Introduction
Tooth loss due to periodontal disease, dental caries
or trauma affects the quality of life of an individual. Attempts to
successfully regenerate lost teeth or their components have long
been an ambition of dentists. Based on stem cells, scaffolds and
growth factors for regenerating missing or damaged tissues, tissue
engineering is one of the latest emerging innovations, aimed at
providing solutions for tissue creation and repair (1). Dental pulp stem cells (DPSCs) are
characterized by their multipotent differentiation, self-renewal
ability, clonogenic capacity and their odontogenic differentiation
potential in particular. Previous studies have shown that DPSCs are
capable of differentiating into odontoblast-like cells in
vitro, and to form the dentin-pulp-like complex when
transplanted into immunocompromised mice in vivo (2). However, despite the promising
characteristics of hDPSCs, there are certain challenges, which
require addressing prior to the routine use of regenerative
techniques involving these cells in clinical applications,
including improving the proliferation capacity and committed
differentiation efficiency of the cells in biomaterials.
Scaffolds are indispensable in tissue engineering,
as they serve as carriers to facilitate the delivery of stem cells
and/or growth factors at a three-dimensional (3D) site to guide
tissue formation by mediating cell survival and cell-scaffold
interactions. Owing to their biodegradability, biocompatibility,
and their nontoxic and nonimmunogenic properties (3–5),
polymers are widely used in medical applications. Poly
(lactic-co-glycolic acid) (PLGA) is a copolymer with desirable
physical and mechanical properties. PLGA is a commonly used
biomaterial for tissue engineering and is approved for clinical use
(6). However, the diffusion of air
and nutrient components in conventional 2D and 3D static culture is
uneven, resulting in reduced cell growth, particularly within the
3D constructs (7,8).
In previous years, with its low hydrodynamic shear
stress and low turbulence, the rotary cell culture system (RCCS)
has been shown to allow the exchange of nutrients and transport of
cellular secretions (9),
contributing to the regulation of the differentiation and
proliferation of stem cells. It has been suggested that RCCS
provides a more controlled dynamic 3D stimulated microgravity (SMG)
environment, which qualifies for improved cell-cell interactions,
and communication associated with proliferation and differentiation
(10–12). Of note, several cell types and
tissues have been successfully cultured under 3D SMG conditions,
including the formation of living organoid-like tissue
architecture, for example, cartilage and bone, in vitro
(13–16). However, there have been few repots
on the proliferation and differentiation of undifferentiated cells
in vivo following culture in 3D SMG.
The present study investigated the proliferation and
odontogenic differentiation of hDPSCs in vivo following the
use of a 3D SMG culture system compared with static 3D culture. The
isolated and identified hDPSCs seeded in PLGA scaffolds were
maintained separately in the 3D SMG culture system and the static
3D culture system with osteogenic medium for 7 days in
vitro. Subsequently, the differentiating cells with scaffolds
were implanted subcutaneously on the backs of nude mice for 6
weeks. Histological and immunohistochemical analyses indicated that
the proliferation and odontogenic differentiation abilities of the
hDPSCs prepared in the 3D SMG culture system were higher, compared
with those prepared in static culture. These results demonstrated
the advantages of the 3D SMG culture system for improving the
proliferation and odontogenic differentiation abilities of hDPSCs
in vivo.
Materials and methods
Isolation and identification of
hDPSCs
All experiments performed in the present study were
approved by the Ethics Committee of the First Affiliated Hospital
of Harbin Medical University (Harbin, China). The isolation and
culturing of the cells were performed as described previously
(2). In brief, fresh dental pulp
tissues were isolated from healthy impacted third molars of donors
(age range, 18–29 years) from the department of the Oral and
Maxillofacial Surgery of the First Affiliated Hospital of Harbin
Medical University (Harbin, China) in July 2014, following the
provision of written informed consent. The dental pulp tissues were
digested with 3 mg/ml collagenase type I (Sigma-Aldrich; Merck
Millipore, Darmstadt,) and 4 mg/ml dispase (BD Biosciences, San
Jose, CA, USA) for 1 h at 37°C, following which the solution was
passed through a 70-µm strainer. The characterizations of the
hDPSCs were based on a previous report (17). The cells between passages two and
five were used in the following experiments.
Cell-PLGA complex culture
The PLGA scaffolds (Synthecon, Inc., Houston, TX,
USA) were pretreated, as previously described (17). The hDPSCs (2×106) were seeded into
each scaffold and cultured in Dulbecco's modified Eagle's medium
(DMEM; Hyclone; GE Healthcare Life Sciences, Chalfont, UK)
supplemented with 10% fetal bovine serum (FBS; Hyclone; GE
Healthcare Life Sciences) for 72 h at 37°C. Subsequently, the
cell-scaffold composites were randomly divided into two groups:
Static 3D culture and 3D SMG culture. The 3D SMG group was
transferred into a 55-ml high-aspect-ratio vessel (Synthecon, Inc.)
filled with osteogenic medium (DMEM supplemented with 10% FBS, 10
nM dexamethasone, 10 mM β-glycerophosphate and 50 µg/ml ascorbic
acid) in the RCCS (Synthecon, Inc.). The rotation speed of the
vessel was adjusted throughout the period of cultivation to
maintain the complexes at a relatively steady position within the
vessel. In parallel, cells cultured in static culture with
osteogenic medium were used as controls.
