Introduction
PCa is the second most frequent cause of
cancer-associated mortality in males in the USA (1). Various growth factors and cytokines
have been reported to be associated with the progress of PCa;
however, the exact underlying molecular mechanism of PCa
development and progression remains to be fully elucidated.
The Wnt/β-catenin signaling pathway regulates
pattern formation during embryogenesis and tumor progression. This
signaling pathway activates various distinct intracellular
pathways, which are involved in the proliferation, differentiation
and polarity of cells. Aberrant activation of the Wnt/β-catenin
signaling pathway has been associated with tumor development and
metastasis in numerous types of cancer (2–4).
During epithelial mesenchymal transition (EMT),
epithelial cells alter morphologically, resulting in a loss of
polarity and cell-to-cell contact. It has previously been
demonstrated have indicated that during tumor progression, EMT is
crucial for the invasion and metastasis of tumor cells (5). Our previous study demonstrated that
the activation of the Wnt/β-catenin signaling pathway is associated
with the process of EMT and its role in the invasion and
proliferation of tumor cells (6).
The Wnt/β-catenin signaling pathway was involved in EMT of human
PCa induced by hypoxia-inducible factor (HIF) 1-α (7). The current hypothesized that the
aberrant activation of the Wnt/β-catenin signaling pathway may be
involved the proliferation and invasive potency of PCa. The present
study aimed to investigate the expression and distribution of
β-catenin in different prostate cancer cell lines. It was
demonstrated β-catenin acted as an adhesion molecule in the LNCaP
and C4-2 cell lines, and as a transcription factor of the
Wnt/β-catenin signaling pathway in the IF11-ARCaP and IA8-ARCaP
cell lines. The results of the present study suggested that the
aberrant activation of the Wnt/β-catenin signaling pathway in
IA8-ARCaP and IF11-ARCaP may be responsible for their high invasive
potency.
RNA interference (RNAi) specifically inhibits the
transcription of target genes, thus, reducing the corresponding
protein levels, and has a high efficiency and specificity. In the
present study, small hairpin (shRNA) targeting β-catenin was used
to investigate the role of the Wnt/β-catenin signaling pathway in
human PCa cells in vitro.
Materials and methods
Cell culture and plasmids
PC-3, LNCaP, C4-2, IA8-ARCaP and IF11-ARCaP human
PCa cell lines were obtained from Professor Dalin He, Xi'an
Jiaotong University (Xi'an, China). PC-3 cell lines were cultured
in Dulbecco's modified Eagle's medium (DMEM), supplemented with 10%
fetal bovine serum (FBS), 100 U/ml penicillin and 100 mg/ml
streptomycin. LNCaP, C4-2, IA8-ARCaP and IF11-ARCaP were cultured
in RPMI-1640 medium supplemented with 10% FBS, 100 U/ml penicillin
and 100 mg/ml streptomycin. DMEM and RPMI-1640 medium were
purchased from Hyclone (GE Healthcare Life Sciences, Logan, UT,
USA), and FBS was purchased from Gibco (Thermo Fisher Scientific,
Inc., Waltham, MA, USA). β-catenin shRNA was constructed according
to the protocol described in our previous study (7). Based on the vector sequence, the
β-catenin shRNA target sequence and a sh-Control sequence (listed
in Table I) were synthesized by
Sunbiotech (Beijing, China). IA8-ARCaP cells were transfected with
β-catenin shRNA using Lipofectamine 2000™ (Invitrogen; Thermo
Fisher Scientific, Inc.) according to the manufacturer's
protocol.
 | Table I.shRNA targeted human β-catenin and
sh-Control sequence. |
Table I.
shRNA targeted human β-catenin and
sh-Control sequence.
