Introduction
Nicotinamide phosphoribosyltransferase
(Nampt) is a novel adipokine, which has been reported to be
expressed in adipose tissue, chondrocytes in the articular
cartilage matrix and peripheral blood mononuclear cells (PBMCs)
(1–4). It has been revealed that Nampt
is closely associated with various biological processes, including
nicotinamide adenine dinucleotide (NAD) biosynthesis, cellular
metabolism and immunomodulatory responses. In the process of NAD
biosynthesis, Nampt regulates the activity of the
NAD-dependent deacetylase silent information regulator 2 (Sir2)
through increasing the cellular level of NAD, and subsequently
promoting Sir2 transcriptional activity in mammalian cells
(5). The potent Nampt
inhibitor FK866 negatively regulates glycolysis by altering the
initial steps in glucose oxidation and leads to changes in
carbohydrate metabolism in cancer cells (6). Furthermore, Nampt is an
essential catabolic mediator of osteoarthritis, which is the most
common form of inflammatory arthritis, and regulates
hypoxia-inducible factor 2α-mediated matrix metalloproteinase
(MMP) expression in chondrocytes, leading to the destruction
of osteoarthritic cartilage (7).
For the treatment of metabolic bone diseases,
including osteoporosis, adipokines are considered to be a
therapeutic target via their effects on two types of bone cell,
osteoclasts and osteoblasts (8).
Osteoclasts are well-characterized cells that are required for bone
resorption and excessive osteoclast differentiation is a
predominant indicator of osteoporosis. Osteoblasts are responsible
for bone formation. A previous study indicated that osteoblast
proliferation and differentiation are enhanced in vitro, and
that acceleration of bone formation and mineral apposition rates
are observed in vivo in the absence of the adipokine apelin,
which is a ligand of the Gi-G protein-coupled receptor APJ. These
data suggest a crucial role of apelin in bone homeostasis as a
physiological antianabolic factor (9). Another adipokine, visceral adipose
tissue-derived serine protease inhibitor (vaspin), has been
reported to suppress receptor activator of nuclear factor-κB ligand
(RANKL)-mediated differentiation of RAW264.7 cells and bone marrow
cells (BMCs) into mature osteoclasts by reducing the expression of
nuclear factor of activated T cells, cytoplasmic 1 (NFATc1)
and the subsequent induction of osteoclast-specific gene markers,
such as MMP-9 and cathepsin K (10). Adiponectin is an important
adipokine that regulates energy homeostasis, which also inhibits
RANKL-induced osteoclastogenesis by decreasing the expression of
several osteoclastogenic factors, including NFATc1, tumor
necrosis factor receptor-associated factor 6, cathepsin K and
tartrate-resistant acid phosphatase (TRAP), and induces
apoptosis in mature osteoclasts (11). It has previously been demonstrated
that Nampt attenuates osteoclast differentiation derived
from PBMCs in patients with multiple myeloma and human
CD14+ monocytes; however, the role of Nampt in the
differentiation of murine bone marrow macrophages (BMMs) into
osteoclasts and its underlying mechanisms have not yet been
revealed (12,13).
The present study investigated the effects of
Nampt on RANKL-mediated osteoclast differentiation and
functional bone-resorbing activity. In addition, the present study
determined whether Nampt is involved in RANKL-dependent
intracellular signaling pathways and the expression of
osteoclast-specific gene markers.
Materials and methods
Preparation of Nampt and reagents
Recombinant mouse Nampt (visfatin/pre-B-cell
colony-enhancing factor) was purchased from Adipogen International,
Inc. (San Diego, CA, USA). Recombinant soluble human macrophage
colony-stimulating factor (M-CSF) and human RANKL were obtained
from PeproTech EC Ltd. (London, UK). Anti-p38 (cat. no. 9212),
anti-phosphorylated (p)-p38 (cat. no. 9211), anti-extracellular
signal-regulated protein kinases (ERK) 1/2 (cat. no. 9102),
anti-p-ERK 1/2 (cat. no. 9101), anti-c-Jun N-terminal kinase (JNK;
cat. no. 9252), anti-p-JNK (cat. no. 9251), anti-Akt (cat. no.
9272), anti-p-Akt (cat. no. 9271), anti-glycogen synthase kinase-3
β (GSK3β; cat. no. 9315), anti-p-GSK3β (cat. no. 9323) and
anti-Bruton's tyrosine kinase (Btk; cat. no. 3533) antibodies were
purchased from Cell Signaling Technology, Inc. (Beverly, MA, USA).
Anti-c-Fos (cat. no. sc-7202), anti-NFATc1 (cat. no. sc-7294),
anti-phospholipase C γ-2 (PLCγ2; cat. no. sc-5283) and anti-p-PLCγ2
(cat. no. sc-101785) antibodies were purchased from Santa Cruz
Biotechnology, Inc. (Dallas, TX, USA). Anti-p-Btk (cat. no.
GTX61792) and monoclonal anti-β-actin (cat. no. GTX109639)
antibodies were obtained from GeneTex, Inc. (Irvine, CA, USA) and
Sigma-Aldrich (Merck Millipore; Darmstadt, Germany), respectively.
Fetal bovine serum (FBS), α-minimum essential medium (α-MEM) and
penicillin/streptomycin were purchased from Gibco (Thermo Fisher
Scientific, Inc., Waltham, MA, USA). All other chemicals were of
analytical grade or complied with the standards required for cell
culture experiments.
Mouse BMM preparation and osteoclast
differentiation
A total of 10 male ICR strain mice (age, 5 weeks;
weight, 30±2 g) were purchased from Samtako (Osan, Korea). During
the experimental period, the mice were maintained in a temperature-
and humidity-controlled environment at 22–24°C and 55–60% humidity,
with a 12-h light/dark cycle and access to sterilized water and
standard rodent chow (Samtako) ad libitum. All experiments
were conducted according to the guidelines of the Institutional
Animal Care and Use Committee of Wonkwang University (WKU-14-23;
Iksan, Korea). BMMs from mice were cultured as described previously
(14). Briefly, to obtain BMMs,
BMCs were cultured in α-MEM supplemented with 10% FBS and M-CSF (10
ng/ml) for 1 day. Non-adherent cells were further cultured in the
presence of M-CSF (30 ng/ml) for 3 days. Subsequently, the adherent
cells were used as BMMs. BMMs were cultured in 48-well plates at
37°C in 5% CO2 for 4 days in the condition of M-CSF (30
ng/ml) and RANKL (100 ng/ml), and pretreated with Nampt
(100, 250 or 500 ng/ml). The cells were fixed in 3.7% formalin,
permeabilized with 0.1% Triton X-100, and stained with TRAP
solution. The stained multinucleated cells (MNCs) with >5 nuclei
were counted to determine the level of osteoclast
differentiation.
