Introduction
Diabetic retinopathy (DR), classified into
nonproliferative diabetic retinopathy and proliferative diabetic
retinopathy (PDR), is an important diabetic microvascular
complication, which has been identified as the leading cause of
blindness in the working-age population worldwide (1). The prevalence of diabetes mellitus
(DM) is growing and the number of patients with DM has been
predicted to increase to 380 million by 2025, with ~4 million
people suffering from DR (2). A
number of risk factors have been reported, including hyperglycemia,
hypertension, dyslipidemia, diabetes duration, ethnic origin,
pregnancy, puberty and cataract surgery (3,4).
Various additional systemic factors, including alcohol consumption,
nephropathy, hypothyroidism, obesity, inflammation and endothelial
dysfunction, have been reported to be associated with the
development and progression of DR (2). Chronic hyperglycemia may initiate a
series of physiological and biochemical changes that may result in
microvascular damage and ultimately DR. Numerous signaling
pathways, including vascular endothelial growth factor (VEGF)
activation, inflammation, oxidative stress, protein kinase C
activation and upregulation of the renin-angiotensin system
(5), have been identified to
contribute the development of DR. The present study focused on the
role of tumor necrosis factor ligand related molecule 1 A (TL1A) in
DR, which is hypothesized to exert its DR effects via
inflammation.
TL1A, also termed tumor necrosis factor superfamily
member 15 and vascular endothelial growth inhibitor, was previously
identified as an anti-angiogenic cytokine that belonged to the TNF
superfamily (6). TL1A may have a
number of functions, including stimulation of dendritic cell
maturation, activation of T-cells, inhibition of endothelial cell
proliferation and endothelial progenitor cell (EPC)
differentiation. In addition, previous studies have reported that
TL1A is involved in inflammatory bowel disease as a Th1-polarizing
cytokine (7), and suppressed tumor
growth, endothelial cell proliferation and angiogenesis (8,9).
Furthermore, reduced expression levels of TL1A have been associated
with unfavorable outcome for breast cancer patients (10). It has previously been demonstrated
that TL1A was an important negative regulator for endothelial cell
proliferation, which may result in inhibition of vasculogenesis.
TL1A may inhibit the proliferation of endothelial cells by two
mechanisms, the induction of early G1 phase arrest and
programmed cell death in proliferating cells (11). In addition, TL1A has been reported
to inhibit vasculogenesis by regulation of soluble isoforms of VEGF
receptor 1 (VEGFR1) (12). DR is a
microvascular DM-associated vision disease with severe negative
effects on the quality of life of patients. During DR, angiogenesis
induces formation of new blood vessels and leads to the breakdown
of the retinal barrier, which may ultimately result in blindness.
As TL1A has been identified to contribute to vasculogenesis, the
present study hypothesized that TL1A level may be important for the
progression of DR and potentially provide a target for the
generation of novel therapeutic and prognostic tools.
VEGF is a key factor that has been investigated in
the pathogenesis of DR. The use of anti-VEGF therapy in diabetic
macular edema, retinal angiogenesis and PDR has been effective;
however, anti-VEGF therapies have transient benefits, as the edema
recurs within a few weeks, and repeated injections are required
(13–15). The role of inflammation in DR has
been investigated extensively and has been demonstrated to be an
important response in the development and progression of the
disease pathology. A previous clinical study identified that
elevated serum concentrations of tumor necrosis factor-α (TNF-α),
interleukin-1β (IL-1β) and VEGF were associated with the presence
and severity of diabetic retinopathy (16). Various VEGF-dependent therapeutic
agents have been used clinically for the treatment of DR patients,
including Macugen, Lucentis, and bevacizumab. However, these
anti-VEGF therapeutic agent regimens included multiple treatments
due to the recurring edema in patients with DR, requiring the
development of novel therapeutic strategies for DR treatment.
Previous studies indicated that VEGF may inhibit the expression of
TL1A in ovarian cancer (17), and
that expression levels of TL1A are upregulated by TNF-α and
interleukin-1 (IL-1) in human umbilical vein endothelial cells
(HUVECs) (18). In addition,
previous studies have reported that TNF-α may contribute to the
pathogenesis of DR, as TNF-α and IL-1β induced leukostatis and
vascular permeability by the activation of macroglia and increased
endothelial adhesion (19,20). Elevated TNF-α and IL-1β expression
levels were identified in the retina of diabetic animals and serum
concentrations of VEGF, TNF-α and IL-1β were associated with the
severity of DR (20,21).
The present study investigated the expression levels
of TL1A, VEGF, TNF-α and IL-1β in serum, the vitreous and retina in
different stages of DR in rats, and discussed the roles of TL1A,
VEGF, TNF-α and IL-1β in the pathogenesis of DR.
Materials and methods
Chemicals
TL1A (cat. no. ab85566), TNF-α (cat. no. ab6671),
IL-1β (cat. no. ab9722) and VEGF (cat. no. ab46154) antibodies were
obtained from Abcam (Cambridge, MA, USA). Enzyme linked
immunosorbent assay (ELISA) kits were obtained from R&D
Systems, Inc. (Minneapolis, MN, USA). The Immunocruz®
staining system was obtained from Santa Cruz Biotechnology, Inc.
(Dallas, TX, USA).
Animal model
A total of 80 male Wistar rats (weight, 200–250 g;
age, 6 weeks) were obtained from Military Medical Academy of China
(Beijing, China). The rats were individually housed in a
temperature (21–23°C)- and humidity (40–70%)-controlled room with a
12-h light/dark cycle, and were provided with food and water. All
the rats were handled according to the National Institutes of
Health Guide for the Care and Use of Laboratory Animals (22) and the procedures were approved by
the Animal Care and Use Committee of Tianjin Medical University
(Tianjin, China). The rats were randomly assigned to four groups:
i) Control group (n=20, no-treatment); ii) DM 1 month group (n=20);
iii) DM 3 month group (n=20); and iv) DM 6 month group (n=20).
Diabetes was induced with an intraperitoneal streptozotocin
injection (60 mg/kg body weight). Rats with blood sugar levels
between 16.5 and 22.5 mM after 3 days of the administration of
streptozotocin were assigned to the diabetic group (n=60) and
included in the experiment. Blood glucose levels were quantified
once a week with a blood glucose monitor (LifeScan, Inc., Milpitas,
CA, USA) and small insulin (Bantus) doses (4 UI/kg) were
administered when necessary, in order to maintain the blood glucose
levels between 16.5 and 22.5 mM.
