Introduction
Allergic rhinitis (AR) is a common global health
problem, which has a severe affect on daily life. The morbidity
rate of AR has increased in previous decades and affects 10–20% of
the population in western countries (1). A report from 2014 from Beijing
Tongren Hospital on the prevalence of allergic rhinitis in China
showed that the morbidity rate of AR in China is also increasing,
as is the prevalence of a ‘western’-type lifestyle (2).
AR has been identified as a chronic inflammatory
disease of the nasal mucosa, which is characterized by symptoms,
including sneezing, watery rhinorrhea, nasal obstruction and nasal
itching. Eosinophils have long been considered to be the prominent
effective cells in allergic inflammation, and eosinophilia has been
suggested to favor the development of allergy (3–5).
Although the mechanisms underlying the pathogenesis and regulation
of AR have been thoroughly investigated, current treatments can
only relieve its symptoms. There is currently no treatment method
able to cure AR, therefore, additional approaches are required for
AR treatment. Genetic therapy offers a promising approach in
treating patients with AR.
In response to a variety of stimuli, eosinophils are
released from bone marrow to inflammatory tissues through cell
surface receptors (4). In
particular, the CC chemokine receptor 3 (CCR3), which is a
cell-surface guanosine-binding protein-coupled receptor containing
a typical motif of seven hydrophobic regions, is primarily
expressed on the cell surface of eosinophils. It has been reported
that CCR3 is activated in response to eotaxin and contributes to
G-protein-dependent intracellular signaling cascades, which leads
to the migration of eosinophils (6,7). The
importance of CCR3 signaling in allergy was demonstrated previously
in studies involving CCR3-deficient mice, which exhibited reduced
Th2 responses and an absence of eosinophilia upon allergen
sensitization and challenge (8,9). In
addition, previous studies have suggested that anti-CCR3 antibody
inhibits eosinophil infiltration in animal models and human samples
(10,11). Thus, the direct inhibition of CCR3
may serve as a novel approach to effectively alleviate eosinophilia
in AR.
The first case of RNA interference was reported in
Caenorhabditis elegans as an endogenous defense mechanism by Fire
et al in 1998 (12). RNA
interference is an effective gene silencing method, achieved
through the transduction of either small interfering RNA (siRNA) or
short hairpin RNA (shRNA) (13).
Using siRNA or shRNA, rather than oligonucleotide antisense and
antibody inhibition, appears to be a more efficient and
long-lasting approach to inhibit certain cellular functions due to
its ability to target mRNA and affect protein expression in cells
(14). Synthetic siRNAs can reduce
gene expression, however, this is transient and dose-dependent. By
contrast, shRNA can be continuously expressed in cells and then
processed by Dicer into siRNA targeting desired genes (15). shRNA carried by a lentivirus can
integrate into the host genome and silence gene expression
permanently (16). In the present
study, the shRNA and lentiviral delivery approach was used for the
construction of a mouse CCR3-shRNA-expressing lentiviral vector.
CCR3 gene silencing is able to reduce the proliferation of
eosinophils and promote eosinophil apoptosis, thereby reducing
eosinophil infiltration, and alleviating the symptoms of allergic
rhinitis. Therefore, the present study evaluated the effects of
this vector on the proliferation and apoptosis of eosinophils.
Materials and methods
Animals
Male BALB/c mice (5–6-week-old) were maintained on
an ovalbumin-free diet under pathogen-free conditions in our animal
experimental institute (the Medical Laboratory Animal Center of
Nanchang University) at room temperature (22–24°C) with a 12-h
dark:light cycle. The study protocol was approved by the
institutional Animal Care and Use Committees of Nanchang University
School of Medicine (Nanchang, China). The present study was
performed in accordance with the ethical guidelines of Directive
2010/63/EU (Comments on the European Directive 2010/63/EU for the
Protection of Laboratory Animals - see http://ec.europa.eu/environment/chemicals/lab_animals/legislation_en.htm).
Culture of bone marrow-derived
eosinophils
The eosinophils originating from bone marrow
pluripotent hematopoietic stem cells were collected from the femurs
and tibias of wild-type BALB/c mice (Laboratory Animal Centre of
Nanchang University School of Medicine), as described previously
(17). Briefly, the BALB/c mice
were sacrificed by cervical dislocation. The separated femurs and
tibias were soaked in 75% ethanol for 5 min, rinsed with 2X
phosphate-buffered saline (PBS) and then the ends of the femurs and
tibias were cut off. The bone marrow was flushed out with
Dulbecco's modified Eagle's medium (DMEM) and collected in a plate.
A single cell suspension of the bone marrow was obtained by
filtering the bone marrow through a syringe with size 7 and size 4
needles. Red blood cell pyrolysis liquid was added to the single
cell suspension to eliminate red blood cells, and the single cell
suspension was centrifuged at 1,500 rev./min for 10 min. The
supernatant was discarded. A cell layer containing eosinophils and
eosinophil stem cells was cultured in eosinophil basic culture
medium, RPMI 1640 medium (Hyclone; GE Healthcare Life Sciences,
Logan, UT, USA), which was supplemented with 20% fetal bovine serum
(FBS; Hyclone; GE Healthcare Life Sciences), 2 mM L-glutamine
(Hyclone; GE Healthcare Life Sciences), 50 µM β-mercaptoethanol
(Gibco; Thermo Fisher Scientific, Inc., Waltham, MA, USA), 10 µg/ml
streptomycin, 100 IU/ml penicillin (Hyclone; GE Healthcare Life
Sciences), 25 mM HEPES, 1 mM sodium pyruvate and 1X non-essential
amino acids (Gibco; Thermo Fisher Scientific, Inc.). Following
resuspension, the cells were cultured with the above medium pulsed
with 100 ng/ml FMS-related tyrosine kinase 3 ligand (FLT3-L;
PeproTech, Inc., Rocky Hill, NJ, USA) and 100 ng/ml stem-cell
factor (SCF; PeproTech, Inc.) for 4 days. On day 4, 10 ng/ml
recombinant mouse interleukin-5 (rmIL-5; PeproTech, Inc.) was added
to replace the FLT3-L and SCF. The cells were then cultured in
medium containing rmIL-5 for 10 days. All cell cultures
(107/ml) were incubated at 37°C in humidified air with
5% CO2. The eosinophils were identified by hematoxylin
and eosin staining for subsequent viral infection.
