Introduction
Glioblastoma multiforme (GBM) is the most common
lethal type of brain cancer characterized by rapid growth and
extensive invasiveness (1). The
prognosis of GBM is poor; it has been reported that the five-year
survival rate is only 9.8% in GBM patients treated with a
combination of temozolomide-based chemotherapy and radiotherapy
(2). The median overall survival
period is 19.6 months following treatment with concurrent adjuvant
chemotherapy and radiotherapy (3).
It is important to identify the key molecular factors contributing
to the aggressive phenotype of glioblastoma cells to develop novel
therapies for the treatment of this disease.
Prospero homeobox protein 1 (PROX1) is a
transcription factor expressed in various tissues, including the
heart, liver, skeletal muscles, pancreas, kidney and brain
(4). This protein is critical for
organ development during embryogenesis (5,6). In
addition, PROX1 is involved in tumorigenesis and progression. For
example, PROX1 has been revealed to promote the survival of colon
cancer cells under metabolic stress and facilitate the spread of
tumor cells (7,8). Similarly, overexpression of PROX1
enhances the migration and invasion of hepatocellular carcinoma
(HCC) cells, and HCC metastasis in nude mice (9). However, ectopic expression of PROX1
exerts anti-proliferative effects against esophageal (10) and pancreatic (11) cancer cells. In high-grade malignant
astrocytic gliomas, PROX1 is highly expressed (12). PROX1 has been identified as an
independent prognostic factor for survival in patients with World
Health Organization grade II gliomas (13). These findings implicate PROX1 in
the pathogenesis of GBM.
The present study overexpressed PROX1 in human GBM
cell lines and examined its roles in glioblastoma cell growth,
tumorigenesis and invasiveness. As oncogenic nuclear factor-κB
(NF-κB) signaling is critical for the growth and progression of GBM
(14), whether the action of PROX1
was mediated through modulation of NF-κB activation was
additionally investigated.
Materials and methods
Cell lines
The LN-229 and U87MG human glioma cell lines were
purchased from the Cell Bank of the Chinese Academy of Sciences
(Shanghai, China). They were cultured in Dulbecco's modified
Eagle's medium (DMEM) supplemented with 10% fetal bovine serum
(FBS), 100 U/ml penicillin and l00 µg/ml streptomycin (Invitrogen;
Thermo Fisher Scientific, Inc., Waltham, MA, USA) at 37°C in a
humidified atmosphere of 5% CO2.
Plasmids and transfection
Full-length human PROX1 cDNA (OriGene Technologies,
Inc., Rockville, MD, USA) was cloned into the expression vector
pcDNA3.1(+) (Invitrogen; Thermo Fisher Scientific, Inc.). The
sequence identity of the pcDNA3.1/PROX1 construct was confirmed.
The NF-κB-luciferase reporter gene plasmid (pNF-κB-luc) was
purchased from Stratagene; Agilent Technologies, Inc. (Santa Clara,
CA, USA) and the Renilla luciferase expression plasmid (pRL-TK) was
purchased from Promega Corporation (Madison, WI, USA). A plasmid
expressing dominant negative inhibitor of κBα (IκBα) containing
serine to alanine mutations at positions 32 and 36 (pCMV-IκBαM) was
purchased from Clontech Laboratories, Inc. (Mountainview, CA,
USA).
At ~80% confluence, U87MG and LN-229 cells were
transfected with 0.5 µg pcDNA3.1(+) empty vector or pcDNA3.1/PROX1
using Lipofectamine® 2000 (Invitrogen; Thermo Fisher
Scientific, Inc.) according to the manufacturer's protocol.
Selection was performed with G418 (Sigma-Aldrich; Merck Millipore,
Darmstadt, Germany) at 800 µg/ml and resistant clones were obtained
and pooled two weeks after transfection. PROX1 overexpression was
confirmed by western blot analysis. For the NF-κB reporter assay,
vector or PROX1 stably-transfected cells or parental cells were
seeded in triplicate at 1×105 cells per well into
12-well plates 24 h prior to transfection. Cells were
co-transfected with 0.5 µg pNF-κB-luc together with 0.1 µg pRL-TK.
Luciferase activity was measured 24 h after transfection with the
Dual-Luciferase Reporter assay system (Promega Corporation)
according to the manufacturer's protocol. The relative luciferase
activity was determined by normalization to Renilla
luciferase activity. For inhibitory studies, vector or PROX1
stably-transfected cells were co-transfected with 0.5 µg pCMV-IκBαM
or pCMV plasmid. At 24 h post-transfection, cells were collected
and subjected to gene expression analysis and cell proliferation
and invasion assays.