Immunohistochemistry
Formalin-fixed paraffin retina sections (5 µm) were
stained with hematoxylin and eosin (H&E). Immunohistochemistry
was performed as previously described (23). Briefly, the staining of TL1A, VEGF,
TNF-α and IL-1β was performed on retina tissue by using a
monoclonal antibody against TL1A, VEGF, TNF-α and IL-1β at a
dilution of 1:100. The Immunocruz® staining system was
used, following the manufacturer's protocol. Staining-positive
cells were analyzed randomly in 6 fields at a magnification of
×400. Results were calculated by counting the positively stained
cells. Images were captured using an Olympus BX51 fluorescent
microscope (Olympus Corporation, Tokyo, Japan).
ELISA
Blood samples from each rat was collected from the
femoral vein and stored in blood collection tubes without
anticoagulants, and placed upright at room temperature for 20 min,
in order to allow the blood to clot naturally and the serum was
separated by centrifugation at 1,000 × g at 20°C for 15 min.
Vitreous samples were collected using 1 ml syringes. Serum and
vitreous samples were maintained at −80°C until biochemical assays
were performed. Serum and vitreous TL1A, VEGF, TNF-α and IL-1β
levels were quantified using ELISA kits according to the
manufacturer's protocol. TL1A, VEGF, TNF-α and IL-1β levels were
determined by reading the absorbance at 450 nm and the
concentration of the cytokines in the samples was demonstrated by
comparing the optical density of the samples to the standard
curve.
Statistical analysis
SPSS version 17.0 (SPSS, Inc., Chicago, IL, USA) was
used for all statistical analyses. Data are presented as the mean ±
standard deviation. Differences between groups were analyzed using
one-way analysis of variance or Student's paired t-test. P<0.05
was considered to indicate a statistically significant
difference.
Results
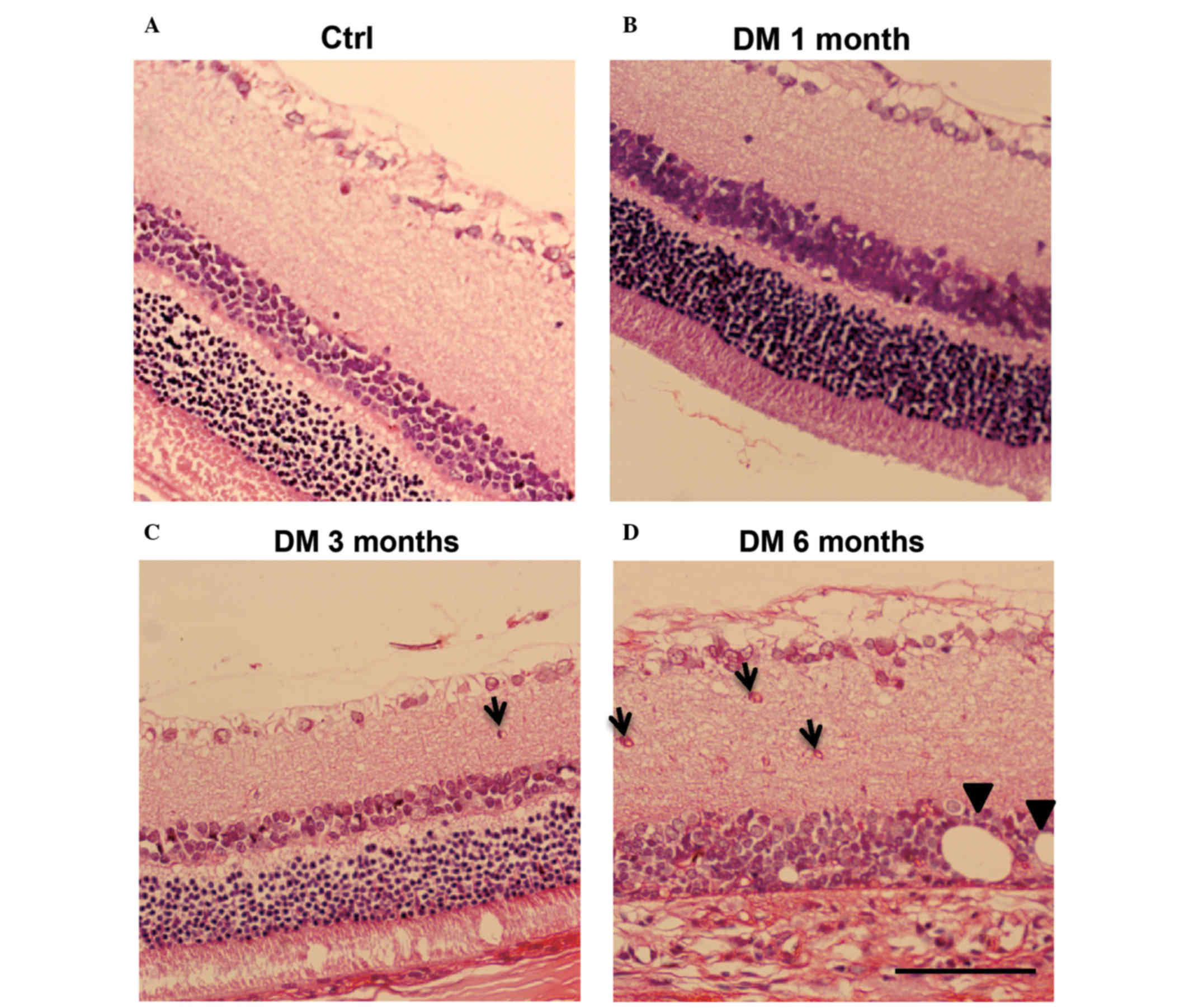
H&E staining of retina DM
model
To confirm that the DM model was successfully
established, H&E staining was performed and the normal
physiology of cell layers were observed in the control (Fig. 1A) and DM 1 month groups (Fig. 1B). By contrast, the DM 3 month
group exhibited signs of retinal edema, such as disorder in cell
layers, decrease of ganglion cells and the presence of microvessels
in the inner plexiform layer (IPL), as denoted by arrows (Fig. 1C). It is of note that the DM 6
month group exhibited severe retina edema, thinner retina, deep
staining of ganglion cells and increased microvessels in the IPL
(Fig. 1D). In addition, the inner
nuclear layer (INL) was in structural disarray, edema was apparent,
fat vacuoles were visible (indicated with arrowheads; Fig. 1D) and layers of the retina were
difficult to distinguish. This demonstrated that the streptozotocin
injection successfully induced the DR model in rats. Notably,
changes to the retina due to the pathology of DR first observed in
DM rats in the 3 month group. Typical pathology changes of DR were
clearly visible in the retina of DM rats at 6 months.