Cell viability assay, western
blotting, reverse transcription-quantitative polymerase chain
reaction (RT-qPCR) analysis and bone resorption assay
The XTT cell viability assay, western blot analysis,
RT-qPCR analysis and the resorption pit assay were performed as
described previously (14).
Resorption pits were imaged and analyzed using Image Pro-Plus
version 4.5 (Media Cybernetics, Inc., Rockville, MD, USA). Primers
used for PCR are summarized in Table
I. The western blots were analyzed using ImageJ (imagej.nih.gov/).
 | Table I.Primer sequences used for reverse
transcription-quantitative polymerase chain reaction analysis. |
Table I.
Primer sequences used for reverse
transcription-quantitative polymerase chain reaction analysis.
| Gene | Primer sequence
(5′-3′) |
|---|
| GAPDH |
|
|
Forward |
5′-TCAAGAAGGTGGTGAAGCAG-3′ |
|
Reverse |
5′-AGTGGGAGTTGCTGTTGAAGT-3′ |
| c-Fos |
|
|
Forward |
5′-GGTGAAGACCGTGTCAGGAG-3′ |
|
Reverse |
5′-TATTCCGTTCCCTTCGGATT-3′ |
| NFATc1 |
|
|
Forward |
5′-GAGTACACCTTCCAGCACCTT-3′ |
|
Reverse |
5′-TATGATGTCGGGGAAAGAGA-3′ |
| TRAP |
|
|
Forward |
5′-TCATGGGTGGTGCTGCT-3′ |
|
Reverse |
5′-GCCCACAGCCACAAATCT-3′ |
| OSCAR |
|
|
Forward |
5′-GGAATGGTCCTCATCTCCTT-3′ |
|
Reverse |
5′-TCCAGGCAGTCTCTTCAGTTT-3′ |
|
DC-STAMP |
|
|
Forward |
5′-TCCTCCATGAACAAACAGTTCCA-3′ |
|
Reverse |
5′-AGACGTGGTTTAGGAATGCAGCTC-3′ |
|
Atp6vOd2 |
|
|
Forward |
5′-GACCCTGTGGCACTTTTTGT-3′ |
|
Reverse |
5′-GTGTTTGAGCTTGGGGAGAA-3′ |
| Cathepsin
K |
|
|
Forward |
5′-CCAGTGGGAGCTATGGAAGA-3′ |
|
Reverse |
5′-CTCCAGGTTATGGGCAGAGA-3′ |
| αv-integrin |
|
Forward | 5′-
ACAAGCTCACTCCCATCACC-3′ |
|
Reverse | 5′-
ATATGAGCCTGCCGACTGAC-3′ |
| β3-integrin |
|
Forward |
5′-GGAGTGGCTGATCCAGATGT-3′ |
|
Reverse |
5′-TCTGACCATCTTCCCTGTCC-3′ |
| CTR |
|
|
Forward |
5′-TCCAACAAGGTGCTTGGGAA-3′ |
|
Reverse |
5′-CTTGAACTGCGTCCACTGGC-3′ |
| Nampt |
|
|
Forward |
5′-ATCCAGGAGGCCAAAGAAGT-3′ |
|
Reverse |
5′-CGGGAGATGACCATCGTATT-3′ |
Retroviral gene transfection
Packaging of the retroviral vectors pMX-IRES-EGFP,
pMX-cFos-IRES-EGFP and pMX-NFATc1-IRES-EGFP was performed using
transient transfection of these pMX vectors (Cell Biolabs, Inc.,
San Diego, CA, USA) into platinum-E (plat-E) retroviral packaging
cells (Cell Biolabs, Inc.) using X-tremeGENE 9 (Roche, Nutley, NJ,
USA) according to the manufacturer's protocol. Following incubation
at 37°C in fresh medium for 2 days, the culture supernatants of the
retrovirus-producing cells were collected. For retroviral
infection, non-adherent BMCs were cultured in M-CSF (30 ng/ml) for
2 days. The BMMs were incubated with viral supernatant medium of
pMX-IRES-EGFP, pMX-cFos-IRES-EGFP and pMX-NFATc1-IRES-EGFP
virus-producing plat-E cells together with polybrene (10 ng/ml) and
M-CSF (30 ng/ml) for 6 h. The infection efficiency of the
retrovirus was determined by green fluorescent protein (GFP)
expression and was always >80%. Post-infection, the BMMs were
induced to differentiate in the presence of M-CSF (30 ng/ml) and
RANKL (100 ng/ml) for 4 days. The expression of each construct was
detected using a fluorescence microscope and osteoclast formation
was determined by fixing in 3.7% formalin, permeabilizing with 0.1%
Triton X-100, and staining with TRAP solution.
Statistical analysis
Each experiment was performed at least three times
and all quantitative data are presented as the mean ± standard
deviation. All statistical analyses were performed using SPSS
(Korean version 14.0; SPSS, Inc., Chicago, IL, USA). Student's
t-test was used to compare the parameters between two groups,
whereas the one-way analysis of variance test, followed by the
Tukey post hoc test, was used to compare the parameters among three
groups. P<0.05 was considered to indicate a statistically
significant difference.
Results
Nampt inhibits RANKL-mediated
osteoclast formation in a dose-dependent manner with no
cytotoxicity
The present study analyzed the expression of
Nampt in BMM cultures treated with M-CSF (30 ng/ml) and
RANKL (100 ng/ml). As shown in Fig.
1A, the mRNA expression levels of Nampt were reduced in
the presence of RANKL. To validate the effects of Nampt on
osteoclast differentiation, mouse primary BMMs were treated with
M-CSF (30 ng/ml) and RANKL (100 ng/ml) in the presence or absence
of various concentrations of Nampt. As expected, the control
untreated group generated TRAP-positive (TRAP+) osteoclasts.