Cell proliferation assay
Cell proliferation was determined using the
3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT)
assay (Sigma-Aldrich; Merck Millipore). Briefly, U87MG and LN-229
cells (3×103) were seeded into 96-well plates and cultured for
three days. MTT solution at a final concentration of 5 mg/ml was
added to each well and incubated at 37°C for 4 h. Dimethyl
sulfoxide was added to dissolve formazan crystals. The number of
viable cells was determined by measuring absorbance at a wavelength
of 570 nm using a microplate reader.
Colony formation assay
For the colony formation assay, U87MG and LN-229
cells (600 per well) were plated into 6-well plates and cultured
for 10 days in DMEM containing 10% FBS. Cells were fixed with
methanol and stained with 0.1% crystal violet (Sigma-Aldrich; Merck
Millipore). Individual colonies consisting of 50 or more cells were
counted.
Xenograft studies
A total of 12 male BALB/c nude mice at 5 weeks of
age were obtained from the Animal Center of Zhengzhou University
(Zhengzhou, China) and housed at 24°C under a 12-h light/dark cycle
with free access to water and standard laboratory food. The
experimental protocols involving animals were approved by the
Institutional Animal Care and Use Committee of Zhengzhou
University. Vector and PROX1 stably-transfected U87MG cells (2×106)
were injected subcutaneously into the right flank of mice (n=6 per
group). Tumor size was measured with calipers every seven days and
the tumor volume was calculated. Animals were anesthetized 35 days
after injection of tumor cells with intraperitoneal injection of
ketamine (60 mg/kg) and xylazine (6 mg/kg), which were purchased
from Shanghai First Biochemical Pharmaceutical Co., Ltd. (Shanghai,
China). Xenograft tumors were extracted and imaged. Tumor volume
and weight were measured.
Transwell invasion assay
Transfected and parental U87MG and LN-229 cells in
serum-free DMEM were plated into the upper chamber of an insert
(8-µm pore size) precoated with Matrigel (BD Biosciences, Franklin
Lakes, NJ, USA). The lower chamber was filled with media containing
10% FBS. Cells were allowed to invade Matrigel-coated inserts at
37°C for 24 h. Subsequently, non-invaded cells were removed with a
cotton swab. The cells that had invaded through the insert were
stained with 0.1% crystal violet in methanol and imaged using an
inverted light microscope. Cells in five random fields per insert
were counted.
Western blot analysis
For the extraction of total cellular proteins, cells
were washed with ice-cold phosphate-buffered saline and lysed with
lysis buffer (50 mM 4-(2-hydroxyethyl)-1-piperazineethanesulphonic
acid at, pH 7.5, with 150 mM NaCl, 1 mM EDTA, 10% glycerol and 1%
Triton X-100) containing a Protease Inhibitor Cocktail
(Sigma-Aldrich; Merck Millipore). Lysates were centrifuged at
12,000 × g for 10 min at 4°C to remove debris, and the supernatants
containing total proteins were collected for further analysis.
Nuclear and cytoplasmic sub-fractions were prepared using a
Nuclear/Cytosol Fractionation kit (BioVision, Inc., Milpitas, CA,
USA) according to the manufacturer's protocol. Equal quantities of
protein samples (50 µg per lane) were separated by 10–12%
SDS-polyacrylamide gel electrophoresis and transferred onto
nitrocellulose membranes. Following the blocking of non-specific
binding sites with 5% non-fat milk, the membranes were incubated
with primary antibodies overnight at 4°C, followed by incubation
with horseradish peroxidase-conjugated goat anti-mouse IgG (catalog
no. sc-2005, Santa Cruz Biotechnology, Inc., Dallas, TX, USA;
1:2,000 dilution) or goat anti-rabbit IgG (catalog no. sc-2004,
Santa Cruz Biotechnology, Inc.; 1:2,000 dilution) for 1 h. The
signal was detected using an Enhanced Chemiluminescence system (GE
Healthcare Life Sciences, Chalfont, UK). The primary antibodies
(all at 1:500 dilution) used were as follows: Rabbit polyclonal
anti-PROX1 (catalog no. ab191019), rabbit monoclonal anti-cyclin D1
(catalog no. ab137875), rabbit monoclonal anti-matrix
metallopeptidase (MMP)-9 (catalog no. ab76003), mouse monoclonal
anti-β-actin (catalog no. ab184220) and rabbit monoclonal
anti-NF-κB p65 (catalog no. ab76311) obtained from Abcam,
Cambridge, MA, USA; and mouse monoclonal anti-histone H3 (catalog
no. 14269), rabbit monoclonal anti-phosphorylated (p)-IκBα (catalog
no. 2859), rabbit monoclonal anti-IκBα (catalog no. 4812), mouse
monoclonal anti-p-protein kinase B (Akt; catalog no. 12694), mouse
monoclonal anti-Akt (catalog no. 2920), rabbit polyclonal
anti-p-extracellular signal-regulated kinase (ERK; catalog no.
9101) and rabbit monoclonal anti-ERK (catalog no. 4695) purchased
from Cell Signaling Technology, Inc., Danvers, MA, USA).
