Introduction
Allergic asthma is a chronic airway disorder
characterized by airway inflammation, mucus hypersecretion, and
airway hyperresponsiveness (AHR) (1). The pro-inflammatory type 2 helper T
(Th2) cell cytokines, interleukin (IL)-4, IL-5 and IL-13, which
trigger the release of IgE from B lymphocytes and airway
eosinophilia (2), may contribute
to AHR in asthma (3). Asthma is
most commonly associated with an aberrant Th2 cell response, but
severe disease is not exclusively associated with the production of
Th2 cell-associated cytokines (4).
It is instead characterized by increased production of the
pro-inflammatory cytokine IL-17. Previous studies have suggested
that IL-17 is involved in the pathogenesis of airway diseases,
including allergic asthma, and IL-17 expression has been revealed
to be upregulated in the airways of mice and humans following
allergen-induced airway inflammation (5–8). A
neutrophilic influx is observed in the lung following IL-17
production, contributing to pulmonary diseases including asthma
(9,10). Therefore, there is accumulating
evidence that IL-17 is associated with allergic asthma.
γδT cells have been reported to be dominant
producers of IL-17 at the site of infection during the early phase
of pulmonary Mycobacterium tuberculosis infection (11). In addition, IL-17-producing γδT
(IL-17+γδT) cells are associated with certain autoimmune
diseases (12).
IL-17+γδT cells are localized in mucosal tissues,
including the lung, intestine, peritoneal cavity and reproductive
organs, that are exposed to exogenous stimuli such as pathogens
(13). Furthermore, several
studies have reported that endogenous IL-23 induces IL-17
production by γδT cells in vivo and in vitro
(14–18).
It has previously been reported that IL-17, an
important pro-inflammatory cytokine, was mainly produced by γδT
cells (19). γδT cells are
generated from naïve T cells, and γδT cell differentiation is
driven by stimuli including IL-23. IL-23- IL-23 receptor (IL-23R)
signaling promotes GATA binding protein 3 (GATA-3) expression and
enhances IL-17 production by γδT cells (20,21).
These cells are the first immune cells found in the fetus and
confer immunity to newborns prior to activation of the adaptive
immune system.
The Bacillus Calmette-Guérin (BCG) vaccine, a
non-specific stimulator of immune function, protects against the
development of asthma in humans and mice via inhibition of Th2
immune responses, which are characteristic of asthma (22–24).
The BCG vaccine is considered safe, with side-effects mainly
including erythema and a papule, ulcer or scar at the immunization
site. These side-effects are mild and do not require treatment.
However, regional suppurative lymphadenitis and osteitis are not
uncommon.
Immunotherapy is the only currently available
treatment with the potential to change the natural history of
allergic disease and delay allergy progression in individuals with
atopic allergies (24). Mucosal
immunotherapy is advantageous due to the non-injection route of
administration and lower side-effect profile (25). Multiple routes for mucosal
immunotherapy have been proposed and investigated, including oral,
nasal, tracheal and sublingual. Atomization delivery is attractive
due to the ease of administration. It has previously been observed
that inhalation of inactivated Mycobacterium phlei (M.
phlei) attenuates airway inflammation via upregulation of IL-10
and interferon (IFN)-γ secretion, which are anti-inflammatory
molecules, and downregulation of IL-4 production (26). γδT cells are generated from native
T cells, and γδT cell differentiation is driven by stimuli such as
IL-23. IL-23-IL-23R signaling promotes GATA-3 expression and
enhances IL-17 production by γδT cells (19,20).
In general, γδT cells account for ~3–5% of all lymphoid cells found
in the secondary lymphoid tissues and the blood. These cells are
the first immune cells found in the fetus and provide immunity to
newborns prior to activation of the adaptive immune system
(27).
Therefore, the present study hypothesized that
inactivated M. phlei, administrated via inhalation, would
exert an antiasthmatic effect in a murine asthma model through
suppression of the pro-inflammatory activity of
IL-17+γδT cells by downregulation of IL-23R
expression.
Materials and methods
Animals
Male BALB/c mice (n=30), 6–8 weeks old, weight 18–22
g, were obtained from the Laboratory Animal Center of Guangxi
Medical University (Nanning, China), and housed under
specific-pathogen-free conditions in a facility with an automatic
12/12 h day/night cycle and fed with a standard laboratory food and
water. Mice were randomly assigned to three experimental groups
(n=10 in each group): The normal control group (group A), the
sensitized/M. phlei untreated group (group B) and the
sensitized/M. phlei treated group (group C). Sensitization
was brought about by challenge with ovalbumin to create a murine
asthma model.