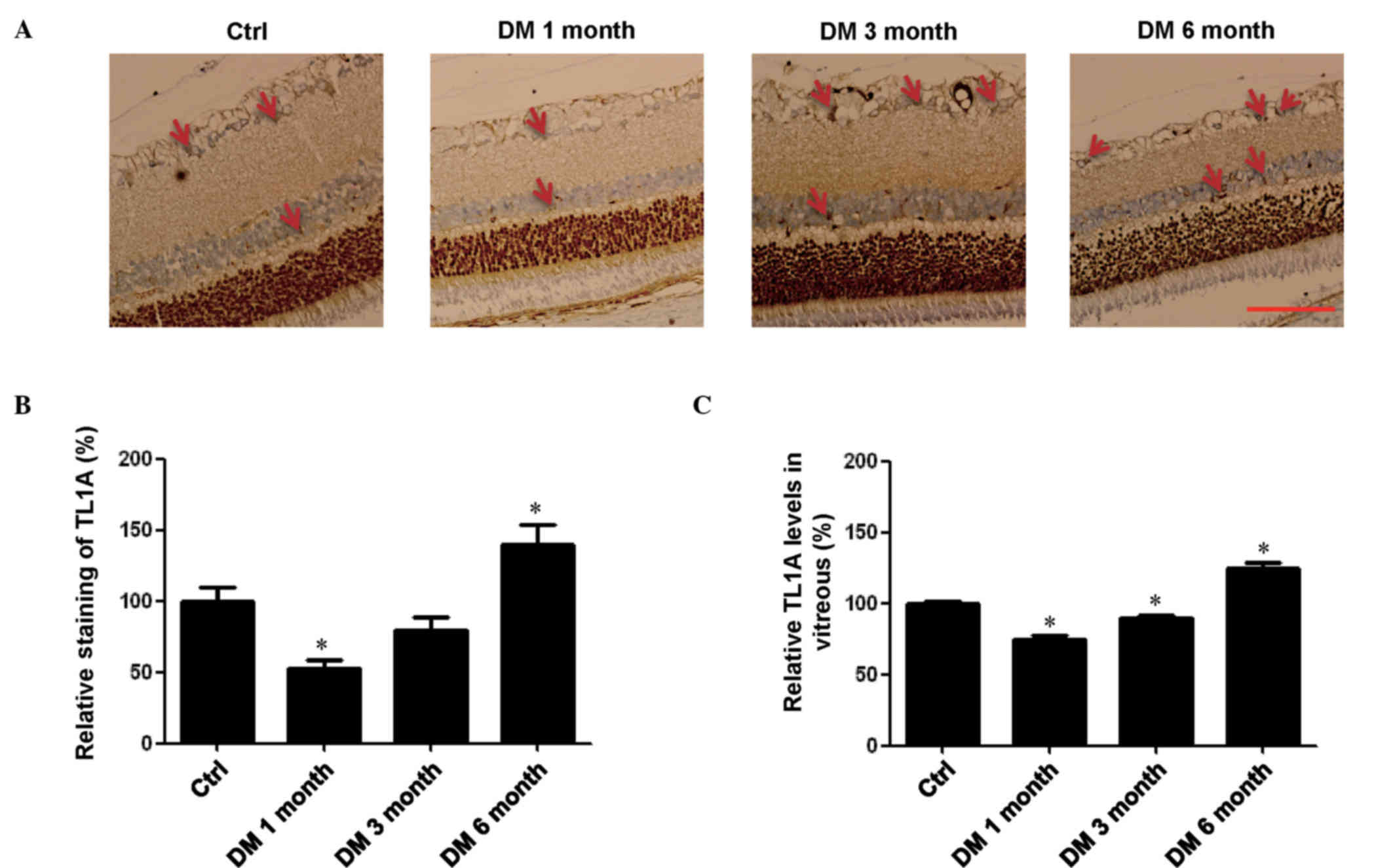
Changes of TL1A levels in retina and
vitreous of rats with different stages of DM
Previous studies have reported TL1A to be a negative
regulator of proliferation for endothelial cells, resulting in
inhibition of vasculogenesis (7,11,12).
As DR is a microvascular DM-associated vision disease involving
vasculogenesis, the present study investigated the levels of TL1A
in the retina of rats with DM by immunohistochemical analysis. It
was demonstrated that TL1A was expressed in retina tissues of the
control groups, including the INL and ganglion cell layer (Fig. 2A). TL1A levels in the DM 1 month
group were significantly lower compared with the control group
(P<0.05; Fig. 2B); however,
they were increased to near baseline levels in the DM 3 month
group. In the DM 6 month group, the TL1A levels were significantly
higher compared with the control group (P<0.05; Fig. 2B). ELISA was used to confirm the
changes of the TL1A expression levels in the vitreous of rats at
different stages of DM. These assays exhibited the same changes of
TL1A expression levels observed in the retina. Briefly, the levels
of TL1A were significantly lower in the DM 1 month and 3 month
groups compared with the control group (P<0.05; Fig. 2C). In addition, TL1A levels were
significantly higher in the DM 6 month group compared with the
control (P<0.05; Fig. 2C).
Thus, it is possible that the expression levels of TL1A may be
regulated by other cytokines.