Statistical analysis
Data are presented as the mean ± standard deviation.
Statistical analyses were performed with SPSS version 16.0 software
(SPSS, Inc., Chicago, IL, USA) using one-way analysis of variance
followed by the Tukey post-hoc test. P<0.05 was considered to
indicate a statistically significant difference.
Results
Overexpression of PROX1 promotes
tumorigenesis of human glioblastoma cells
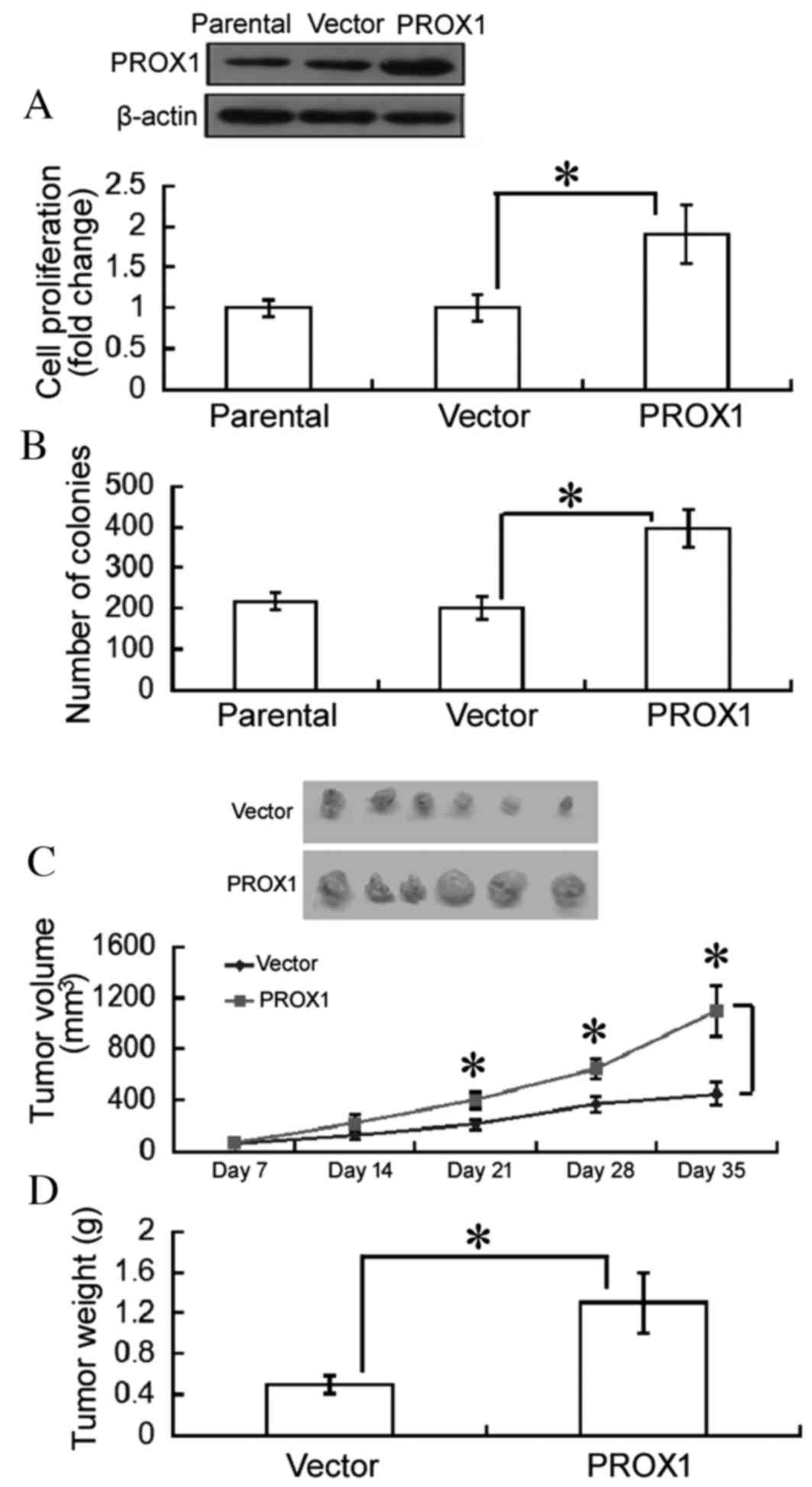
To determine the role of PROX1 in glioblastoma cell
growth, PROX1 was overexpressed in U87MG and LN-229 cells. An MTT
assay revealed that overexpression of PROX1 significantly enhanced
the proliferation of U87MG cells following a 3-day culture,
compared with empty vector-transfected cells (P=0.0342; Fig. 1A). The numbers of colonies were
significantly increased in PROX1-overexpressing U87MG cells, as
assessed by colony formation assays (P=0.0206; Fig. 1B). Similar results were observed in
LN-229 cells (data not shown).
