release (73–77).
The reduction in PP cell density observed in the present
investigation is in line with what has been reported in UC and CD
(10). Although the reduction in
somatostatin cell density in DSS-induced colitis observed in the
current study is also in line with previous publications on UC and
CD (28,29), it is in disagreement with a study
in which the density of somatostatin cells was observed to be
unchanged in these conditions (10). As for the other endocrine cell
types assessed in the current study, the correlation between the
alterations in the PP and somatostatin cell densities points to
their involvement in the inflammatory process.
A potential interaction is suggested between
inflammation as indicated by the increase in immune cells and the
colonic endocrine cells. It is possible that the increase in
serotonin and the reduction in somatostatin cell densities results
from inflammation, and that the changes in CgA, PYY, oxyntomodulin
and PP cells are secondary responses to the changes in serotonin
and somatostatin. Cytokines appear to serve a significant role in
the proliferation and differentiation of intestinal stem cells
(78–80). It is suggested that inflammation
with increased cytokine production increases the serotonin and
reduces the somatostatin cell densities by affecting their early
progenitors, and that these alterations would result in increased
gastrointestinal motility and secretion in addition to visceral
hypersensitivity. As a compensatory defense, an increase in PYY and
oxyntomodulin, and a reduction in PP would slow gastrointestinal
motility and reduce gastrointestinal secretions. The increase in
CgA, which appears to have anti-inflammatory effects, may simply
reflect the total increase in colonic endocrine cells or another
defensive action against inflammation (30).
The induction of colitis by DSS in rats affects all
of the colonic endocrine cells. Given the available data on the
interactions between hormones and the immune system, it can be
hypothesized that inflammation induces the proliferation of
serotonin cells and inhibits that of somatostatin cells, in
response to which there is a secondary change in the densities of
CgA, PYY, oxyntomodulin and PP cells. The close correlation between
the changes in all endocrine cell types and immune cells emphasizes
the importance of the role of interactions between the intestinal
hormone and immune systems in the pathophysiology of intestinal
inflammation.
Acknowledgements
The current study was supported by grants from
Helse-Fonna (grant no. 40415), and Helse-Vest (grant no. 911978),
Norway.
References
|
1
|
Danese S and Fiocchi C: Etiopathogenesis
of inflammatory bowel diseases. World J Gastroenterol.
12:4807–4812. 2006.PubMed/NCBI
|
|
2
|
Nunes T, Fiorino G, Danese S and Sans M:
Familial aggregation in inflammatory bowel disease: Is it genes or
environment? World J Gastroenterol. 17:2715–2722. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
El-Salhy M, Gundersen D, Hatlebakk JG and
Hausken T: Clinical presentation, diagnosis, pathogenesis and
treatment options for lymphocytic colitis (Review). Int J Mol Med.
32:263–270. 2013.PubMed/NCBI
|
|
4
|
Prantera C and Marconi S:
Glucocorticosteroids in the treatment of inflammatory bowel disease
and approaches to minimizing systemic activity. Therap Adv
Gastroenterol. 6:137–156. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Podolsky DK: Inflammatory bowel disease. N
Engl J Med. 347:417–429. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Podolsky DK: The current future
understanding of inflammatory bowel disease. Best Pract Res Clin
Gastroenterol. 16:933–943. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Carter MJ, Lobo AJ and Travis SP: IBD
Section, British Society of Gastroenterology: Guidelines for the
management of inflammatory bowel disease in adults. Gut. 53:Suppl
5. V1–16. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
El-Salhy M, Seim I, Chopin L, Gundersen D,
Hatlebakk JG and Hausken T: Irritable bowel syndrome: The role of
gut neuroendocrine peptides. Front Biosci (Elite Ed). 4:2783–2800.
2012.PubMed/NCBI
|
|
9
|
Wu T, Rayner CK, Young RL and Horowitz M:
Gut motility and enteroendocrine secretion. Curr Opin Pharmacol.
13:928–934. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
El-Salhy M, Danielsson A, Stenling R and
Grimelius L: Colonic endocrine cells in inflammatory bowel disease.