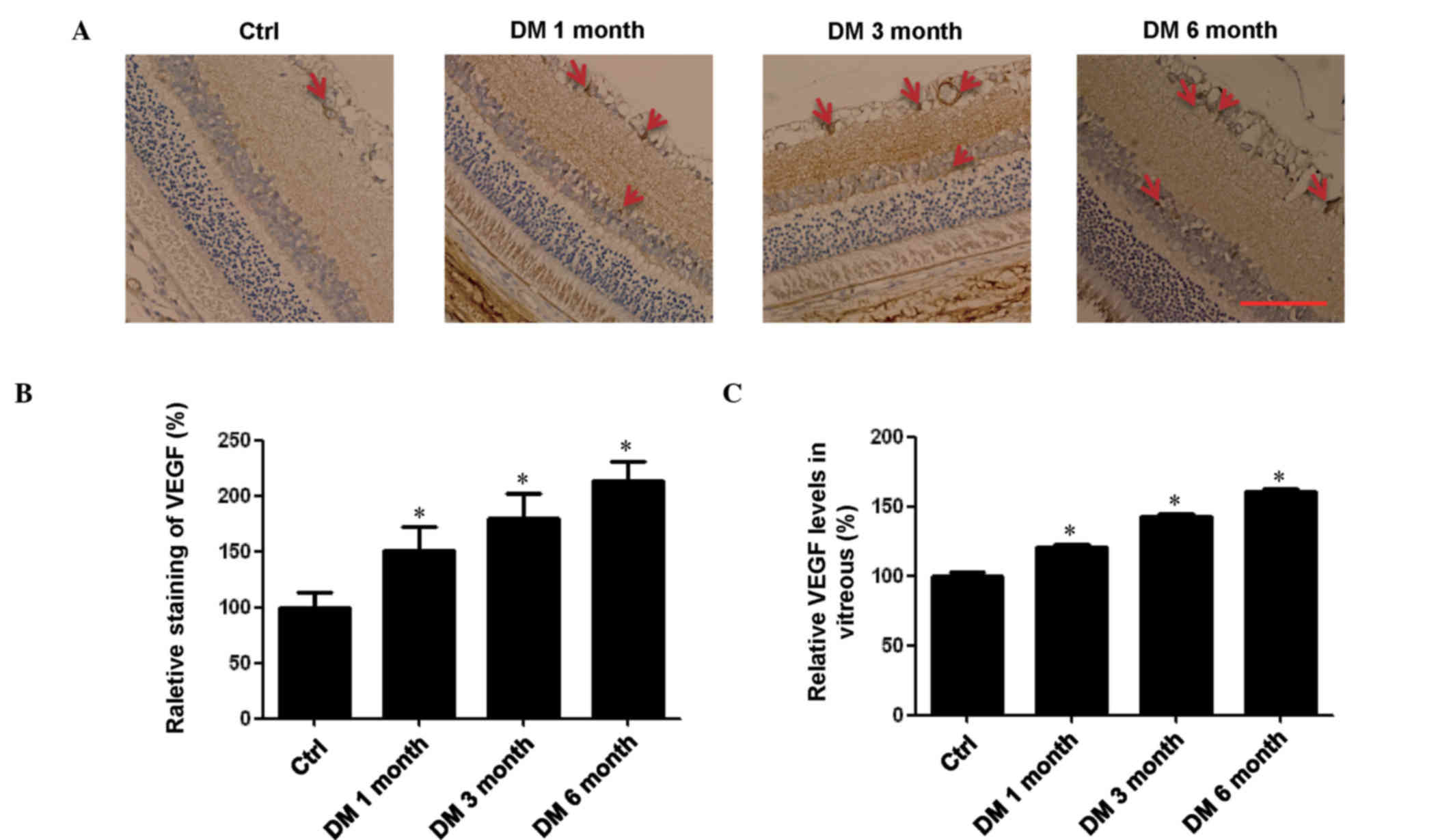
Levels of VEGF are upregulated in the
retina and vitreous of rats with DM
It has previously been reported that VEGF may
inhibit the expression of TL1A (17). Therefore, the present study
conducted immunohistochemistry experiments in the retina and ELISA
in the vitreous to determine the expression levels of VEGF at
different stages of DM. Expression levels of VEGF in the DM groups
were all significantly higher compared with the control group, and
the levels increased with the progression of DR in the retina and
vitreous (P<0.05; Fig. 3). This
result was consistent with our previous study (24), which demonstrated that the level of
VEGF in patients with DR was significantly higher compared with
healthy persons. However, in the present study, this trend did not
negatively correlate with TL1A levels in the retina and vitreous,
suggesting that the expression of TL1A may be regulated by
additional factors.
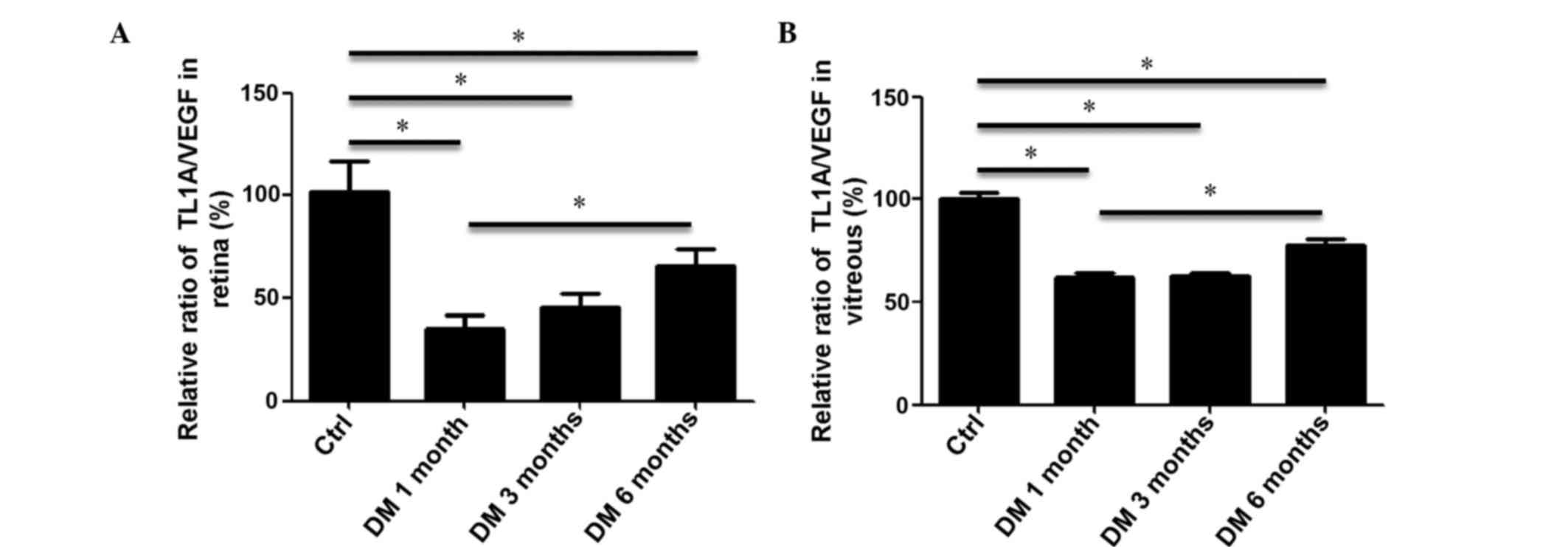
Ratios of TL1A/VEGF in retina and
vitreous of rats with DM
As TL1A and VEGF have opposing functions in
microvascular endothelial cells, the ratio of TL1A/VEGF in the
retina and vitreous are important to the development and
progression of DR. Therefore, quantification of the
immunohistochemistry experiments in retina and ELISA assays in
vitreous were used to calculate the ratio of TL1A/VEGF. It was
demonstrated that the ratio of TL1A/VEGF in the retina of DM 1
month rat group was significantly reduced to 35.37% compared with
the control (P<0.05; Fig. 4A).