To further confirm the oncogenic role of PROX1 in
vivo, tumor xenografts from vector- or PROX1-transfected U87MG
cells were generated. Growth of PROX1 tumor xenografts was
significantly increased compared with empty vector tumors
(P<0.05; Fig. 1C). At 35 days,
tumor weight was significantly greater in the PROX1 group compared
with the empty vector group (P=0.0379; Fig. 1D).
PROX1 enhances glioblastoma cell
invasion
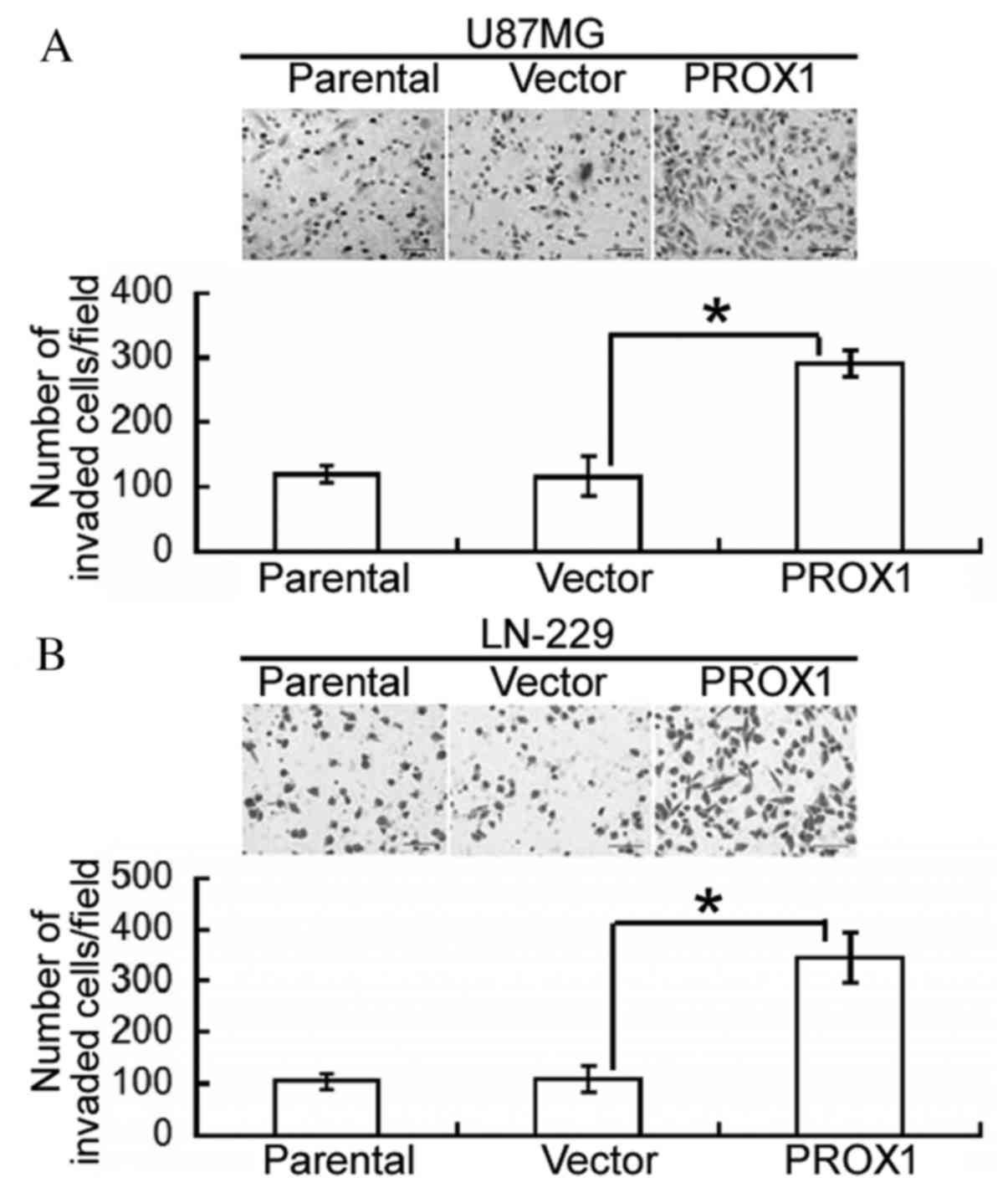
Following this, the effect of PROX1 overexpression
on the invasiveness of glioblastoma cells was assessed. Compared
with vector-transfected U87MG cells, PROX1 overexpression
significantly increased the number of invading cells following a
24-h incubation (P=0.0074; Fig.
2A). Similarly, PROX1-overexpressing LN-229 cells demonstrated
a significantly greater invasive capacity compared with control
cells (P=0.0125; Fig. 2B).