However, the presence of Nampt suppressed the formation of
TRAP+ multinucleated osteoclasts in a dose-dependent manner
(Fig. 1B and C). Subsequently, XTT
cell viability assays were conducted to ascertain whether
Nampt induced cytotoxicity during RANKL-induced osteoclast
differentiation. The addition of Nampt did not affect cell
viability at any of the concentrations used in the present study
(Fig. 1D).
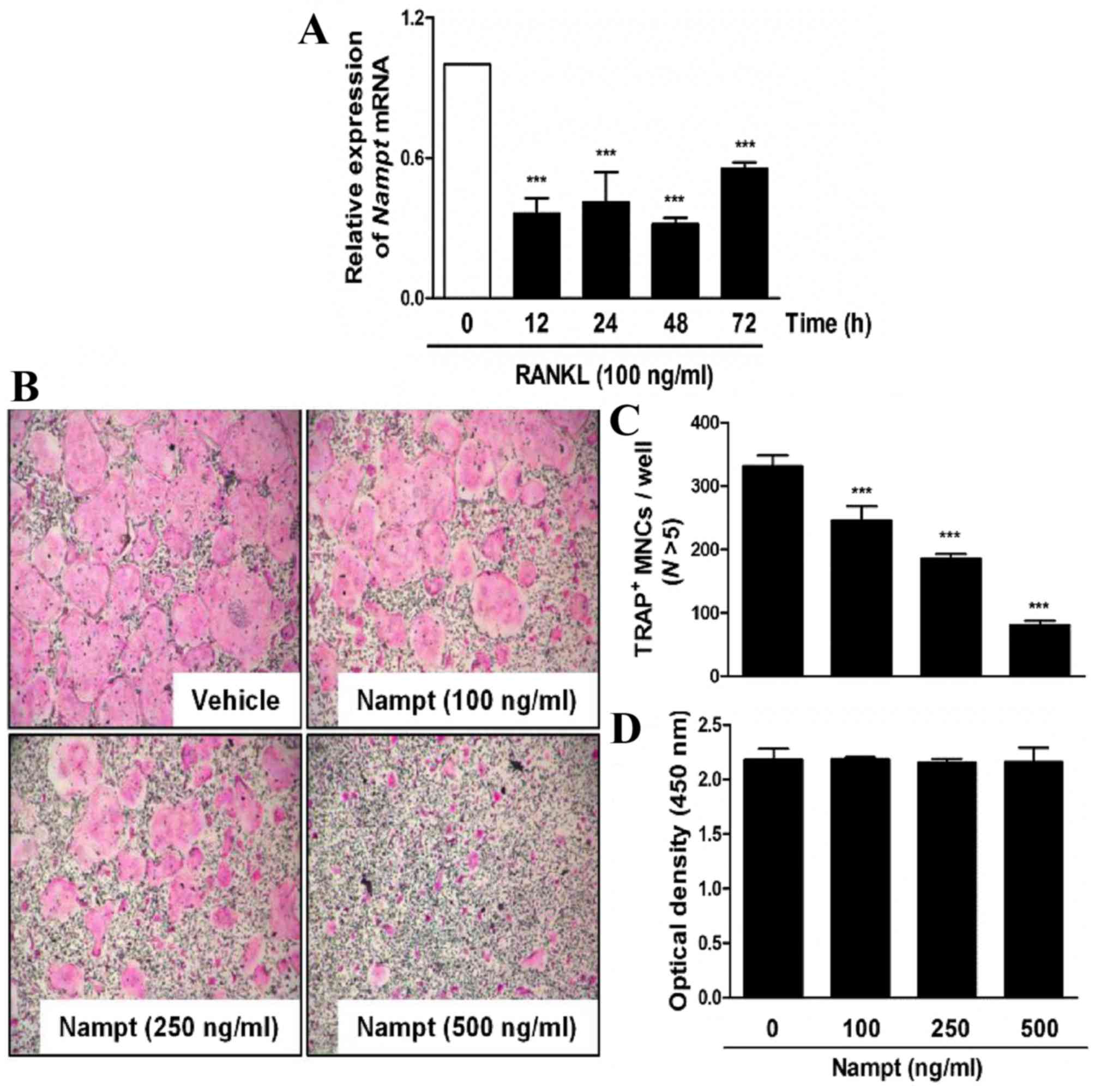 | Figure 1.Nampt attenuates TRAP-positive
osteoclast formation without cytotoxicity. (A) BMMs were cultured
in the presence of M-CSF (30 ng/ml) and then stimulated with RANKL
(100 ng/ml) for the indicated durations. Total RNA was isolated
from cells using QIAzol reagent and Nampt mRNA levels were
evaluated by reverse transcription-quantitative polymerase chain
reaction. ***P<0.001 vs. control group. (B) BMMs were cultured
for 3 days in the presence of M-CSF (30 ng/ml) and RANKL (100
ng/ml), with or without the indicated concentrations of Nampt.
Cells were fixed, permeabilized and stained with TRAP solution.
Images of TRAP+ cells were captured under a light
microscope (magnification, ×5). (C) BMMs were seeded into a 96-well
plate and cultured for 3 days in the presence of M-CSF (30 ng/ml)
with the indicated concentrations of Nampt (100, 250 or 500 ng/ml).
After 3 days, cell viability was analyzed by the XTT assay. (D)
TRAP+ MNCs with >5 nuclei were counted as
osteoclasts. ***P<0.001 vs. control group. BMMs, bone marrow
macrophages; M-CSF, macrophage colony-stimulating factor; MNCs,
mononucleated cells; N, nuclei; Nampt, nicotinamide
phosphoribosyltransferase; RANKL, receptor activator of nuclear
factor-κB ligand; TRAP, tartrate-resistant acid phosphatase. |
Nampt regulates osteoclastogenesis via
mediating RANKL-dependent early signaling pathways
To elucidate a molecular mechanism that underlies
the inhibitory effects of Nampt on osteoclastogenesis,
Nampt was added to BMM cultures treated with M-CSF (30
ng/ml) and RANKL (100 ng/ml), at three different time points after
RANKL treatment. The results indicated that Nampt (500
ng/ml) significantly blocked osteoclast differentiation when the
cells were exposed on days 0–1 after RANKL treatment but not on
days 1–2 or 2–3 (Fig. 2A and B).