Establishment of a murine model of
asthma
A murine model of asthma was established according
to a modification of previous methods (26). Mice were sensitized via
intraperitoneal injections of 25 µg ovalbumin (OVA) and 1 mg
Al(OH)3 suspended in 0.2 ml saline on days 0, 7 and 14.
Following initial sensitization the mice were challenged for 20 min
with 2% OVA once per day using an ultrasonic nebulizer (Model
WH-2000; Guangdong Yuehua Medical Instrument Factory Co., Ltd.,
Guangdong, China) in a closed chamber on days 21-28. Group A mice
received saline in place of OVA at the sensitization and challenge
stages.
Following the challenge, the treatment group inhaled
a solution of inactivated M. phlei (1.72 µg ampule M.
phlei dissolved in 10 ml saline; cat. no. S20040067; Chengdu
Jinxing Jiankang Pharmaceutical Co., Ltd., Chengdu, China)
administered by nebulizer once per day for 5 days. The normal
control group and asthma model group (groups A and B) were sham
treated with 10 ml atomized saline instead. The animals were
sacrificed by cervical dislocation 24 h after the final inactivated
M. phlei treatment. Lung tissue was subsequently harvested:
Left lobes were fixed with 10% formalin for hematoxylin and eosin
(H&E) staining and immunohistochemistry, while right lungs were
stored at −80°C until further use for fluorescence-activated cell
sorting (FACS).
Measurement of AHR
Total lung resistance (RL), dynamic
compliance (Cdyn) and peak expiratory flow (PEF) were assessed via
a tracheostomy tube 3 h following the inhalation of saline or
multiplied methacholine treatment as previously described, using a
computerized small animal ventilator (Data Sciences International,
Minneapolis, MN, USA) (28).
Methacholine is used to diagnose asthma by inducing
bronchoconstriction. Mice were allowed to stabilize on the
ventilator for 5 min prior to measurements. Once stabilized, dose
responsiveness to methacholine (6.25, 12.5, 25 and 50 mg/ml) was
measured and reported as total lung resistance.
Pulmonary histological analysis
Lungs were harvested from the mice. Left lobes were
fixed with 10% formalin for 24 h and embedded in paraffin for
histopathology analysis. 4–5 µm sections were cut. The tissue
sections underwent H&E staining to visualise airway
inflammation changes through light microscopy (Olympus Corporation,
Tokyo, Japan).
Bronchoalveolar lavage fluid cell
counting
Bronchoalveolar lavage fluid (BALF) was isolated as
previously described (2). BALF was
centrifuged at 600 × g for 5 min, and the supernatant was
discarded. The cell pellet was resuspended in 200 µl of RPMI-1640
medium (cat. no. 11875093; Thermo Fisher Scientific, Inc., Waltham,
MA, USA), and the red blood cells were lysed using 200 µl Red Blood
Cell Lysis Buffer (cat. no. R1010; Beijing Solarbio Science and
Technology Co., Ltd., Beijing, China). The cells were subsequently
adhered to a hemocytometer slide and counted at ×100 magnification
with a light microscope. The absolute cell counts per BALF sample
were calculated for neutrophils and eosinophils.
Immunohistochemistry examination of
IL-17 and IL-23 receptor (IL-23R)
For immunohistochemical detection of IL-17 and
IL-23R in the airway, formalin-fixed, paraffin-embedded sections
were stained with biotinylated polyclonal antibodies specific for
IL-17 (cat. no. 500-P07Bt; PeproTech, Inc., Rocky Hill, NJ, USA)
and IL-23R (cat. no. BAF1400; R&D Systems, Inc., Minneapolis,
MN, USA). Negative control experiments were performed by omitting
the primary antibodies. Sections were blocked with 3% bovine serum
albumin and 0.4% Triton X-100 in TBS buffer for 30 min at room
temperature, then incubated overnight at 4°C with IL-17 antibody
and IL-23R antibodies at 1:50 dilutions, with the subsequent
addition of a peroxidase complex prepared according to the
manufacturer's instructions. Image analysis was then performed and
analysed with Lecia LAS AF software version 2.6.0 (Leica
Microsystems GmbH, Wetzlar, Germany).
Flow cytometric analysis
The following antibodies were used for flow
cytometric analysis of BALF-derived T cells: PERCP-CY5.5-conjugated
IL-17 antibody (cat. no. TC11-18H10; BD Pharmingen, San Diego, CA,
USA), IL-23R polyclonal antibody (cat. no. 06-1331; Merck
Millipore, Darmstadt, Germany) and goat anti-rabbit IgG-PE (cat.
no. sc-3739; Santa Cruz Biotechnology, Inc., Dallas, TX, USA).