PROX1 overexpression induces sustained
NF-κB activation
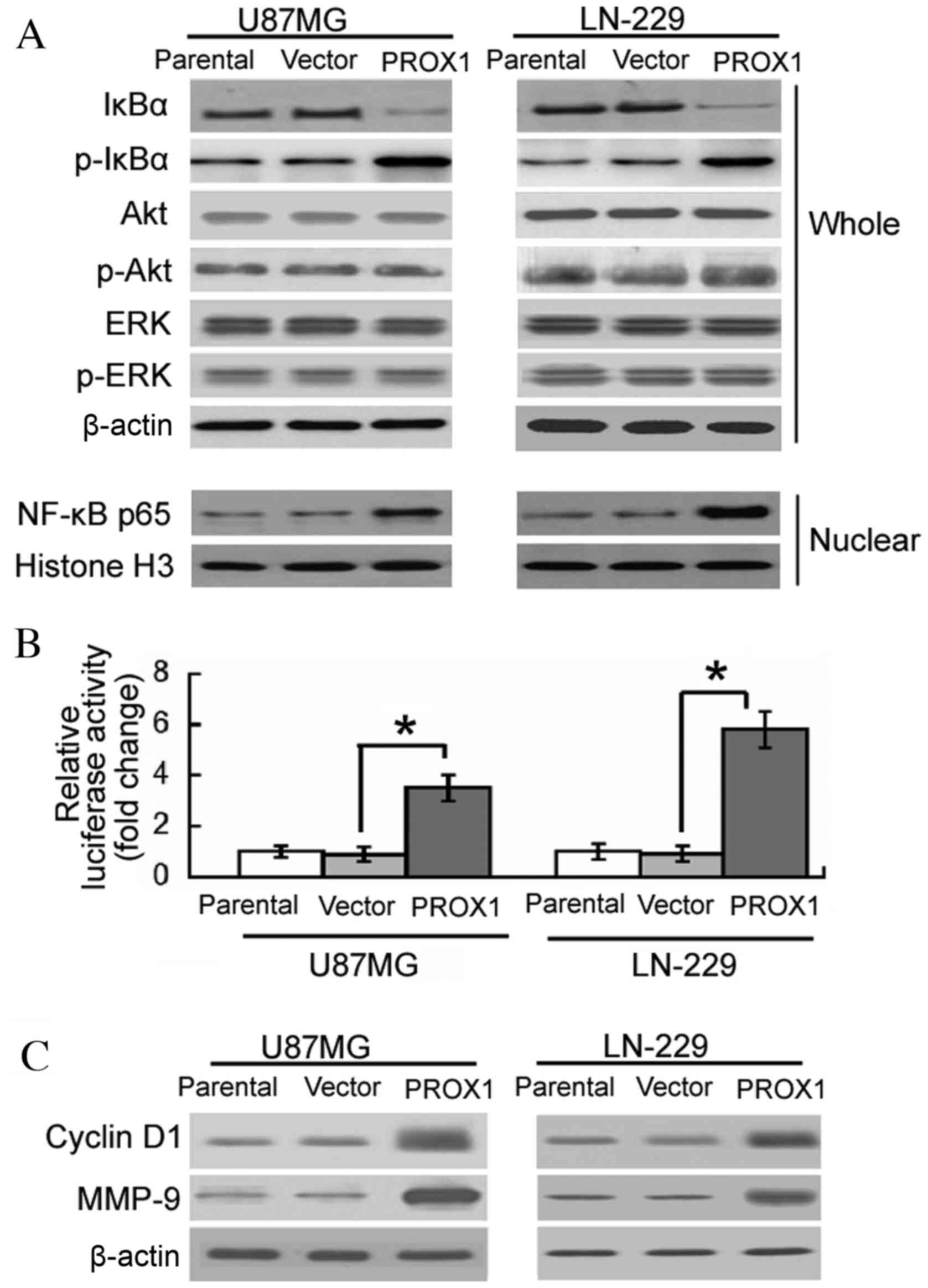
The involvement of signaling pathways in the
oncogenic activity of PROX1 was subsequently investigated. Western
blot analysis revealed that PROX1 overexpression induced the
phosphorylation of IκBα and decreased the level of IκBα in U87MG
and LN-229 cells (Fig. 3A).
Nuclear accumulation of NF-κB p65 was detected in
PROX1-overexpressing cells. However, the phosphorylation levels of
Akt and ERK were not altered by PROX1 overexpression. To confirm
the effect of PROX1 overexpression on NF-κB activation, vector or
PROX1 stably-transfected U87MG and LN-229 cells were co-transfected
with an NF-κB-dependent reporter gene and a pRL-TK control vector.