Construction of the murine CCR3
(mCCR3)-shRNA-expressing lentiviral vector
The mCCR3 sequence was obtained from GeneBank
(accession no. NM_009914.4). Our previous studies revealed that the
formed shuttle plasmid (pLVX-mCCR3-1+2+3+4-shRNA) had a more marked
effect on silencing the CCR3 gene (18,19).
In the present study, four short tandemly arranged fragments of
CCR3 shRNA were subcloned into lentiviruses to form
pLVX-mCCR3-1+2+3+4-shRNA (18).
This lentiviral vector can deliver a substantial quantity of viral
RNA into the DNA of the host cell, and this viral RNA can be then
integrated into the DNA of host cells. According to the mCCR3
sequence, four pairs of primers of CCR3 shRNA were designed to
amplify four CCR3 shRNA fragments by polymerase chain reaction
(PCR). The primer sequences are summarized in Table I. The PCR reaction comprised (in a
total volume of 50 µl): 5 µl 10X short hairpin (sh)DNA annealing
buffer, 5 µl sense and antisense strands (100 µM) and 35 µl double
distilled H2O. The PCR cycling steps were 95°C for 5 min; 85°C for
5 min; 75°C for 5 min and 70°C for 5 min; and samples were stored
at 4°C.
 | Table I.Primers for CCR3 short hairpin RNA
amplification. |
Table I.
Primers for CCR3 short hairpin RNA
amplification.
| mCCR3-1F |
5′-CACCGGTTGTGTTGATCCTCATAAATTCAAGAGATTTATGAGGATCAACACAACCTTTTTTG-3′ |
| mCCR3-1R |
5′-AGCTCAAAAAAGGTTGTGTTGATCCTCATAAATCTCTTGAATTTATGAGGATCAACACAACC-3′ |
| mCCR3-2F |
5′-TTTGGCTGACAATTGACAGATACCTTTCAAGAGAAGGTATCTGTCAATTGTCAGCTTTTTTG-3′ |
| mCCR3-2R |
5′-AGCTCAAAAAAGCTGACAATTGACAGATACCTTCTCTTGAAAGGTATCTGTCAATTGTCAGC-3′ |
| mCCR3-3F |
5′-CCTCGCAGCATTGCCTGAATTTATCTTCAAGAGAGATAAATTCAGGCAATGCTGCTTTTTTG-3′ |
| mCCR3-3R |
5′-AGCTCAAAAAAGCAGCATTGCCTGAATTTATCTCTCTTGAAGATAAATTCAGGCAATGCTGC-3′ |
| mCCR3-4F |
5′-TCCCGACCACACCCTATGAATATGATTCAAGAGATCATATTCATAGGGTGTGGTCTTTTTTG-3′ |
| mCCR3-4R |
5′-AGCTCAAAAAAGACCACACCCTATGAATATGATCTCTTGAATCATATTCATAGGGTGTGGTC-3′ |
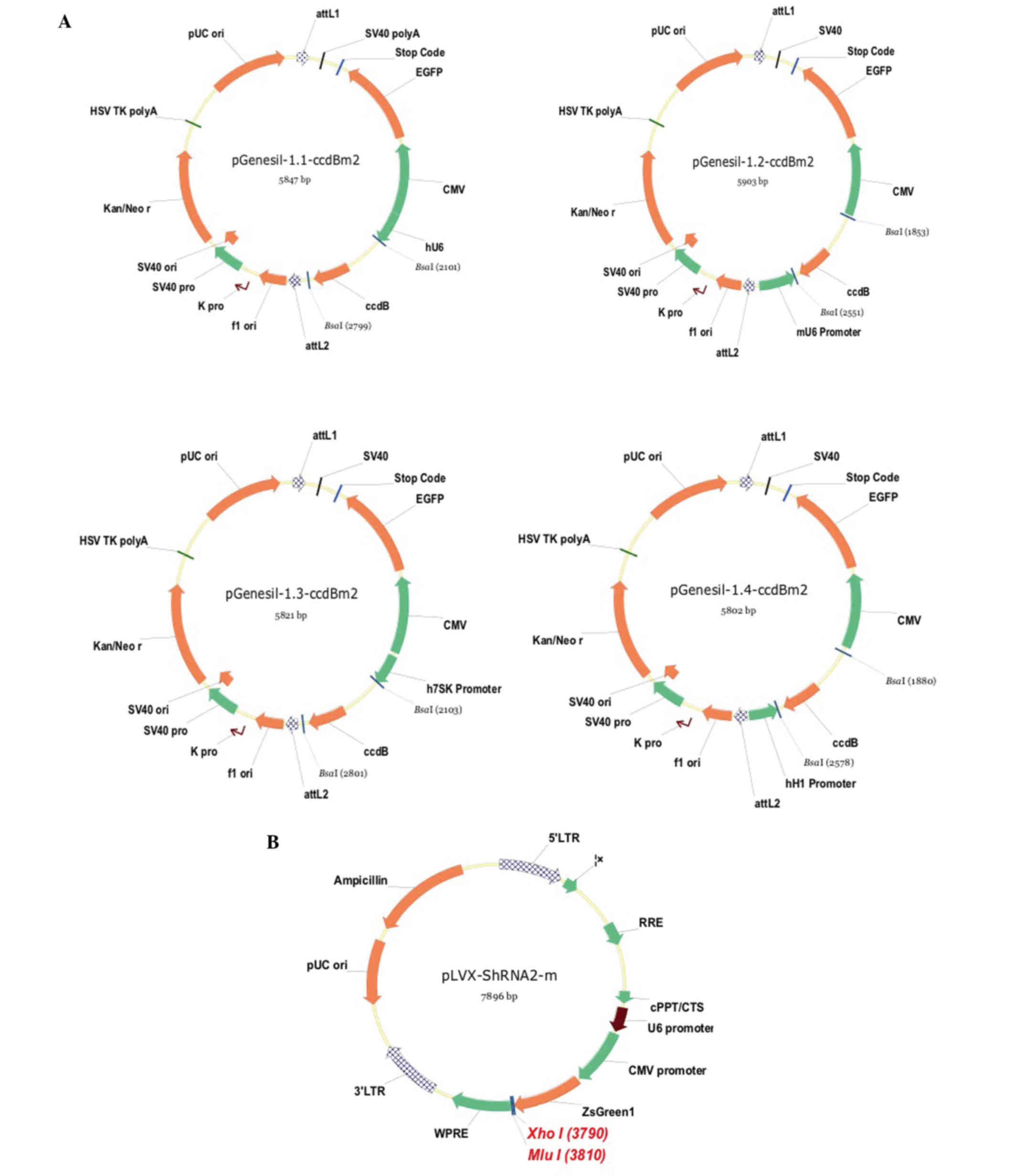
pGenesil1.1, pGenesil1.2, pGenesil1.3 and
pGenesil1.4 were used as vectors for subcloning the four fragments
described above. The restriction enzyme, BsaI (New England BioLabs,
Inc., Ipswich, MA, USA), was utilized to cut pGenesil1.1 (bp
2101–2799), pGenesil1.2 (bp 1853–2551), pGenesil1.3 (bp 2103–2801)
and pGenesil1.4 (bp 1880–2578). The four fragments of CCR3 shRNA
were ligated to the four cut pGenesil vectors using T4DNA ligase
separately. The pGenesil vector containing the mCCR3 shRNA was then
transformed into recombinant cells, replicated as the recombinant
cells proliferated and was extracted from the recombinant cells
using a DNA extraction kit. Sequence correct pGenesil-mCCR3-1-shRNA
and pGenesil-mCCR3-2-shRNA were cut using HindIII and BamH I