As shown in Fig. 2C and D, Nampt
negatively affected the phosphorylation of JNK, Akt and GSK3β. In
addition, Nampt downregulated the phosphorylation of Btk and
PLCγ2, which are required for calcium signaling during osteoclast
differentiation (Fig. 2E and F).
These results indicated that Nampt is involved in the early
stages of osteoclast differentiation by inducing dephosphorylation
of JNK, Akt and its downstream target GSK3β, Btk and PLCγ2.
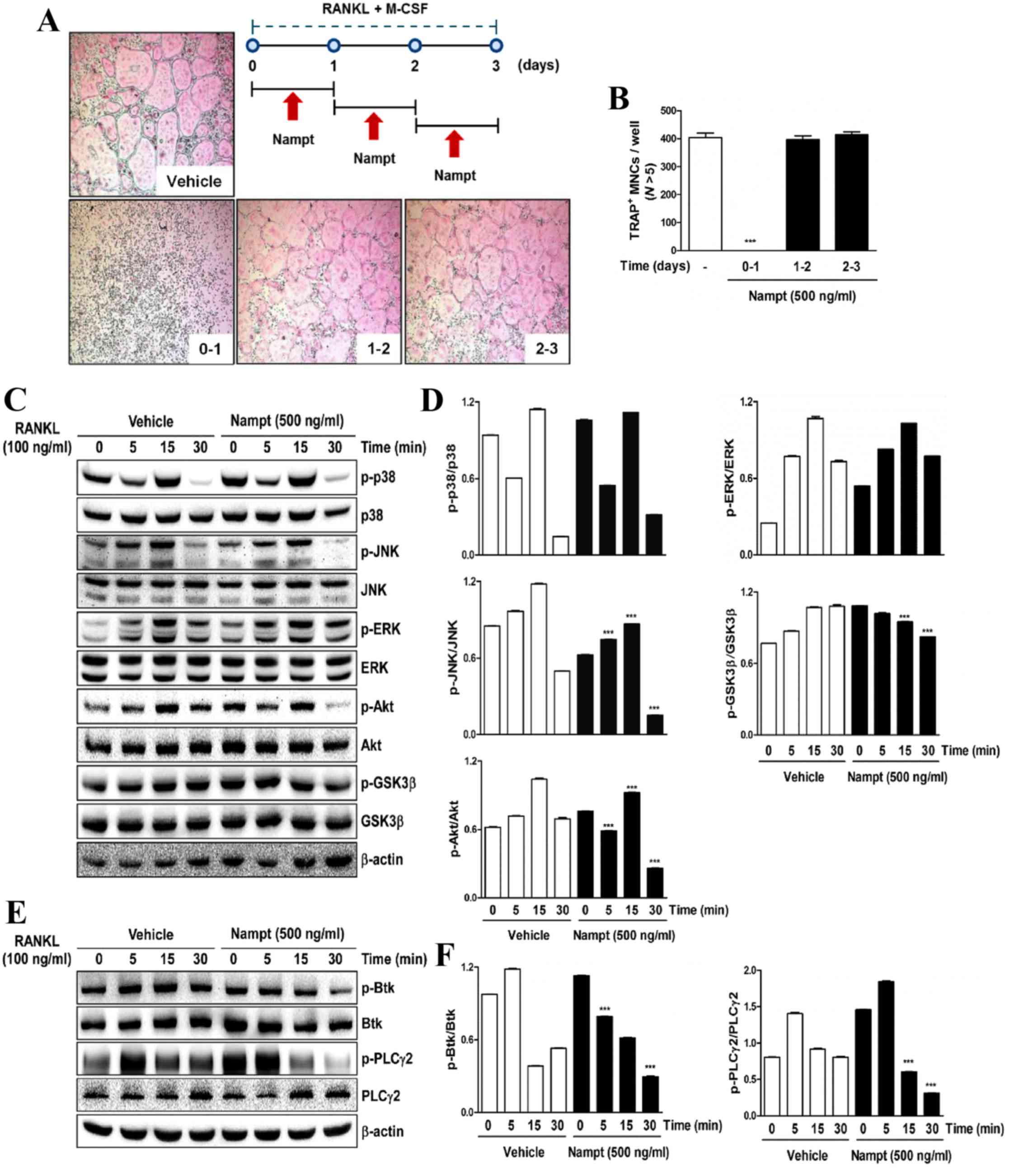 | Figure 2.Nampt inhibits the early stages of
osteoclastogenesis by downregulating RANKL-dependent early
signaling pathways. (A) BMMs were cultured for 3 days in the
presence of M-CSF (30 ng/ml) and RANKL (100 ng/ml) with or without
Nampt (500 ng/ml) for the indicated durations. Cells were then
stained with TRAP solution and images of TRAP+ cells
were captured under a light microscope (magnification, 5x). The
diagram shows the time period of Nampt treatment. (B)
TRAP+ MNCs with >5 nuclei were counted as
osteoclasts. ***P<0.001 vs. control group. (C) BMMs were
pretreated with or without Nampt (500 ng/ml) for 1 h in the
presence of M-CSF (30 ng/ml) prior to RANKL (100 ng/ml) stimulation
at the indicated time points. Whole-cell lysates were analyzed by
western blotting with the indicated antibodies. β-actin was used as
the internal control. (D) Semi-quantification of blots was
performed using ImageJ. ***P<0.001 vs. the control group (E)
BMMs were pretreated with or without of Nampt (500 ng/ml) for 1 h
in the presence of M-CSF (30 ng/ml) prior to RANKL (100 ng/ml)
stimulation at the indicated time points. Whole-cell lysates were
analyzed by western blotting with the indicated antibodies. β-actin
was used as the internal control. (F) Semi-quantification of
western blot bands was performed using ImageJ. ***P<0.001 vs.
the control group. BMMs, bone marrow macrophages; Btk, Bruton's
tyrosine kinase; ERK, extracellular signal-regulated protein
kinases; GSK3β, glycogen synthase kinase-3 β; JNK, c-Jun N-terminal
kinase; M-CSF, macrophage colony-stimulating factor; MNCs,
mononucleated cells; N, nuclei; Nampt, nicotinamide
phosphoribosyltransferase; p-, phosphorylated; PLCγ2, phospholipase
C γ-2 RANKL, receptor activator of nuclear factor-κB ligand; TRAP,
tartrate-resistant acid phosphatase. |
Nampt downregulates the expression
levels of c-Fos, NFATc1 and osteoclast-specific marker genes
To examine whether Nampt regulates
RANKL-induced osteoclast differentiation by downregulating the
activation of c-Fos and NFATc1, the present study
evaluated the effects of Nampt on RANKL-induced c-Fos
and NFATc1 expression. When BMMs were stimulated for 12–48 h
with RANKL (100 ng/ml), the mRNA expression levels of c-Fos
and NFATc1 were increased in the control group, whereas
Nampt treatment reduced their expression (Fig. 3A). Similarly, western blot analysis
demonstrated that Nampt significantly reduced the protein
levels of c-Fos and NFATc1 (Fig.
