Introduction
Glioblastoma multiforme (GBM) is the most malignant
type of primary adult brain tumor (1). Patients with GBMs have a median
survival of only 12–15 months (2).
Surgery with maximum feasible resection, radiotherapy and treatment
with alkylating agent temozolomide (TMZ) are standard treatments
for GBM (3). The rapid growth and
high rate of recurrence of GBM make surgical resection non-curative
and leads to poor prognosis of this disease (4). Although TMZ has been identified to
improve the survival rates of patients with GBM, a majority
experience disease progression within one year (3). Therefore, it is important to identify
ways to promote the efficacy of TMZ for GBM.
Paired box 6 (PAX6), a transcription factor critical
for the development of the brain, is additionally expressed in the
adult brain (5). Previous studies
have demonstrated that PAX6 functions as a tumor suppressor that
markedly limits the growth of GBM cells (6). The expression level of PAX6
reportedly is inversely correlated with the tumorigenicity and
invasion of GBM cell lines and is significantly reduced in GBMs
(7,8).
MicroRNAs (miRNAs) are small non-coding RNA
molecules (containing ~22 nucleotides) that function in RNA
silencing and post-transcriptional regulation of gene expression.
miRNAs normally cleave mRNA by base-pairing to the 3′-untranslated
region (UTR) of the target genes (9). Previously, it has been identified
that miRNAs are involved in the progression and chemoresistance of
numerous types of cancer, including GBM (10,11).
Previous studies have suggested that miRNAs may be important
regulators of the therapeutic efficacy of TMZ for GBMs (12–14).
The present study aimed to investigate the potential
interaction among TMZ, PAX6 and miRNAs in GBM cells and assess its
impact on GBM cell proliferation for, to the best of our knowledge,
the first time.
Materials and methods
Cells lines and reagents
U251 and U118 human GBM cell lines were purchased
from the Cell Center of Peking Union Medical University (Beijing,
China) and American Type Culture Collection (ATCC; Manassas, VA,
USA), respectively. Human PAX6-short hairpin RNA (shRNA) plasmid
(sc-36195-SH), control shRNA plasmid (sc-108060), mouse monoclonal
anti-PAX6 (AD2, 35) (sc-53108) and mouse monoclonal anti-β-actin
(C4) (sc-47778) antibodies were purchased from Santa Cruz
Biotechnology, Inc. (Santa Cruz, CA, USA). miR-223 mimic and
miR-223 antagomir [a 20-O-methyl (20-OMe) modified antisense
oligonucleotide 5′-GGGGUAUUUGACAAACUGACA-3′] were purchased from
Shanghai GenePharma Co., Ltd. (Shanghai, China). Lipofectamine 2000
transfection reagent was purchased from Life Technologies (Thermo
Fisher Scientific, Inc., Waltham, MA, USA). The bromodeoxyuridine
(BrdU) Cell Proliferation ELISA kit (colorimetric; ab126556) was
purchased from Abcam (Cambridge, MA, USA) and the dual-luciferase
reporter assay system was purchased from Promega Corp. (Madison,
WI, USA). Human full length PAX6 cDNA clone (SC109551) was
purchased from OriGene Technologies, Inc. (Beijing, China) and
subcloned into pcDNA 3.1 expression vector (Life Technologies;
Thermo Fisher Scientific, Inc.). The PAX6-3′UTR-luciferase reporter
was generated by subcloning human PAX6 3′-UTR into the psiCheck2
vector (Promega Corp.) downstream of the Renilla luciferase
gene. miRNAs potentially able to suppress PAX6 expression were
selected using TargetScan prediction software version 6.0
(www.targetscan.org). TMZ and all
chemicals of reagent grade were purchased from Sigma-Aldrich (Merck
Millipore, Darmstadt, Germany). TMZ was dissolved in dimethyl
sulfoxide at a stock concentration of 100 mM and stored at
−20°C.
Transfection
Plasmids, miR-223 mimic and antagomir were
respectively transfected into cells using Lipofectamine 2000
transfection reagent (Life Technologies; Thermo Fisher Scientific,
Inc.) according to the manufacturer's instructions. The cells were
subject to analysis 48 h after transfection.
Western blot analysis
Cells were lysed with a hypotonic buffer containing
2% Nonidet-P and a protease inhibitor cocktail (Sigma-Aldrich;
Merck Millipore) by sonication three times for 3 sec on ice. The
supernatant obtained after centrifugation at 2,000 × g for 15 min
at 4°C was used for protein concentration determination by the
Coomassie blue method and for subsequent steps. Equal amounts of
protein (5 µg) for each sample were separated using a 10%
SDS-polyacrylamide gel and blotted onto a polyvinylidene difluoride
microporous membrane (EMD Millipore, Billerica, MA, USA). Membranes
were incubated for 1 h at room temperature with a 1:1,000 dilution
of the primary antibody and then washed and revealed using
incubation with bovine anti-mouse secondary antibody conjugated
with horseradish peroxidase conjugate (1:5,000; Santa Cruz
Biotechnology, Inc.; cat. no. sc-2371) at room temperature for 1 h.
Peroxidase was observed using a GE Healthcare ECL kit (RPN2235; GE
Healthcare Life Sciences, Shanghai, China). Three independent
experiments were performed.
Reverse transcription-quantitative
polymerase chain reaction (RT-qPCR)
RNA was prepared from cells using TRIzol reagent and
cDNAs were synthesized using SuperScript II reverse transcriptase
(Invitrogen; Thermo Fisher Scientific, Inc.). RT-qPCR was performed
on an ABI-Prism 7700 Sequence Detection system, with use of the
fluorescent dye SYBR-Green Master Mix (Applied Biosystems, Thermo
Fisher Scientific, Inc., Beijing, China) as described by the
manufacturer. The results were normalized against that of the
reference gene glyceraldehyde-3-phosphate dehydrogenase (GAPDH) in
the same sample. The primers used were as follows: Human PAX6,
5′-AGACACAGCCCTCACAAAC-3′ (forward) and 5′-ATCATAACTCCGCCCATTC-3′
(reverse); human GAPDH, 5′-GACTCATGACCACAGTCCATGC-3′ (forward) and
5′-AGAGGCAGGGATGATGTTCTG-3′ (reverse). The PCR reaction mixture
contained 12.5 µl SYBR-Green Master Mix (Thermo Fisher Scientific,
Inc.), 500 ng template cDNA, forward and reverse primers (0.25 µM
each) and 12 µl nuclease-free water (Thermo Fisher Scientific,
Inc.). The thermocycling conditions were as follows: 20 sec at
95°C; followed by 40 cycles of 5 sec at 95°C and 30 sec at 60°C.
Each experiment was repeated three times in duplicate.
Luciferase assay
Cells were transfected with the human
PAX6-3′UTR-luciferase reporter plasmid using Lipofectamine 2000
transfection reagent (Life Technologies; Thermo Fisher Scientific,
Inc.) and then cultured for 48 h. Luciferase assays were performed
with the Dual-Luciferase Reporter Assay system (Promega Corp.)
according to the manufacturer's instructions. Each experiment was
repeated three times in duplicate.
BrdU cell proliferation assay
Cells were cultured at 2×105 cells per well in
96-well tissue culture plates and treated with TMZ (400 µmol/l) for
48 h at 37°C. Cell proliferation was measured at 48 h with a
colorimetric BrdU Cell Proliferation ELISA kit (Abcam) (15,16).
BrdU was added 4 h before the end of the incubation period. The
cells were then fixed, the DNA was denatured, and BrdU content was
assessed using a monoclonal anti-BrdU antibody following the
manufacturer's instructions (Abcam). Cell proliferation was
presented as the optical density values at 450 nm. Each experiment
was repeated three times in duplicate.