enzymes. The large cut fragment of pGenesil-mCCR3-1-shRNA
containing the promoter and mCCR3-1-shRNA was ligated with the
small fragment of pGenesil-mCCR3-2-shRNA, (280 bp mCCR3-2-shRNA),
to construct pGenesil-mCCR3-1+2-shRNA. Similarly, sequence correct
pGenesil-mCCR3-3-shRNA and pGenesil-mCCR3-4-shRNA were cut using
EcoRI and SalI. The large cut fragment of pGenesil-mCCR3-3-shRNA
containing the promoter and mCCR3-3-shRNA was ligated with the
small cut fragment of pGenesil-mCCR3-4-shRNA (380 bp mCCR3-4-shRNA)
to form pGenesil-mCCR3-3+4-shRNA. pGenesil-mCCR3-1+2-shRNA and
pGenesil-mCCR3- 3+4-shRNA were cut using BamH I and SalI. The large
cut fragment of pGenesil-mCCR3-1+2-shRNA containing the promoter
and mCCR3-1+2-shRNA was ligated with the small cut fragment of
pGenesil-mCCR3-3+4-shRNA to form pGenesil-mCCR3-1+2+3+4-shRNA. To
construct the lentiviral mCCR3-1+2+3+4-shRNA, a pLVX-shRNA2-m
lentiviral vector was used. The pLVX-ShRNA2-m vector (Biowit
Technologies Ltd., Quincy, MA, USA) was cut using MluI and XhoI
enzymes. pGenesil-mCCR3-1+2+3+4-shRNA were cut using MulI and SalI
enzymes. The large fragment of the cut pLVX-shRNA2-m containing a
promotor was ligated with the small fragment of the cut
pGenesil-mCCR3-1+2+3+4-shRNA, to form the target plasmid,
pLVX-mCCR3-1+2+3+4-shRNA. Validation of the sequences of
mCCR3-1+2+3+4-shRNA were confirmed using DNA sequencing and are
shown in Table II.
 | Table II.Sequences of four shRNAs for plasmid
construction |
Table II.
Sequences of four shRNAs for plasmid
construction
| mCCR3-1 shRNA |
5′-GGTTGTGTTGATCCTCATAAA-3′ |
| mCCR3-2 shRNA |
5′-GCTGACAATTGACAGATACCT-3′ |
| mCCR3-3 shRNA |
5′-GCAGCATTGCCTGAATTTATC-3′ |
| mCCR3-4 shRNA | 5′-
GACCACACCCTATGAATATGA-3 |
Packaging of the pLVX-ShRNA2-m vector
and the constructed pLVX-mCCR3-1+2+3+4-shRNA plasmid, and the
culture of eosinophils with viral infection
To ensure the safety and the titer of the
pLVX-ShRNA2-m vector and the constructed pLVX-mCCR3-1+2+3+4-shRNA,
these two lentiviruses were separately co-transfected with the
packaging plasmids, Baculo p35, pCMV R8.2 and VSV (quantities: 2 µg
Baculo p35, 2 µg VSV plasmid, 4.7 µg pCMV R8.2 plasmid and 2.3 µg
Lentiviral vector), into 293T cells in 100-mm tissue culture dishes
of DMEM containing 10% FBS without antibiotics at 37°C. The medium
was replaced 24 h later, and the virus-containing medium was
harvested 48 h following transduction. The supernatants were
filtered through a 0.22 µm syringe filter (EMD Millipore,
Billerica, MA, USA). The eosinophil cultures on day 10 were
infected with the supernatants at a multiplicity of infection of
50, and polybrene (Sigma-Aldrich; Thermo Fisher Scientific, Inc.)