| Group | Sequence |
|---|
| shRNA |
5n'-GATCCCCACAGTCTTACCTGGACTCTTTCAAGAGAAGAGTCCAGGTAAGACTGTTTTTTA-3′ |
|
|
3′-GGGTGTCAGAATGGACCTGAGAAAGTTCTCTTCTCAGGTCCATTCTGACAAAAAATTCGA-5′ |
| sh-Control |
5′-GATCCCCAACGAGTGTGCCTACATCCTTCAAGAGAGGATGTAGGCACACTCGTTTTTTTA-3′ |
|
|
3′-GGGTTGCTCACACGGATGTAGGAAGTTCTCTCCTACATCCGTGTGAGCAAAAAAATTCGA-5′ |
Western blotting
Total protein was isolated from cultured cells using
300 ml ice cold lysis buffer containing 1% NP-40, 50 mmol/l Tris
(pH 7.4), 150 mmol/l NaCl, 0.1% sodium dodceyl sulfate, 0.5%
deoxycholate, 200 mg/ml phenylmethanesulfonyl fluoride and 50 mg/ml
aprotinin. Insoluble materials were removed by ultracentrifugation
at 15,000 × g for 30 min at 4°C. The concentration of the extracted
protein was measured spectrophotometrically with Coomassie G-250.
Total protein (50 µg) was loaded in each lane and resolved on a
denaturing 12% SDS-PAGE gel. The proteins were transferred onto
polyvinylidene fluoride membranes (PVDF) using a wet transfer
method following polyacrylamide gel electrophoresis, then blocked
with 3% bovine serum albumin (Santa Cruz Biotechnology, Inc.,
Dallas, TX, USA) for 1 h at room temperature and washed with
Tris-buffered saline and Tween-20 three times. Antibodies specific
for β-catenin (mouse anti-human IgG; 1:500; cat. no. sc-7963) and
GAPDH (mouse anti-human IgG; 1:10,000; cat. no. sc-47724) were used
to probe membranes, followed by peroxidase-conjugated secondary
antibodies (goat anti-mouse IgG; 1:5,000; cat. no. sc-3697) and
enhanced chemiluminescence detection (Sigma-Aldrich; Merck
Millipore, Darmstadt, Germany). All primary antibodies were
obtained from Santa Cruz Biotechnology, Inc. For quantification of
band intensity, appropriate films were scanned and band densities
were determined using Quantity One software (version 4.6.6; Bio-Rad
Laboratories, Inc., Hercules, CA, USA), normalized against GAPDH,
and presented as a ratio of control.
Reverse transcription-polymerase chain
reaction (RT-PCR)
Total RNA was extracted from the cell lines at
80–90% confluence using TRIzol® (Invitrogen; Thermo
Fisher Scientific, Inc.). RNA (1 µg) was reverse transcribed using
a RevertAid First Strand cDNA Synthesis kit (Fermentas; Thermo
Fisher Scientific, Inc.). Amplification of 2 µl cDNA was performed
using Taq polymerase (Takara Bio, Inc., Otsu, Japan) with specific
primers for β-catenin and β-actin (Table II). All primers were obtained from
Sunbiotech. All PCR reactions were initiated with an incubation at
94°C for 2 min, followed by 29 cycles of 94°C for 30 sec, 58°C for
30 sec and 72°C for 2 min. Reactions were finished with a 72°C, 10
min extension step. PCR products were visualized by electrophoresis
on 1.2% agarose gels and quantified with Quantity One analyzing
software (version 4.6.6), normalized against β-actin, and presented
as a ratio of control. All assays were performed at least 3
times.
 | Table II.Specific primers for β-catenin and
β-actin. |
Table II.
Specific primers for β-catenin and
β-actin.
| Gene | Direction | Primer sequence
(5′-3′) | Size (bp) |
|---|
| β-catenin | Forward |
ACTAAACAGGAAGGGATGGAAGG | 236 |
| β-catenin | Reverse |
AGATGACGAAGAGCACAGATGG |
|
| β-actin | Forward |
ATCGTGCGTGACATTAAGGAGAAG | 179 |
| β-actin | Reverse |
AGGAAGGAAGGCTGGAAGAGTG |
|
Indirect immunofluorescence
To detect the expression and distribution of
β-catenin, PCa cells were fixed in 10% paraformaldehyde for 30 min
and blocked with goat serum (Santa Cruz Biotechnology, Inc.) for 30
min. Cells were then incubated at 37°C for 1 h with a mouse
anti-human β-catenin monoclonal antibody (1:200; Santa Cruz
Biotechnology, Inc.; sc-7963). Following three washes with PBS,
cells were incubated with a fluorescence isothiocyanate-conjugated
goat anti-mouse antibody (1:100; Santa Cruz Biotechnology, Inc.;
sc-3692) at 37°C for 1 h. The fluorescence staining intensity and
intracellular location were examined by fluorescence
microscopy.
