Introduction
Androgen and the androgen receptor (AR) are
essential in male spermatogenesis and fertility (1–3). AR
mediates physiological and pathophysiological effects of androgens
by binding to genomic androgen response elements (AREs) of target
genes (4,5). In human, mutations in the AR
gene can result in male infertility and cancer, including androgen
insensitivity and testicular feminization syndrome, breast
carcinoma and prostate cancer (6–8).
Similarly, constitutive AR knockout mice exhibit complete
androgen insensitivity with testes that are larger and located at
the abdomen, and their germ-cell development is severely disrupted
(9).
Previous studies have demonstrated that germ cells
do not express AR, and the effect of androgens on spermatogenesis
is predominantly mediated via Sertoli cells (SCs). Sertoli
cell-selective AR knockout (SCARKO) mice exhibit a complete
disruption of meiosis, which demonstrates that SCs are important in
regulating spermatogenesis (10–12).
Although gene expression profile analysis demonstrated that a
number of genes were regulated by androgens in mouse SCs, only a
few genes have been identified as candidate target genes for AR,
including ubiquitin conjugating enzyme E2 B, heat shock factor
protein 1, reproductive homeobox 5 (Rhox5) and tubulin β3
class III (Tubb3) (13–16).
However, AR function during spermatogenesis is still not well
understood. Further research is essential to determine which target
genes are physiologically relevant and may be useful as diagnostic
or therapy targets to modulate the effects of ARs in
spermatogenesis.
The mouse alanine and arginine rich domain
containing protein (Aard) gene was first identified as a
gene exhibiting sexually dimorphic expression in fetal gonads using
polymerase chain reaction (PCR)-based cDNA subtraction analysis. A
previous study demonstrated that AARD was specifically expressed at
higher levels in the testes of the developing mouse embryo relative
to the ovaries and other tissues. Strong testis-specific expression
of AARD was also detected in the adult mouse (17). Furthermore, AARD is exclusively
located in SCs of XY gonads during sex differentiation (17). Notably, a previous study using
digital gene expression analysis data also demonstrated that the
Aard gene was markedly downregulated in SCARKO mouse testes
compared with wild-type (WT) mice (18). This suggests that Aard may
be a critical target gene of AR involved in spermatogenesis.
The present study identified the AARD was
specifically expressed in Sertoli cells (SCs) of the mouse testis
and increased in an age-dependent manner, and its expression was
markedly downregulated in SCARKO mouse testes. The chromatin
immunoprecipitation and electrophoretic mobility shift assays
demonstrated that Aard was a novel direct target gene of AR
in mouse SCs. These observations suggest that the low expression of
Aard in the testes of SCARKO mouse testes may be one of the
factors that impair spermatogenesis and result in male
infertility.
Materials and methods
Animals
C57BL/6 (n=30) and SCARKO (n=30) mice (8 weeks old)
were obtained from the Model Animal Research Center of Nanjing
University (Nanjing, China). Mice were housed in a pathogen-free
environment at ~22°C under a 12 h light/dark cycle. All the animals
had free access to standard water and chow. All mice were treated
according to the National Institutes of Health Guide for the Care
and Use of Laboratory Animals. The study was approved by the ethics
committee of Xiyuan Hospital of China Academy of Chinese Medical
Science (Beijing, China).
Primary SCs cultures and
transfection
Primary SCs were separated from 19-day-old mouse
testes as previously described (19). Cells were cultured at 37°C in a 5%
CO2 humidified atmosphere with Dulbecco's modified
Eagle's medium (Invitrogen; Thermo Fisher Scientific, Inc.,
Waltham, MA, USA) and 10% fetal bovine serum (Invitrogen; Thermo
Fisher Scientific, Inc.), and supplemented with 100 U/ml penicillin
and 100 U/ml streptomycin (Invitrogen; Thermo Fisher Scientific,
Inc.).
Primary SCs were seeded in a 6 or 24-well plate and
transfected using Lipofectamine 2000 (Invitrogen; Thermo Fisher
Scientific, Inc.) according to the manufacturer's protocols.
AR siRNA (siAr) was obtained from Shanghai GenePharma, Co.,
Ltd. (Shanghai, China). The sequences were as follows:
5′-AGGACUUGCUGUCAUUGAAAUGGA-3′ for siAR; and
5′-CAGCAUAUUAGAAUAGCGCGACA-3′ for siNC. Ar overexpression
plasmid (pCDNA4.1-AR) and corresponding empty vector (pCDNA4.1)
were synthesized by Thermo Fisher Scientific, Inc.