Scanning electron microscopy (SEM)
observation
The cell-scaffold complexes of the static 3D culture
and 3D SMG culture systems were gently rinsed three times with PBS.
The samples were then fixed with 2% glutaraldehyde and dehydrated
using a graded ethanol series of 30, 50, 70, 90 and 100%. Following
being dipped into isoamyl acetate and dried in a critical-point
dryer, the samples were observed under an SEM.
In vivo transplantation
A total of 20 female nude mice (6–8 weeks old)
(Weitonglihua Experimental Animal Technology Co., Ltd., Beijing,
China) were randomly assigned into the two groups. All the animals
were housed under standard conditions of 12 h light/dark cycles and
fed an autoclaved laboratory rodent diet. Following culture in the
3D static or 3D SMG rotating culture systems with osteogenic medium
for 7 days in vitro, the cell-PLGA complexes were implanted
subcutaneously onto the backs of the nude mice for 4 weeks. In each
mouse, one control scaffold and one SMG scaffold was present on
either side of the spine. At 4 weeks post-transplantation, the mice
were sacrificed by cervical dislocation followed by extraction of
the implants. The implants were fixed in formalin, embedded in
paraffin and cut into sections measuring 5 µm in thickness for
histological and immunohistochemical examinations.
Histology
The sample sections were deparaffinized in xylene,
rehydrated through a gradient of ethanol solutions, stained with
hematoxylin and eosin (H&E), Masson's trichrome staining and
von Kossa staining, and viewed using a light microscope (Olympus
Corporation, Tokyo, Japan).
Immunohistochemistry
Immunohistochemical analyses of the retrieved
implants were performed using the streptavidin-biotin complex
method, according to the manufacturer's recommended protocol. The
deparaffinized sections were treated with 100 µl 3%
H2O2 for 10 min at room temperature to
suppress endogenous peroxidase activity. The sections were then
blocked in 5% normal goat serum (Beijing Zhongshan Golden Bridge
Biotechnology, Co.; OriGene Technologies, Inc., Rockville, MD, USA)
for 1 h at room temperature and incubated with primary antibodies
(1:100-1:500 dilutions) overnight at 4°C. The following primary
monoclonal antibodies were used: Ki-67 and type I collagen (rabbit
anti-mouse, cat. no. ab16667, diluted 1:500 and goat anti-mouse,
cat. no. ab34710, diluted 1:100; Abcam, Cambridge, MA USA), and
dentin sialoprotein (DSP) and DMP-1 (goat anti-mouse, cat. nos.
sc-18328 and sc-54181, diluted 1:100 and 1:200; Santa Cruz
Biotechnology, Inc. Dallas, TX, USA). Incubation in PBS alone
instead of primary antibodies served as negative controls. The
sections were rinsed in PBST and incubated in biotinylated
secondary antibodies (anti-goat IgG, cat. no. sc-2042 and
anti-rabbit IgG, cat. no. sc-2040, all purchased from Santa Cruz
Biotechnology, Inc.; diluted 1:400) for 45 min at room temperature.
The sections were then washed three times in PBST, incubated in
streptavidin-biotin complex for 30 min at room temperature and
stained with 100 µl DAB solution. When brown coloration was
detected, the slides were rinsed and then counterstained with
hematoxylin for 1 min and observed under a light microscope.
Statistical analysis
The numbers of hDPSCs were counted three times (n=3)
in each field of view and section, with three samples for each
group. Immunohistochemical analyses were performed using three
samples for each group, and calculated three times with ImageJ
software (National Institutes of Health, Bethesda, MD, USA). Values
are presented as the means ± standard deviation. Statistical
analyses were performed using Student's t-test with SPSS version
16.0 software. (SPSS, Inc., Chicago, IL, USA) P<0.05 was
considered to indicate a statistically significant difference.