TMZ chemosensitivity assay
Cells were plated in duplicate in 96-well plates at
a density of 5,000 cells per well. Transfection of plasmids,
miR-223 mimic and antagomir were performed 6 h later. After 24 h of
incubation, the medium was replaced by fresh medium with or without
various concentrations (0.1, 0.15, 0.20, 0.25, 0.3, 0.5, 1.0, 1.5,
3.0, 6.0 or 15.0 mM) of TMZ (Sigma-Aldrich; Merck Millipore). Then
cell viability was assayed 72 h later using a modified MTT assay as
previously described (17). The
half maximal inhibitory concentration (IC50) values were
defined as the concentrations resulting in a 50% reduction in
growth compared with control cell growth.
Statistical analysis
Statistical analyses were performed with SPSS for
Windows, version 10.0 (SPSS Inc., Chicago, IL, USA). All data
values were expressed as the mean ± standard deviation. Comparisons
of the means among multiple groups were performed with one-way
analysis of variance followed by post hoc pairwise comparisons
using Tukey's tests. A two-tailed P<0.05 was considered to
indicate a statistically significant difference.
Results
TMZ induces expression of PAX6 in GBM
cells
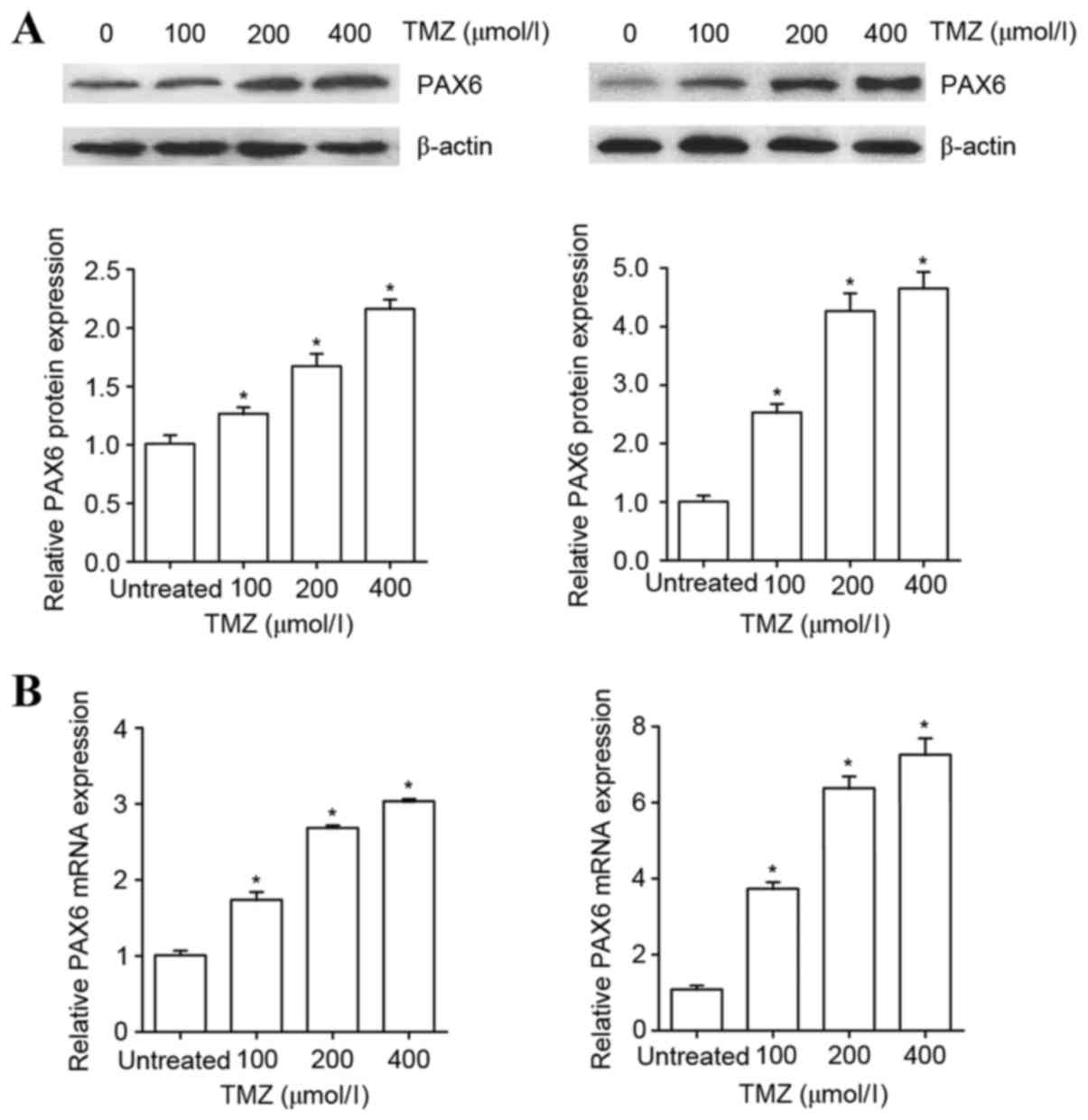
To examine the effect of TMZ on the expression of
PAX6 in GBM cells, human U251 and U118 GBM cells were treated with
TMZ in increasing concentrations (100, 200 or 400 µM) for 48 h. As
presented in Fig. 1, TMZ in the