Quantitative PCR (qPCR)
RNA was extracted from mouse tissues using TRIzol
(Invitrogen; Thermo Fisher Scientific, Inc.). Total RNA extraction
and qPCR reactions were performed as previously described (20) using the following kits: Reverse
transcription, ReverPrimeScript RT Enzyme Mix I kit (Takara Bio,
Inc., Otsu, Japan); qPCR, SYBR® Premix EX Taq™II PCR kit
(Takara Bio, Inc.). The primers were as follows: Sense,
5′-AGAGCCCGCAGGATAAGGAGAT-3′ and antisense,
5′-AGTGTTAATGCTAGGAGGGTTTCA-3′ for mouse Aard; sense,
5′-CTCTTTCAAGGGAGGTTACGCC-3′ and antisense,
5′-CTGGTATGCTGCTGCCTCGTCT-3′ for AR; and sense,
5′-TTCCAGCCTTCCTTCTTGGGTAT-3′ and antisense,
5′-GTTGGCATAGAGGTCTTTACGG-3′ for Actb, which served as an
internal control. The relative expression levels of Aard and
AR were evaluated according to the 2−ΔΔCq method
(21).
Western blotting
Protein extracts of different mouse tissues and
primary SCs using radioimmunoprecipitation assay buffer
(Invitrogen; Thermo Fisher Scientific, Inc.). The protein
concentration was quantified using a bicinchoninic acid assay kit
(Pierce; Thermo Fisher Scientific, Inc.). Samples (20 µg) were
subjected to 12% sodium dodecyl sulfate polyacrylamide gel
electrophoresis and transferred to polyvinylidene fluoride
membranes (EMD Millipore, Billerica, MA, USA). Following blocking
with 5% non-fat milk in Tris-buffered saline with Tween 20, the
membrane was then incubated with anti-AARD antibody (1:500; Santa
Cruz Biotechnology, Dallas, TX, USA; cat. no. sc-86960), anti-AR
antibody (1:1,000; Abcam, Cambridge, UK; cat. no. ab133273), and
anti-ACTB (1:10,000; Abcam; cat. no. ab8226) overnight at 4°C,
followed by horseradish peroxidase (HRP)-conjugated corresponding
secondary antibody (1:2,000; Abcam; cat. no. ab97051) for 1 h at
37°C. A chemiluminescence phototube-HRP kit (EMD Millipore,
Billerica, MA, USA) was used to visualize the immunoreactive
bands.
Immunofluorescence double
staining
Adult mice were sacrificed using an intraperitoneal
injection of 3% pentobarbital sodium (50 mg/kg) and the testes were
fixed in 4% paraformaldehyde solution for 48 h at 37°C. Tissues
were processed into paraffin using standard techniques and
sectioned at 3–4 µm thickness. Following blocking in 10% bovine
serum albumin (Beyotime Institute of Biotechnology, Haimen, China),
the sections were then incubated with the goat anti-AARD antibody
(1:200) and rabbit anti-transcription factor SOX-9 (SOX9; 1:300;
Abcam; cat. no. ab185230) antibody overnight at 4°C. Sections were
subsequently washed with phosphate-buffered saline and performed
with appropriate fluorescein isothiocyanate- or
tetramethylrhodamine-conjugated secondary antibodies (1:500; Abcam;
cat. nos. ab6717 and ab6718, respectively) for 1 h at 37°C.
Finally, the sections were stained with Hoechst 33342 (1 mg/ml;
Invitrogen; Thermo Fisher Scientific, Inc.). Representative
sections were photographed using a laser scanning confocal
microscope (Zeiss AG, Oberkochen, Germany) and images were analyzed
using the Coreldraw 9 (Corel Corporation, Ottawa, Canada).
Plasmid construction and
dual-luciferase reporter assay
Bioinformatics analysis was performed in order to
identify the potential ARE region in the Aard promoter. The
promoter sequences of Aard were obtained using the National
Center for Biotechnology Information (www.ncbi.nlm.nih.gov/) and the UCSC Genome
bioinformatics brwoser (genome.ucsc.edu/). Subsequently, an ARE structure
(TGTTCT) at position 1637 to 1632 in the region extending from
−2000 bp relative to the Aard transcription initiation site
was identified. Luciferase reporter plasmid assay was performed
using the psiCHECK-2 as a basic vector. The Aard promoter
(WT) was sub-cloned into psiCHECK-2 vector by KpnI and
NheI sites. The primers for the Aard promoter was as
follows: Forward, 5′-CGGGGTACCAGGTATGAGCTCCACTCAGTATTT-3′ and
reverse, 5′-CTAGCTAGCGCGGGGGCAGTTAACGGAACAGGCA-3′. The mutant
Aard promoter (MUT) has the same sequence as the WT
Aard promoter excluding the mutation at position −1637 to
−1632 (TGT TCT to CAC CTC) was conducted using the QuickChange II
site-directed mutagenesis kit (Stratagene; Agilent Technologies,
Inc., Santa Clara, CA, USA) with appropriate primers according to
the manufacturer's instructions. All recombinant plasmids were
verified by DNA sequencing performed by Invitrogen (Thermo Fisher
Scientific, Inc).