PROX1-overexpressing U87MG and LN-229 cells demonstrated a 3.5- and
5.8-fold increase in NF-κB-dependent reporter activity,
respectively, compared with vector-transfected controls (Fig. 3B). The expression of various NF-κB
responsive genes, including cyclin D1 and MMP-9, was subsequently
determined. Enforced expression of PROX1 resulted in a marked
elevation in the protein expression levels of cyclin D1 and MMP-9,
compared with the vector-transfected controls (Fig. 3C).
Inhibition of NF-κB activity
attenuates the oncogenic activity of PROX1 in glioblastoma
cells
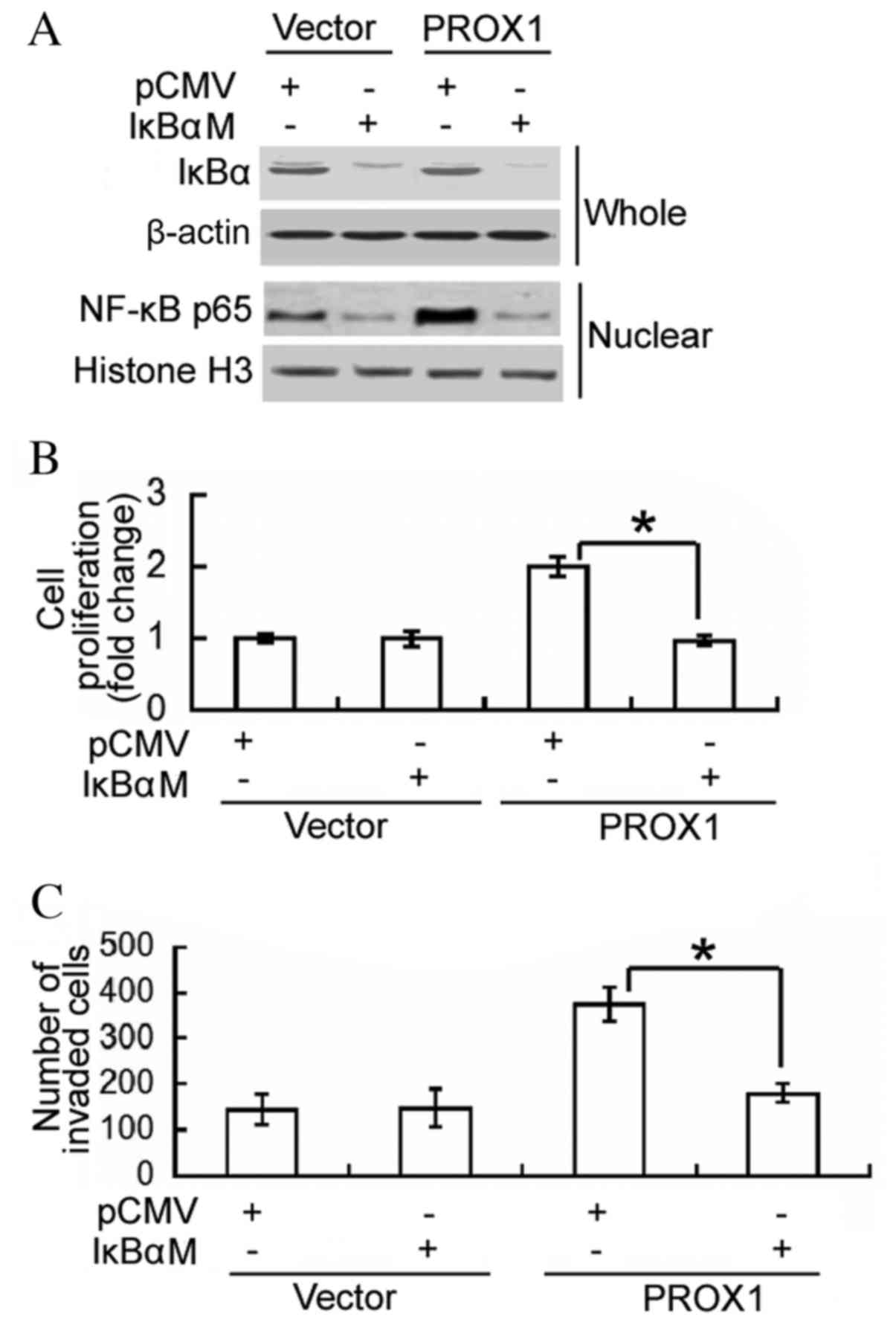
To determine whether the oncogenic role of PROX1 in
glioblastoma cells is mediated via activation of NF-κB, a dominant
IκBα mutant was transfected into PROX1-overexpressing U87MG cells,
and cell proliferation and invasion were assessed. Western blot
analysis confirmed the overexpression of the IκBα mutant in
IκBαM-transfected cells, which was accompanied by reduced nuclear
accumulation of NF-κB p65 (Fig.