was added to a final concentration of 8 µg/ml. The harvested
eosinophils were transfected with either a blank control (RPMI 1640
medium), empty vector (pLVX-shRNA2-m) or the constructed target
plasmid (pLVX-mCCR3-1+2+3+4-shRNA), respectively. The culture
medium was aspirated 48 h following transduction and the cells were
washed with PBS for the subsequent quantitative (q)PCR and western
blot analyses, and terminal deoxynucleotidyl transferase dUTP nick
end labeling (TUNEL) and
3-(4,5-dimethylthiazol-2-yl)-5-(3-carboxymethoxyphenyl)-2-(4-sulfophenyl)-2H-tetrazolium
(MTT) assays.
qPCR analysis
The eosinophils were suspended in TRIzol
(Invitrogen; Thermo Fisher Scientific, Inc.) and RNA was extracted
according to the manufacturer's protocol. The RNA was converted
into cDNA using a High Capacity cDNA Reverse Transcription kit
(Applied Biosystems; Thermo Fisher Scientific, Inc.) according to
the manufacturer's protocol. The cDNA was used with
SYBR® Green qPCR SuperMix (Invitrogen; Thermo Fisher
Scientific, Inc.). For the detection of CCR3, GAPDH was used as a
control. The primers of CCR3 (ID: NM_009914.4) and GAPDH (ID:
NM_017008.4) were designed as follows: CCR, forward 5′-CTG GCA CAC
AGA CCC TAG AA-3′ and reverse 5′-TTG AGT CTC TGA ACG CAT CA-3′; and
GAPDH, forward 5′-GGC CTC CAA GGA GTA AGA AA-3′ and reverse 5′-GCC
CCT CCT GTT ATT ATG G-3′. The total reaction mixture was run on a
7500 Real-Time system (Applied Biosystems; Thermo Fisher
Scientific, Inc.) with relative quantitation according to the
manufacturer's protocol. The following thermocycling steps were
used: 95°C denaturation for 10 sec, one cycle; 95°C denaturation
for 5 sec; 54°C annealing extension for 30 sec, a total of 40
cycles; 95°C for 1 min, one cycle, and 55°C for 30 sec, 41
cycles.
Western blot analysis
The eosinophils were homogenized in RIPA lysis
buffer (Pierce Biotechnology, Inc., Rockford, IL, USA). After 20
min on ice, insoluble materials were removed by centrifugation at
4°C at 14,000 × g. The supernatants were mixed with SDS sample
buffer and boiled for 5 min. The proteins were separated on
SDS-polyacrylamide (10%) gels, following which they were blotted
onto PVDF membranes (EMD Millipore). Non-specific protein binding
sites were blocked by incubation with 5% bovine serum albumin in
TBST buffer (20 mM Tris-HCl, 137 mM NaCl and 0.05% Tween 20) at pH
7.6 for 1 h, followed by incubation with rabbit polyclonal
anti-CCR3 primary antibody (cat. no. AJ1417a; 1:200 dilution; Santa
Cruz Biotechnology, Inc.) overnight at 4°C. The membranes were
washed three times with TBST, followed by incubated with
horseradish peroxidase-conjugated goat anti-rabbit IgG secondary
antibody (cat. no. C1508; 1:20,000 dilution; SouthernBiotech,
Burmingham, AL, USA) for 1 h at room temperature. The blots were
visualized using an enhanced chemiluminescence system (GE
Healthcare Life Sciences) according to the manufacturer's protocol.
To normalize for protein content, the blots were stripped and
stained with GAPDH antibody (cat. no. KC-5G5; 1:10,000 dilution;
Abcam, Cambridge, MA, USA) overnight at 4°C. The concentration of
each target protein was normalized against that of GAPDH.
TUNEL assay
Quantitative assessment of apoptosis in the
eosinophils was performed using a TUNEL method according to the
manufacturer's protocol (Promega, Madison, WI, USA). Briefly, the
eosinophils were incubated with either the blank control (RPMI 1640
medium), empty vector (pLVX-shRNA2-m) or the constructed target
plasmid (pLVX-mCCR3-1+2+3+4-shRNA), respectively, for 48 h. The
cells were then trypsinized, fixed with 4% paraformaldehyde and
permeabilized with 0.1% Triton-X-100 in 0.1% sodium citrate.
Following washing with PBS, the cells were incubated with the
reaction mixture for 60 min at 37°C. The stained cells were then
analyzed using a FACScan cytometer (BD Biosciences, Franklin Lakes,
NJ, USA).
Cell proliferation assay
The eosinophils were plated at a density of
1×105 per well (100 µl) in 96-well plates and treated
with either blank control (RPMI 1640 medium), empty vector
(pLVX-shRNA2-m) or the constructed target plasmid
(pLVX-mCCR3-1+2+3+4-shRNA) for different durations (0, 24, 48 and
72 h). The culture media were then removed and the cells were
washed with PBS. An MTS assay was performed using a kit from
Promega in accordance with the manufacturer's protocol. The
absorbance was measured at a fixed wavelength of 490 nm on a
microplate reader (VersaMax; Molecular Devices, LLC, Sunnyvale, CA,
USA). Each data point was normalized to the value of their
corresponding control samples.
Statistical analysis
The results obtained from the blank control, empty
vector (pLVX-shRNA2-m) and constructed target plasmid
(pLVX-mCCR3-1+2+3+4-shRNA) groups were analyzed using one-way
analysis of variance using SPSS 18 software (SPSS, Inc., Chicago,
IL, USA). Each experiment was repeated three times. Data are
presented as the mean ± standard error of the mean of triplicate
samples. P<0.05 was considered to indicate a statistically
significant difference.