MTT assay
The MTT assay was used to determine the
proliferation of human PCa cells. PCa cells were seeded in 96-well
plates and transfected with β-catenin shRNA. At various time points
(24, 48 and 72 h), 50 ml 2.5 ng/ml thiazolyl blue tetrazolium
bromide (MTT) solution was added into the cell culture plate and
incubated with cells for an additional 4 h. The media was collected
separately from each chamber, and cell-associated MTT crystal was
dissolved separately in 15 µl/well dimethyl sulfoxide on a shaker
at room temperature. Absorbance at a wavelength of 570 nm
(proportional to viable cell number) was subsequently measured
using a multiplate reader (Bio-Rad Laboratories, Inc.).
Matrigel Transwell assay
Transwell polycarbonate filters (8-mm pore size; EMD
Millipore, Billerica, MA, USA) were coated with 50 ml Matrigel
(1:5; Sigma-Aldrich; Merck Millipore) in serum-free medium and
air-dried for 12 h. Following this, 1×105 cells in serum-free
RPMI-1640 were seeded into the upper chamber and 1 ml RPMI-1640
with 20% FBS was added to the lower chamber. Human PCa cells:
IA8-ARCaP cells, IA8-β-catenin, IA8-ARCaP cells transfected with
β-catenin shRNA and IA8-shControl (IA8-ARCaP cells transfected with
β-catenin sh-Control plasmid) were seeded into the upper chamber
and allowed to migrate in a 5% CO2 incubator at 37°C for
48 h. Following incubation, a cotton swab was used to remove cells
on the upper surface, and any invaded cells attached to the lower
surface of the membrane were subsequently fixed with 4%
paraformaldehyde and visualized with Giemsa stain (1:100) for 2 h.
Cell numbers were counted under a light microscope in 3 random
microscopic fields (magnification, ×10) per membrane.
Statistical analysis
Data are presented as the mean ± standard deviation.
All data analyses were performed using SPSS software version 13.0
for Windows (SPSS, Inc., Chicago, IL, USA). One-way analysis of
variance (ANOVA) and independent-samples t-tests were conducted.
Tukey's test was used for post hoc test following the ANOVA. All
experiments were performed at least three times with similar
results. P<0.05 was considered to indicate a statistically
significant difference.
Results
Expression and distribution of
β-catenin in different PCa cell lines
β-catenin is a multifunctional protein involved in
two processes, cell-to-cell adhesion and the Wnt/β-catenin
signaling pathway. To investigate the functional activities of the
Wnt/β-catenin signaling pathway in different PCa cells, the present
study performed indirect immunofluorescence to detect the
expression and distribution of β-catenin protein in PC-3, LNCaP,
C4-2, IA8-ARCaP and IF11-ARCaP cell lines.
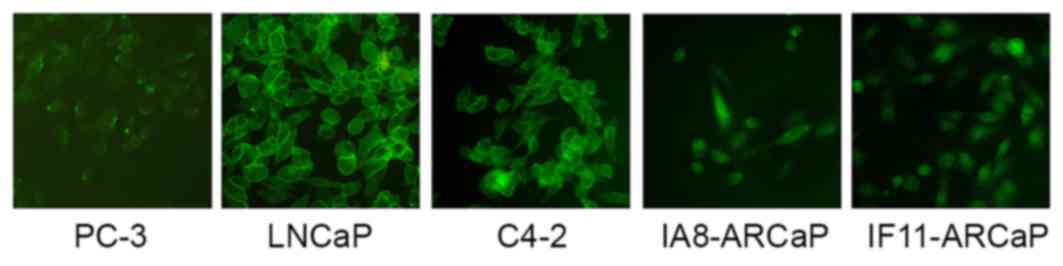
As presented in Fig.