3B). Subsequently, the present study examined whether ectopic
expression of c-Fos or NFATc1 is sufficient to rescue
the inhibitory effects of Nampt on osteoclastogenesis using
a retroviral system. BMMs were infected with c-Fos or
NFATc1-encoding retroviruses and cultured with M-CSF (30
ng/ml) and RANKL (100 ng/ml) in the presence or absence of
Nampt (500 ng/ml). Indeed, the overexpression of
c-Fos or NFATc1 rescued the anti-osteoclastogenic
effect of Nampt (Fig. 3C).
In addition, it was determined whether Nampt regulates the
mRNA expression of various osteoclast-specific transcription
factors, including TRAP, osteoclast associated receptor
(OSCAR), cathepsin K, calcitonin receptor (CTR),
Atp6v0d2, dendritic cell-specific transmembrane protein
(DC-STAMP), αv-integrin and β3-integrin. These genes are
associated with osteoclast formation and function during
RANKL-induced osteoclast differentiation. The mRNA expression
levels of TRAP, OSCAR, cathepsin K, CTR, Atp6v0d2,
DC-STAMP, αv-integrin and β3-integrin were significantly
decreased by Nampt (Fig.
4). These results suggested that Nampt efficiently
inhibits c-Fos and NFATc1 activation, leading to the downregulation
of osteoclast marker gene expression during RANKL-mediated
osteoclast differentiation.
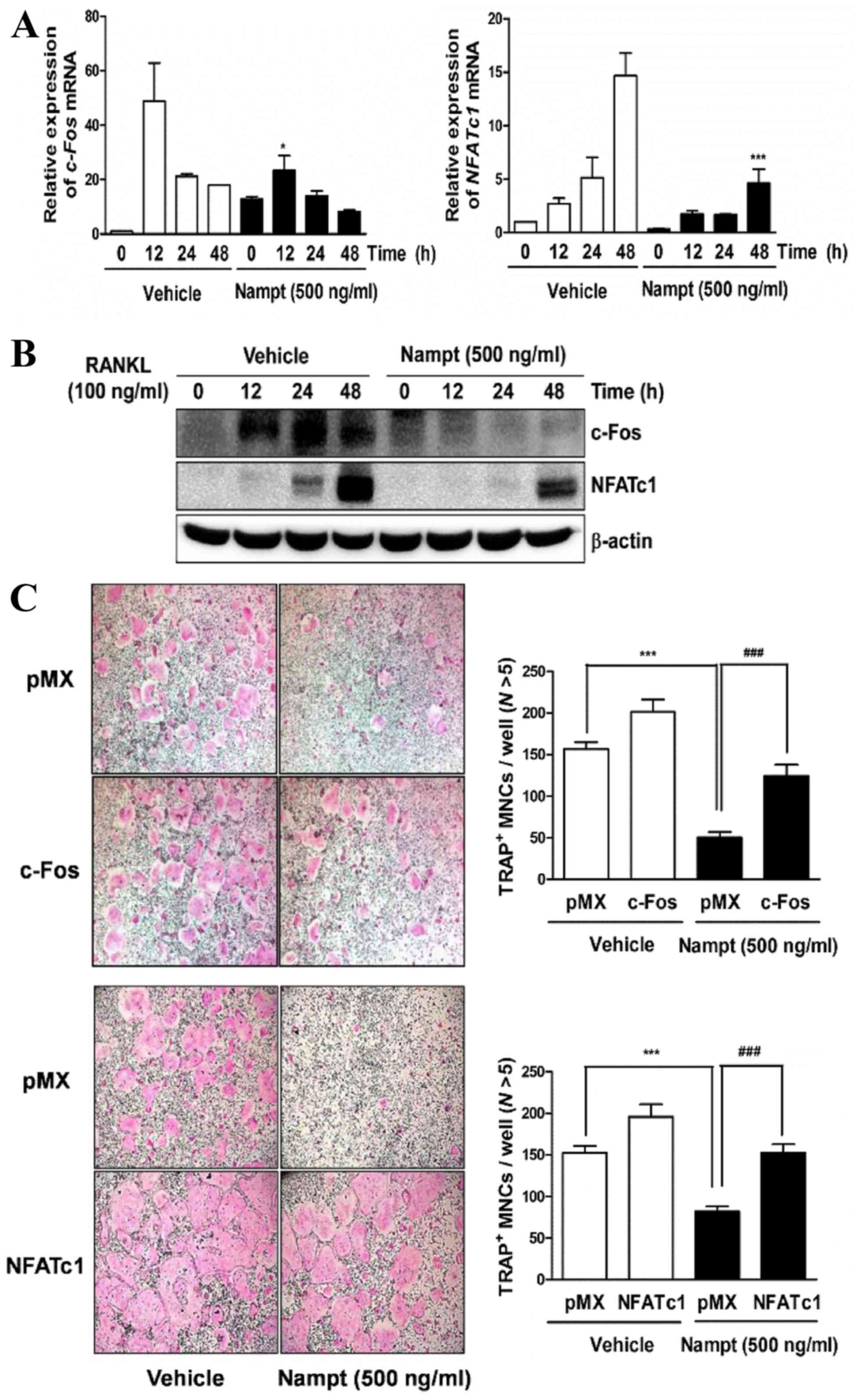 | Figure 3.Nampt reduces the expression of c-Fos
and NFATc1. (A) BMMs were pretreated with or without Nampt (500
ng/ml) for 1 h in the presence of M-CSF (30 ng/ml), and then
stimulated with RANKL (100 ng/ml) for the indicated times. The mRNA
expression levels of c-Fos and NFATc1 were analyzed by reverse
transcription-quantitative polymerase chain reaction. *P<0.05,
***P<0.001 vs. control group at the indicated time points. (B)
The effects of Nampt on protein levels of c-Fos and NFATc1 were
evaluated by western blot analysis with the indicated antibodies.