J Intern Med. 242:413–419. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
El-Salhy M, Gundersen D, Hatlebakk JG and
Hausken T: Chromogranin a cell density as a diagnostic marker for
lymphocytic colitis. Dig Dis Sci. 57:3154–3159. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
El-Salhy M, Gundersen D, Hatlebakk JG and
Hausken T: High densities of serotonin and peptide YY cells in the
colon of patients with lymphocytic colitis. World J Gastroenterol.
18:6070–6075. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
El-Salhy M, Lomholt-Beck B and Gundersen
TD: High chromogranin A cell density in the colon of patients with
lymphocytic colitis. Mol Med Rep. 4:603–605. 2011.PubMed/NCBI
|
|
14
|
Moran GW, Pennock J and McLaughlin JT:
Enteroendocrine cells in terminal ileal Crohn's disease. J Crohns
Colitis. 6:871–880. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Moran GW, Leslie FC and McLaughlin JT:
Crohn's disease affecting the small bowel is associated with
reduced appetite and elevated levels of circulating gut peptides.
Clin Nutr. 32:404–411. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Besterman HS, Mallinson CN, Modigliani R,
Christofides ND, Pera A, Ponti V, Sarson DL and Bloom SR: Gut
hormones in inflammatory bowel disease. Scand J Gastroenterol.
18:845–852. 1983. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
El-Salhy M, Mazzawi T, Gundersen D,
Hatlebakk JG and Hausken T: The role of peptide YY in
gastrointestinal diseases and disorders (review). Int J Mol Med.
31:275–282. 2013.PubMed/NCBI
|
|
18
|
Hirotani Y, Mikajiri K, Ikeda K, Myotoku M
and Kurokawa N: Changes of the peptide YY levels in the intestinal
tissue of rats with experimental colitis following oral
administration of mesalazine and prednisolone. Yakugaku Zasshi.
128:1347–1353. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Vona-Davis LC and McFadden DW: NPY family
of hormones: Clinical relevance and potential use in
gastrointestinal disease. Curr Top Med Chem. 7:1710–1720. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
20
|
El-Salhy M, Suhr O and Danielsson A:
Peptide YY in gastrointestinal disorders. Peptides. 23:397–402.
2002. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Tari A, Teshima H, Sumii K, Haruma K,
Ohgoshi H, Yoshihara M, Kajiyama G and Miyachi Y: Peptide YY
abnormalities in patients with ulcerative colitis. Jpn J Med.
27:49–55. 1988. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Sciola V, Massironi S, Conte D, Caprioli
F, Ferrero S, Ciafardini C, Peracchi M, Bardella MT and Piodi L:
Plasma chromogranin a in patients with inflammatory bowel disease.
Inflamm Bowel Dis. 15:867–871. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Bishop AE, Pietroletti R, Taat CW,
Brummelkamp WH and Polak JM: Increased populations of endocrine
cells in Crohn's ileitis. Virchows Arch A Pathol Anat Histopathol.
410:391–396. 1987. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Manocha M and Khan WI: Serotonin and GI
disorders: An update on clinical and experimental studies. Clin
Transl Gastroenterol. 3:e132012. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Stoyanova II and Gulubova MV: Mast cells
and inflammatory mediators in chronic ulcerative colitis. Acta
Histochem. 104:185–192. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Yamamoto H, Morise K, Kusugami K, Furusawa
A, Konagaya T, Nishio Y, Kaneko H, Uchida K, Nagai H, Mitsuma T and
Nagura H: Abnormal neuropeptide concentration in rectal mucosa of
patients with inflammatory bowel disease. J Gastroenterol.
31:525–532. 1996. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Payer J, Huorka M, Duris I, Mikulecky M,
Kratochvílová H, Ondrejka P and Lukác L: Plasma somatostatin levels
in ulcerative colitis. Hepatogastroenterology. 41:552–553.
1994.PubMed/NCBI
|
|
28
|
Watanabe T, Kubota Y, Sawada T and Muto T:
Distribution and quantification of somatostatin in inflammatory
disease. Dis Colon Rectum. 35:488–494. 1992. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Koch TR, Carney JA, Morris VA and Go VL:
Somatostatin in the idiopathic inflammatory bowel diseases. Dis
Colon Rectum. 31:198–203. 1988. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Khan WI and Ghia JE: Gut hormones:
Emerging role in immune activation and inflammation. Clin Exp
Immunol. 161:19–27. 2010.PubMed/NCBI
|
|
31
|
Margolis KG and Gershon MD: Neuropeptides
and inflammatory bowel disease. Curr Opin Gastroenterol.