Results
Construction of the mCCR3-shRNA
plasmid
As described in the previous section,
mCCR3-1+2+3+4-shRNA, the sequence of which was confirmed using DNA
sequencing, was successfully ligated using a sub-cloning technique
with the pGenesil1 vectors, pGenesil1.1, pGenesil1.2, pGenesil1.3
and pGenesil1.4 (Fig. 1A). The
recombinant fragment of the mCCR3-1+2+3+4-shRNA was successfully
inserted into the pLVX-shRNA2-m lentiviral vector, to obtain the
pLVX-CCR3-1+2+3+4-shRNA vector (Fig.
1B). This novel lentiviral vector was then packaged into 293T
cells by co-transfection with the Baculo p35, pCMV R8.2 and VSV
packaging plasmids. In a pilot experiment, the highest transduction
efficiency of the pLVX-mCCR3-1+2+3+4-shRNA virus was observed at a
multiplicity of infection of 10.
Detection of the expression levels of
mCCR3 in eosinophils
The preliminary experiment demonstrated that the
pLVX-mCCR3-1+2+3+4-shRNA, which contained four different
interfering shRNAs against mCCR3, had higher gene silencing
efficiency, compared with any single shRNA of mCCR3 in the
eosinophils, determined using qPCR. As shown in Fig. 2A, the mRNA level of mCCR3 was
significantly inhibited only by transduction with
pLVX-mCCR3-1+2+3+4-shRNA. The mRNA levels of mCCR3 were not
affected by transduction with the negative control shRNA vector
(Fig. 2A). In addition, the
protein level of mCCR3 was markedly inhibited by transduction with
pLVX-mCCR3-1+2+3+4-shRNA, as determined using western blot