Results
Cell morphology of hDPSCs in PLGA
under static 3D culture and 3D SMG culture in vitro
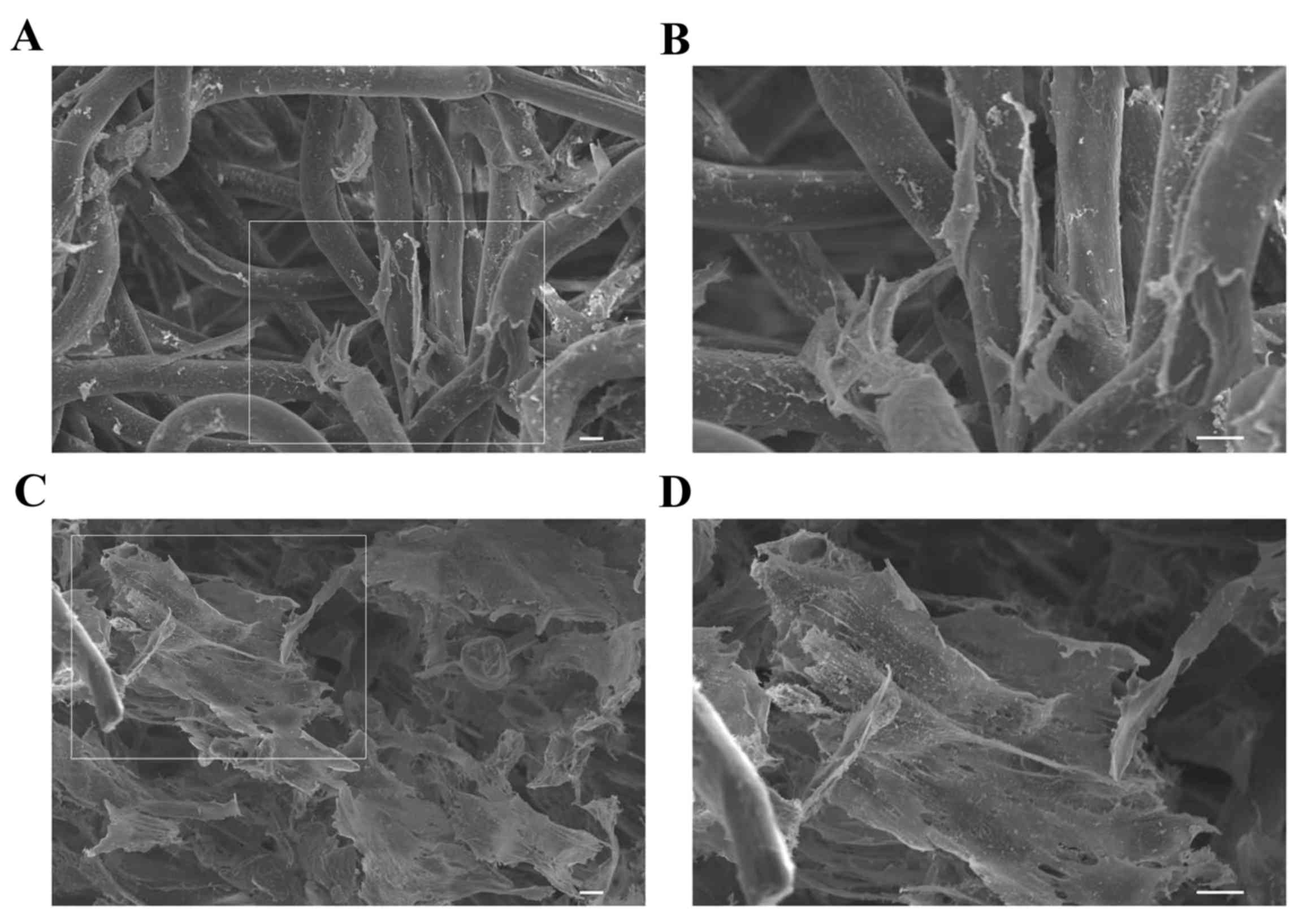
To investigate the cellular interaction of hDPSC
within PLGA scaffolds under static 3D culture and 3D SMG culture,
cell growth and morphology were observed using an electron
microscope. As shown in Fig. 1A and
B, the cells under static 3D culture were attached to the inner
surface of the scaffold in vitro. The cells in the 3D SMG
culture system grew tightly to each other with abundant
extracellular matrix deposited on the scaffolds (Fig. 1C and D). These results indicated
that the scaffolds were suitable for the following in vivo
experiments.