Primary SCs were seeded in a 24-well plate at
~4×104 cells/well, and transiently co-transfected with
pcDNA4.1-AR or pcDNA4.1 empty vector together with recombinant
psiCHECK-2 WT or MUT reporter plasmids using Lipofectamine 2000.
After 6–8 h transfection, cells were treated with 10 nM
testosterone or ethanol vehicle for 24 h. Firefly luciferase and
Renilla activities were detected using the dual luciferase
reporter system (Promega Corporation, Madison, WI, USA) with a
Modulus™ Single Tube Multimode Reader (Bio-Systems International,
Beloit, WI, USA). Firefly luciferase activity was used to normalize
Renilla luciferase activity. Following normalization for
transfection efficiency, induction factors were analyzed as the
ratios of the mean value of the luciferase signal in the
testosterone-stimulated samples compared with untreated samples.
The mouse mammary tumor virus long terminal repeat served as a
positive control.
Electrophoretic mobility shift assay
(EMSA)
EMSA was conducted using a Light-Shift
Chemiluminescent EMSA kit (Thermo Fisher Scientific, Inc.),
according to the manufacturer's protocols. Primary SCs were
cultured in 100-mm dishes and transiently transfected with
pcDNA4.1-AR or pcDNA4.1 empty vector for 24 h using Lipofectamine
2000. Subsequently, cells were treated with 10 nM testosterone for
24 h prior to the EMSA. The WT probe
5′-CACCCCTGCCCCTGTTCTGTGTGCACACGT-3′ and MUT probe
5′-CACCCCTGCCCCCACCTCGTGTGCACACGT-3′ from the mouse
Aard promoter, including the potential ARE structure (in
bold), were obtained from Thermo Fisher Scientific, Inc., and
treated using Biotin 3′ End DNA Labeling kit (Pierce; Thermo Fisher
Scientific, Inc.). Binding reactions were performed at 37°C for 20
min in the binding buffer according to the manufacturer's
protocols. Another 50- or 200-fold molar excess of the unlabeled WT
probe was used for the competitive assay. Subsequently, the AR
antibody (5 µl; 1:200) was added for AR super-shift analysis and
incubated for 2 h at 37°C. Finally, the DNA-protein complexes were
separated in a 6% non-denaturing polyacrylamide gel by
electrophoresis, transferred to nylon membranes and detected using
enhanced chemiluminescence detection kit (Pierce; Thermo Fisher
Scientific, Inc.) and a FluorChem M imaging system (ProteinSimple,
San Jose, CA, USA).
Chromatin immunoprecipitation (ChIP)
assay
Primary SCs were treated as described above for the
EMSA assay. Samples without testosterone treatment were regarded as
the negative control. The detailed procedure for extraction of
chromatin from cells and subsequent chromatin immunoprecipitation
reaction was performed as previously (22). Immunoprecipitated DNA fragments
were analyzed by semi-quantitative PCR amplification using primers
for Aard. The primers for the Aard ARE bind region
were as follows: Sense, 5′-CAGGTGCCAGCACTACAGAACCAGT-3′ and
antisense, 5′-ACTGGTTCTGTAGTGCTGGCACCTG-3′. The AR target genes,
Rhox5 and Tubb3, served as positive controls. The
primers for were as follows: Forward, 5′-GGAGGGCAACACCAGTCCCTG-3′
and reverse, 5′-CTCGGTGTCGCAAAAGGGCA-3′ for Rhox5 ARE bind region;
and forward, 5′-TGGCCCCCAGAACAGAAG-3′ and reverse,
5′-TGGTGTTCCCACTCTGTACAATG-3′ for Tubb3. The PCR was
performed using EmeraldAmp PCR Master Mix kit (Takara Bio, Inc.)
and the following cycling conditions: 98°C for 2 min; 32 cycles of
98°C for 10 sec, 60°C for 30 sec and 72°C for 30 sec; and 72°C for
5 min. The amplified products were separated on a 2% agarose gel
and visualized with ultraviolet imaging system (Bio-Rad
Laboratories, Inc., Hercules, CA, USA).
Statistical analysis
Each experiment was repeated at least three times.
Data were expressed as the mean ± standard error of the mean.
Statistical analysis was analyzed using SSPS 16.0 (SPSS, Inc.,
Chicago, IL, USA). Student's t-test was used to compare the
difference between different groups. P<0.05 was considered to
indicate a statistically significant difference.