analysis. Similarly, the protein expression of mCCR3 was not
altered by transduction with the negative control shRNA vector
(Fig. 2B).
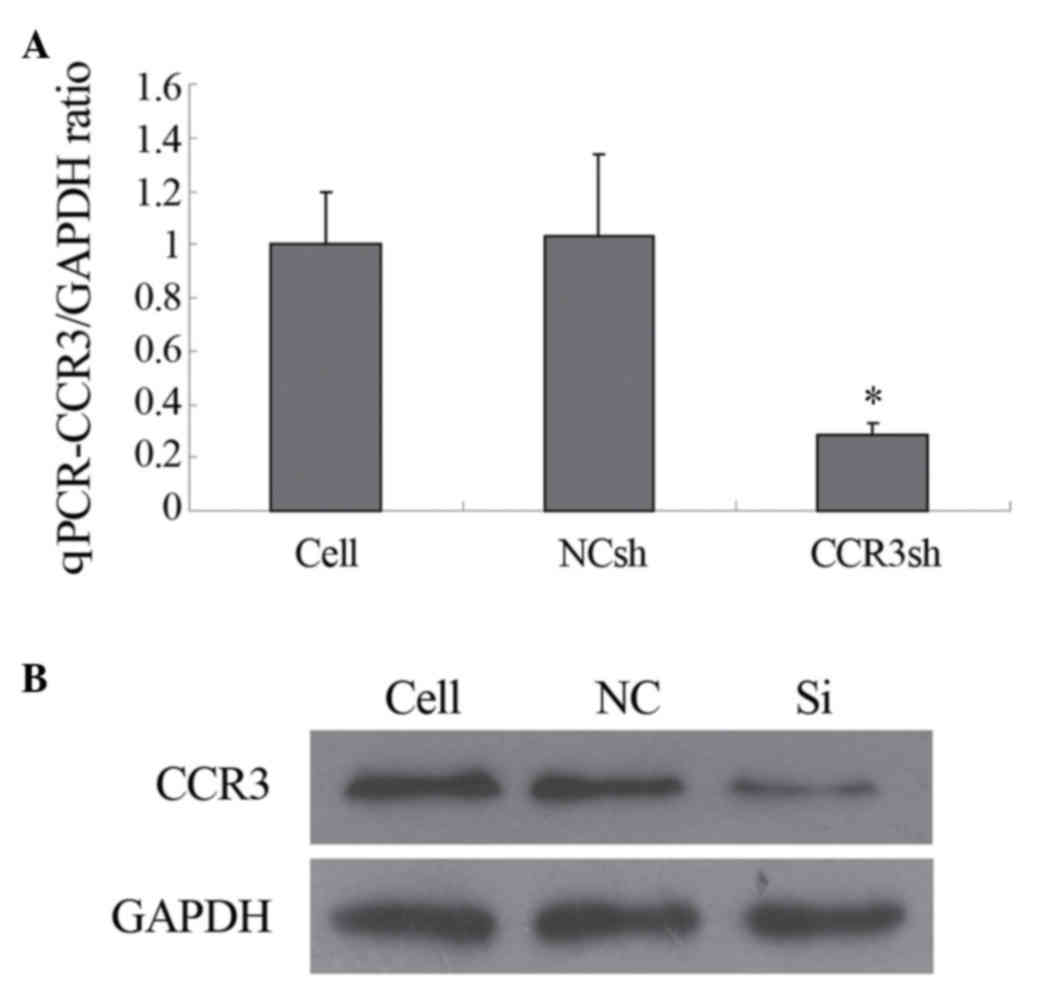 | Figure 2.Expression of CCR3. (A) qPCR
determination of cellular CCR3/GAPDH ratio. The expression of
CCR3mRNA was significantly inhibited by
pLVX-shRNA2-mCCR3-1+2+3+4shRNA, but not by the NCsh vector
(*P<0.05). (B) Expression of CCR3, determined using western blot
analysis. GAPDH, a house-keeping protein was used as a control. The
expression of CCR3 was significantly inhibited by
pLVX-shRNA2-mCCR3-1+2+3+4shRNA, but not by NC. CCR3, murine CC
chemokine receptor 3; shRNA, short hairpin RNA; Cell, blank control
(cells only); NCsh/NC, cells transfected with empty vector;
CCR3sh/Si, cells transfected with pLVX-shRNA2-mCCR3-1+2+3+4shRNA;
qPCR, quantitative polymerase chain reaction. |
Silencing of mCCR3 with lentiviral
shRNA promotes apoptosis of eosinophils
To investigate whether the downregulation of mCCR3
with lentiviral shRNA can induce apoptosis of eosinophils, the
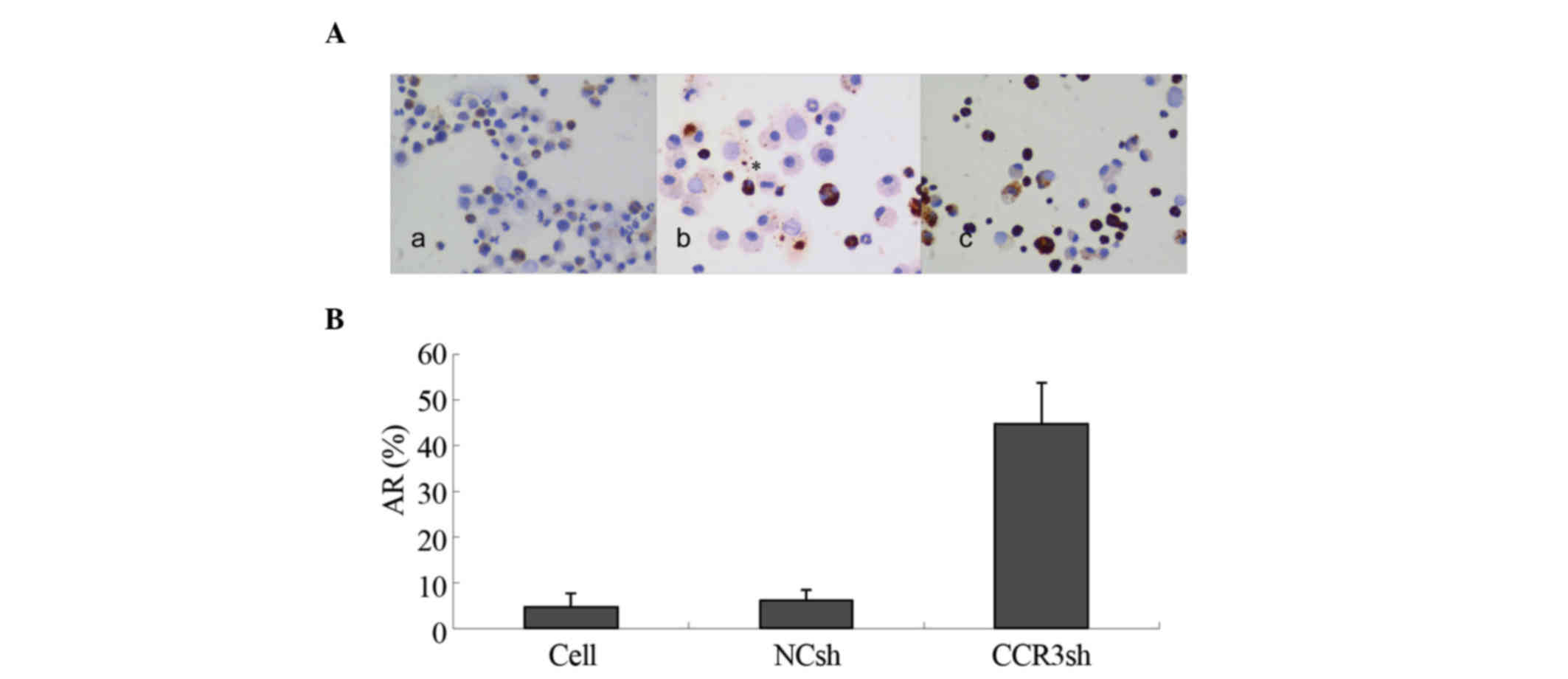
TUNEL method was used. The results showed that <8% of the
eosinophils showed apoptosis in the blank control- and empty
vector-transduced cells. However, 45% of the
pLVX-mCCR3-1+2+3+4shRNA-transduced eosinophils exhibited apoptotic
characteristics (Fig. 3A and B).
This result suggested that expression of mCCR3 was critical to the
survival of the eosinophils.