Intracellular cytokine detection of BALF-derived T cells was
performed as previously described (29).
Statistical analysis
Data are expressed as the mean ± standard deviation.
Statistical analysis was performed via one-way analysis of variance
for multiple comparisons, followed by Fisher's Least Significant
Difference test for comparisons between groups. P<0.05 was
considered to indicate a statistically significant difference.
Results
Effects of inactivated M. phlei on the
pulmonary pathology of OVA-induced asthmatic mice
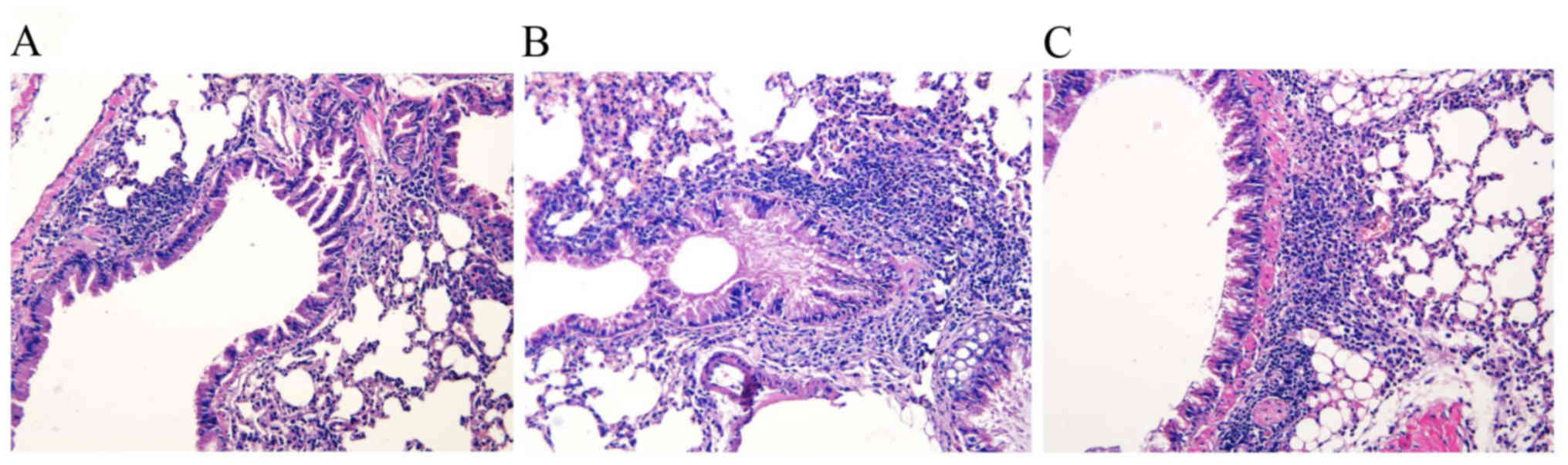
To determine the effect on the lung parenchyma
following inactivated M. phlei treatment, formalin-fixed,
paraffin-embedded whole lungs were sectioned and stained with
H&E. The lung histology demonstrated increased numbers of
inflammatory cells within the bronchiolar and alveolar
compartments, as well cell hyperplasia, in the two sensitized
groups compared with the normal control group. Predominately
perivascular and peribronchiolar mixed eosinophil and lymphocyte
cellular aggregates were consistently observed following OVA
challenge and were not observed in the normal control group.
Thickened basement membranes were present in the sensitized groups
vs. the normal control group (Fig. 1A
and B). The administration of inactivated M. phlei
attenuated the infiltration of inflammatory cells in the
peribronchial and perivascular areas as compared with the asthma
model mice, with fewer inflammatory eosinophil and lymphocyte
cellular aggregates in the sensitized/M. phlei treated group
compared with the sensitized/M. phlei untreated group
(Fig. 1B and C).