concentration range of 100–400 µM increasingly augmented the
protein and mRNA levels of PAX6 in U251 and U118 cells within 48 h
of treatment. The results indicate that TMZ induces the expression
of PAX6 in GBM cells.
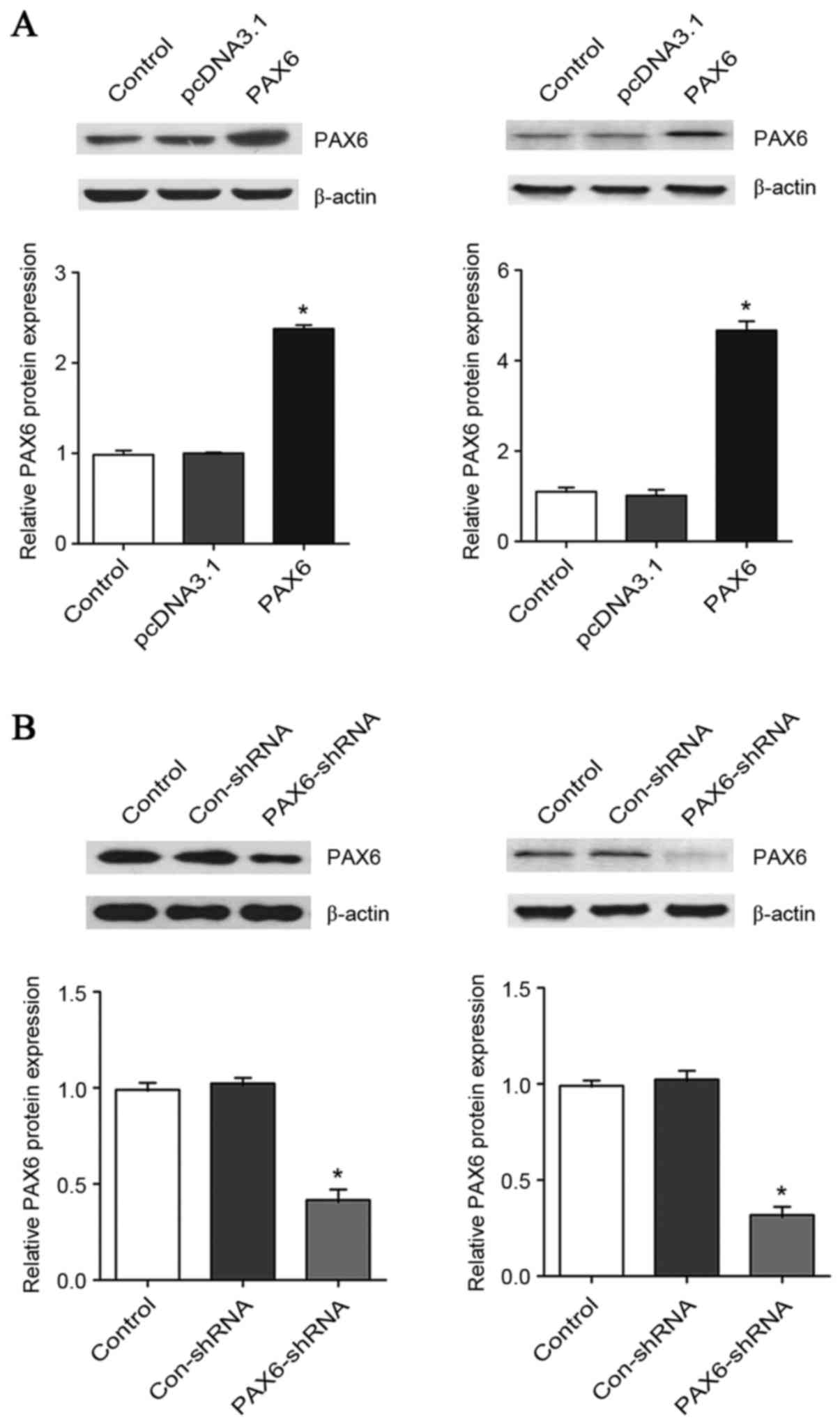
PAX6 is critical for the inhibitory
effect of TMZ on GBM cell proliferation
To explore the functional role of PAX6 in the
inhibitory effect of TMZ on GBM cell proliferation, PAX6 was
overexpressed and knocked down in U251 and U118 cells. As presented
in Fig. 2, transfection of the
cells with a PAX6 expression vector increased the protein level of
PAX6 by ~2.4-fold in U251 cells and 4.7-fold in U118 cells. On the
other hand, transfection of the cells with PAX6-shRNA plasmids
knocked down the protein level of PAX6 by ~60% in U251 cells and
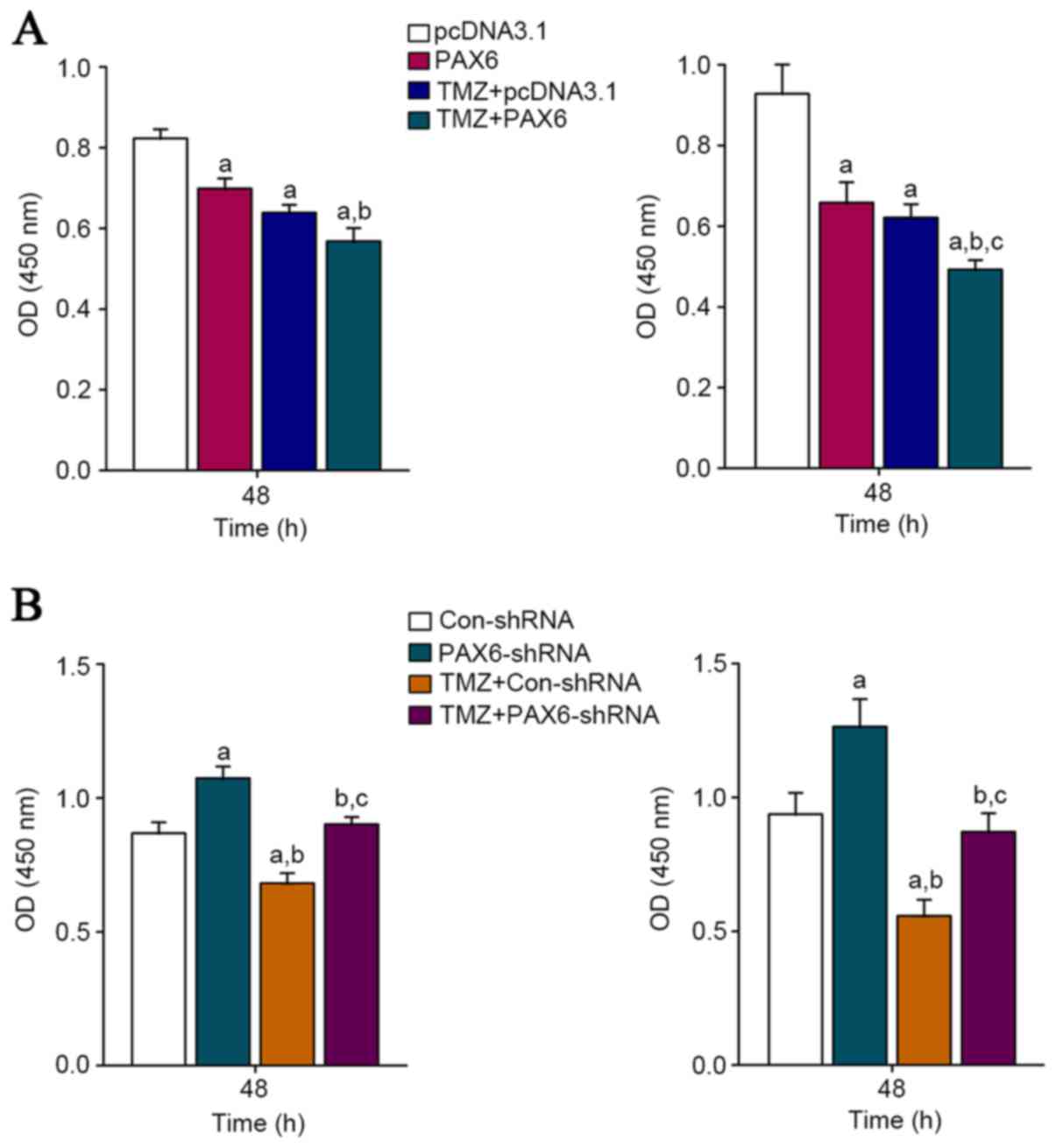
70% in U118 cells. The proliferation of U251 and U118 cells with or
without TMZ (400 µM) treatment was measured after this. BrdU cell
proliferation assays demonstrated that 48 h of TMZ treatment
significantly inhibited GBM cell proliferation in both U251 and
U118 cells (Fig. 3).
Overexpression of PAX6 increased the inhibitory effect of TMZ on
GBM cell proliferation; on the other hand, knockdown of PAX6
abolished the inhibitory effect of TMZ on GBM cell proliferation
(Fig. 3). The results suggest that
PAX6 is an essential mediator of the inhibitory effect of TMZ on
GBM cell proliferation.