25:503–511. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Elson CO, Sartor RB, Tennyson GS and
Riddell RH: Experimental models of inflammatory bowel disease.
Gastroenterology. 109:1344–1367. 1995. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Grimstad T, Bjørndal B, Cacabelos D,
Aasprong OG, Omdal R, Svardal A, Bohov P, Pamplona R, Portero-Otin
M, Berge RK and Hausken T: A salmon peptide diet alleviates
experimental colitis as compared with fish oil. J Nutr Sci.
2:e22013. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Stucchi AF, Shofer S, Leeman S, Materne O,
Beer E, McClung J, Shebani K, Moore F, O'Brien M and Becker JM:
NK-1 antagonist reduces colonic inflammation and oxidative stress
in dextran sulfate-induced colitis in rats. Am J Physiol
Gastrointest Liver Physiol. 279:G1298–G1306. 2000.PubMed/NCBI
|
|
35
|
El-Salhy M, Mazzawi T, Umezawa K and Gilja
OH: Enteroendocrine cells, stem cells and differentiation
progenitors in rats with TNBS-induced colitis. Int J Mol Med. Oct
24–2016.(Epub ahead of print).
|
|
36
|
Saleh M and Elson CO: Experimental
inflammatory bowel disease: Insights into the host-microbiota
dialog. Immunity. 34:293–302. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Sands BE: New therapies for the treatment
of inflammatory bowel disease. Surg Clin North Am. 86:1045–1064.
2006. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Lopez A, Billioud V, Peyrin-Biroulet C and
Peyrin-Biroulet L: Adherence to anti-TNF therapy in inflammatory
bowel diseases: A systematic review. Inflamm Bowel Dis.
19:1528–1533. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Danese S, Semeraro S, Armuzzi A, Papa A
and Gasbarrini A: Biological therapies for inflammatory bowel
disease: Research drives clinics. Mini Rev Med Chem. 6:771–784.
2006. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Motomura Y, Ghia JE, Wang H, Akiho H,
El-Sharkawy RT, Collins M, Wan Y, McLaughlin JT and Khan WI:
Enterochromaffin cell and 5-hydroxytryptamine responses to the same
infectious agent differ in Th1 and Th2 dominant environments. Gut.
57:475–481. 2008.PubMed/NCBI
|
|
41
|
Wirtz S, Neufert C, Weigmann B and Neurath
MF: Chemically induced mouse models of intestinal inflammation. Nat
Protoc. 2:541–546. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Dieleman LA, Palmen MJ, Akol H, Bloemena
E, Peña AS, Meuwissen SG and Van Rees EP: Chronic experimental
colitis induced by dextran sulphate sodium (DSS) is characterized
by Th1 and Th2 cytokines. Clin Exp Immunol. 114:385–391. 1998.
View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Low D, Nguyen DD and Mizoguchi E: Animal
models of ulcerative colitis and their application in drug
research. Drug Des Devel Ther. 7:1341–1357. 2013.PubMed/NCBI
|
|
44
|
Öhman L, Tornblom H and Simrén M:
Crosstalk at the mucosal border: Importance of the gut
microenvironment in IBS. Nat Rev Gastroenterol Hepatol. 12:36–49.
2015.PubMed/NCBI
|
|
45
|
Buffa R, Maré P, Gini A and Salvadore M:
Chromogranins A and B and secretogranin II in hormonally identified
endocrine cells of the gut and the pancreas. Basic Appl Histochem.
32:471–484. 1988.PubMed/NCBI
|
|
46
|
Eiden LE: Is chromogranin a prohormone?
Nature. 325:3011987. View Article : Google Scholar : PubMed/NCBI
|
|
47
|
Buffa R, Capella C, Fontana P, Usellini L
and Solcia E: Types of endocrine cells in the human colon and
rectum. Cell Tissue Res. 192:227–240. 1978.PubMed/NCBI
|
|
48
|
Curry WJ, Johnston CF, Hutton JC, Arden
SD, Rutherford NG, Shaw C and Buchanan KD: The tissue distribution
of rat chromogranin A-derived peptides: Evidence for differential
tissue processing from sequence specific antisera. Histochemistry.