Silencing of mCCR3 with lentiviral
shRNA reduces the proliferation of eosinophils
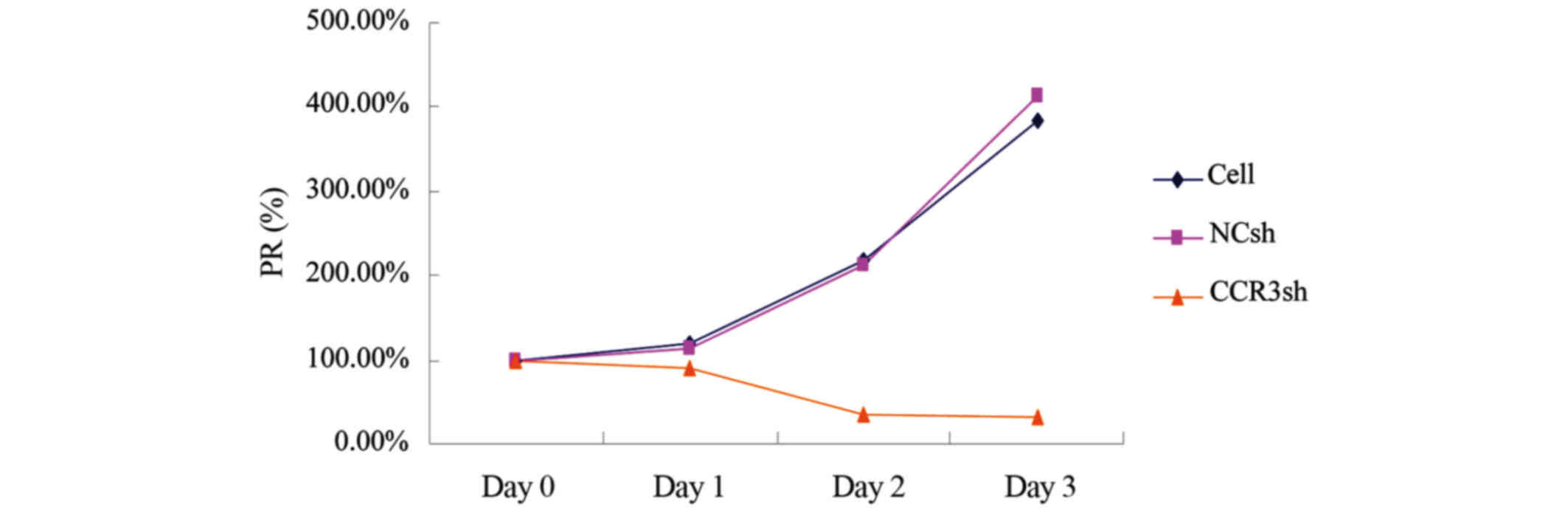
The present study used an MTS assay to investigate
the effect of mCCR3-shRNA on the proliferation of eosinophils. The
cells were incubated with MTS reagents 0, 24, 48 and 72
post-transduction with virons carrying pLVX-mCCR3-1+2+3+4-shRNA. As
shown in Fig. 4, transduction with
pLVX-mCCR3-1+2+3+4-shRNA started to inhibit cell proliferation at
24 h (80%), and the cell proliferation rate decreased to 20% at 48
h and to 19% at 72 h (Fig. 4). By
contrast, cell proliferation rates in the blank control group and
empty vector control group were increased, reaching 200% at 48 h
and 400% at 72 h (Fig. 4).
Discussion
Eosinophils are considered to be a critical factor
in the induction of inflammation and allergy by releasing reactive
oxygen species and cytotoxic molecules, including major basic
protein, eosinophilic peroxidase, eosinophil-derived neurotoxin and
eosinophil cationic protein (5).
Eosinophils develop from CD34+ hematopoietic progenitor cells
within the bone marrow under the stimulation of cytokines,
including granulocyte-macrophage colony-stimulating factor
(GM-CSF), IL-3 and IL-5 (20).
IL-5 is predominantly expressed in white blood cells and is a key
modulatory cytokine, which is important in regulating the
proliferation, differentiation and activation of eosinophils
(21). Allergic IL-5-deficiency in
mice leads to reduced numbers of eosinophilia in the bone marrow
and blood, and eosinophils are recruited to the tissue in reduced
numbers in response to allergen exposure. However, treating
patients with anti-IL-5 monoclonal antibody only partially reduced
eosinophilia in airway tissues and bone marrow (22,23),
suggesting that other factors contribute to eosinophil survival in
these tissues. With the exception of IL-5, GM-CSF and IL-3 have
also been known to have growth factor effects on eosinophils
(20). The present study revealed
that knockdown of CCR3 by specific shRNA efficiently inhibited
eosinophil proliferation and promoted eosinophil apoptosis.
Although the mechanism underlying these effects were not
investigated, there are several possible mechanisms. The FBS used
in the culture medium contains the eosinophil-associated
growth-factors IL-5, IL-3 and GM-CSF. As CCR3 protein was expressed
in the eosinophils in control group, IL-5-, IL-3- and
GM-CSF-induced eosinophil growth may require CCR3 in its growth
signaling pathway. When CCR3 was silenced by CCR3 shRNA, the growth
pathway involving IL-5, IL-3 and GM-CSF was not active, therefore,
the proliferation rate of the eosinophils declined rapidly. In
terms of why silencing CCR3 resulted in apoptosis, CCR3 may be
associated with factors involved in the apoptotic signaling
pathway, including p53, p73, B cell lymphoma-2-associated X protein
(BAX), phorbol-12-myristate-13-acetate-induced protein 1 (Noxa) and
p53 upregulated modulator of apoptosis (PUMA). The present study
hypothesized that CCR3 inhibits the above factors and inhibits
apoptosis, and when CCR3 was silenced by CCR3 shRNA, the above
factors in the apoptotic signaling pathway were activated, causing
eosinophils to undergo apoptosis.
In conclusion, using the techniques described,
pLVX-mCCR3-1+2+3+4shRNA was successfully constructed in the present
study. The results demonstrated that virions
pLVX-mCCR3-1+2+3+4shRNA significantly reduced the mRNA and protein
expression levels of CCR3, promoted eosinophil apoptosis and
inhibited eosinophil proliferation. This may have contributed to
the inhibition of eosinophil infiltration in the airway. However,
the mechanism underlying the eosinophil apoptosis and proliferation
inhibition induced by CCR3 silencing requires further
investigation. An understanding of the fundamental causes of
regulating eosinophil apoptosis may lead to novel strategies for
the treatment of allergic inflammation. Subsequent investigations
aim to use a single shRNA-expressing lentiviral vector targeting
IL-5 and CCR3 to affect eosinophil infiltration in the airway
tissues in vitro and in vivo.
Acknowledgements
The authors would like to thank the Molecular
Biology Center in Jiangxi province for its continuing support. This
study was supported by grants from the National Natural Science
Foundation of China (grant no. 81060084), the Jiangxi Provincial
Natural Science Foundation (grant no. 2010GZY0251) and the Jiangxi
Provincial Department of Science and Technology project (grant no.