Effect of inhaled inactived M. phlei
on neutrophils and eosinophils in BALF
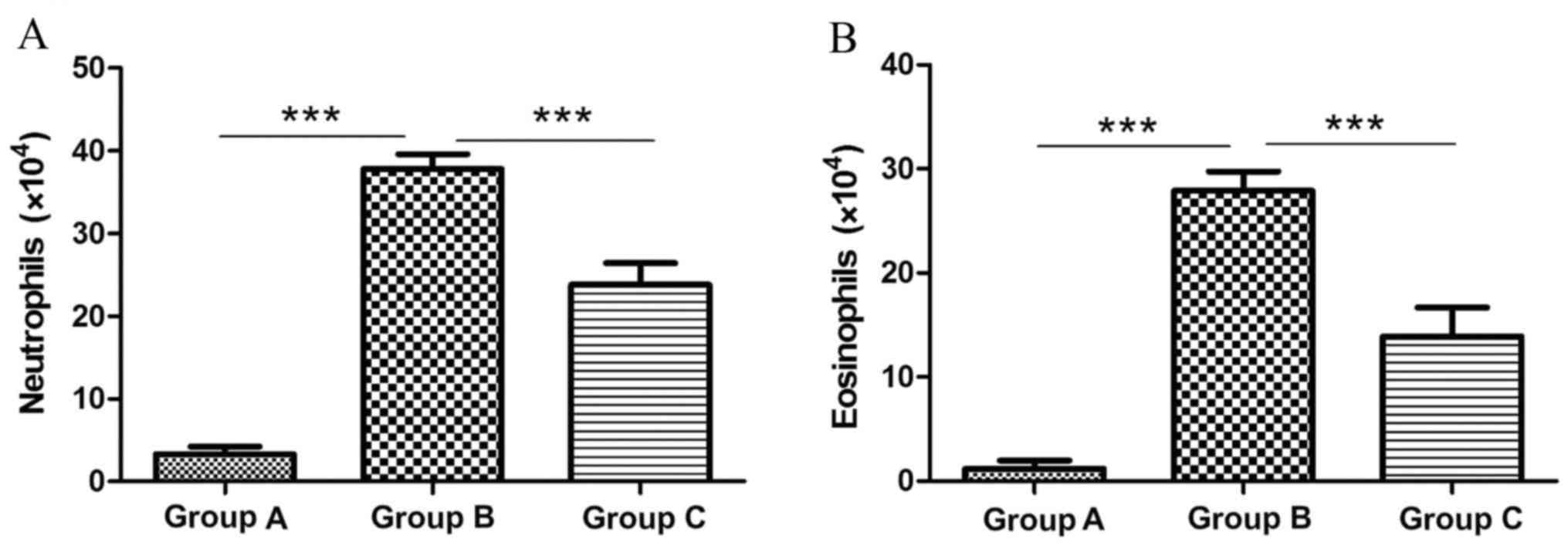
Neutrophil numbers were significantly elevated in
the sensitized/M. phlei untreated group (37.8×104; Fig. 2A) compared with the normal control
group (3.3×104; 10.45-fold; P<0.0001; Fig. 2A). However neutrophil numbers were
significantly decreased in the sensitized/M. phlei treated
group compared with the sensitized/M. phlei untreated group
(1.59-fold difference; P<0.0001; Fig. 2A). Eosinophil numbers were
significantly increased in sensitized/M. phlei untreated
mice (27.9×104; Fig. 2B) compared
with the normal control group (1.17×104; 23.8 fold difference;
P<0.0001; Fig. 2B). A 2-fold
decrease in eosinophil numbers was observed in the sensitized/M.
phlei treated group (13.9×104; Fig. 2B) compared with the
sensitized/M. phlei untreated group (P<0.0001; Fig. 2B). The results suggest that
inactived M. phlei may attenuate the airway inflammation of
mice with asthma.