4A). Notably, the delivery of the negative IκBα mutant
significantly impaired the proliferation and invasiveness of
PROX1-overexpressing cells (P<0.0001 and P=0.0042, respectively;
Fig. 4B and C). These results
support an important role for NF-κB activation in PROX1-mediated
induction of cell proliferation and invasion.
Discussion
PROX1 acts as a tumor suppressor or tumor promoter
in different types of cancers. For example, in HCC PROX1
facilitates cancer cell invasion and metastasis (9), whereas in pancreatic cancer cells
this protein induces growth-suppressive effects (1). Although previous studies have
documented the high expression of PROX1 in GBM (12,13),
the biological functions of PROX1 in the tumorigenesis and
invasiveness of glioblastoma cells remain unclear. The results of
the present study revealed that overexpression of PROX1
significantly increased the proliferation and colony formation of
GBM cells. In vivo studies further confirmed that PROX1
overexpression enhanced tumor xenograft growth. These results
provide, to the best of our knowledge, the first evidence for the
tumor-promoting role of PROX1 in GBM.
Glioblastoma cells possess a high invasiveness
potential, which is an important factor contributing to poor
prognosis of GBM (15,16). Therefore, the effect of the
overexpression of PROX1 on the invasiveness of glioblastoma cells
was examined. An in vitro Transwell assay revealed that
enforced expression levels of PROX1 significantly increased
invasiveness through the Matrigel layer in U87MG and LN-229 cells.
The pro-invasive activity of PROX1 in glioblastoma cells may
provide an explanation for the significant association between high
expression levels of PROX1 and poor prognosis in patients with
grade II gliomas (13). In
addition, PROX1 demonstrates the ability to modulate cell invasion
in various other types of cancer cells. For example, ectopic
expression of PROX1 has been revealed to promote colon cancer cell
invasion via induction of epithelial-mesenchymal transition
(17).
To determine the underlying mechanism of the
tumor-promoting activities of PROX1, the effect of PROX1
overexpression on NF-κB activation was investigated. It was
identified that PROX1-overexpressing cells had increased activation
of NF-κB, as evidenced by reduced IκBα levels and nuclear
accumulation of NF-κB p65. Additionally, there was a significant
increase in NF-κB-dependent transcriptional activity in
PROX1-overexpressing cells. Cyclin D1 and MMP-9 are two downstream
genes of NF-κB and serve important roles in tumor growth and
progression (18,19). Accompanying the activation of
NF-κB, PROX1 overexpression increased the protein expression levels
of cyclin D1 and MMP-9 in glioblastoma cells. To confirm the
involvement of NF-κB signaling in the action of PROX1, a dominant
IκBα mutant was transfected into PROX1-overexpressing U87MG cells.
Notably, overexpression of the IκBα mutant interfered with NF-κB
activation and hindered the proliferation and invasiveness of
PROX1-overexpressing cells. Taken together, these results suggested
that the oncogenic role of PROX1 in glioblastoma cells may be at
least partially mediated through the activation of the NF-κB
signaling pathway. PROX1 has been demonstrated to promote HCC
metastasis via upregulation of hypoxia-inducible factor 1α
(9). Therefore, other signaling
pathways may additionally mediate the action of PROX1 in
glioblastoma cells.
The present study has various limitations. Firstly,
overexpression studies do not fully address the pathophysiological
roles of PROX1 in GBM. Knockdown experiments may confirm the
requirement for PROX1 in the growth and progression of GBM.
Secondly, there is a lack of information surrounding the
association between PROX1 expression and NF-κB activation in GBM
patients. Finally, the underlying mechanism of the regulation of
NF-κB activation by PROX1 in glioblastoma cells remains to be fully
elucidated.
In conclusion, the results of the present study
demonstrated an oncogenic role for PROX1 in GBM. Overexpression of
PROX1 was revealed to promote the tumorigenesis and invasiveness of
glioblastoma cells, which was primarily associated with activation
of the NF-κB signaling pathway. PROX1 may represent a potential
therapeutic target for the treatment of GBM; however, additional
studies are required to confirm the impact of PROX1 in the
pathogenesis of GBM.
References
|
1
|
Fukushima T, Tezuka T, Shimomura T, Nakano
S and Kataoka H: Silencing of insulin-like growth factor-binding
protein-2 in human glioblastoma cells reduces both invasiveness and
expression of progression-associated gene CD24. J Biol Chem.
282:18634–18644. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Stupp R, Hegi ME, Mason WP, van den Bent
MJ, Taphoorn MJ, Janzer RC, Ludwin SK, Allgeier A, Fisher B,
Belanger K, et al: Effects of radiotherapy with concomitant and
adjuvant temozolomide versus radiotherapy alone on survival in
glioblastoma in a randomised phase III study: 5-year analysis of
the EORTC-NCIC trial. Lancet Oncol. 10:459–466. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Lai A, Tran A, Nghiemphu PL, Pope WB,
Solis OE, Selch M, Filka E, Yong WH, Mischel PS, Liau LM, et al:
Phase II study of bevacizumab plus temozolomide during and after
radiation therapy for patients with newly diagnosed glioblastoma
multiforme. J Clin Oncol. 29:142–148. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Zinovieva RD, Duncan MK, Johnson TR,
Torres R, Polymeropoulos MH and Tomarev SI: Structure and
chromosomal localization of the human homeobox gene Prox 1.