1, there were marked differences in the expression and
distribution of β-catenin between these cell lines. β-catenin was
observed in the cytoplasm and nuclei of IA8-ARCaP and IF11-ARCaP
cells, whereas it appeared to be present in the membrane of LNCaP
and C4-2 cells. There was low expression of β-catenin in the PC-3
cell line. Therefore, β-catenin may act as an adhesion molecule in
the LNCaP and C4-2 cell lines, and as a transcription factor of the
Wnt/β-catenin signaling pathway in the IF11-ARCaP and IA8-ARCaP
cell lines. This data indicated that the functional activity of
Wnt/β-catenin signaling pathway in IF11-ARCaP and IA8-ARCaP cells
was greater compared with PC-3, LNCaP and C4-2 cells. IA8-ARCaP was
therefore used as the cell model to further investigate the role of
the Wnt/β-catenin signaling pathway in PCa.
Inhibition of mRNA expression by
β-catenin shRNA
Our previous study successfully constructed
β-catenin shRNA using gene recombination technology, which
significantly decreased the expression of β-catenin in HEK-293 cell
lines (6). In the present study,
the effects of β-catenin shRNA on the expression of β-catenin mRNA
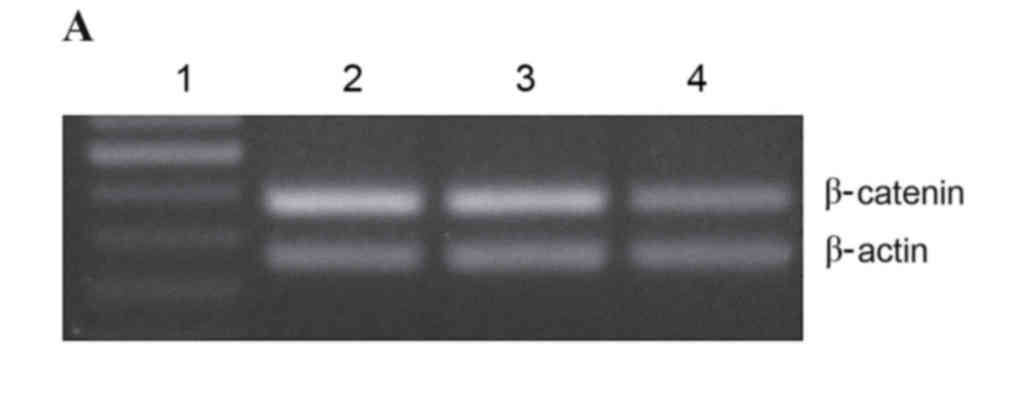
in IA8-ARCaP cell lines were investigated. Fig. 2A reveals the levels of β-catenin
mRNA in IA8, IA8-shControl and IA8/β-catenin(−) cell lines, as
measured by RT-PCR. Statistical analysis demonstrated that the
β-catenin mRNA expression levels in the IA8/β-catenin(−) cell line
was significantly downregulated compared with IA8 cells (P=0.002;
Fig. 2B). There was no significant
difference between the IA8-shControl and IA8 groups.
Inhibition of β-catenin protein
expression by β-catenin shRNA
To further investigate the inhibitory effects of
β-catenin shRNA on the Wnt/β-catenin signaling pathway, the present
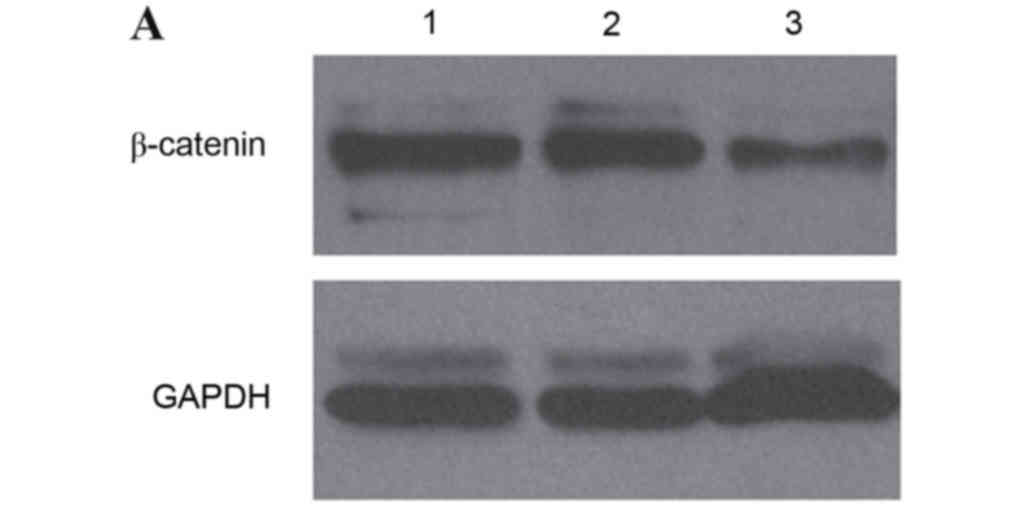
study detected the expression of β-catenin protein using western
blot analysis. The level of β-catenin protein in IA8, IA8-shControl
and IA8/β-catenin(−) cell lines is presented in Fig. 3A. Statistical analysis revealed
that the protein expression levels of β-catenin in IA8/β-catenin(−)
was significantly decreased compared with IA8 and IA8-shControl
group (P=0.029; Fig. 3B). There
was no significant difference between the IA8-shControl and IA8
groups.