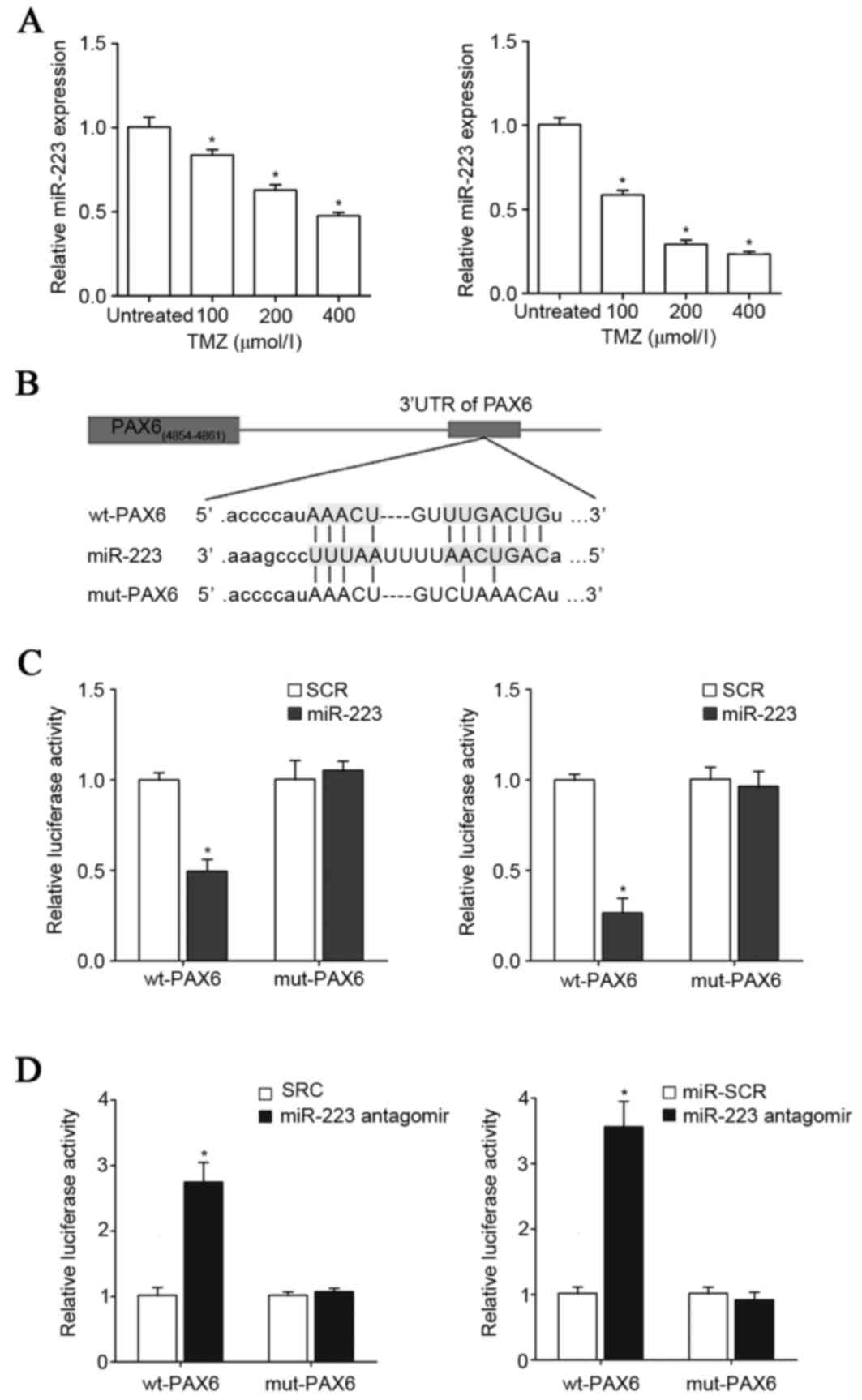
TMZ inhibits expression of miR-223,
which targets PAX6 in GBM cells
Next the TargetScan prediction software was employed
to analyze the 3′-UTR of the PAX6 gene, which revealed that
miR-223, miR-590-3p, miR-190, miR-190b and miR-7 would be most
likely to target PAX6. As presented in Fig. 4A, TMZ (100–400 µM)
concentration-dependently reduced the expression of miR-223 in U251
and U118 cells in a concentration-dependent manner within 48 h of
treatment. Compared with that of miR-223, the expression levels of
miR-590-3p, miR-190, miR-190b and miR-7 were not as significantly
regulated by TMZ in GBM cells (data not shown). Thus, the
interaction among TMZ, PAX6 and miR-223 in GBM cells was further
explored. To demonstrate a direct interaction between miR-223 and
PAX6, the 3′-UTR of the PAX6 gene was inserted downstream of the
Renilla luciferase gene in the psiCheck2 vector to generate
a wild type (wt)-PAX6-3′UTR-luciferase reporter. Meanwhile, the
potential binding sequence for miR-223 in the 3′-UTR of the PAX6
gene, as predicted by TargetScan, was mutated to generate a
mut-PAX6-3′UTR-luciferase reporter (Fig. 4B). U251 and U118 cells were
co-transfected with miR-223 mimic or antagomir together with either
the wt-PAX6-3′UTR-luciferase reporter or the mut-PAX6-luciferase
reporter. As presented in Fig. 4C and
D, compared with the scrambled control, miR-223 mimic and
antagomir decreased and increased the luciferase activity of the
wt-PAX6-3′UTR respectively, however did not affect that of the
mut-PAX6-3′UTR reporter. This suggests that miR-223 could suppress
the expression of PAX6 by directly binding to the 3′-UTR of the
PAX6 gene. RT-qPCR assays demonstrated that miR-223 mimic and
antagomir decreased and increased the mRNA levels of PAX6,
respectively, in U251 and U118 cells (Fig. 5), confirming that miR-223 targets
PAX6 in GBM cells.
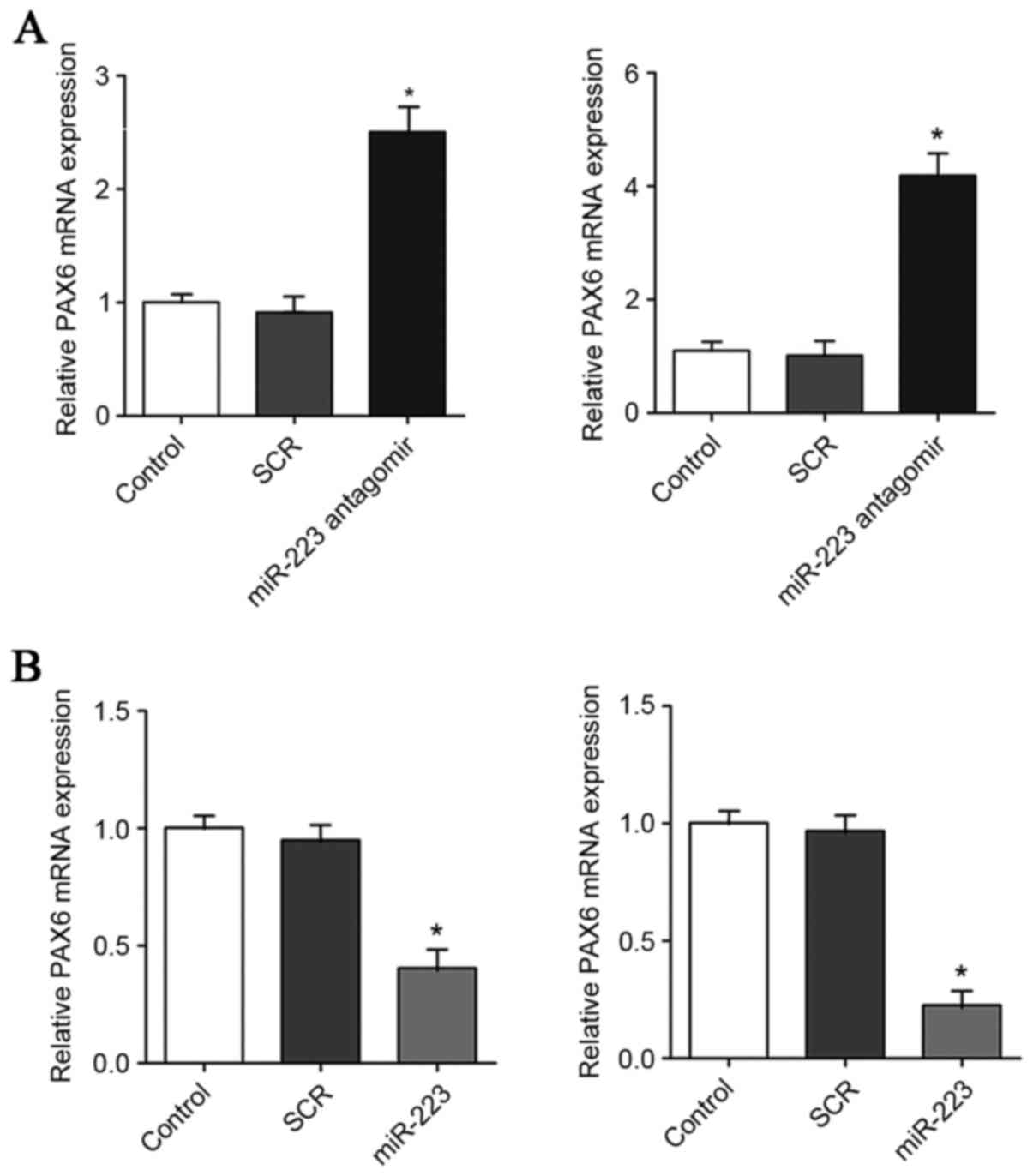 | Figure 5.Effect of miR-223 on the mRNA level of
PAX6 in GBM cells. (A) In U251 (left panel) and U118 (right panel)
GBM cells, the mRNA levels of PAX6 were determined with RT-qPCR in
control cells, cells transfected with scrambled control (SCR), and
cells transfected with miR-223 antagomir. (B) In U251 (left panel)
and U118 (right panel) GBM cells, the mRNA levels of PAX6 were
determined by RT-qPCR in control cells, cells transfected with SCR
and cells transfected with miR-223 mimic. The mRNA level of PAX6
was expressed as fold changes to that of control (designated as 1).