3D SMG culture promotes the growth of
hDPSCs in vivo
Following 3D static or 3D SMG culture for 7 days
with osteogenic medium in vitro, the differentiating cells
within the scaffolds were implanted subcutaneously on the backs of
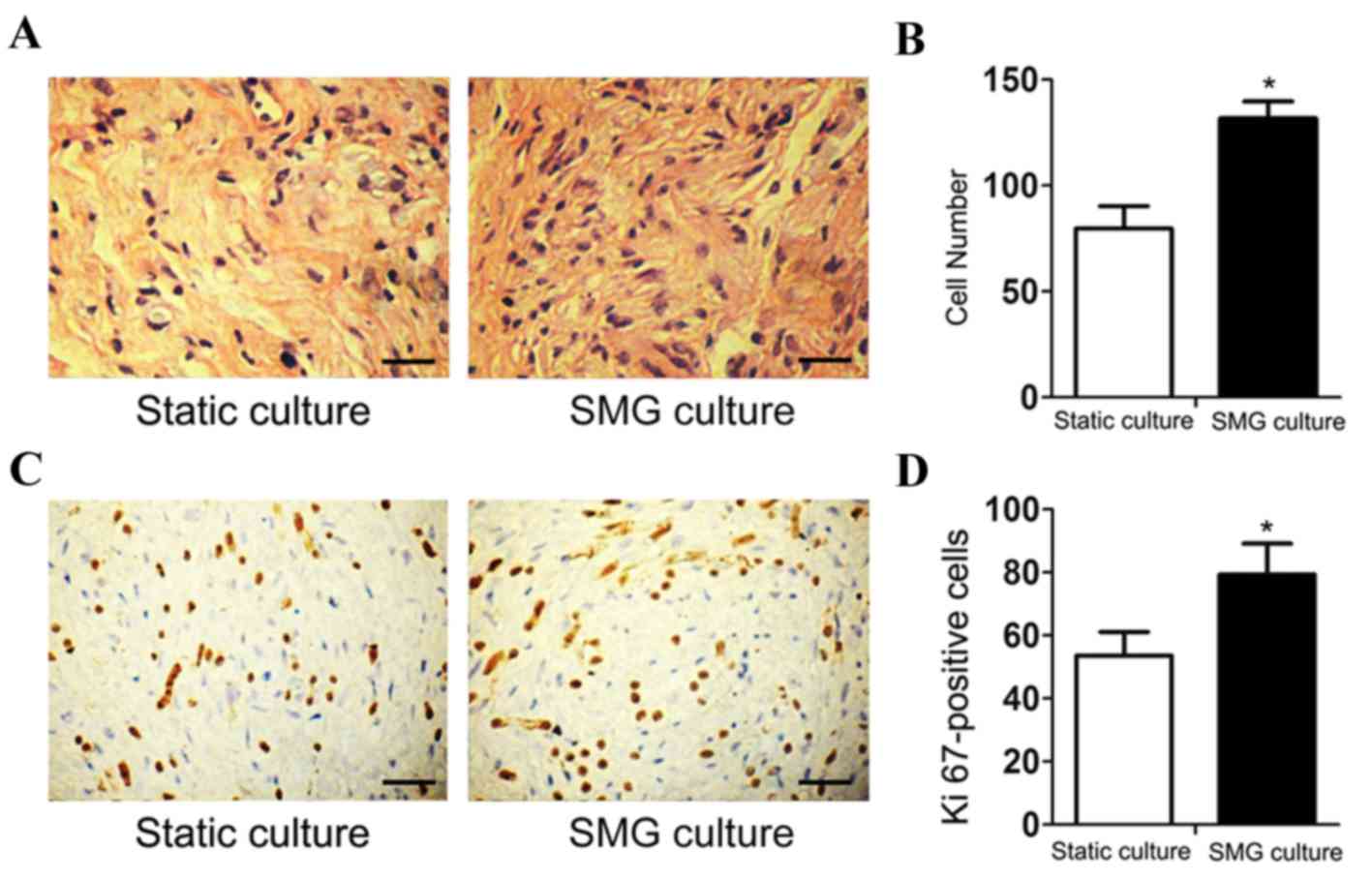
nude mice. Subsequent H&E staining showed that the number of
cells cultured in the SMG system was higher, compared with that in
the static culture system (Fig. 2A and
B). Immunohistochemical analysis of the endogenous
proliferation marker, Ki-67, showed an increase in cell
proliferation in the SMG group (Fig.
2C and D).
3D SMG culture induces increased
collagen fibrils, calcium phosphate formation and the expression of
DMP-1 and dentin sialoprotein (DSP) in vivo
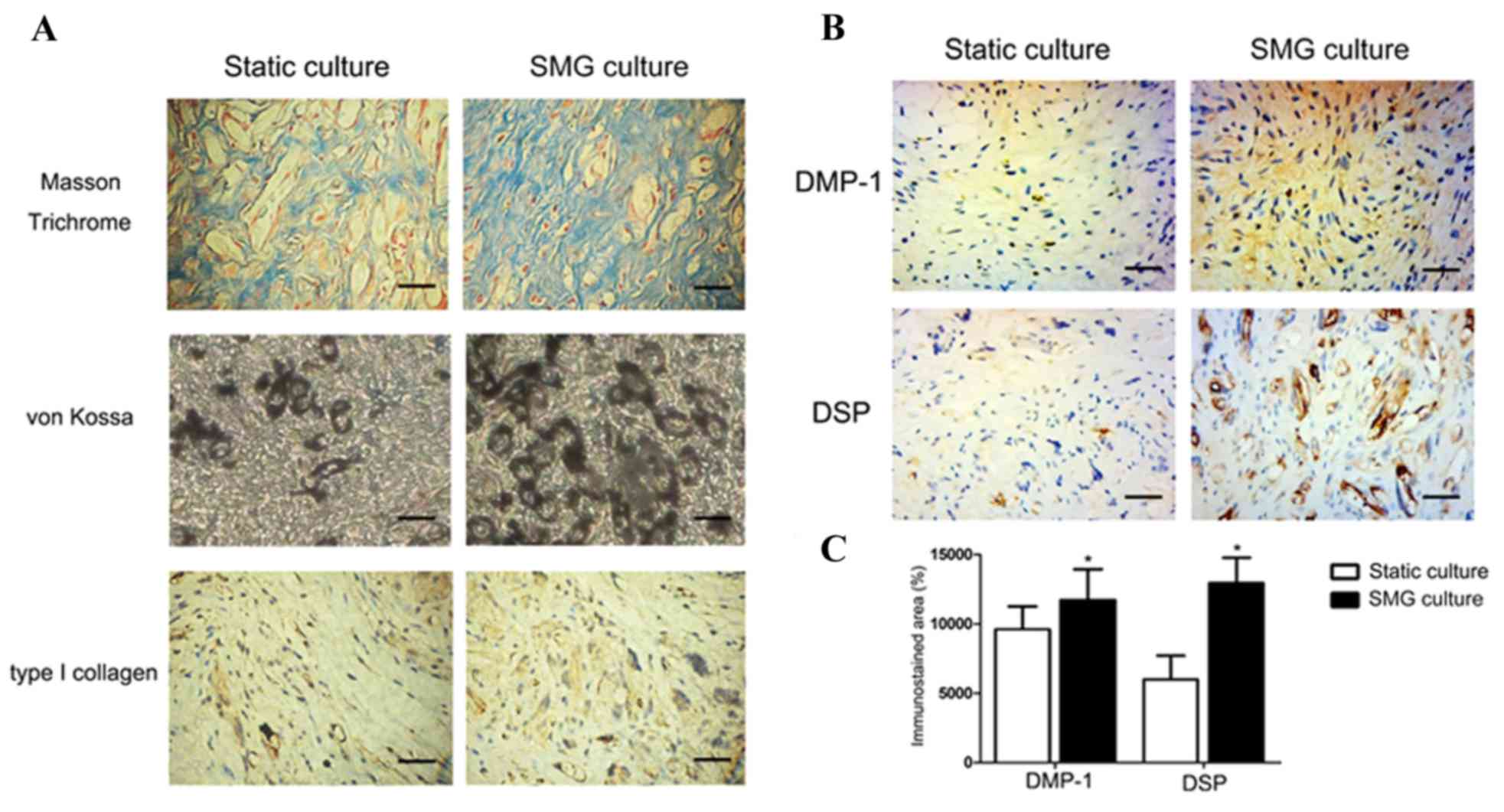
The tissue sections were stained with Masson's
trichrome and von Kossa to identify evidence of collagen fiber
formation and mineralization, respectively. The Masson's trichrome
staining showed a higher number of collagen fibers stained blue in
the SMG culture, compared with the static culture (Fig. 3A). An increase of von Kossa
staining was observed in the SMG culture, suggesting that the 3D
SMG culture enhanced matrix mineralization. The positive staining
of type I collagen observed in the static culture was also reduced,
compared with the SMG culture, consistent with the results of the
Masson's trichrome staining (Fig.
3A). The immunohistochemical data showed a marginal increase in
the expression of DMP-1 (Fig. 3B).
However, a significant increase in the expression of DSP was
observed in the 3D SMG culture, compared with the static culture
(Fig. 3C).
Discussion
In the present study, a 3D dynamic system consisting
of an SMG rotary bioreactor, biodegradable polymer scaffolds and
osteogenic medium, was successfully established. Following the
culture of DPSCs in this system for 7 days in vitro,
post-transplantation analysis indicated that the proliferation and
odontogenic differentiation abilities of the hDPSCs were increased
compared with those of cells cultured in the static culture system.