β-catenin regulates cell
proliferation
As malignancy is associated with increasing cancer
cell proliferation and invasion, the present study investigated the
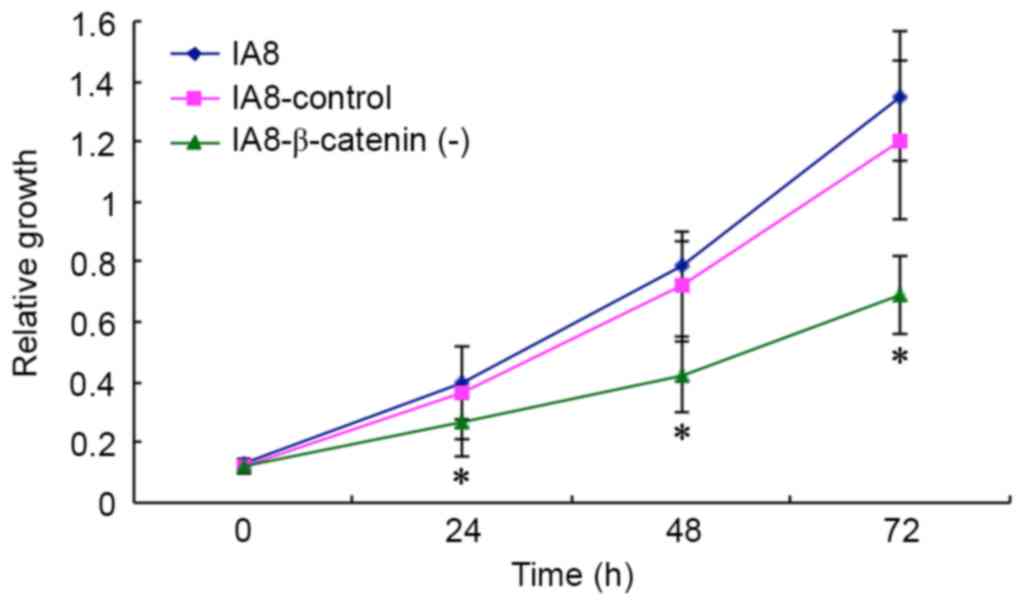
effect of β-catenin knockdown on proliferation in IA8-ARCaP cell
lines. The growth potency of IA8, IA8-β-catenin(−) and
IA8-shControl cells were assessed by MTT assay. As presented in
Fig. 4, IA8-β-catenin(−) cells
exhibited a reduced growth potency compared with IA8-shControl and
IA8 cells (P=0.479 at 24 h, P=0.037 at 48 h, P=0.020 at 72 h),
suggesting that β-catenin shRNA had an inhibitory effect on the
proliferation of the IA8-ARCaP cell line. Suppressing the activity
of the Wnt/β-catenin signaling pathway via β-catenin shRNA resulted
in growth inhibition of IA8-ARCaP cells in vitro.