*P<0.05 vs. control. PAX6, paired box 6; GBM, glioblastoma
multiforme; RT-qPCR, reverse transcription-quantitative polymerase
chain reaction; SCR, scrambled control; miR, miRNA. |
TMZ/miR-223 signaling inhibits GBM
cell proliferation through inducing expression of PAX6
The above observations indicate that TMZ inhibits
the expression of miR-223, which targets PAX6 in GBM cells. To
explore the effect of TMZ/miR-223/PAX6 signaling on GBM cell
proliferation, it was first examined how TMZ/miR-223 signaling
would regulate the expression of PAX6 in GBM cells. In the absence
of TMZ treatment, compared with scrambled control, miR-223 mimic
decreased the expression of PAX6 by approximately 60% in U251 cells
and 70% in U118 cells, while miR-223 antagomir increased the
expression of PAX by approximately 2.1-fold in U251 cells and
2.7-folds in U118 cells (Fig. 6).
In the presence of TMZ treatment (400 µM for 48 h), compared with
scrambled control, miR-223 mimic decreased the expression of PAX6
by approximately 45% in U251 cells and 75% in U118 cells, while
miR-223 antagomir increased the expression of PAX by approximately
2.5-fold in U251 cells and 3.5-fold in U118 cells (Fig. 6). Regardless of TMZ treatment,
overexpression and knockdown of PAX6 respectively abolished the
effects of miR-223 mimic and antagomir (Fig. 6). In the presence of TMZ treatment
(400 µM for 48 h), compared with the controls, miR-223 mimic
increased proliferation by approximately 1.3-fold in U251 cells and
1.6-fold in U118 cells, while miR-223 antagomir decreased
proliferation by approximately 30% in U251 cells and 43% in U118
cells (Fig. 7). Overexpression and
knockdown of PAX6, respectively, abolished the effects of miR-223
mimic and antagomir (Fig. 7).
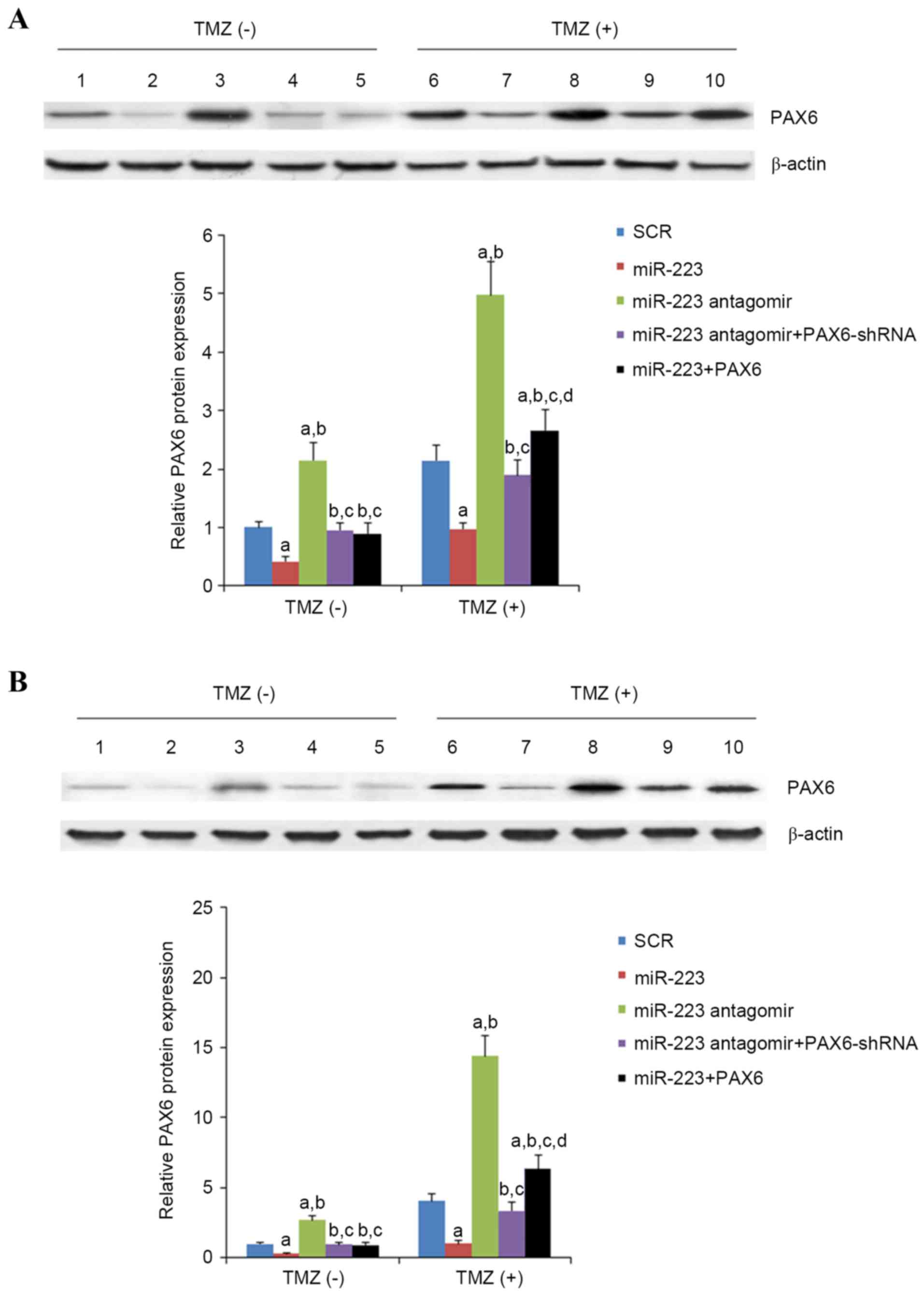 | Figure 6.Effect of TMZ/miR-223 signaling on the
protein level of PAX6 in GBM cells. In (A) U251 and (B) U118 GBM
cells with or without TMZ treatment (400 µmol/l) for 48 h, the
protein levels of PAX6 were determined with western blot analysis
in cells transfected with SCR (lanes 1 and 6), cells transfected
with miR-223 mimic (lanes 2 and 7), cells transfected with miR-223
antagomir (lanes 3 and 8), cells co-transfected with miR-223
antagomir and PAX6-shRNA (lanes 4 and 9) and cells co-transfected
with miR-223 mimic and PAX6 (lanes 5 and 10). β-actin blotting was
used as a loading control. Density of the PAX6 blot was normalized
against that of the β-actin blot to obtain a relative blot density,
which was then expressed as fold changes to that of cells
transfected with SCR without TMZ treatment (designated as 1).