96:531–538. 1991. View Article : Google Scholar : PubMed/NCBI
|
|
49
|
Portela-Gomes GM and Stridsberg M:
Selective processing of chromogranin A in the different islet cells
in human pancreas. J Histochem Cytochem. 49:483–490. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
50
|
Portela-Gomes GM and Stridsberg M:
Chromogranin A in the human gastrointestinal tract: An
immunocytochemical study with region-specific antibodies. J
Histochem Cytochem. 50:1487–1492. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
51
|
Taupenot L, Harper KL and O'Connor DT: The
chromogranin-secretogranin family. N Engl J Med. 348:1134–1149.
2003. View Article : Google Scholar : PubMed/NCBI
|
|
52
|
Wiedenmann B and Huttner WB: Synaptophysin
and chromogranins/secretogranins-widespread constituents of
distinct types of neuroendocrine vesicles and new tools in tumor
diagnosis. Virchows Arch B Cell Pathol Incl Mol Pathol. 58:95–121.
1989. View Article : Google Scholar : PubMed/NCBI
|
|
53
|
Ferrero E, Magni E, Curnis F, Villa A,
Ferrero ME and Corti A: Regulation of endothelial cell shape and
barrier function by chromogranin A. Ann N Y Acad Sci. 971:355–358.
2002. View Article : Google Scholar : PubMed/NCBI
|
|
54
|
Spiller R: Serotonin and GI clinical
disorders. Neuropharmacology. 55:1072–1080. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
55
|
Egger M, Beer AG, Theurl M, Schgoer W,
Hotter B, Tatarczyk T, Vasiljevic D, Frauscher S, Marksteiner J,
Patsch JR, et al: Monocyte migration: A novel effect and signaling
pathways of catestatin. Eur J Pharmacol. 598:104–111. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
56
|
Feistritzer C, Mosheimer BA, Colleselli D,
Wiedermann CJ and Kahler CM: Effects of the neuropeptide
secretoneurin on natural killer cell migration and cytokine
release. Regul Pept. 126:195–201. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
57
|
El-Salhy M, Gundersen D, Hatlebakk JG and
Hausken T: Irritable bowel syndrome: Diagnosis, pathogenesis and
treatment options. Nova Science Publishers, Inc.; New York:
2012
|
|
58
|
Bertrand PP and Bertrand RL: Serotonin
release and uptake in the gastrointestinal tract. Auton Neurosci.
153:47–57. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
59
|
Qian BF, El-Salhy M, Melgar S, Hammarstrom
ML and Danielsson A: Neuroendocrine changes in colon of mice with a
disrupted IL-2 gene. Clin Exp Immunol. 120:424–433. 2000.
View Article : Google Scholar : PubMed/NCBI
|
|
60
|
Oshima S, Fujimura M and Fukimiya M:
Changes in number of serotonin-containing cells and serotonin
levels in the intestinal mucosa of rats with colitis induced by
dextran sodium sulfate. Histochem Cell Biol. 112:257–263. 1999.
View Article : Google Scholar : PubMed/NCBI
|
|
61
|
Coates MD, Mahoney CR, Linden DR, Sampson
JE, Chen J, Blaszyk H, Crowell MD, Sharkey KA, Gershon MD, Mawe GM
and Moses PL: Molecular defects in mucosal serotonin content and
decreased serotonin reuptake transporter in ulcerative colitis and
irritable bowel syndrome. Gastroenterology. 126:1657–1664. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
62
|
Sjolund K, Schaffalitzky OB, Muckadell DE,
Fahrenkrug J, Håkanson R, Peterson BG and Sundler F:
Peptide-containing nerve fibres in the gut wall in Crohn's disease.
Gut. 24:724–733. 1983. View Article : Google Scholar : PubMed/NCBI
|
|
63
|
Wang H, Steeds J, Motomura Y, Deng Y,
Verma-Gandhu M, El-Sharkawy RT, McLaughlin JT, Grencis RK and Khan
WI: CD4+ T cell-mediated immunological control of
enterochromaffin cell hyperplasia and 5-hydroxytryptamine
production in enteric infection. Gut. 56:949–957. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
64
|
Cloëz-Tayarani I and Changeux JP: Nicotine
and serotonin in immune regulation and inflammatory processes: A
perspective. J Leukoc Biol. 81:599–606. 2007.PubMed/NCBI
|
|
65
|
Stefulj J, Cicin-Sain L, Schauenstein K
and Jernej B: Serotonin and immune response: Effect of the amine on
in vitro proliferation of rat lymphocytes. Neuroimmunomodulation.