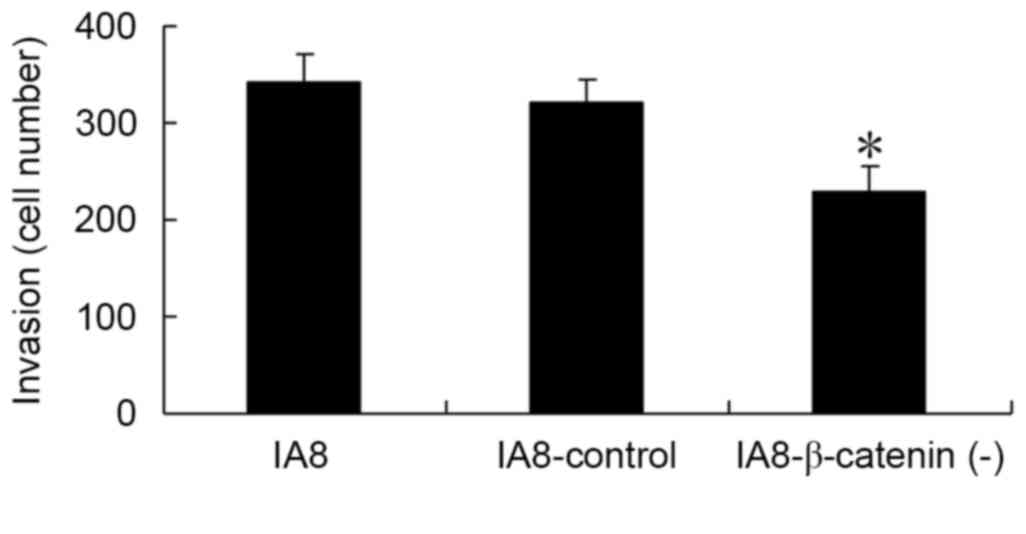
β-catenin regulates cell invasion
To further examine the role of the Wnt/β-catenin
signaling pathway in cell invasion of human PCa cells, a Matrigel
Transwell assay was performed in vitro. The IA8,
IA8/β-catenin(−) and IA8-shControl cells were seeded into the upper
chambers and 1 ml RPMI-1640 containing 20% FBS was added to the
lower chambers. Cells were allowed to migrate for 48 h. As
presented in Fig. 5, the number of
invading cells in the IA8/β-catenin(−) group was significantly
reduced compared with the IA8 and IA8-shControl groups (P=0.002).
The results of the Matrigel Transwell assay demonstrated that
inhibition of the Wnt/β-catenin signaling pathway via β-catenin
shRNA may result in an inhibition of PCa cell invasion in
vitro.
Discussion
β-catenin exhibits a dual function in epithelial
cells, depending on the intracellular localization. At the plasma
membrane, β-catenin is a constituent of adherens junctions, and
aids in cell-to-cell adhesion via binding to E-cadherin, and along
with α-catenin to the actin cytoskeleton. In addition, β-catenin
acts as a key component of the Wnt/β-catenin signaling cascade in
the nucleus. Wnt protein binding to the seven-pass transmembrane
receptors known as Frizzled proteins activates the signaling
pathway, leading to β-catenin stabilization in the cytoplasm.
Stabilized β-catenin subsequently translocates to the nucleus,
where it associates with T cell factor/lymphoid enhancer factor-1
and promotes specific gene expression. Multiple key oncogenic
proteins, including c-Myc, cyclin D1 and cyclooxyenase-2 have been
reported to be regulated by the Wnt/β-catenin signaling pathway
(8).
The aberrant activation of the Wnt/β-catenin
signaling pathway in premalignant and malignant cells results in
uncontrolled cell proliferation, growth and survival of cancer
cells, and therefore cancer progression. The Wnt/β-catenin
signaling pathway is a direct driver of thyroid transcription
factor-1 expression, which is a tissue-specific transcription
factor essential for thyroid differentiation (9). Dickkopf-related protein-1 (DKK-1), a
secreted protein that binds to low density lipoprotein-related
receptors 5/6, blocks Wnt-1 protein signaling. Accumulation of DKK1
and cytoplasmic/nuclear β-catenin in triple negative breast cancers
is indicative of poor prognosis. DKK1 expression alone or in
conjunction with β-catenin may identify patients who may benefit
from early systemic treatment (10). In addition, the canonical
Wnt/β-catenin signaling pathway regulates self-renewal of cancer
stem cells and drug resistance of cancer cells (11,12).