These findings indicated that the 3D SMG dynamic system offers
potential for use as a potent method for tooth tissue
regeneration.
Due to their odontogenic differentiation potential,
DPSCs been used as effective seed cells for dental tissue
engineering and regeneration. The application of DPSCs in dental
tissue engineering provides a significant enhancement to dental
regeneration; however, sufficient cell numbers are required,
leading to the formation of 3D mineralized, dentin tissue-like
constructs. However, limitations in the quantity and committed
differentiation efficiency of DPSCs inevitably introduce challenges
to dental regeneration. Microgravity and polymer scaffolds have
been confirmed to offer significant advantages in cell culture by
providing a dynamic 3D microenvironment with low-shear forces and
high-mass transfer (18–23).
Previous studies have indicated that a variety of 3D
biomaterials are suitable for the proliferation and differentiation
of DPSCs (24–26). However, due to the effect of
gravity, cells seeded in 3D static culture systems preferentially
fall down to the base of the scaffolds, rather than scattering
evenly (27). In addition, air,
nutrient components and metabolic wastes are also distributed
unevenly. Cellular metabolic waste is difficult to transport out of
the scaffolds, and the concentration of growth/differentiation
factors is usually confined to the surface of the scaffold,
resulting in decreased cell proliferation and lineage-specific
differentiation (28,29). Fortunately, these problems can be
overcome by the dynamic system of the SMG rotary bioreactor, which
creates a suspension culture environment contributing to supply of
oxygen and nutrients, and the transport of metabolic waste from the
cells. In the present study, a 3D dynamic system of SMG was
prepared for 7 days in vitro, in which the DPSCs grew
tightly to each other with abundant extracellular matrix, and a
higher number of cells were observed in vivo (Fig. 2). As previous experiments have
reported, undergoing 3D dynamic SMG culture leads to the promotion
of cell proliferation (18,30–32).