9:103–108. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
66
|
Betten A, Dahlgren C, Hermodsson S and
Hellstrand K: Serotonin protects NK cells against oxidatively
induced functional inhibition and apoptosis. J Leukoc Biol.
70:65–72. 2001.PubMed/NCBI
|
|
67
|
Laberge S, Cruikshank WW, Beer DJ and
Center DM: Secretion of IL-16 (lymphocyte chemoattractant factor)
from serotonin-stimulated CD8+ T cells in vitro. J
Immunol. 156:310–315. 1996.PubMed/NCBI
|
|
68
|
Soga F, Katoh N, Inoue T and Kishimoto S:
Serotonin activates human monocytes and prevents apoptosis. J
Invest Dermatol. 127:1947–1955. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
69
|
Spångéus A, Forsgren S and el-Salhy M:
Does diabetic state affect co-localization of peptide YY and
enteroglucagon in colonic endocrine cells? Histol Histopathol.
15:37–41. 2000.PubMed/NCBI
|
|
70
|
Pyarokhil AH, Ishihara M, Sasaki M and
Kitamura N: The developmental plasticity of colocalization pattern
of peptide YY and glucagon-like peptide-1 in the endocrine cells of
bovine rectum. Biomed Res. 33:35–38. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
71
|
El-Salhy M, Gundersen D, Gilja OH,
Hatlebakk JG and Hausken T: Is irritable bowel syndrome an organic
disorder? World J Gastroenterol. 20:384–400. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
72
|
El-Salhy M, Gundersen D, Hatlebakk JG and
Hausken T: Diet and irritable bowel syndrome, with a focus on
appetite-regulating hormonesNutrition in the prevention and
treatment of abdominal obesity. Watson RR: Elsevier; San Diego: pp.
5–16G. 2014
|
|
73
|
Payan DG, Hess CA and Goetzl EJ:
Inhibition by somatostatin of the proliferation of T-lymphocytes
and Molt-4 lymphoblasts. Cell Immunol. 84:433–438. 1984. View Article : Google Scholar : PubMed/NCBI
|
|
74
|
Adeyemi EO, Savage AP, Bloom SR and
Hodgson HJ: Somatostatin inhibits neutrophil elastase release in
vitro. Peptides. 11:869–871. 1990. View Article : Google Scholar : PubMed/NCBI
|
|
75
|
Stanisz AM, Befus D and Bienenstock J:
Differential effects of vasoactive intestinal peptide, substance P,
and somatostatin on immunoglobulin synthesis and proliferations by
lymphocytes from Peyer's patches, mesenteric lymph nodes, and
spleen. J Immunol. 136:152–156. 1986.PubMed/NCBI
|
|
76
|
Scicchitano R, Dazin P, Bienenstock J,
Payan DG and Stanisz AM: Distribution of somatostatin receptors on
murine spleen and Peyer's patch T and B lymphocytes. Brain Behav
Immun. 1:173–184. 1987. View Article : Google Scholar : PubMed/NCBI
|
|
77
|
Scicchitano R, Stanisz AM, Payan DG,
Kiyono H, McGhee JR and Bienenstock J: Expression of substance P
and somatostatin receptors on a T helper cell line. Adv Exp Med
Biol 216A. 185–190. 1987.
|
|
78
|
Montgomery RK and Breault DT: Small
intestinal stem cell markers. J Anat. 213:52–58. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
79
|
Potten CS: Stem cells in gastrointestinal
epithelium: Numbers, characteristics and death. Philos Trans R Soc
Lond B Biol Sci. 353:821–830. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
80
|
Potten CS: Interleukin-11 protects the
clonogenic stem cells in murine small-intestinal crypts from
impairment of their reproductive capacity by radiation. Int J
Cancer. 62:356–361. 1995. View Article : Google Scholar : PubMed/NCBI
|