Previous studies have suggested that the
Wnt/β-catenin signaling pathway may be important in PCa. Chen et
al (4) demonstrated that high
levels of Wnt-1 and β-catenin expression were associated with
advanced, metastatic, hormone-refractory PCa, and may serve as
biomarkers of disease progression. Another study indicated that
promoting Wnt/β-catenin activity via blocking DKK-1 promoted
osteoblastic activity in osteolytic PC-3 cells, which suggested
that the Wnt/β-catenin signaling pathway contributed to the
osteoblastic phenotype of PCa cell bone metastasis (13).
EMT is important for cancer growth and invasion. Our
previous study demonstrated that the Wnt/β-catenin signaling
pathway is involved in EMT in human PCa and may be induced by
HIF-1α (5). In the current study,
the role of the Wnt/β-catenin signaling pathway in human PCa growth
and invasion was investigated. Indirect immunofluorescence was used
to detect the expression and distribution of β-catenin, the key
component of the Wnt/β-catenin signaling pathway in different human
PCa cell lines.
The results of the present study indicated that in
the LNCaP and C4-2 cell lines, β-catenin localized in the membrane,
whereas in the IA8-ARCaP and IF11-ARCaP cell lines, it was
localized in the cytoplasm and nucleus. There was low expression of
β-catenin in the PC-3 cell line. The EMT-positive cell lines
IA8-ARCaP and IF11-ARCaP exhibited a greater level of cytoplasmic
and nuclear β-catenin compared with the EMT-negative cell lines
PC-3, LNCaP and C4-2. The activity of the Wnt/β-catenin signaling
pathway in the more invasive PCa cells IA8-ARCaP and IF11-ARCaP was
markedly greater compared with LNCaP and C4-2 cells. IA8-ARCaP and
IF11-ARCaP were established from the ascites of a patient with
widely disseminated disease that represented a lethal form of human
PCa with the ability to invade and metastasize aggressively to bone
and soft tissue (14). The results
of the present study suggested that the high functional activity of
the Wnt/β-catenin signaling pathway in IA8-ARCaP and IF11-ARCaP may
be responsible for their high invasive potency.
RNA interference is the process of
post-transcriptional, sequence-specific gene silencing in plants
and animals, which was first introduced by Fire et al
(15) in 1998, via experimentation
in Caenorhabditis elegans. The process is initiated by shRNA
homologous in sequence to the particular gene to be silenced. RNA
interference is now a tool extensively employed in genetic
engineering as a simple and effective gene knockdown technique.
shRNA is an artificial RNA molecule with a tight hairpin turn that
may be used to silence target gene expression via RNAi. shRNA is an
advantageous mediator of RNAi in that it has a relatively low rate
of degradation and turnover (15,16).
To further investigate the role of the Wnt/β-catenin signaling
pathway in PCa cells, β-catenin shRNA was used to suppress the
activity of the Wnt/β-catenin signaling pathway in IA8-ARCaP cell
lines. The results demonstrated that the β-catenin-specific shRNA
significantly suppressed the mRNA and protein expression levels of
β-catenin, confirming that β-catenin shRNA may significantly
inhibit the activity of the Wnt/β-catenin signaling pathway.
As malignancy has been associated with increased
cancer cell proliferation and invasion, the present study
investigated the effects of β-catenin shRNA on proliferation and
invasion. An MTT assay indicated that knockdown of β-catenin
significantly inhibited the proliferation of the IA8-ARCaP cell
line. A Matrigel Transwell assay revealed that β-catenin knockdown
significantly suppressed the invasive activity of the IA8-ARCaP
cell line, which suggested that the Wnt/β-catenin signaling pathway
regulated the proliferation and invasion of the IA8-ARCaP cell
line. The data indicated that the high functional activity of
Wnt/β-catenin signaling in IA8-ARCaP cells was responsible for its
high invasive potency.
In conclusion, the present study demonstrated that
suppressing the functional activity of the Wnt/β-catenin signaling
pathway via β-catenin shRNA results in an inhibition of PCa
proliferation and invasion. The results suggested that disrupting
the Wnt/β-catenin signaling pathway may represent an opportunity
for rational and novel drug design for the treatment and prevention
of PCa.
Acknowledgements
The present study was supported by the program of
the Science and Technology Development Foundation of Beijing Anzhen
Hospital (grant no. 2013Z03).