Ki-67, used as a biomarker for the proliferation of cells, showed
an increase in cell proliferation in the dynamic system (Fig. 2) as a result of DPSCs obtaining
sufficient nutrition and the prompt delivery of metabolic waste in
the suspension culture environment. Following transplantation in
vivo, the optimal viability and state of the DPSCs were
observed. The increased proliferation of the DPSCs in vivo
is important for dental tissue engineering and regeneration, as
this is limited in autologous or allogenic seeding of cells.
The most notable feature of hDPSCs is their
odontogenic differentiation potential for dental tissue engineering
(33). In the present study,
Masson's trichrome staining and the immunohistochemical analysis of
type I collagen were applied to determine the collagen fibers in
the tissue sections. Type I collagen is the most important
constituent of the extracellular matrix of dental pulp connective
tissue (34). It has been
suggested that the synthesis of type I collagen is an important
step in the odontoblast differentiation process (35). Previous studies have shown that
type I collagen may be a component of the predentin secreted by
polarized odontoblasts (36), and
it has been found to be associated with the production and
mineralization of dentine (37).
In the present study, increased collagen was produced in the
dynamic system group, which indicated that dynamic culture
triggered the deposition of oriented collagen fibers, which in turn
suggested the possibility of the formation of dentin. Von Kossa
staining is usually used to identify the existence and formation of
calcium phosphate (38). As the
primary component of teeth is calcium phosphate, the results
indicating a higher level of calcium phosphate formation in the
implanted cells from the dynamic SMG system, compared with that in
static culture 4 weeks post-transplantation ex vivo
suggested that the dynamic SMG system promoted the mineralization
of DPSCs. DSP and DMP-1, the major noncollagenous proteins
synthesized by odontoblasts, are well-known markers of odontogenic
differentiation. DSP, which is expressed at high levels in
odontoblasts, is essential to the formation and calcification of
dentin (39,40). Expressed prior to DSP, DMP-1
regulates the mineralization of dentin (41) and is involved in the
differentiation of odontoblasts (42–44).
Thus DSP and DMP-1 are usually selected as specific markers of
differentiation to detect the odontogenic potential of DPSCs. The
upregulation of DSP and DMP-1 in the DPSCs induced by the dynamic
SMG system, indicated the promotion of odontogenesis of the DPSCs.
The dynamic system of SMG upregulated the mineralization capacity
and expression levels of DSP and DMP-1 in the DPSCs, which
supported the idea that the dynamic SMG system was more suitable
for odontogenic differentiation of DPSCs. There is substantial
evidence, which shows that SMG promotes the differentiation of stem
cells in vitro (18,30–32)
and, consistent with these reports, the present study found that
the dynamic SMG system increased the odontogenic differentiation of
DPSCs in vivo. This may also be due, in part, to the prompt
delivery of factors in osteogenic medium and interactions with the
microenvironment in the nude mice. The DPSCs under the dynamic
culture system, which contributed to the sufficient transfer of
nutrients and factors in osteogenic medium, were maintained in good
condition throughout the entire treatment process prior to in
vivo implantation. With DPSCs in a preferable condition, the
interaction between cells and the microenvironment in vivo
may be improved, which may have a positive effect on the committed
differentiation of stem cells.
In conclusion, the present study showed that the
dynamic system combining SMG with scaffolds and osteogenic medium
significantly improved the proliferation and odontogenic
differentiation of DPSCs by improving their metabolism and
microenvironment. These results further indicated the potential of
the dynamic SMG system in dentin regeneration, and provided novel
insight into tooth engineering.
Acknowledgements
This study was supported by grants from The Nature
Science Foundation of China (grant nos. 81271132 and 81570963) and
the Nature Science Foundation of Heilongjiang Province (grant no.
H201440).
References
|
1
|
Langer R and Vacanti JP: Tissue
engineering. Science. 260:920–926. 1993. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Gronthos S, Mankani M, Brahim J, Robey PG
and Shi S: Postnatal human dental pulp stem cells (DPSCs) in vitro
and in vivo. Proc Natl Acad Sci USA. 97:13625–13630. 2000.
View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Rutzky LP, Bilinski S, Kloc M, Phan T,
Zhang H, Katz SM and Stepkowski SM: Microgravity culture condition
reduces immunogenicity and improves function of pancreatic islets1.
Transplantatio. 74:13–21. 2002. View Article : Google Scholar
|
|
4
|
Vilos C and Velasquez LA: Therapeutic
strategies based on polymeric microparticles. J Biomed Biotechnol.
2012:6727602012. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Lesman A, Koffler J, Atlas R, Blinder YJ,
Kam Z and Levenberg S: Engineering vessel-like networks within
multicellular fibrin-based constructs. Biomaterials. 32:7856–7869.