β-actin was used as the internal control. (C) BMMs were infected
with retroviruses expressing pMX-IRES-EGFP (pMX),
pMX-cFos-IRES-EGFP (c-Fos) or pMX-NFATc1-IRES-EGFP (NFATc1).
Infected BMMs were cultured with or without Nampt (500 ng/ml) in
the presence of M-CSF (30 ng/ml) and RANKL (100 ng/ml) for 4 days.
After culturing, the cells were stained with TRAP solution. Images
of TRAP+ cells were captured under a light microscope
(magnification, 5x). TRAP+ MNCs with >5 nuclei were
counted as osteoclasts. ***P<0.001 vs. control group and
###P<0.001 vs. Nampt group. BMMs, bone marrow
macrophages; M-CSF, macrophage colony-stimulating factor; MNCs,
mononucleated cells; N, nuclei; Nampt, nicotinamide
phosphoribosyltransferase; NFATc1, nuclear factor of activated T
cells, cytoplasmic 1; RANKL, receptor activator of nuclear
factor-κB ligand; TRAP, tartrate-resistant acid phosphatase. |
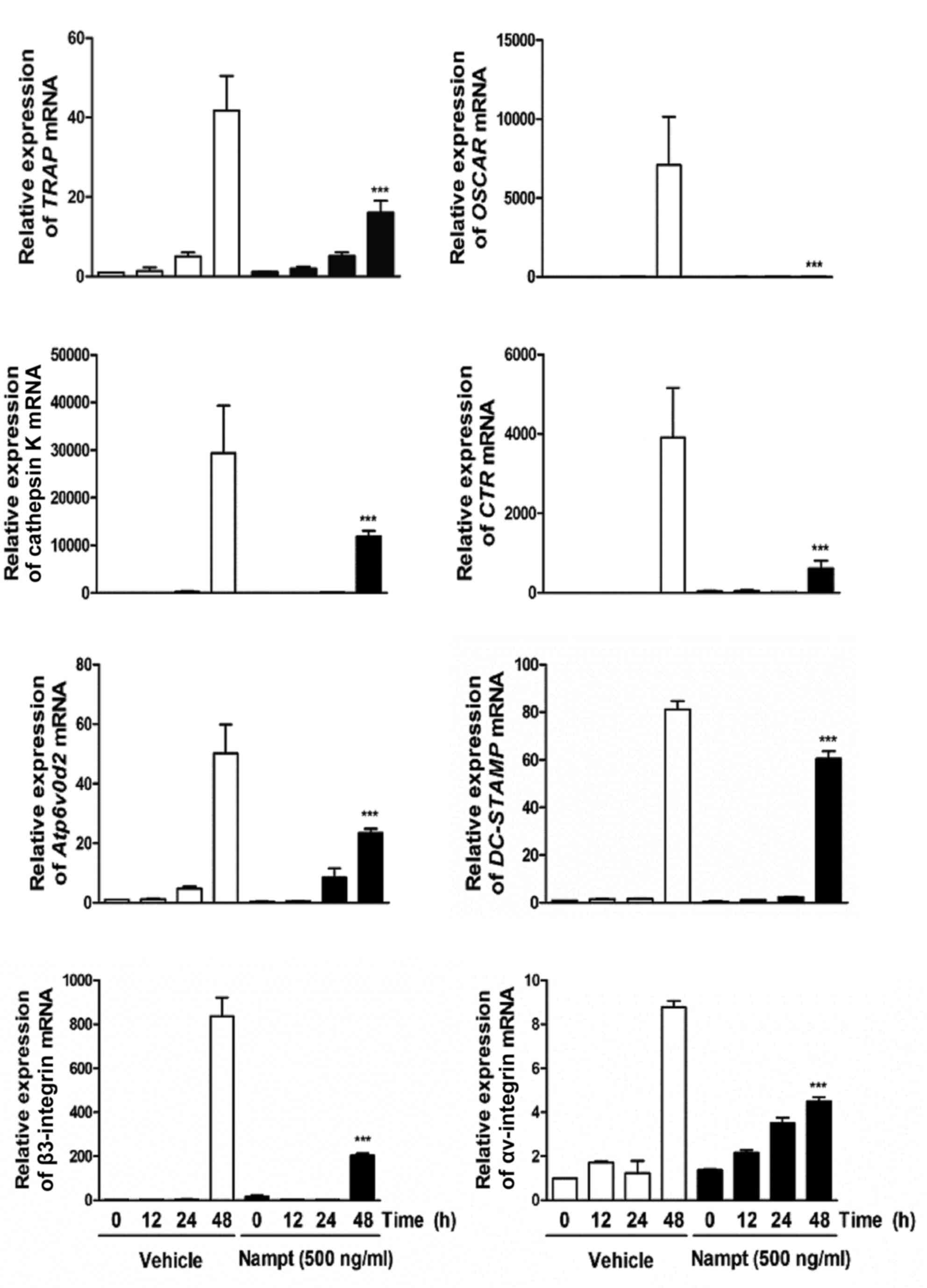 | Figure 4.Nampt reduces the expression of
osteoclast-specific genes. BMMs were pretreated with or without
Nampt (500 ng/ml) for 1 h in the presence of M-CSF (30 ng/ml) and
then stimulated with RANKL (100 ng/ml) for the indicated times.
Total RNA was isolated from cells using QIAzol reagent and the mRNA
expression levels of TRAP, OSCAR, cathepsin K, CTR, Atp6vOd2,
DC-STAMP, β3-integrin and αv-integrin were evaluated by reverse
transcription-quantitative polymerase chain reaction. ***P<0.001
vs. control group at the indicated time. BMMs, bone marrow
macrophages; CTR, calcitonin receptor; DC-STAMP, dendritic
cell-specific transmembrane protein; M-CSF, macrophage
colony-stimulating factor; Nampt, nicotinamide
phosphoribosyltransferase; OSCAR, osteoclast-associated receptor;
RANKL, receptor activator of nuclear factor-κB ligand; TRAP,
tartrate-resistant acid phosphatase. |
Nampt is not associated with the
bone-resorbing activity of mature osteoclasts
The present study subsequently examined whether
Nampt regulates osteoclastic bone-resorptive functions. To
investigate this role, mature osteoclasts were seeded onto the top
of hydroxyapatite-coated plates in the presence or absence of
Nampt (500 ng/ml). However, the number and area of
resorption pits were unaffected by Nampt treatment (Fig. 4), suggesting that Nampt does
not have a role in the resorbing activity of mature
osteoclasts.