Results
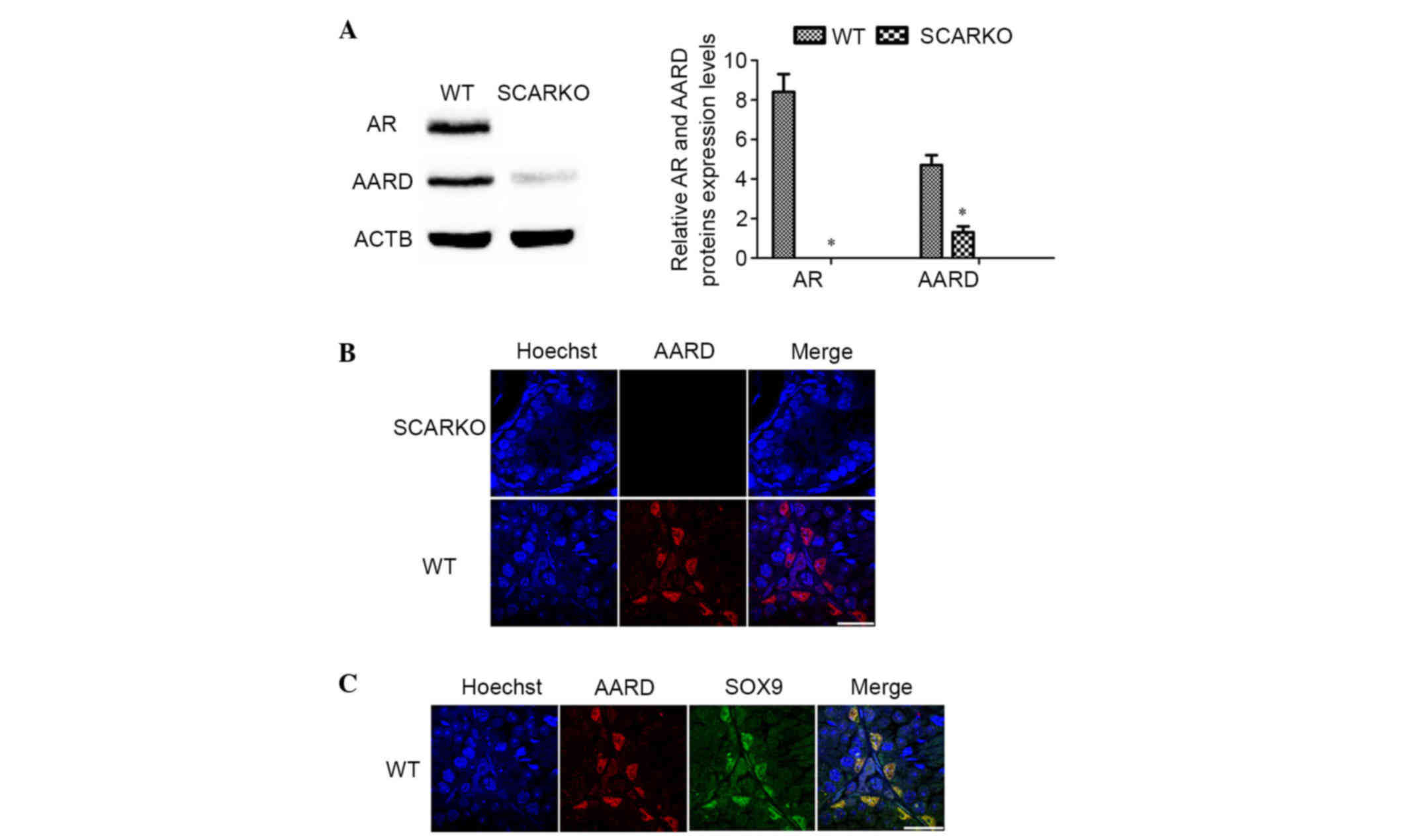
Expression of AARD in SCARKO and WT
mouse testes
Previous microarray analysis data indicated that
AARD was downregulated in SCARKO mouse testis (18). Thus, AARD expression was first
demonstrated in adult SCARKO and WT mouse testes using western blot
and immunofluorescence analysis. Consistent with the expectations
of the present study, the results demonstrated that AARD was
significantly decreased in the testes of adult SCARKO mice compared
with WT mice (P<0.05; Fig. 1A).
As presented in Fig. 1B, AARD
protein was also downregulated in SCARKO mouse testes. AARD was
exclusively located in the nucleus of SCs (Fig. 1C), which was verified by
co-immunostaining with SOX9, an approved SCs marker.
Temporal expression pattern of AARD
during mouse testis development
To investigate the function and underlying molecular
mechanisms of AARD during mouse testis development, qPCR and
western blotting were performed to evaluate the distribution of
AARD expression in different adult mouse tissues. The results
demonstrated that Aard mRNA and protein was predominantly
expressed in mouse testis (Fig.