aP<0.05 vs. SCR; bP<0.05 vs. miR-223;
cP<0.05 vs. miR-223 antagomir; dP<0.05
vs. miR-223 antagomir + PAX6-shRNA. TMZ, temozolomide; miR, miRNA;
PAX6, paired box 6; GBM, glioblastoma multiforme; SCR, scrambled
control; shRNA, short hairpin RNA. |
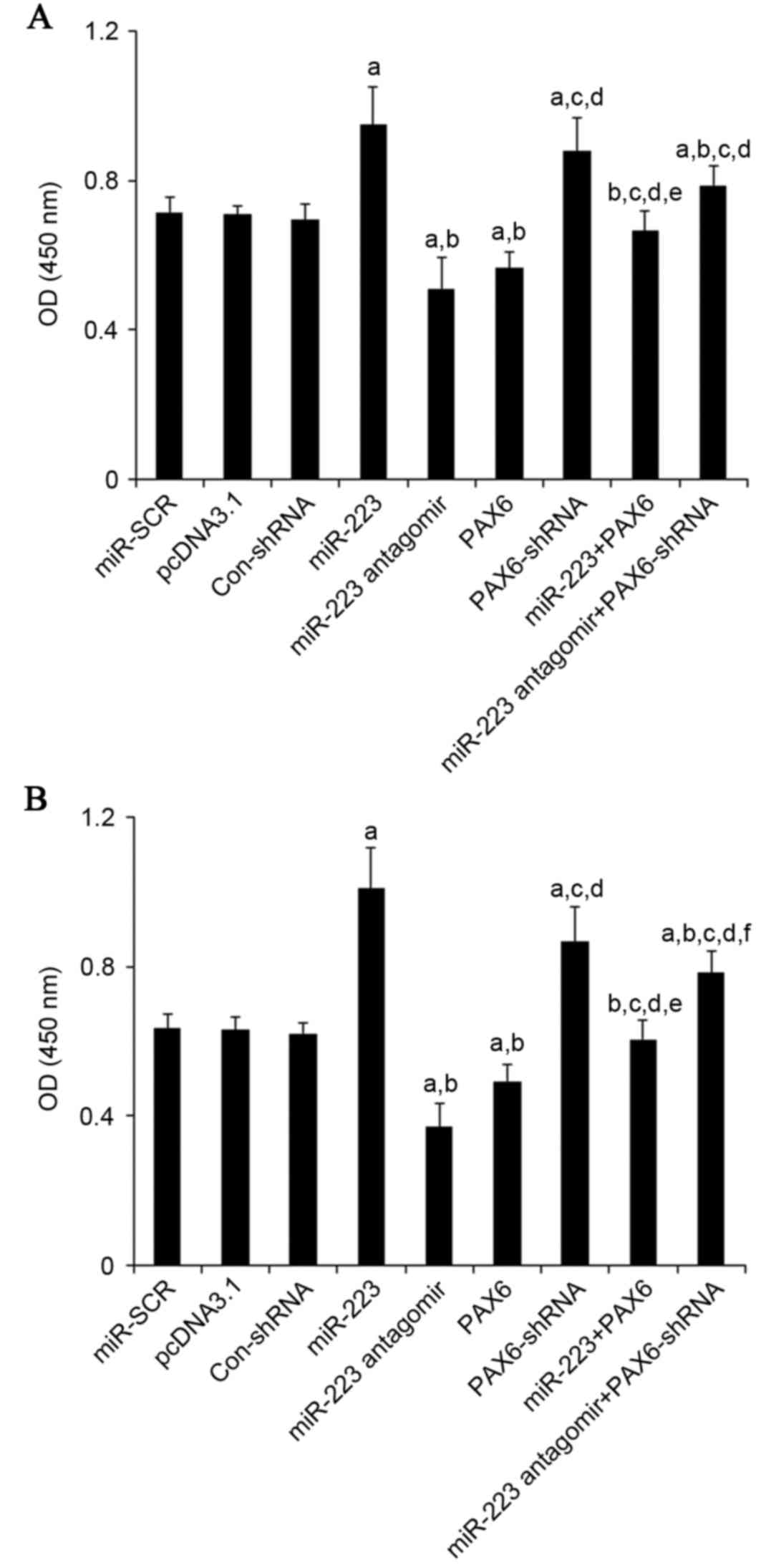 | Figure 7.Effect of TMZ/miR-223/PAX6 signaling
on proliferation of GBM cells. In (A) U251 and (B) U118 GBM cells
with TMZ treatment (400 µM) for 48 h, cell proliferation was
measured at 48 h of culture with a microplate reader-based BrdU
cell proliferation ELISA kit in cells transfected with SCR, cells
transfected with the empty pcDNA3.1 plasmid, cells transfected with
Con-shRNA, cells transfected with miR-223 mimic, cells transfected
with miR-223 antagomir, cells transfected with PAX6, cells
transfected with PAX6-shRNA, cells co-transfected with miR-223
mimic and PAX6 and cells co-transfected with miR-223 antagomir and
PAX-shRNA. Cell proliferation was indicated by OD values at 450 nm.
aP<0.05 vs. controls (SCR, pcDNA3.1 and Con-shRNA);
bP<0.05 vs. miR-223; cP<0.05 vs.
miR-223 antagomir; dP<0.05 vs. PAX6;
eP<0.05 vs. PAX6-shRNA; fP<0.05 vs.
miR-223 + PAX6. TMZ, temozolomide; miR, miRNA; PAX6, paired box 6;
GBM, glioblastoma multiforme; SCR, scrambled control; Con, control;
shRNA, short hairpin RNA; OD, optical density. |
Inhibition of miR-223/PAX6 signaling
increases TMZ chemoresistance in GBM cells
To explore the effect of inhibition of miR-223/PAX6
signaling on TMZ chemoresistance in GBM cells, TMZ IC50
values were examined. A higher IC50 value was considered
to correspond with clinical chemoresistance to TMZ. U251 and U118
GBM cells were plated in 96-well plates. Transfection of plasmids,
miR-223 mimic and antagomir were performed 6 h later. After 24 h of
incubation, the medium was replaced by fresh medium with or without
various concentrations of TMZ, and cell viability was assayed 72 h
later. As presented in Fig. 8, the
TMZ IC50 values in U251 and U118 cells were 240 and 970
µM, respectively; miR-223 mimics increased the TMZ IC50
to 2,549 and 8,900 µM, respectively, which was abolished by
overexpression of PAX6. On the other hand, inhibiting miR-223 with
antagomir decreased TMZ IC50 to 45 and 170 µM in U251
and U118 cells, respectively, which was abolished by knockdown of
PAX6 (Fig. 8).