2011. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Lü JM, Wang X, Marin-Muller C, Wang H, Lin
PH, Yao Q and Chen C: Current advances in research and clinical
applications of PLGA-based nanotechnology. Expert Rev Mol Diagn.
9:325–341. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Holy CE, Shoichet MS and Davies JE:
Engineering three-dimensional bone tissue in vitro using
biodegradable scaffolds: Investigating initial cell-seeding density
and culture period. J Biomed Mater Res. 51:376–382. 2000.
View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Inanc B, Elcin AE and Elcin YM: Osteogenic
induction of human periodontal ligament fibroblasts under two- and
three-dimensional culture conditions. Tissue Eng. 12:257–266. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Rutzky LP, Bilinski S, Kloc M, Phan T,
Zhang H, Katz SM and Stepkowski SM: Microgravity culture condition
reduces immunogenicity and improves function of pancreatic islets1.
Transplantation. 74:13–21. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Hammond TG and Hammond JM: Optimized
suspension culture: The rotating-wall vessel. Am J Physiol Renal
Physiol. 281:F12–F25. 2001.PubMed/NCBI
|
|
11
|
Klement BJ, Young QM, George BJ and
Nokkaew M: Skeletal tissue growth, differentiation and
mineralization in the NASA rotating wall vessel. Bone. 34:487–498.
2004. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Goodwin TJ, Prewett TL, Wolf DA and
Spaulding GF: Reduced shear stress: A major component in the
ability of mammalian tissues to form three-dimensional assemblies
in simulated microgravity. J Cell Biochem. 51:301–311. 1993.
View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Ohyabu Y, Kida N, Kojima H, Taguchi T,
Tanaka J and Uemura T: Cartilaginous tissue formation from bone
marrow cells using rotating wall vessel (RWV) bioreactor.
Biotechnol Bioeng. 95:1003–1008. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Qiu QQ, Ducheyne P and Ayyaswamy PS:
Fabrication, characterization and evaluation of bioceramic hollow
microspheres used as microcarriers for 3-D bone tissue formation in
rotating bioreactors. Biomaterials. 20:989–1001. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Khaoustov VI, Darlington GJ, Soriano HE,
Krishnan B, Risin D, Pellis NR and Yoffe B: Induction of
three-dimensional assembly of human liver cells by simulated
microgravity. In Vitro Cell Dev Biol Anim. 35:501–509. 1999.
View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Martin A, Zhou A, Gordon RE, Henderson SC,
Schwartz AE, Schwartz AE, Friedman EW and Davies TF: Thyroid
organoid formation in simulated microgravity: Influence of
keratinocyte growth factor. Thyroid. 10:481–487. 2000.PubMed/NCBI
|
|
17
|
He L, Pan S, Li Y, Zhang L, Zhang W, Yi H,
Song C and Niu Y: Increased proliferation and adhesion properties
of human dental pulp stem cells in PLGA scaffolds via simulated
microgravity. Int Endod J. 49:161–173. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Lei XH, Ning LN, Cao YJ, Liu S, Zhang SB,
Qiu ZF, Hu HM, Zhang HS, Liu S and Duan EK: NASA-approved rotary
bioreactor enhances proliferation of human epidermal stem cells and
supports formation of 3D epidermis-like structure. PLoS One.
6:e266032011. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Wu C, Guo X, Wang F, Li X, Tian XC, Li L,
Wu Z and Zhang S: Simulated microgravity compromises mouse oocyte
maturation by disrupting meiotic spindle organization and inducing
cytoplasmic blebbing. PLoS One. 6:e222142011. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Freed LE, Hollander AP, Martin I, Barry
JR, Langer R and Vunjak-Novakovic G: Chondrogenesis in a
cell-polymer-bioreactor system. Exp Cell Res. 240:58–65. 1998.
View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Pan H, Jiang H and Chen W: Interaction of
dermal fibroblasts with electrospun composite polymer scaffolds
prepared from dextran and poly lactide-co-glycolide. Biomaterials.
27:3209–3220. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
McBane JE, Battiston KG, Wadhwani A,
Sharifpoor S, Labow RS and Santerre JP: The effect of degradable
polymer surfaces on co-cultures of monocytes and smooth muscle
cells. Biomaterials. 32:3584–3595. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Xue Y, Dånmark S, Xing Z, Arvidson K,
Albertsson AC, Hellem S, Finne-Wistrand A and Mustafa K: Growth and
differentiation of bone marrow stromal cells on biodegradable
polymer scaffolds: An in vitro study. J Biomed Mater Res A.