20133BBG70071).
References
|
1
|
Brozek JL, Bousquet J, Baena-Cagnani CE,
Bonini S, Canonica GW, Casale TB, van Wijk RG, Ohta K, Zuberbier T
and Schünemann HJ: Allergic rhinitis and its impact on asthma
(ARIA) guidelines: 2010 revision. J Allergy Clin Immunol.
126:466–476. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Zhang Y and Zhang L: Prevalence of
allergic rhinitis in China. Allergy Asthma Immunol Res. 6:105–113.
2014. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Plaut M and Valentine MD: Clinical
practice. Allergic rhinitis. N Engl J Med. 353:1934–1944. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Weller PF: The immunobiology of
eosinophils. N Engl J Med. 324:1110–1118. 1991. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Stone KD, Prussin C and Metcalfe DD: IgE,
mast cells, basophils, and eosinophils. J Allergy Clin Immunol.
125(2 Suppl 2): S73–S80. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Ponath PD, Qin S, Post TW, Wang J, Wu L,
Gerard NP, Newman W, Gerard C and Mackay CR: Molecular cloning and
characterization of a human eotaxin receptor expressed selectively
on eosinophils. J Exp Med. 183:2437–2448. 1996. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Sallusto F, Mackay CR and Lanzavecchia A:
Selective expression of the eotaxin receptor CCR3 by human T helper
2 cells. Science. 277:2005–2007. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Fulkerson PC, Fischetti CA, McBride ML,
Hassman LM, Hogan SP and Rothenberg ME: A central regulatory role
for eosinophils and the eotaxin/CCR3 axis in chronic experimental
allergic airway inflammation. Proc Natl Acad Sci USA.
103:16418–16423. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Pope SM, Zimmermann N, Stringer KF, Karow
ML and Rothenberg ME: The eotaxin chemokines and CCR3 are
fundamental regulators of allergen-induced pulmonary eosinophilia.
J Immunol. 175:5341–5350. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Chuang CC, Su KE, Chen CW, Fan CK, Lin FK,
Chen YS and Du WY: Anti-CCR3 monoclonal antibody inhibits
eosinophil infiltration in Angiostrongylus cantonensis-infected ICR
mice. Acta Trop. 113:209–213. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Heath H, Qin S, Rao P, Wu L, LaRosa G,
Kassam N, Ponath PD and Mackay CR: Chemokine receptor usage by
human eosinophils. The importance of CCR3 demonstrated using an
antagonistic monoclonal antibody. J Clin Invest. 99:178–184. 1997.
View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Fire A, Xu S, Montgomery MK, Kostas SA,
Driver SE and Mello CC: Potent and specific genetic interference by
double-stranded RNA in Caenorhabditis elegans. Nature. 391:806–811.
1998. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
DeVincenzo JP: The promise, pitfalls and
progress of RNA-interference-based antiviral therapy for
respiratory viruses. Antivir Ther. 17:213–225. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Karimi MH, Ebadi P, Pourfathollah AA,
Moazzeni M, Soheili ZS and Samiee S: Comparison of three techniques
for generation of tolerogenic dendritic cells: siRNA,
oligonucleotide antisense and antibody blocking. Hybridoma
(Larchmt). 29:473–480. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Paddison PJ, Caudy AA, Bernstein E, Hannon
GJ and Conklin DS: Short hairpin RNAs (shRNAs) induce
sequence-specific silencing in mammalian cells. Genes Dev.
16:948–958. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Rubinson DA, Dillon CP, Kwiatkowski AV,
Sievers C, Yang L, Kopinja J, Rooney DL, Zhang M, Ihrig MM, McManus
MT, et al: A lentivirus-based system to functionally silence genes
in primary mammalian cells, stem cells and transgenic mice by RNA
interference. Nat Genet. 33:401–406. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Dyer KD, Moser JM, Czapiga M, Siegel SJ,
Percopo CM and Rosenberg HF: Functionally competent eosinophils
differentiated ex vivo in high purity from normal mouse bone
marrow. J Immunol. 181:4004–4009. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Zhu XH, Liao B, Wang XY, Liu K and Liu YH:
Construction and identification of mouse eosinophils CCR3gene RNA
interference lentiviral vector. Zhonghua Er Bi Yan Hou Tou Jing Wai
Ke Za Zhi. 48:316–321. 2013.PubMed/NCBI
|
|
19
|
Zhu XH, Liao B, Liu K and Liu YH: Effect
of RNA interference therapy on the mice eosinophils CCR3 gene and
granule protein in the murine model of allergic rhinitis. Asian Pac
J Trop Med. 7:226–230. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Tai PC, Sun L and Spry CJ: Effects of
IL-5, granulocyte/macrophage colony-stimulating factor (GM-CSF) and
IL-3 on the survival of human blood eosinophils in vitro. Clin Exp
Immunol. 85:312–316. 1991. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Coffman RL, Seymour BW, Hudak S, Jackson J
and Rennick D: Antibody to interleukin-5 inhibits helminth-induced
eosinophilia in mice. Science. 245:308–310. 1989. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Cho JY, Miller M, Baek KJ, Han JW, Nayar
J, Lee SY, McElwain K, McElwain S, Friedman S and Broide DH:
Inhibition of airway remodeling in IL-5-deficient mice. J Clin
Invest. 113:551–560. 2004. View
Article : Google Scholar : PubMed/NCBI
|
|
23
|
Leckie MJ, ten Brinke A, Khan J, Diamant
Z, O'Connor BJ, Walls CM, Mathur AK, Cowley HC, Chung KF,
Djukanovic R, et al: Effects of an interleukin-5 blocking
monoclonal antibody on eosinophils, airway hyper-responsiveness,
and the late asthmatic response. Lancet. 356:2144–2148. 2000.
View Article : Google Scholar : PubMed/NCBI
|