The ratio of retinal TL1A/VEGF was increased in the DM 3 month
group and peaked in the DM 6 month group. However, this peak was
significantly lower compared with the control group (P<0.05;
Fig. 4A). Consistent with the
observations obtained from the retina, the ratio of TL1A/VEGF in
the vitreous of rats with DM 1 month significantly decreased to
61.77% compared with the control group (P<0.05; Fig. 4B). The ratio of vitreous TL1A/VEGF
was significantly increased only in the DM 6 month group, which
remained significantly lower than that of the control group
(P<0.05; Fig. 4B). These data
demonstrated that although the ratio of TL1A/VEGF increased during
the development and progression of DR, it was consistently lower in
DM groups compared with the control group. Therefore, it is
possible that additional factors are involved in the
pathophysiology of DR.
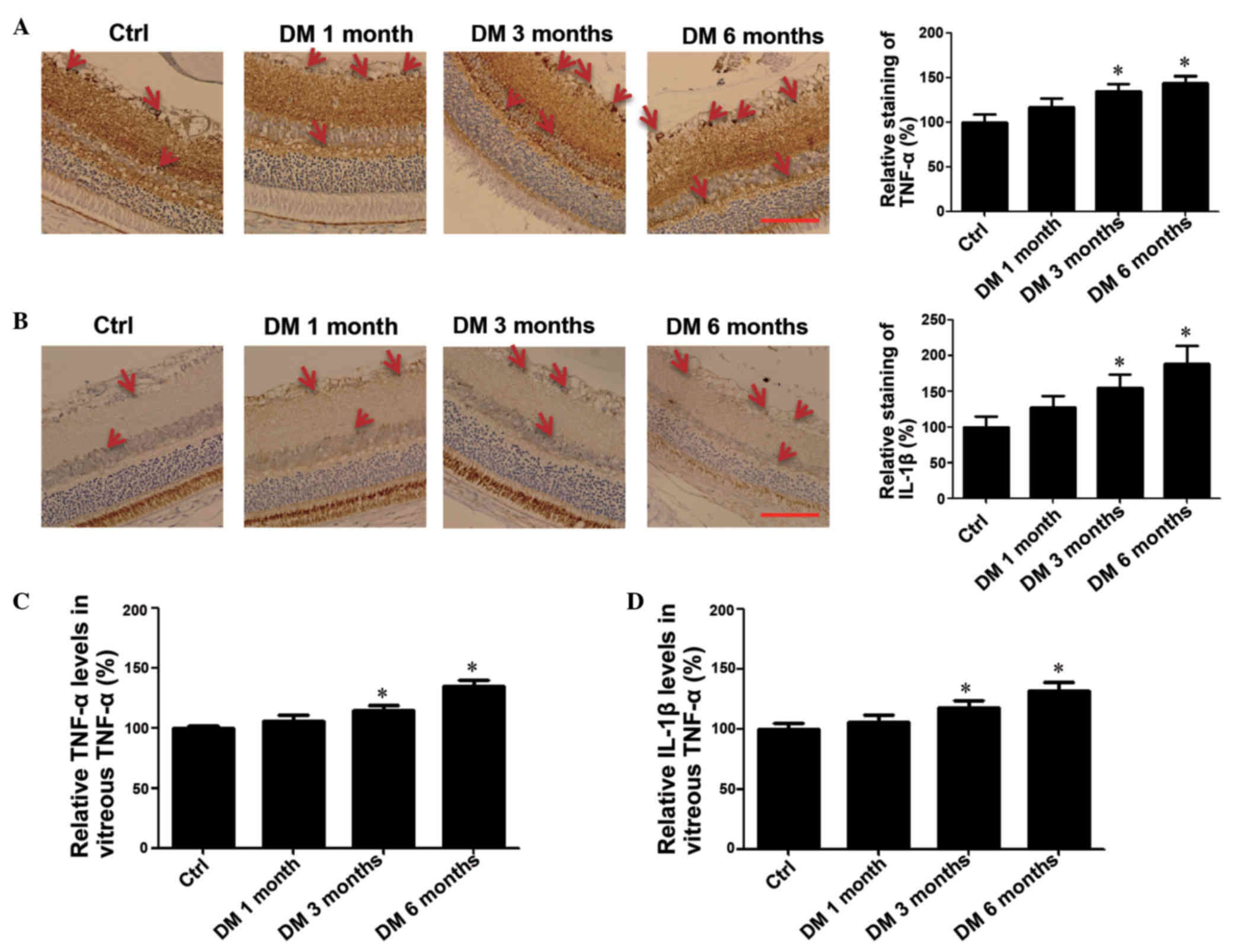
Levels of TNF-α and IL-1β are
upregulated in the retina and vitreous of rats with DM
Previous studies have reported that DM increased the
release of retinal inflammatory mediators, including IL-1β, TNF-α,
VEGF, intercellular adhesion molecule 1 (ICAM-1) and angiotensin II
(16), and the expression of TL1A
was upregulated by TNF-α and IL-1β (18). To investigate the association
between the expression of TL1A and TNF-α and IL-1β levels,
immunohistochemistry analysis of the retina and ELISA assays in
vitreous were performed. The immunochemistry result indicated a
time-dependent increase in TNF-α expression levels (Fig. 5A) and IL-1β (Fig. 5B) during the development and
progression of DR. The ELISA assays were consistent with the
immunohistochemistry as the expression levels of TNF-α and IL-1β
were significantly higher in DM 3 month and 6 month groups compared
with the control group (P<0.05; Fig. 5C and D). These data suggested that
the increased TNF-α and IL-1β levels may contribute to the
increased expression of TL1A.