2A). The temporal expression of AARD was further analyzed
during postnatal testis development at mRNA and protein levels. As
presented in the Fig. 2B, the data
indicated that AARD is expressed in the testes during all stages
and increased from the postnatal 1 week to 8 weeks, which suggested
Aard may be important during the process of
spermatogenesis.
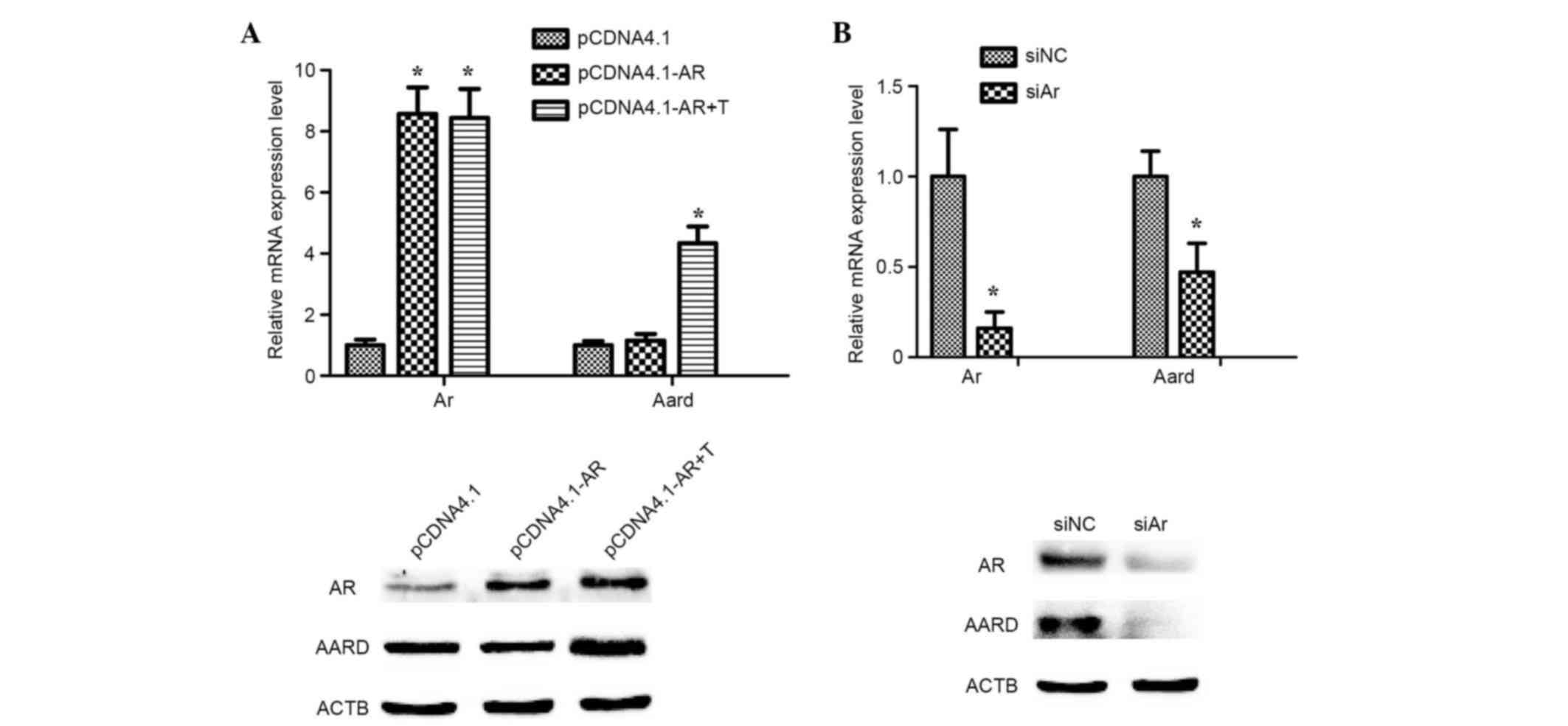
Activation of AARD expression induced
by testosterone in SCs in vitro
To investigate whether testosterone can promote AARD
expression, mediated via AR, primary SCs were transiently
transfected with a pcDNA4.1-AR overexpression plasmid and treated
with 10 nM testosterone for 24 h. The results indicated that AARD
expression was significantly upregulated at the mRNA level
(P<0.05) and markedly upregulated at the protein level, as
compared with testosterone untreated samples (Fig. 3A). However, the mRNA expression
level of AARD was significantly downregulated (P<0.05) and the
protein level notably downregulated following treatment of SCs with
siAr oligonucleotides (Fig. 3B).
These results strongly indicated that AARD expression was activated
by testosterone and mediated via AR.