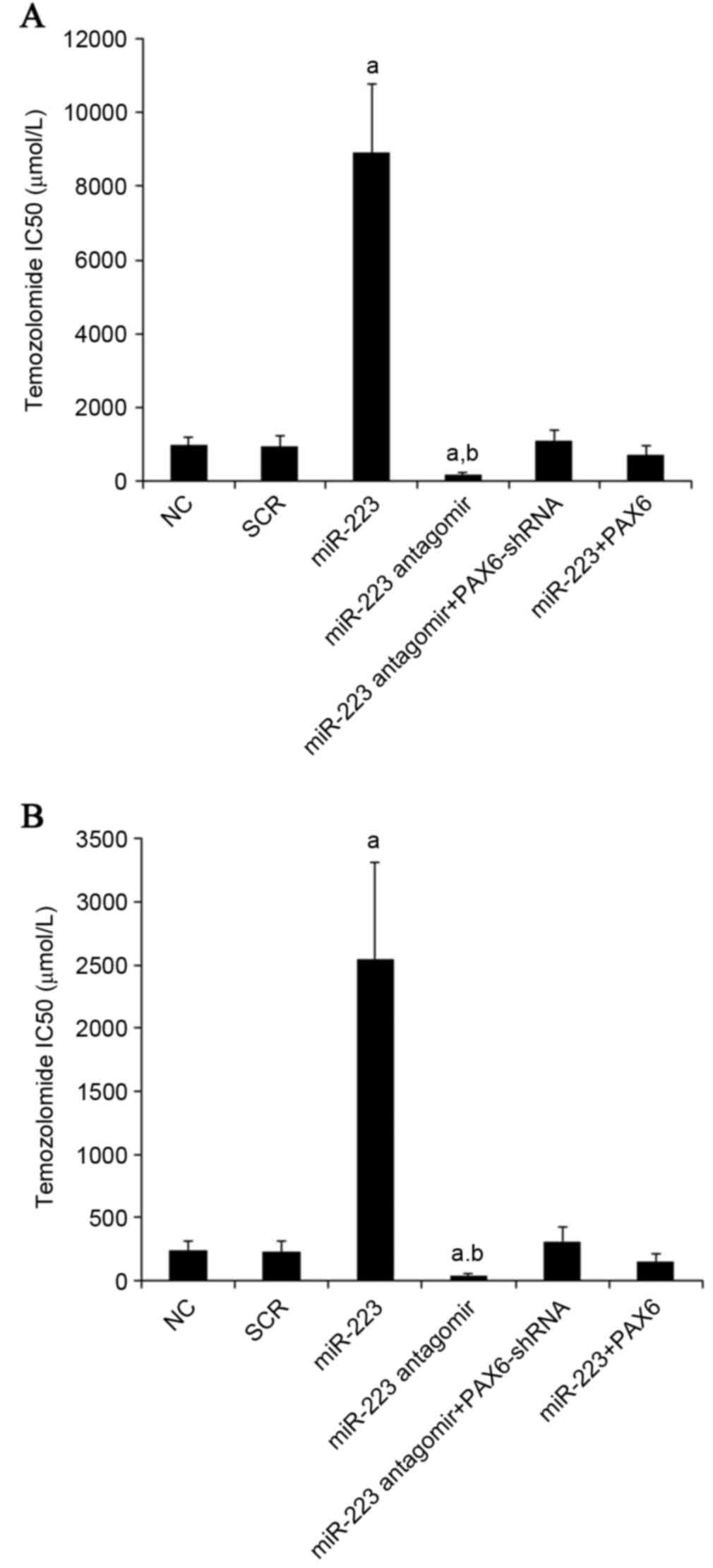 | Figure 8.Effect of miR-223/PAX6 signaling on
TMZ chemoresistance in GBM cells. (A) U251 and (B) U118 GBM cells
were plated into 96-well plates. Transfection of plasmids, miR-223
mimic and antagomir were performed 6 h later. After 24 h of
incubation, the medium was replaced by fresh medium with or without
various concentrations of TMZ, and cell viability was assayed 72 h
later. The half maximal inhibitory concentrations (IC50)
of TMZ were determined in NC cells, cells transfected with SCR,
cells transfected with miR-223 mimic, cells transfected with
miR-223 antagomir, cells co-transfected with miR-223 antagomir and
PAX6-shRNA and cells co-transfected with miR-223 mimic and PAX6.
aP<0.05 vs. NC and SCR; bP<0.05 vs.
miR-223. miR, miRNA; PAX6, paired box 6; TMZ, temozolomide; NC,
normal control; SCR, scrambled control. |
Discussion
GBM is a fatal adult brain tumor (18), and the prognosis of GBM remains
poor despite advances in surgery, chemotherapy and radiotherapy
(2,4). It is common that patients with GBM
show innate or acquired resistance to TMZ, a standard
chemotherapeutic agent for GBM (3). PAX6 is reported as an independent
prognostic marker for GBM (5).
There is accumulating evidence that PAX6 functions as a tumor
suppressor and suppresses growth of GBM cells (6). A recent study has demonstrated that
miR-223 promotes the growth and invasion of GBM cells by targeting
PAX6 (8). The present study
confirms that miR-223 directly targets PAX6 in GBM cells.
Nevertheless, the highlight of the present study is providing the
first evidence that TMZ inhibits the proliferation of GBM cells by
increasing the expression of PAX6 through inhibiting miR-223. In
addition, it demonstrates that inhibiting miR-223 can markedly
decrease TMZ chemoresistance in GBM cells, suggesting that
miR-223/PAX6 signaling could be a potential target for overcoming
TMZ chemoresistance in GBM.
In the present study, two GBM cell lines (U251 and
U118) were used as the cell models. In both cell lines, TMZ
concentration-dependently decreased the expression of miR-223,
which led to increased expression of PAX6 and decreased
proliferation of GBM cells. The TMZ/miR-223/PAX signaling axis
provides novel insight into the pharmacological mechanisms of TMZ.
The molecular mechanism underlying TMZ-induced inhibition of
miR-223 requires further investigation.
TMZ IC50 was employed as a measure of TMZ
chemoresistance in GBM cells. An increased IC50 was
considered to correspond with clinical chemoresistance to TMZ.
Overexpression and inhibition of miR-223, respectively, decreased
and increased the expression of PAX6, which markedly altered TMZ
IC50 by ~1 order of magnitude in GBM cells. In the
presence of TMZ, miR-223 antagomir significantly enhanced
TMZ-induced inhibition on GBM cell proliferation and decreased TMZ
chemoresistance, suggesting that inhibition of miR-223 may be a
potential novel strategy to enhance the therapeutic effects of TMZ
on GBM. The effects of miR-223 antagomir was respectively abolished
and enhanced by knockdown and overexpression of PAX6, confirming
that miR-223 promotes GBM resistance to TMZ predominantly by
downregulating PAX6, or miR-223 antagomir decreases TMZ
chemoresistance by upregulating PAX6.
miR-223 is a highly conserved miRNA, that was
originally identified to be crucial for myeloid differentiation of
progenitor cells (19). The
expression levels of miR-223 are reportedly decreased in chronic
lymphocytic leukemia (CLL) and could predict treatment-free
survival and overall survival for CLL (20). It is also commonly repressed in
hepatocellular carcinoma (21). A
previous study has demonstrated that miR-223 inhibits non-small
cell lung cancer cell proliferation and invasion, suggesting that
it functions as a tumor suppressor (22). On the other hand, miR-223 is
upregulated in bladder cancer (23) and esophageal squamous cell
carcinoma (24), and it has been
identified to promote tumor cell proliferation and invasion in
gastric cancer and GBM (8,25). In this study, it is demonstrated
that miR-223 antagonizes the inhibitory effects of TMZ on GBM cells
by inhibiting the expression of PAX6. Taken together, the
observations suggest that miR-223 likely serves a dual role in
cancer malignancy and chemoresistance, depending on tissue
specificity, and possibly, tissue-specific expression of PAX6. Due
to the fact that PAX6 is expressed in the eye, brain and pancreas
in healthy adults (5), the
exploration of the role of miR-223/PAX6 signaling in the
pathogenesis and chemoresistance of retinoblastoma and pancreatic
cancer besides GBM may be beneficial.
Similar to that of several other solid tumor types,
GBM is considered to be driven by a small sub-population of cells
known as glioma stem cells (26).
miR-223 reportedly functions as an essential regulator of human
embryonic stem cell differentiation (27). Previous studies have indicated that
PAX6 serves an important role in maintaining retinal stem cell
properties (28) and is
overexpressed in cancer stem-like cells in retinoblastoma (29), suggesting that PAX6 may be closely
involved in maintenance of cancer stem cells. Thus, the examination
of the role of miR-223/PAX6 signaling in the biology and
chemoresistance of glioma stem cells may be beneficial in future
studies.