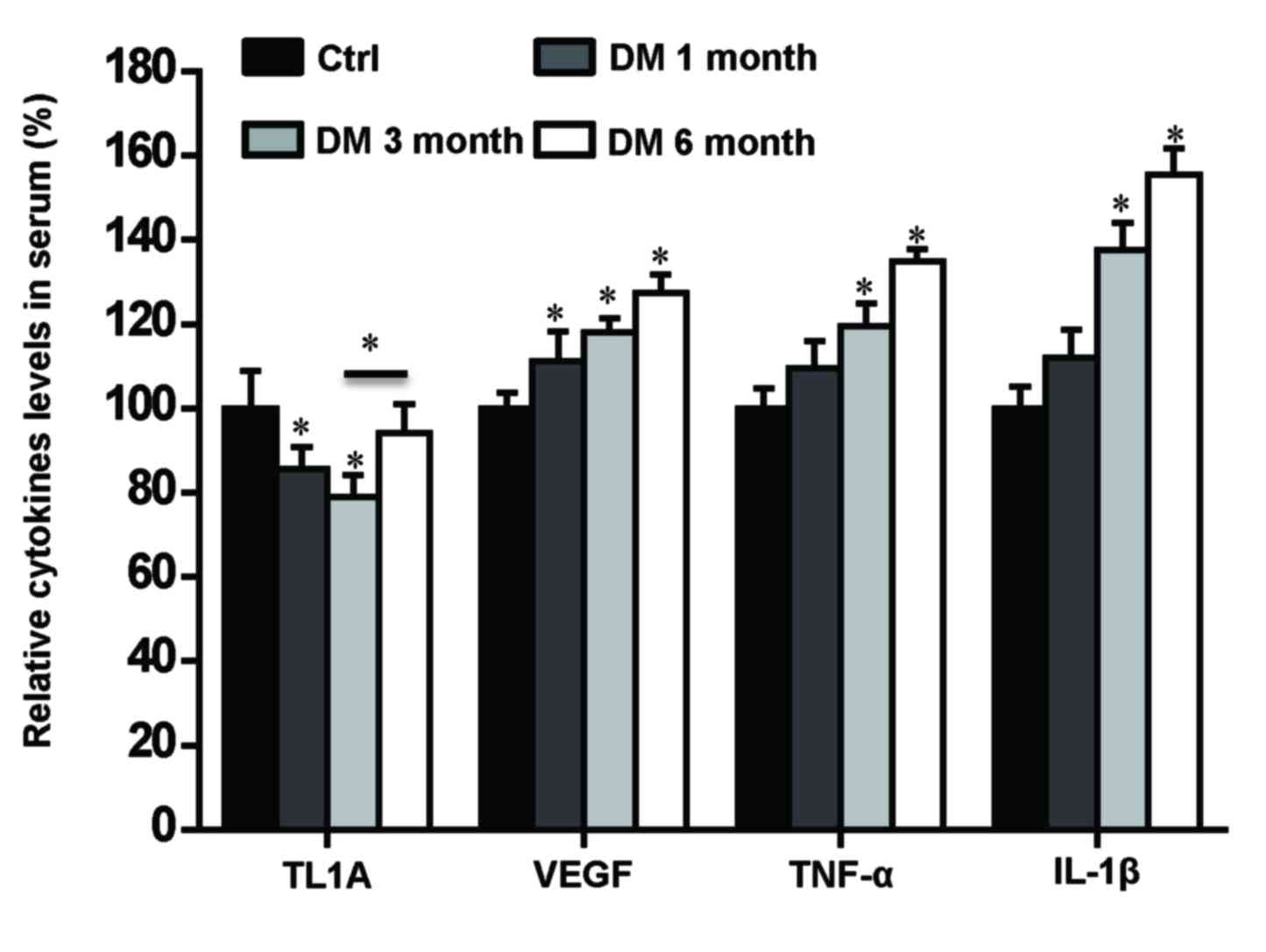
Levels of TL1A, VEGF, TNF-α and IL-1β
in serum samples from rats with DM
Previous studies have reported elevated serum
concentrations of IL-1β, TNF-α, and VEGF correlate with the
severity of DR (19,21). In order to investigate the
association between levels of TL1A, VEGF, TNF-α and IL-1β and the
pathogenesis of DR, their expression levels in serum from DM rats
were quantified using ELISA assays. The changes of the TL1A, VEGF,
TNF-α and IL-1β levels in serum indicated similar expression change
dynamics as those observed in the retina and vitreous.
Specifically, the expression levels of TL1A were significantly
lower in DM 1 and 3 month groups compared with the control group
(P<0.05; Fig. 6). However, they
significantly increased in DM 6 month group compared with the DM 3
month group (Fig. 6), which was
different from the changes of TL1A levels in the retina and
vitreous. The VEGF expression levels were significantly higher in
all three DM groups compared with the control group (P<0.05;
Fig. 6), which was consistent with
VEGF changes observed in the retina and vitreous. The expression
levels of TNF-α and IL-1β were significantly higher in DM 3 month
and 6 month groups compared with the control group, which was
consistent with changes observed in the retina and vitreous
(P<0.05; Fig. 6). These results
suggest that the levels of TL1A, VEGF, TNF-α and IL-1β in the serum
may be associated with the changes observed in the retina and
vitreous.
Discussion
Previous studies have reported that the pathogenesis
of DR may be associated with microvascular occlusion and leakage,
neuronal dysfunction and inflammation (4,16).
VEGF has been identified as a proinflammatory factor that affected
endothelial tight junction proteins, resulting in the extravasation
of fluid and retinal edema (25),
and has been considered to be a key molecule for the pathogenesis
of DR. TL1A has been identified as an important cytokine that may
be involved in various inflammatory responses and hyperplasia of
microvascular diseases (9,26). The present study demonstrated that
TL1A may act as a protective factor in the pathogenesis of DR. The
retina histopathology (H&E stain) results revealed that retina
pathology changes that may be attributed to DR were evident in the
rats from the DM 3 month group and typical pathology changes of DR
were also observed in DM 6 month group. Subsequently, it was
determined that the expression levels of TL1A were lower in the
early stages of DM compared with the control group, and increased
in groups with DR (DM 3 and 6 month groups). Consistent with
disease progression, levels of VEGF were higher in the DM groups
compared with the control group. Additionally, the levels of TNF-α
and IL-1β, which had been reported to upregulate the expression of
TL1A were investigated in the DM groups. The present study
determined that the expression levels of TNF-α and IL-1β were
higher in the DM groups compared with the control group. Therefore,
it is possible that the ratio of TL1A/VEGF may contribute to the
development and progression of DR. Furthermore, the expression of
TL1A may be regulated by various factors, including VEGF, TNF-α and
IL-1β.