Discussion
The present study demonstrated that Nampt
attenuated RANKL-mediated differentiation of primary mouse BMMs
into TRAP+ MNCs in a dose-dependent manner without
cytotoxic effects. During this process, Nampt decreased the
phosphorylation of various early signal transducers, including JNK
and Akt, and its downstream target, GSK3β, as well as
calcium-dependent signaling pathways, including PLCγ2 and Btk.
Furthermore, the mRNA and protein expression levels of two master
regulators of osteoclastogenesis, c-Fos and NFATc1,
were significantly decreased by Nampt treatment, leading to
decreased expression levels of various key transcription factors in
osteoclast differentiation including TRAP, OSCAR,
cathepsin K, CTR, Atp6vOd2, DC-STAMP, β3- and
αv-integrin.
The differentiation of monocyte/macrophage lineage
precursors into bone-resorbing osteoclasts is initiated in response
to two important cytokines, M-CSF and RANKL, resulting in the
activation of early downstream pathways (15). During this process, the
phosphorylation of numerous signal transducers, including
mitogen-activated protein kinases (MAPKs), which are comprised of
p38, ERK and JNK; nuclear factor-κB; phosphatidylinositol
3-kinase/Akt; PLCγ2 and Btk occurs (16–19).
RANKL-mediated activation of JNK is known to have an anti-apoptotic
function in osteoclastogenesis, and Akt is a potent inducer of
osteoclast differentiation by promoting the formation of an
inactive form of GSK3β (p-GSK3β) and the nuclear translocation of
NFATc1 (20,21). In addition, it has been well
established that calcium signaling is crucial for RANKL-dependent
osteoclastogenesis. PLCγ2 activation requires phosphorylation of
its tyrosine residues to induce calcium oscillations and the
translocation of NFATc1 by forming a complex with regulatory
adapter molecule GRB2-associated binding protein 2 and modulating
its recruitment to RANK (22,23).
PLCγ2 is regulated by the upstream tyrosine kinase Btk, which is
involved in osteoclast differentiation by linking RANK and
immunoreceptor tyrosine-based activation motif signaling, with
subsequent regulation of the formation of Btk/BLNK-containing
complex and activation of PLCγ2-dependent calcium signaling
(19). The results of the present
study revealed that Nampt suppressed RANKL-induced
osteoclast differentiation by interfering with survival-related
signaling pathways that contain JNK and Akt, as well as
Btk-PLCγ2-dependent intracellular calcium signaling. Since
Nampt affected several signals associated with the early
stages of osteoclastogenesis, the present study examined whether
Nampt is involved in the expression of late-stage
transcription factors, c-Fos and NFATc1. A previous
report indicated that c-Fos knock-out mice exhibit
morphological characteristics of osteopetrosis, owing to osteoclast
malfunction, whereas impaired osteoclastogenesis in murine BMMs is
completely rescued by exogenous overexpression of c-Fos
(24,25). In response to the activation of
c-Fos, another master regulator, NFATc1, serves a
crucial role in osteoclast differentiation. NFATc1
inhibition in embryonic stem cells suppresses their ability to
differentiate into normal osteoclasts, and this phenomenon is
reversed by ectopic expression of NFATc1 even in the absence
of RANKL (26,27). This c-Fos-NFATc1 activation cascade
leads to the elevated expression of osteoclast-specific gene
markers, such as TRAP, OSCAR, CTR, cathepsin
K, DC-STAMP and β3-integrin. The present data revealed that
Nampt expression significantly decreased mRNA and protein
levels of c-Fos and NFATc1 compared with the control, resulting in
the downregulation of various transcription factors associated with
osteoclast formation and function.
Although the relationship between bone regulation
and Nampt has previously been reported, the present study is
the first, to the best of our knowledge, to identify the effects of
Nampt on mouse BMM-derived osteoclastogenesis and its
molecular mechanisms. In conclusion, the present study demonstrated
that the adipokine Nampt effectively interferes with
RANKL-mediated osteoclast differentiation by inactivating several
early signal transducers. These signaling molecules include JNK,
Akt and GSK3β, as well as Btk-PLCγ2-calcium signaling. Furthermore,
Nampt treatment decreases c-Fos and NFATc1 mRNA and protein
levels, resulting in the downregulation of various target gene mRNA
levels. Nampt does not influence the bone-resorbing activity
of mature osteoclasts; instead, Nampt exerts its
anti-osteoclastogenic effects by targeting osteoclast precursors
rather than mature multinucleated osteoclasts. Although further
studies are required to reveal the restorative effect of
Nampt on osteoporotic bone loss in mouse models, it may be
suggested that increased Nampt is a potential target for the
treatment of metabolic bone diseases, such as osteoporosis, by
suppressing osteoclast differentiation and function.
Acknowledgments
The present study was supported by a grant from the
Basic Science Research Program through the National Research
Foundation of Korea (NRF) funded by the Ministry of Education
(grant no. NRF-2016R1D1A1B03933712).
References
|
1
|
Samal B, Sun Y, Steams G, Xie C, Suggs S
and McNiece I: Cloning and characterization of the cDNA encoding a
novel human pre-B-cell colony-enhancing factor. Mol Cell Biol.
14:1431–1437. 1994. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Luk T, Malam Z and Marshall JC: Pre-B cell
colony-enhancing factor (PBEF)/visfatin: A novel mediator of innate
immunity. J Leukoc Biol. 83:804–816. 2006. View Article : Google Scholar
|
|
3
|
Gosset M, Berenbaum F, Salvat C, Sautet A,
Pigenet A, Tahiri K and Jacques C: Crucial role of visfatin/pre-B
cell colony-enhancing factor in matrix degradation and
prostaglandin E2 synthesis in chondrocytes: Possible influence on
osteoarthritis. Arthritis Rheum. 58:1399–1409. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Moschen AR, Kaser A, Enrich B, Mosheimer
B, Theurl M, Niederegger H and Tilg H: Visfatin, an adipocytokine
with proinflammatory and immunomodulating properties. J Immunol.