Promotion of Aard promoter activity by
testosterone in SCs
In order to assess the function of testosterone in
the activation of the transcription of Aard, a
dual-luciferase reporter assay was performed. Primary SCs were
transfected with WT or MUT Aard promoter luciferase
plasmids, and then were conducted with 10 nM testosterone. Notably,
it was observed that the relative luciferase activity of WT
Aard promoter was significantly promoted by testosterone
(P<0.05), while sequences change from TGTTCT (−1637 to −1632) to
CACCTC in the ARE blocked Aard promoter regulation by
testosterone in SCs (Fig. 4).
Collectively, these results suggest that testosterone activated
Aard, which is mediated via the AR by directly binding to
the ARE in the Aard promoter.
 | Figure 4.Promotion of Aard promoter
activity by testosterone in SCs. A dual-luciferase reporter assay
was used to detect the activation of Aard promoter activity
by testosterone in SCs cells. Positive control samples were
performed with the testosterone-inducible MMTV recombination
vector. (A) SCs were transfected with an AR expression plasmid and
treated with 10 nM testosterone for 24 h. Results were presented as
fold-change of testosterone-treated samples relative to
testosterone-untreated samples. *P<0.05 vs. pcDNA4.1-AR. (B) The
luciferase activity was evaluated following treatment of SCs with
siAr oligonucleotides. *P<0.05 vs. siNC. Data were presented as
the mean ± standard deviation. LUC, luciferase; WT, wild-type; MUT,
mutant; T, testosterone; SCs, Sertoli cells; AR, androgen receptor;
AARD, alanine and arginine rich domain containing protein; siNC,
negative control small interfering RNA; siAR, AR small interfering
RNA; MMTV, mouse mammary tumor virus long terminal repeat. |
Directly binding of AR to the ARE in
Aard promoter
To determine whether AR could directly bind to the
ARE in Aard promoter, EMSA and ChIP assays were performed
(Fig. 5A). A putative ARE was
observed in the Aard promoter using bioinformatics analysis.
As presented in Fig. 5B, lane 1
was used as a negative control, the nuclear extracts of AR
overexpressing SCs treated with 10 nM testosterone could notably
bind to the WT ARE probe (lane 2). In addition, this binding
ability was fully inhibited by addition of 50- or 200-fold molar
excess of unlabeled ARE probe (cold probe; lane 3). By contrast,
the binding was not eliminated by addition of the MUT ARE probe
(lane 4). Furthermore, AR was identified in the ARE-Aard/AR complex
by supershift analysis with an anti-AR antibody (lane 5).
 | Figure 5.Direct binding of AR to the ARE in
Aard promoter. (A) Sequences of putative ARE in the
Aard promoter. (B) Electrophoretic mobility shift assay
competition experiments. Lane 1, the mobility of the labeled WT
probe without nuclear extracts; lane 2, the mobile ability of the
labeled WT probe with nuclear extracts; lane 3, sample treated with
50- or 200-fold molar excess of unlabeled ARE-like probe (cold
probe); lane 4, a similar excess of MUT probe, which the AR does
not bind with; lane 5, super-shift assays with WT probe and nuclear
extracts in the presence of anti-AR antibody. (C) Chromatin
immunoprecipitation analysis demonstrated that AR could directly
bind to the Aard promoter ARE in SCs in the presence of
testosterone. Polymerase chain reaction amplification of eluted DNA
was performed using specific primers for the Aard promoter
region. WT, wide type; MUT, mutant; AR, androgen receptor; ARE,
androgen-responsive element; IgG, immunoglobulin G; Aard, alanine
and arginine rich domain containing protein; Tubb3, tubulin β3
class III; Rhox5, reproductive homeobox 5. |
A ChIP assay was also conducted to assess the
binding capacity of the AR to the ARE in the Aard promoter.
SCs were transiently transfected with pcDNA4.1-AR overexpression
plasmid and then treated with 10 nM testosterone. AR binding to the
ARE of the Aard promoter (−1637 to-1632) was confirmed by
semi-quantitative PCR amplification using primers for Aard
in anti-AR antibody-treated samples compared with anti-IgG antibody
(Fig. 5B). These results indicate
that AR has the ability to directly bind the Aard promoter
DNA sequence.
Discussion
Normal spermatogenesis requires the action of
androgen and the AR (1,23,24).
Although androgens are sufficient to drive spermatogenesis, the
underlying molecular mechanisms require further elucidation. The
present study identified Aard as a novel target of the AR in
mouse SCs. The present study demonstrated that the AR upregulated
Aard expression by directly binding to its promoter, which
may provide information regarding the molecular mechanism for
abnormal spermatogenesis and male infertility.
Aard is specifically located in mouse SCs.