TL1A, which belongs to TNF superfamily, has been
expressed primarily in endothelial cells as a negative modulator of
angiogenesis and may be induced in response to inflammatory stimuli
(9,26,27).
Previous studies have reported that TL1A suppressed the ability of
capillary formation in vitro, which was induced by
fibroblast growth factor-2 (FGF2) or VEGF in adult bovine aortic
endothelial cells (27).
Additionally, TL1A inhibited ~50% of the neovascularization induced
by either FGF2 or VEGF in vivo. A recent study Qi et
al (12) determined that TL1A
inhibited vasculogenesis by regulating the expression levels of the
VEGFR1. They also reported that TL1A inhibited VEGF-driven,
EPC-supported vasculogenesis in a murine matrigel implant model
(12). Therefore, it is possible
that TL1A may be important in the development and progression of
DR, which may be characterized by inflammation and
neovascularization driven by pro-angiogenesis growth factors and
cytokines. The current study investigated the association between
the expression of TL1A and the development and progression of DR,
by quantifying the expression levels of TL1A in the retina and
vitreous of DM rats. It was determined that TL1A levels were
significantly lower in the early stages of DM and started to
increase in the DM 3 month group, which was consistent with the
features of the early stage of DR observed in histological
staining. The levels peaked in the DM 6 month group and were
significantly higher compared with the control group. VEGF has been
previously reported to be secreted by cancer cells and may
downregulate TL1A production by endothelial cells in vitro,
and recombinant TL1A has been demonstrated to effectively suppress
the angiogenesis and growth of tumors (17,28).
Therefore, VEGF may downregulate the expression of TL1A. The
present study demonstrated that the levels of VEGF in the retina
and vitreous were higher in DM groups compared with the control
group. These data were consistent with previous studies (24,29),
which determined that VEGF also stimulated increased leukostasis in
the microvessels of the retina and the sticky leukocytes released
cytokines or may migrate via the transendothelial route, which may
result in a breakdown of the blood-retinal barrier (BRB) and VEGF
appeared to be important for promoting intraocular
neovascularization in PDR. The present study revealed the ratio of
TL1A/VEGF was lower in DM groups compared with the control group.
However, the ratio of the DM 6 month group was significantly higher
compared with the DM 1 month group. These findings suggested that
increased levels of VEGF downregulated the expression levels of
TL1A in the early stage of DM (DM 1 month), whilst the organism may
overexpress TL1A, as a negative regulator of DM, in order to limit
the development and progression of DR (DM 3 month and 6 month
groups). Cytokine levels were also investigated in order to
determine whether they regulated the expression of TL1A.
Diabetes has been identified to increase the release
of retinal inflammatory mediators, such as IL-1β, TNF-α, VEGF,
ICAM-1 and angiotensin II and activate microglial cells in the
early stage of DR. TNF-α and IL-1β may induce leukostatis and
vascular permeability via activation of the microglia and increased
endothelial adhesion (19,30,31).
It has been reported that TNF-α is important for the BRB breakdown
during DR pathogenesis (32). A
previous study determined that anti-angiogenic dose of TNF-α (30
ng/ml) upregulated the expression of TL1A in endothelial cells
(10). Notably, there is clear
evidence that the expression of TL1A may be upregulated by TNF-α
and IL-1 in HUVECs (18).
Therefore, the present study assessed the levels of TNF-α and IL-1β
in the different stages of DM. It was determined that the levels of
TNF-α and IL-1β were significantly higher in DM 3 month and 6 month
groups compared with the control group. Therefore, the levels of
TNF-α and IL-1β were higher in DR rats, resulting in increased
levels of TL1A.
Previous studies have identified that the elevated
serum concentrations of IL-1β, TNF-α, and VEGF correlated with the
presence and severity of DR (19,21).
It was suggested that the increase of these cytokines may be an
important systemic factor of DR development. However, another study
revealed that the vitreous levels of VEGF were not influenced by
the VEGF serum concentrations in DR and were not correlated with
the presence and severity of DR (33). The results of the current study
were consistent with the first study (19), in which levels of VEGF, TNF-α, and
IL-1β were higher in the DR groups compared with the control group.
Changes in TL1A expression levels were partly consistent with those
in the retina and vitreous. Briefly, levels of TL1A were
significantly lower in the DM 1 and 3 month groups compared with
the control group, and significantly increased in the DM 6 month
group compared with the DM 3 month group, which was different from
the changes of TL1A levels observed in the retina and vitreous.
This indicated that the changes of TL1A levels in the serum
occurred after those in retina and vitreous. The findings of the
present study may provide the rationale required for the
development of novel diagnostic strategies of DR.
In conclusion, the present study determined the
changes of TL1A, VEGF, TNF-α, and IL-1β expression levels, which
occur in the retina, vitreous and serum of rats at different stages
of DR. These results suggested that TL1A may be a negative
regulator in the development and progression of DR and may be
regulated by VEGF, TNF-α, and IL-1β, and provide additional
therapeutic targets for the treatment of DR.
Acknowledgements
The present study was supported by the National
Natural Science Foundation of China (grant nos. 81371038 and
91442124) and the Tianjin Application Infrastructure and
Cutting-edge Technology Research Program of China (grant no
10JCZDJC20300) to Dr Hua Yan, and the National Natural Science
Foundation of China (grant no. 81500743) to Dr Zhu-Hong Zhang. The
views presented in this article do not necessarily reflect those of
the U.S. Food and Drug Administration.
References
|
1
|
Ebneter A and Zinkernagel MS: Novelties in
diabetic retinopathy. Endocr Dev. 31:84–96. 2016.PubMed/NCBI
|
|
2
|
Tarr JM, Kaul K, Chopra M, Kohner EM and
Chibber R: Pathophysiology of diabetic retinopathy. ISRN
Ophthalmol. 2013:3435602013. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Ahsan H: Diabetic retinopathy-biomolecules
and multiple pathophysiology. Diabetes Metab Syndr. 9:51–54. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Cheung N, Mitchell P and Wong TY: Diabetic
retinopathy. Lancet. 376:124–136. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Tang J and Kern TS: Inflammation in
diabetic retinopathy. Prog Retin Eye Res. 30:343–358. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Hsu H and Viney JL: The tale of TL1A in
inflammation. Mucosal Immunol. 4:368–370. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Zhang Z and Li LY: TNFSF15 Modulates
neovascularization and inflammation. Cancer Microenviron.