178:1748–1758. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Revollo JR, Grimm AA and Imai S: The NAD
biosynthesis pathway mediated by nicotinamide
phosphoribosyltransferase regulates Sir2 activity in mammalian
cells. J Biol Chem. 279:50754–50763. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Tan B, Dong S, Shepard RL, Kays L, Roth
KD, Geeganage S, Kuo MS and Zhao G: Inhibition of nicotinamide
phosphoribosyltransferase (NAMPT), an enzyme essential for NAD+
biosynthesis, leads to altered carbohydrate metabolism in cancer
cells. J Biol Chem. 290:15812–15824. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Yang S, Ryu JH, Oh H, Jeon J, Kwak JS, Kim
JH, Kim HA, Chun CH and Chun JS: NAMPT (visfatin), a direct target
of hypoxia-inducible factor-2α, is an essential catabolic regulator
of osteoarthritis. Ann Rheum Dis. 74:595–602. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Liu Y, Song CY, Wu SS, Liang QH, Yuan LQ
and Liao EY: Novel adipokine and bone metabolism. Int J Endocrinol.
2013:8950452013. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Wattanachanya L, Lu WD, Kundu RK, Wang L,
Abbott MJ, O'Carroll D, Quertermous T and Nissenson RA: Increased
bone mass in mice lacking the adipokine apelin. Endocrinology.
154:2069–2080. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Kamio N, Kawato T, Tanabe N, Kitami S,
Morita T, Ochiai K and Maeno M: Vaspin attenuates RANKL-induced
osteoclast formation in RAW264.7 cells. Connect Tissue Res.
54:147–152. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Tu Q, Zhang J, Dong LQ, Saunders E, Luo E,
Tang J and Chen J: Adiponectin inhibits osteoclastogenesis and bone
resorption via APPL1-mediated suppression of Akt1. J Biol Chem.
286:12542–12553. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Venkateshaiah SU, Khan S, Ling W, Bam R,
Li X, van Rhee F, Usmani S, Barlogie B, Epstein J and Yaccoby S:
NAMPT/PBEF1 enzymatic activity is indispensable for myeloma cell
growth and osteoclast activity. Exp Hematol. 41:547.e2–557.e2.
2013. View Article : Google Scholar
|
|
13
|
Moschen AR, Geiger S, Germer R and Tilg H:
Pre-B cell colony enhancing factor/NAMPT/visfatin and its role in
inflammation-related bone disease. Mutat Res. 690:95–101. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Kim JY, Cheon YH, Oh HM, Rho MC,
Erkhembaatar M, Kim MS, Lee CH, Kim JJ, Choi MK, Yoon KH, et al:
Oleanolic acid acetate inhibits osteoclast differentiation by
downregulating PLCγ2-Ca(2+)-NFATc1 signaling, and suppresses bone
loss in mice. Bone. 60:104–111. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Glantschnig H, Fisher JE, Wesolowski G,
Rodan GA and Reszka AA: M-CSF, TNFalpha and RANK ligand promote
osteoclast survival by signaling through mTOR/S6 kinase. Cell Death
Differ. 10:1165–1177. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Li X, Udagawa N, Itoh K, Suda K, Murase Y,
Nishihara T, Suda T and Takahashi N: p38 MAPK-mediated signals are
required for inducing osteoclast differentiation but not for
osteoclast function. Endocrinology. 143:3105–3113. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Kobayashi N, Kadono Y, Naito A, Matsumoto
K, Yamamoto T, Tanaka S and Inoue J: Segregation of TRAF6-mediated
signaling pathways clarifies its role in osteoclastogenesis. EMBO
J. 20:1271–1280. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Gingery A, Bradley E, Shaw A and Oursler
MJ: Phosphatidylinositol 3-kinase coordinately activates the
MEK/ERK and AKT/NFkappaB pathways to maintain osteoclast survival.
J Cell Biochem. 89:165–179. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Shinohara M, Koga T, Okamoto K, Sakaguchi
S, Arai K, Yasuda H, Takai T, Kodama T, Morio T, Geha RS, et al:
Tyrosine kinases Btk and Tec regulate osteoclast differentiation by
linking RANK and ITAM signals. Cell. 132:794–806. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Ikeda F, Matsubara T, Tsurukai T, Hata K,
Nishimura R and Yoneda T: JNK/c-Jun signaling mediates an
anti-apoptotic effect of RANKL in osteoclasts. J Bone Miner Res.
23:907–914. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Moon JB, Kim JH, Kim K, Youn BU, Ko A, Lee
SY and Kim N: Akt induces osteoclast differentiation through
regulating the GSK3β/NFATc1 signaling cascade. J Immunol.
188:163–169. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Wilde JI and Watson SP: Regulation of
phospholipase C gamma isoforms in haematopoietic cells: Why one,
not the other? Cell Signal. 13:691–701. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Mao D, Epple H, Uthgenannt B, Novack DV
and Faccio R: PLCgamma2 regulates osteoclastogenesis via its
interaction with ITAM proteins and GAB2. J Clin Invest.
116:2869–2879. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Wisdon R and Verma IM: Transformation by
Fos proteins requires a C-terminal transactivation domain. Mol Cell
Biol. 13:7429–7438. 1993. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Grigoriadis AE, Wang ZQ, Cecchini MG,
Hofstetter W, Felix R, Fleisch HA and Wagner EF: c-Fos: A key
regulator of osteoclast-macrophage lineage determination and bone
remodeling. Science. 266:443–448. 1994. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Takayanagi H: The role of NFAT in
osteoclast formation. Ann N Y Acad Sci. 1116:227–237. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Takayanagi H, Kim S, Koga T, Nishina H,
Isshiki M, Yoshida H, Saiura A, Isobe M, Yokochi T, Inoue J, et al:
Induction and activation of the transcription factor NFATc1 (NFAT2)
integrate RANKL signaling in terminal differentiation of
osteoclasts. Dev Cell. 3:889–901. 2002. View Article : Google Scholar : PubMed/NCBI
|


