The presence of a predicted leucine-zipper domain and a
phosphorylation site in the AARD protein domain suggested that AARD
may be involved in transcriptional regulation or intracellular
signal transduction pathways (25). Based on analysis of SCARKO and WT
mouse testes, the present study demonstrated that AARD had
significantly reduced expression in SCARKO mouse testes compared
with WT mouse. These functions of androgen on AARD expression were
also observed following restoration or knockdown expression of
AR in primary SCs-treated with testosterone in vitro.
These data demonstrated that AR-regulated Aard expression
was critical for spermatogenesis.
To distinguish between the possibilities that the
effect of androgens involved a direct interaction of the AR with
the Aard gene or was indirectly mediated via an
androgen-regulated intermediary transcription factor, functional
AREs were searched for in the promoter region extending from −2000
bp relative to the Aard transcription initiation site.
Previous studies demonstrated that the canonical ARE binding site
is composed of a dimer to an inverted repeat segregated by three
oligonucleotides (AGAACAnnnTGTTCT). The atypical AREs contain the
half-site ARE sequence TGTTCT or the inverted complement AGAACA;
head-to-head AREs [AGAACA (0–8 n) TGTTCT, n≠3]; tail-to-tail AREs
[TGTTCT (0–8 n) AGAACA]; or ARE direct repeats [AGAACA (0–8 n)
AGAACA] (26,27). Based on the above principles and
combined with the matrix-scan tool of the regulatory sequences
analysis tools software package (rsat.ulb.ac.be/rsat/), a potential
ARE sequence (−1637 to-1632, TGTTCT) was observed to be located in
the Aard promoter. This ARE binds to AR in vitro and
is crucial for androgen-dependent reporter expression, based on the
dual-luciferase reporter assay. Furthermore, EMSA and ChIP assays
further proved that AR can directly bind to DNA in the Aard
promoter.
In conclusion, the results of the present study
indicate that Aard is a target of AR action in mouse SCs and
suggest a novel finding by which the loss of AR function in SCs
blocks spermatogenesis and results in male infertility. In normal
mouse SCs, AR promotes AARD expression by directly binding to its
promoter region. The loss of AR function resulted in a decrease in
AARD expression in SCARKO mice. The relatively low expression in
SCARKO mice may impair normal spermatogenesis, leading to male
infertility. These results support a role for the AR-AARD axis in
spermatogenesis, and further implicate the AR-AARD axis as a
potential therapeutic target, particularly for those with male
infertility resulting from aberrations in AR expression.
References
|
1
|
Heemers HV and Tindall DJ: Androgen
receptor (AR) coregulators: A diversity of functions converging on
and regulating the AR transcriptional complex. Endocr Rev.
28:778–808. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Patrão MT, Silva EJ and Avellar MC:
Androgens and the male reproductive tract: An overview of classical
roles and current perspectives. Arq Bras Endocrinol Metabol.
53:934–945. 2009.PubMed/NCBI
|
|
3
|
Lazaros L, Xita N, Takenaka A, Sofikitis
N, Makrydimas G, Stefos T, Kosmas I, Zikopoulos K, Hatzi E and
Georgiou I: Semen quality is influenced by androgen receptor and
aromatase gene synergism. Hum Reprod. 27:3385–3392. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Heinlein CA and Chang C: Androgen receptor
(AR) coregulators: An overview. Endocr Rev. 23:175–200. 2002.
View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Nantermet PV, Xu J, Yu Y, Hodor P, Holder
D, Adamski S, Gentile MA, Kimmel DB, Harada S, Gerhold D, et al:
Identification of genetic pathways activated by the androgen
receptor during the induction of proliferation in the ventral
prostate gland. J Biol Chem. 279:1310–1322. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Marcelli M, Ittmann M, Mariani S,
Sutherland R, Nigam R, Murthy L, Zhao Y, DiConcini D, Puxeddu E,
Esen A, et al: Androgen receptor mutations in prostate cancer.
Cancer Res. 60:944–949. 2000.PubMed/NCBI
|
|
7
|
Chen MJ, Vu BM, Axelrad M, Dietrich JE,
Gargollo P, Gunn S, Macias CG, McCullough LB, Roth DR, Sutton VR
and Karaviti LP: Androgen Insensitivity Syndrome: Management
Considerations from Infancy to Adulthood. Pediatr Endocrinol Rev.