Genomics. 35:517–522. 1996. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Kim H, Cruz M, Bourdeau A and Dumont DJ:
Cell-cell interactions influence vascular reprogramming by Prox1
during embryonic development. PLoS One. 8:e521972013. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Sosa-Pineda B, Wigle JT and Oliver G:
Hepatocyte migration during liver development requires Prox1. Nat
Genet. 25:254–255. 2000. View
Article : Google Scholar : PubMed/NCBI
|
|
7
|
Ragusa S, Cheng J, Ivanov KI, Zangger N,
Ceteci F, Bernier-Latmani J, Milatos S, Joseph JM, Tercier S,
Bouzourene H, et al: PROX1 promotes metabolic adaptation and fuels
outgrowth of Wnt(high) metastatic colon cancer cells. Cell Rep.
8:1957–1973. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Wiener Z, Högström J, Hyvönen V, Band AM,
Kallio P, Holopainen T, Dufva O, Haglund C, Kruuna O, Oliver G, et
al: Prox1 promotes expansion of the colorectal cancer stem cell
population to fuel tumor growth and ischemia resistance. Cell Rep.
8:1943–1956. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Liu Y, Zhang JB, Qin Y, Wang W, Wei L,
Teng Y, Guo L, Zhang B, Lin Z, Liu J, et al: PROX1 promotes
hepatocellular carcinoma metastasis by way of up-regulating
hypoxia-inducible factor 1α expression and protein stability.
Hepatology. 58:692–705. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Akagami M, Kawada K, Kubo H, Kawada M,
Takahashi M, Kaganoi J, Kato S, Itami A, Shimada Y, Watanabe G and
Sakai Y: Transcriptional factor Prox1 plays an essential role in
the antiproliferative action of interferon-γ in esophageal cancer
cells. Ann Surg Oncol. 18:3868–3877. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Takahashi M, Yoshimoto T, Shimoda M, Kono
T, Koizumi M, Yazumi S, Shimada Y, Doi R, Chiba T and Kubo H: Loss
of function of the candidate tumor suppressor prox1 by RNA mutation
in human cancer cells. Neoplasia. 8:1003–1010. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Elsir T, Eriksson A, Orrego A, Lindström
MS and Nistér M: Expression of PROX1 is a common feature of
high-grade malignant astrocytic gliomas. J Neuropathol Exp Neurol.
69:129–138. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Elsir T, Qu M, Berntsson SG, Orrego A,
Olofsson T, Lindström MS, Nistér M, von Deimling A, Hartmann C,
Ribom D and Smits A: PROX1 is a predictor of survival for gliomas
WHO grade II. Br J Cancer. 104:1747–1754. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Cherry EM, Lee DW, Jung JU and Sitcheran
R: Tumor necrosis factor-like weak inducer of apoptosis (TWEAK)
promotes glioma cell invasion through induction of NF-κB-inducing
kinase (NIK) and noncanonical NF-κB signaling. Mol Cancer.
14:92015. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Han S, Xia J, Qin X, Han S and Wu A:
Phosphorylated SATB1 is associated with the progression and
prognosis of glioma. Cell Death Dis. 4:e9012013. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Siebzehnrubl FA, Silver DJ, Tugertimur B,
Deleyrolle LP, Siebzehnrubl D, Sarkisian MR, Devers KG, Yachnis AT,
Kupper MD, Neal D, et al: The ZEB1 pathway links glioblastoma
initiation, invasion and chemoresistance. EMBO Mol Med.
5:1196–1212. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Lu MH, Huang CC, Pan MR, Chen HH and Hung
WC: Prospero homeobox 1 promotes epithelial-mesenchymal transition
in colon cancer cells by inhibiting E-cadherin via miR-9. Clin
Cancer Res. 18:6416–6425. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Bera A, Ghosh-Choudhury N, Dey N, Das F,
Kasinath BS, Abboud HE and Choudhury GG: NFκB-mediated cyclin D1
expression by microRNA-21 influences renal cancer cell
proliferation. Cell Signal. 25:2575–2586. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Lee GR, Jang SH, Kim CJ, Kim AR, Yoon DJ,
Park NH and Han IS: Capsaicin suppresses the migration of
cholangiocarcinoma cells by down-regulating matrix
metalloproteinase-9 expression via the AMPK-NF-κB signaling
pathway. Clin Exp Metastasis. 31:897–907. 2014. View Article : Google Scholar : PubMed/NCBI
|