References
|
1
|
Keller ET, Zhang J, Cooper CR, Smith PC,
McCauley LK, Pienta KJ and Taichman RS: Prostate carcinoma skeletal
metastases: Cross-talk between tumor and bone. Cancer Metastasis
Rev. 20:333–349. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Fodde R and Brabletz T: Wnt/beta-catenin
signaling in cancer stemness and malignant behavior. Curr Opin Cell
Biol. 19:150–158. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Guturi KK, Mandal T, Chatterjee A, Sarkar
M, Bhattacharya S, Chatterjee U and Ghosh MK: Mechanism of
β-catenin-mediated transcriptional regulation of epidermal growth
factor receptor expression in glycogen synthase kinase 3
β-inactivated prostate cancer cells. J Biol Chem. 287:18287–18296.
2012. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Chen G, Shukeir N, Potti A, Sircar K,
Aprikian A, Goltzman D and Rabbani SA: Up-regulation of Wnt-1 and
beta-catenin production in patients with advanced metastatic
prostate carcinoma: Potential pathogenetic and prognostic
implications. Cancer. 101:1345–1356. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Luo Y, He DL, Ning L, Shen SL, Li L and Li
X: Hypoxia-inducible factor-1alpha induces the
epithelial-mesenchymal transition of human prostatecancer cells.
Chin Med J (Engl). 119:713–718. 2006.PubMed/NCBI
|
|
6
|
Zhao JH, Luo Y, Jiang YG, He DL and Wu CT:
Knockdown of β-catenin through shRNA cause a reversal of EMT and
metastatic phenotypes induced by HIF-1α. Cancer Invest. 29:377–382.
2011. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Jiang YG, Luo Y, He DL, Li X, Zhang LL,
Peng T, Li MC and Lin YH: Role of Wnt/beta-catenin signaling
pathway in epithelial-mesenchymal transition of human prostate
cancer induced by hypoxia-inducible factor-1alpha. Int J Urol.
14:1034–1039. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Schmalhofer O, Brabletz S and Brabletz T:
E-cadherin, beta-catenin, and ZEB1 in malignant progression of
cancer. Cancer Metastasis Rev. 28:151–166. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Gilbert-Sirieix M, Makoukji J, Kimura S,
Talbot M, Caillou B, Massaad C and Massaad-Massade L: Wnt/β-catenin
signaling pathway is a direct enhancer of thyroid transcription
factor-1 in human papillary thyroid carcinoma cells. PLoS One.
6:e222802011. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Xu WH, Liu ZB, Yang C, Qin W and Shao ZM:
Expression of dickkopf-1 and beta-catenin related to the prognosis
of breast cancer patients with triple negative phenotype. PLoS One.
7:e376242012. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Cai C and Zhu X: The Wnt/β-catenin pathway
regulates self-renewal of cancer stem-like cells in human gastric
cancer. Mol Med Report. 5:1191–1196. 2012.
|
|
12
|
Cui J, Jiang W, Wang S, Wang L and Xie K:
Role of Wnt/β-catenin signaling in drug resistance of pancreatic
cancer. Curr Pharm Des. 18:2464–2471. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Hall CL, Bafico A, Dai J, Aaronson SA and
Keller ET: Prostate cancer cells promote osteoblastic bone
metastases through Wnts. Cancer Res. 65:7554–7560. 2005.PubMed/NCBI
|
|
14
|
Zhau HE, Odero-Marah V, Lue HW, Nomura T,
Wang R, Chu G, Liu ZR, Zhou BP, Huang WC and Chung LW: Epithelial
to mesenchymal transition (EMT) in human prostate cancer: Lessons
learned from ARCaP model. Clin Exp Metastasis. 25:601–610. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Fire A, Xu S, Montgomery MK, Kostas SA,
Driver SE and Mello CC: Potent and specific genetic interference by
double-stranded RNA in Caenorhabditis elegans. Nature. 391:806–811.
1998. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Elbashir SM, Harborth J, Lendeckel W,
Yalcin A, Weber K and Tuschl T: Duplexes of 21-nucleotide RNAs
mediate RNA interference in cultured mammalian cells. Nature.
411:494–498. 2001. View
Article : Google Scholar : PubMed/NCBI
|