12:373–387. 2015.PubMed/NCBI
|
|
8
|
Kim Y, Jae E and Yoon M: Influence of
Androgen Receptor Expression on the Survival Outcomes in Breast
Cancer: A Meta-Analysis. J Breast Cancer. 18:134–142. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Yeh S, Tsai MY, Xu Q, Mu XM, Lardy H,
Huang KE, Lin H, Yeh SD, Altuwaijri S, Zhou X, et al: Generation
and characterization of androgen receptor knockout (ARKO) mice: An
in vivo model for the study of androgen functions in selective
tissues. Proc Natl Acad Sci USA. 99:13498–13503. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
De Gendt K, Swinnen JV, Saunders PT,
Schoonjans L, Dewerchin M, Devos A, Tan K, Atanassova N, Claessens
F, Lécureuil C, et al: A Sertoli cell-selective knockout of the
androgen receptor causes spermatogenic arrest in meiosis. Proc Natl
Acad Sci USA. 101:1327–1332. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Wang RS, Yeh S, Tzeng CR and Chang C:
Androgen receptor roles in spermatogenesis and fertility: Lessons
from testicular cell-specific androgen receptor knockout mice.
Endocr Rev. 30:119–132. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Chang C, Lee SO, Wang RS, Yeh S and Chang
TM: Androgen receptor (AR) physiological roles in male and female
reproductive systems: Lessons learned from AR-knockout mice lacking
AR in selective cells. Biol Reprod. 89:212013. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Mou L, Zhang Q, Wang Y, Zhang Q, Sun L, Li
C, Huang W, Yuan Y, Duan Y, Diao R, et al: Identification of Ube2b
as a novel target of androgen receptor in mouse sertoli cells. Biol
Reprod. 89:322013. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Yang L, Wang Y, Zhang Q, Lai Y, Li C,
Zhang Q, Huang W, Duan Y, Jiang Z, Li X, et al: Identification of
Hsf1 as a novel androgen receptor-regulated gene in mouse Sertoli
cells. Mol Reprod Dev. 81:514–523. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Bhardwaj A, Rao MK, Kaur R, Buttigieg MR
and Wilkinson MF: GATA factors and androgen receptor collaborate to
transcriptionally activate the Rhox5 homeobox gene in Sertoli
cells. Mol Cell Biol. 28:2138–2153. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
De Gendt K, Denolet E, Willems A, Daniels
VW, Clinckemalie L, Denayer S, Wilkinson MF, Claessens F, Swinnen
JV and Verhoeven G: Expression of Tubb3, a beta-tubulin isotype, is
regulated by androgens in mouse and rat Sertoli cells. Biol Reprod.
85:934–945. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Svingen T, Beverdam A, Verma P, Wilhelm D
and Koopman P: Aard is specifically up-regulated in Sertoli cells
during mouse testis differentiation. Int J Dev Biol. 51:255–258.
2007. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Zhang QX, Zhang XY, Zhang ZM, Lu W, Liu L,
Li G, Cai ZM, Gui YT and Chang C: Identification of
testosterone-/androgen receptor-regulated genes in mouse Sertoli
cells. Asian J Androl. 14:294–300. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Verhoeven G and Cailleau J: Testicular
peritubular cells secrete a protein under androgen control that
inhibits induction of aromatase activity in Sertoli cells.
Endocrinology. 123:2100–2110. 1988. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Lutfalla G and Uze G: Performing
quantitative reverse-transcribed polymerase chain reaction
experiments. Methods Enzymol. 410:386–400. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Kim JM, Hwang SH, Song EJ, Lee SY, Kim YD,
Lee CH, Lee MK, Chang CL and Lee EY: Comparative quantification of
plasma hnRNP B1 mRNA in non-small cell lung cancer patients by
real-time PCR. Korean J Lab Med. 29:249–255. 2009.(In Korean).
View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Fujii H and Fujita T: Isolation of
Specific Genomic Regions and Identification of Their Associated
Molecules by Engineered DNA-Binding Molecule-Mediated Chromatin
Immunoprecipitation (enChIP) Using the CRISPR System and TAL
Proteins. Int J Mol Sci. 16:21802–21812. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Smith LB and Walker WH: The regulation of
spermatogenesis by androgens. Semin Cell Dev Biol. 30:2–13. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Walker WH: Testosterone signaling and the
regulation of spermatogenesis. Spermatogenesis. 1:116–120. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Blomberg LA, Chan WY, Clerch LB and
Massaro D: Molecular cloning and characterization of a novel gene
upregulated early during postnatal rat lung development. Biochim
Biophys Acta. 1574:391–398. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Wang Q, Li W, Liu XS, Carroll JS, Jänne
OA, Keeton EK, Chinnaiyan AM, Pienta KJ and Brown M: A hierarchical
network of transcription factors governs androgen
receptor-dependent prostate cancer growth. Mol Cell. 27:380–392.
2007. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Spillane M, Schwarz N and Willoughby DS:
Upper-body resistance exercise augments vastus lateralis androgen
receptor-DNA binding and canonical Wnt/β-catenin signaling compared
to lower-body resistance exercise in resistance-trained men without
an acute increase in serum testosterone. Steroids. 98:63–71. 2015.
View Article : Google Scholar : PubMed/NCBI
|