In conclusion, the present study demonstrates that
TMZ inhibits GBM cell proliferation by inhibiting the expression of
miR-223, which leads to increased expression of tumor suppressor
PAX6. While overexpression of miR-223 increases TMZ
chemoresistance, inhibition of miR-223 with antagomir markedly
decreases TMZ chemoresistance in GBM cells. The present study
provides novel insight into the molecular mechanisms underlying the
pharmacological effects and chemoresistance of TMZ for GBM.
References
|
1
|
Zhang Z and Lin CC: Taking advantage of
neural development to treat glioblastoma. Eur J Neurosci.
40:2859–2866. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Wen PY and Kesari S: Malignant gliomas in
adults. N Engl J Med. 359:492–507. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
van den Bent MJ, Hegi ME and Stupp R:
Recent developments in the use of chemotherapy in brain tumours.
Eur J Cancer. 42:582–588. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Giese A, Bjerkvig R, Berens ME and
Westphal M: Cost of migration: Invasion of malignant gliomas and
implications for treatment. J Clin Oncol. 21:1624–1636. 2003.
View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Zhou YH, Tan F, Hess KR and Yung WK: The
expression of PAX6, PTEN, vascular endothelial growth factor, and
epidermal growth factor receptor in gliomas: Relationship to tumor
grade and survival. Clin Cancer Res. 9:3369–3375. 2003.PubMed/NCBI
|
|
6
|
Zhou YH, Wu X, Tan F, Shi YX, Glass T, Liu
TJ, Wathen K, Hess KR, Gumin J, Lang F and Yung WK: PAX6 suppresses
growth of human glioblastoma cells. J Neurooncol. 71:223–229. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Mayes DA, Hu Y, Teng Y, Siegel E, Wu X,
Panda K, Tan F, Yung WK and Zhou YH: PAX6 suppresses the
invasiveness of glioblastoma cells and the expression of the matrix
metalloproteinase-2 gene. Cancer Res. 66:9809–9817. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Huang BS, Luo QZ, Han Y, Li XB, Cao LJ and
Wu LX: microRNA-223 promotes the growth and invasion of
glioblastoma cells by targeting tumor suppressor PAX6. Oncol Rep.
30:2263–2269. 2013.PubMed/NCBI
|
|
9
|
Ma R, Jiang T and Kang X: Circulating
microRNAs in cancer: Origin, function and application. J Exp Clin
Cancer Res. 31:382012. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Jones KB, Salah Z, Del Mare S, Galasso M,
Gaudio E, Nuovo GJ, Lovat F, LeBlanc K, Palatini J, Randall RL, et
al: miRNA signatures associate with pathogenesis and progression of
osteosarcoma. Cancer Res. 72:1865–1877. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Hermansen SK and Kristensen BW: MicroRNA
biomarkers in glioblastoma. J Neurooncol. 114:13–23. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Shi L, Chen J, Yang J, Pan T, Zhang S and
Wang Z: MiR-21 protected human glioblastoma U87MG cells from
chemotherapeutic drug temozolomide induced apoptosis by decreasing
Bax/Bcl-2 ratio and caspase-3 activity. Brain Res. 1352:255–264.
2010. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Zhang S, Wan Y, Pan T, Gu X, Qian C, Sun
G, Sun L, Xiang Y, Wang Z and Shi L: MicroRNA-21 inhibitor
sensitizes human glioblastoma U251 stem cells to chemotherapeutic
drug temozolomide. J Mol Neurosci. 47:346–356. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Wang J, Sai K, Chen FR and Chen ZP:
miR-181b modulates glioma cell sensitivity to temozolomide by
targeting MEK1. Cancer Chemother Pharmacol. 72:147–158. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Mesti T, Savarin P, Triba MN, Le Moyec L,
Ocvirk J, Banissi C and Carpentier AF: Metabolic impact of
anti-angiogenic agents on U87 glioma cells. PLoS One. 9:e991982014.
View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Olivares S, Green RM and Henkel AS:
Endoplasmic reticulum stress activates the hepatic activator
protein 1 complex via mitogen activated protein kinase-dependent
signaling pathways. PLoS One. 9:e1038282014. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Ding X, Zhang Z, Li S and Wang A:
Combretastatin A4 phosphate induces programmed cell death in
vascular endothelial cells. Oncol Res. 19:303–309. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Louis DN, Ohgaki H, Wiestler OD, Cavenee
WK, Burger PC, Jouvet A, Scheithauer BW and Kleihues P: The 2007
WHO classification of tumours of the central nervous system. Acta
Neuropathol. 114:97–109. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Fazi F, Rosa A, Fatica A, Gelmetti V, De
Marchis ML, Nervi C and Bozzoni I: A minicircuitry comprised of
microRNA-223 and transcription factors NFI-A and C/EBPalpha
regulates human granulopoiesis. Cell. 123:819–831. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Stamatopoulos B, Meuleman N, Haibe-Kains
B, Saussoy P, Van Den Neste E, Michaux L, Heimann P, Martiat P,
Bron D and Lagneaux L: microRNA-29c and microRNA-223
down-regulation has in vivo significance in chronic lymphocytic
leukemia and improves disease risk stratification. Blood.
113:5237–5245. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Wong QW, Lung RW, Law PT, Lai PB, Chan KY,
To KF and Wong N: MicroRNA-223 is commonly repressed in
hepatocellular carcinoma and potentiates expression of Stathmin1.
Gastroenterology. 135:257–269. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Nian W, Ao X, Wu Y, Huang Y, Shao J, Chen
Z, Chen F and Wang D: miR-223 functions as a potent tumor
suppressor of the Lewis lung carcinoma cell line by targeting
insulin-like growth factor-1 receptor and cyclin-dependent kinase
2. Oncol Lett. 6:359–366. 2013.PubMed/NCBI
|
|
23
|
Gottardo F, Liu CG, Ferracin M, Calin GA,
Fassan M, Bassi P, Sevignani C, Byrne D, Negrini M, Pagano F, et
al: Micro-RNA profiling in kidney and bladder cancers. Urol Oncol.
25:387–392. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Kurashige J, Watanabe M, Iwatsuki M,
Kinoshita K, Saito S, Hiyoshi Y, Kamohara H, Baba Y, Mimori K and
Baba H: Overexpression of microRNA-223 regulates the ubiquitin
ligase FBXW7 in oesophageal squamous cell carcinoma. Br J Cancer.
106:182–188. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Li X, Zhang Y, Zhang H, Liu X, Gong T, Li
M, Sun L, Ji G, Shi Y, Han Z, et al: miRNA-223 promotes gastric
cancer invasion and metastasis by targeting tumor suppressor
EPB41L3. Mol Cancer Res. 9:824–833. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Singh SK, Hawkins C, Clarke ID, Squire JA,
Bayani J, Hide T, Henkelman RM, Cusimano MD and Dirks PB:
Identification of human brain tumour initiating cells. Nature.
432:396–401. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Yu YH, Zhang L, Wu DS, Zhang Z, Huang FF,
Zhang J, Chen XP, Liang DS, Zeng H and Chen FP: MiR-223 regulates
human embryonic stem cell differentiation by targeting the
IGF-1R/Akt signaling pathway. PLoS One. 8:e787692013. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Bhattacharya S, Das A, Mallya K and Ahmad
I: Maintenance of retinal stem cells by Abcg2 is regulated by notch
signaling. J Cell Sci. 120:2652–2662. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Ma B, Lei X, Guan Y, Mou LS, Yuan YF, Yue
H, Lu Y, Xu GT and Qian J: Maintenance of retinal cancer stem
cell-like properties through long-term serum-free culture from
human retinoblastoma. Oncol Rep. 26:135–143. 2011.PubMed/NCBI
|