5:237–247. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Sethi G, Sung B and Aggarwal BB:
Therapeutic potential of VEGI/TL1A in autoimmunity and cancer. Adv
Exp Med Biol. 647:207–215. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Chew LJ, Pan H, Yu J, Tian S, Huang WQ,
Zhang JY, Pang S and Li LY: A novel secreted splice variant of
vascular endothelial cell growth inhibitor. FASEB J. 16:742–744.
2002.PubMed/NCBI
|
|
10
|
Parr C, Gan CH, Watkins G and Jiang WG:
Reduced vascular endothelial growth inhibitor (VEGI) expression is
associated with poor prognosis in breast cancer patients.
Angiogenesis. 9:73–81. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Yu J, Tian S, Metheny-Barlow L, Chew LJ,
Hayes AJ, Pan H, Yu GL and Li LY: Modulation of endothelial cell
growth arrest and apoptosis by vascular endothelial growth
inhibitor. Circ Res. 89:1161–1167. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Qi JW, Qin TT, Xu LX, Zhang K, Yang GL, Li
J, Xiao HY, Zhang ZS and Li LY: TNFSF15 inhibits vasculogenesis by
regulating relative levels of membrane-bound and soluble isoforms
of VEGF receptor 1. Proc Natl Acad Sci USA. 110:13863–13868. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Stefanini FR, Arevalo JF and Maia M:
Bevacizumab for the management of diabetic macular edema. World J
Diabetes. 4:19–26. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Gupta N, Mansoor S, Sharma A, Sapkal A,
Sheth J, Falatoonzadeh P, Kuppermann B and Kenney M: Diabetic
retinopathy and VEGF. Open Ophthalmol J. 7:4–10. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Ho AC, Scott IU, Kim SJ, Brown GC, Brown
MM, Ip MS and Recchia FM: Anti-vascular endothelial growth factor
pharmacotherapy for diabetic macular edema: A report by the
american academy of ophthalmology. Ophthalmology. 119:2179–2188.
2012. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Rao RC and Dlouhy BJ: Diabetic
retinopathy. N Engl J Med. 367:1842012. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Deng W, Gu X, Lu Y, Gu C, Zheng Y, Zhang
Z, Chen L, Yao Z and Li LY: Down-modulation of TNFSF15 in ovarian
cancer by VEGF and MCP-1 is a pre-requisite for tumor
neovascularization. Angiogenesis. 15:71–85. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Kim S and Zhang L: Identification of
naturally secreted soluble form of TL1A, a TNF-like cytokine. J
Immunol Methods. 298:1–8. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Kaul K, Hodgkinson A, Tarr JM, Kohner EM
and Chibber R: Is inflammation a common retinal-renal-nerve
pathogenic link in diabetes? Curr Diabetes Rev. 6:294–303. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Krady JK, Basu A, Allen CM, Xu Y, LaNoue
KF, Gardner TW and Levison SW: Minocycline reduces proinflammatory
cytokine expression, microglial activation and caspase-3 activation
in a rodent model of diabetic retinopathy. Diabetes. 54:1559–1565.
2005. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Koleva-Georgieva DN, Sivkova NP and
Terzieva D: Serum inflammatory cytokines IL-1beta, IL-6, TNF-alpha
and VEGF have influence on the development of diabetic retinopathy.
Folia Med (Plovdiv). 53:44–50. 2011.PubMed/NCBI
|
|
22
|
National Research Council (US) Committee
for the Update of the Guide for the Care and Use of Laboratory
Animals, . Guide for the Care and Use of Laboratory Animals. 8th
edition. National Academies Press; Washington (DC): 2011,
PubMed/NCBI
|
|
23
|
Zheng H, Zhang Z, Luo N, Liu Y, Chen Q and
Yan H: Increased Th17 cells and IL17 in rats with traumatic optic
neuropathy. Mol Med Rep. 10:1954–1958. 2014.PubMed/NCBI
|
|
24
|
Wang J, Chen S, Jiang F, You C, Mao C, Yu
J, Han J, Zhang Z and Yan H: Vitreous and plasma VEGF levels as
predictive factors in the progression of proliferative diabetic
retinopathy after vitrectomy. PLoS One. 9:e1105312014. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Antcliff RJ and Marshall J: The
pathogenesis of edema in diabetic maculopathy. Semin Ophthalmol.
14:223–232. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Zhai Y, Ni J, Jiang GW, Lu J, Xing L,
Lincoln C, Carter KC, Janat F, Kozak D, Xu S, et al: VEGI, a novel
cytokine of the tumor necrosis factor family, is an angiogenesis
inhibitor that suppresses the growth of colon carcinomas in vivo.
FASEB J. 13:181–189. 1999.PubMed/NCBI
|
|
27
|
Zhai Y, Yu J, Iruela-Arispe L, Huang WQ,
Wang Z, Hayes AJ, Lu J, Jiang G, Rojas L, Lippman ME, et al:
Inhibition of angiogenesis and breast cancer xenograft tumor growth
by VEGI, a novel cytokine of the TNF superfamily. Int J Cancer.
82:131–136. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Hou W, Medynski D, Wu S, Lin X and Li LY:
VEGI-192, a new isoform of TNFSF15, specifically eliminates tumor
vascular endothelial cells and suppresses tumor growth. Clin Cancer
Res. 11:5595–5602. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Penn JS, Madan A, Caldwell RB, Bartoli M,
Caldwell RW and Hartnett ME: Vascular endothelial growth factor in
eye disease. Prog Retin Eye Res. 27:331–371. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Zeng HY, Green WR and Tso MO: Microglial
activation in human diabetic retinopathy. Arch Ophthalmol.
126:227–232. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Wilkinson-Berka JL, Tan G, Jaworski K and
Miller AG: Identification of a retinal aldosterone system and the
protective effects of mineralocorticoid receptor antagonism on
retinal vascular pathology. Circ Res. 104:124–133. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Huang H, Gandhi JK, Zhong X, Wei Y, Gong
J, Duh EJ and Vinores SA: TNFalpha is required for late BRB
breakdown in diabetic retinopathy and its inhibition prevents
leukostasis and protects vessels and neurons from apoptosis. Invest
Ophthalmol Vis Sci. 52:1336–1344. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Burgós R, Simo R, Audí L, Mateo C, Mesa J,
García-Ramírez M and Carrascosa A: Vitreous levels of vascular
endothelial growth factor are not influenced by its serum
concentrations in diabetic retinopathy. Diabetologia. 40:1107–1109.
1997. View Article : Google Scholar : PubMed/NCBI
|