95:1244–1251. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Karadzic I, Vucic V, Jokanovic V,
Debeljak-Martacic J, Markovic D, Petrovic S and Glibetic M: Effects
of novel hydroxyapatite-based 3D biomaterials on proliferation and
osteoblastic differentiation of mesenchymal stem cells. J Biomed
Mater Res A. 103:350–357. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Miyashita S, Ahmed NE, Murakami M, Iohara
K, Yamamoto T, Horibe H, Kurita K, Takano-Yamamoto T and Nakashima
M: Mechanical forces induce odontoblastic differentiation of
mesenchymal stem cells on three-dimensional biomimetic scaffolds. J
Tissue Eng Regen Med. Jun 12–2014.(Epub ahead of print). PubMed/NCBI
|
|
26
|
Roozafzoon R, Lashay A, Vasei M, Ai J,
Khoshzaban A, Keshel SH, Barabadi Z and Bahrami H: Dental pulp stem
cells differentiation into retinal ganglion-like cells in a three
dimensional network. Biochem Biophys Res Commun. 457:154–160. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Boukhechba F, Balaguer T, Michiels JF,
Ackermann K, Quincey D, Bouler JM, Pyerin W, Carle GF and Rochet N:
Human primary osteocyte differentiation in a 3D culture system. J
Bone Miner Res. 24:1927–1935. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Hassell T, Gleave S and Butler M: Growth
inhibition in animal cell culture. The effect of lactate and
ammonia. Appl Biochem Biotechnol. 30:29–41. 1991. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Glowacki J, Mizuno S and Greenberger JS:
Perfusion enhances functions of bone marrow stromal cells in
three-dimensional culture. Cell Transplant. 7:319–326. 1998.
View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Yuge L, Kajiume T, Tahara H, Kawahara Y,
Umeda C, Yoshimoto R, Wu SL, Yamaoka K, Asashima M, Kataoka K and
Ide T: Microgravity potentiates stem cell proliferation while
sustaining the capability of differentiation. Stem Cells Dev.
15:921–929. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Li S, Ma Z, Niu Z, Qian H, Xuan D, Hou R
and Ni L: NASA-approved rotary bioreactor enhances proliferation
and osteogenesis of human periodontal ligament stem cells. Stem
Cells Dev. 18:1273–1282. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Qiu Q, Ducheyne P, Gao H and Ayyaswamy P:
Formation and differentiation of three-dimensional rat marrow
stromal cell culture on microcarriers in a rotating-wall vessel.
Tissue Eng. 4:19–34. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
About I, Bottero MJ, De Denato P, Camps J,
Franquin JC and Mitsiadis TA: Human dentin production in vitro. Exp
Cell Res. 258:33–41. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Karjalainen S, Söderling E, Pelliniemi L
and Foidart JM: Immunohistochemical localization of types I and III
collagen and fibronectin in the dentine of carious human teeth.
Arch Oral Biol. 31:801–806. 1986. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Andujar MB, Couble P, Couble ML and
Magloire H: Differential expression of type I and type III collagen
genes during tooth development. Development. 111:691–698.
1991.PubMed/NCBI
|
|
36
|
Mao YQ, Ohsaki Y and Kurisu K:
Immunohistochemical study of the relationship between extracellular
matrix and root bifurcation in the mouse molar. Arch Oral Biol.
35:583–591. 1990. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Garcia JM, Martins MD, Jaeger RG and
Marques MM: Immunolocalization of bone extracellular matrix
proteins (type I collagen, osteonectin and bone sialoprotein) in
human dental pulp and cultured pulp cells. Int Endod J. 36:404–410.
2003. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Hao J, Narayanan K, Ramachandran A, He G,
Almushayt A, Evans C and George A: Odontoblast cells immortalized
by telomerase produce mineralized dentin-like tissue both in vitro
and in vivo. J Biol Chem. 277:19976–19981. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
McKnight DA, Simmer JP, Hart PS, Hart TC
and Fisher LW: Overlapping DSPP mutations cause dentin dysplasia
and dentinogenesis imperfecta. J Dent Res. 87:1108–1111. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Lee SK, Lee KE, Jeon D, Lee G, Lee H, Shin
CU, Jung YJ, Lee SH, Hahn SH and Kim JW: A novel mutation in the
DSPP gene associated with dentinogenesis imperfecta type II. J Dent
Res. 88:51–55. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
He G, Dahl T, Veis A and George A: Dentin
matrix protein 1 initiates hydroxyapatite formation in vitro.
Connect Tissue Res. 44 Suppl 1:240–245. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Almushayt A, Narayanan K, Zaki AE and
George A: Dentin matrix protein 1 induces cytodifferentiation of
dental pulp stem cells into odontoblasts. Gene Ther. 13:611–620.
2006. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Narayanan K, Srinivas R, Ramachandran A,
Hao J, Quinn B and George A: Differentiation of embryonic
mesenchymal cells to odontoblast-like cells by overexpression of
dentin matrix protein 1. Proc Natl Acad Sci USA. 98:4516–4521.
2001. View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Chaussain C, Eapen AS, Huet E, Floris C,
Ravindran S, Hao J, Menashi S and George A: MMP2-cleavage of DMP1
generates a bioactive peptide promoting differentiation of dental
pulp stem/progenitor cell. Eur Cell Mater. 18:84–95. 2009.
View Article : Google Scholar : PubMed/NCBI
|