Effect of inhaled inactived M. phlei
on lung function alongside methacholine treatment in asthmatic
mice
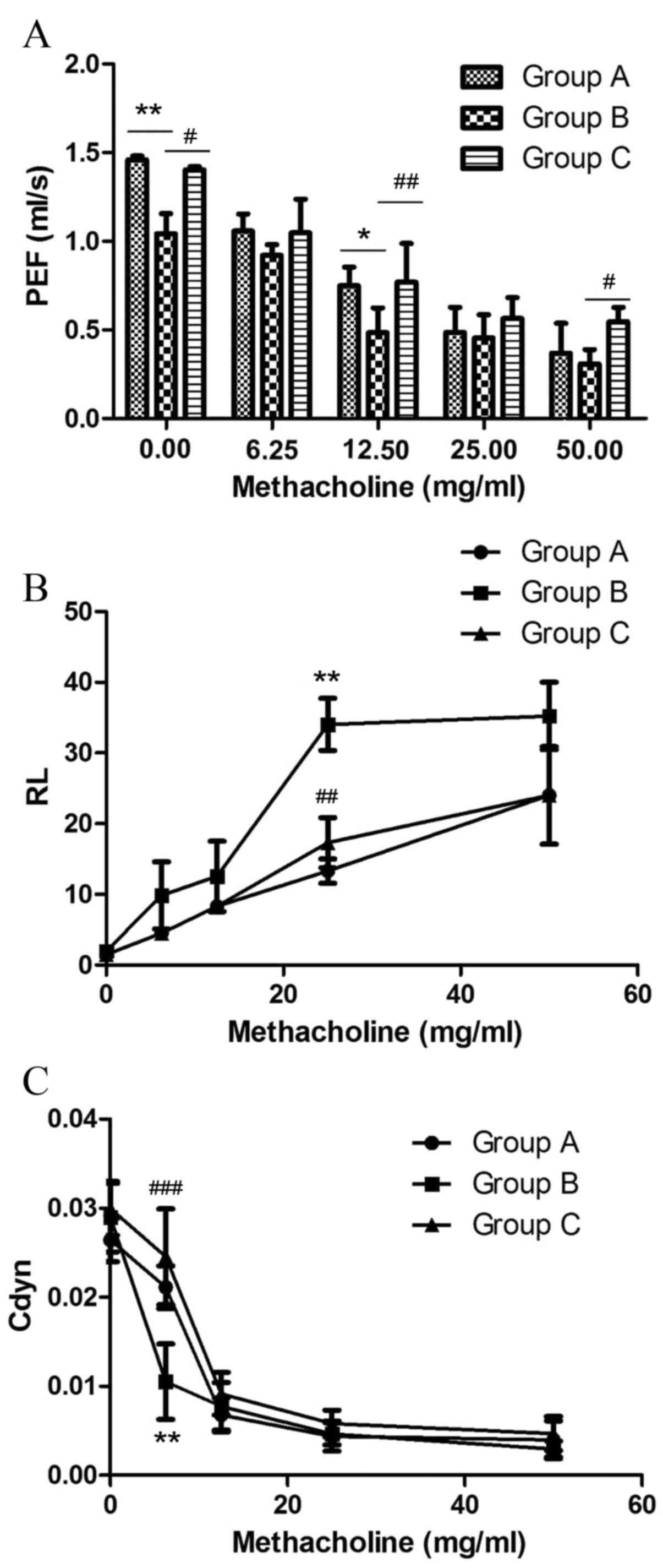
The effect of inhaled inactived M. phlei on
AHR to methacholine in asthmatic mice was evaluated through
measuring changes in RL, Cdyn and PEF.
PEF is the maximum flow rate during expiration,
measured in ml/s (Fig. 3A). OVA
challenge significantly decreased PEF in the sensitized/M.
phlei untreated group compared with the normal control group at
0 mg/ml methacholine (P=0.0038; Fig.
3A), and at 12.5 mg/ml methacholine (P=0.0146; Fig. 3A). Sensitized/M. phlei
treated mice demonstrated significantly elevated PEF compared with
sensitized/M. phlei untreated mice at 0 mg/ml methacholine
(P=0.0139; Fig. 3A), 12.5 mg/ml
methacholine (P=0.00375; Fig. 3A)
and 50 mg/ml methacholine (P=0.0142; Fig. 3A). No significant difference ws
observed in PEF between sensitized/M. phlei treated and
normal control groups (Fig. 3A).
These results demonstrate that inhaled inactived M. phlei
attenuates the impairment to PEF caused by methacholine in a mouse
model of asthma.
OVA challenge significantly increased RL
at all 4 methacholine doses tested in sensitized/M. phlei
untreated mice, with the maximum increase at 25 mg/ml (P=0.001 vs.
normal control group; P=0.06 vs. sensitized/M. phlei treated
group; Fig. 3B). The RL
of the normal control group and the sensitized/M. phlei
treated group also increased in response to methacholine doses, but
there was no significant difference between these two groups
(Fig. 3B).
A dose of 6.25 mg/ml methacholine significantly
decreased Cdyn in the sensitized/M. phlei untreated group
compared with the normal control group (P=0.02; Fig. 3C) and the sensitized/M.
phlei treated group (P<0.0001; Fig. 3C) at a dose of 6.25 mg/ml
methacholine. Other methacholine doses demonstrated no significant
difference among the 3 groups. There was also no significant
difference between the normal control group and the
sensitized/M. phlei treated group at any dose (Fig. 3C).
These results demonstrate that an atomized solution
of inactivated M. phlei treatment restored these 3 aspects
close to the levels recorded in healthy control mice. The atomized
solution of inactivated M. phlei can suppress the adverse
impact of methacholine, and recover pulmonary function almost to
the healthy level.
Effects of inhaled inactived M. phlei
on inflammatory cytokine levels in lung tissues, visualized with
immunofluorescence
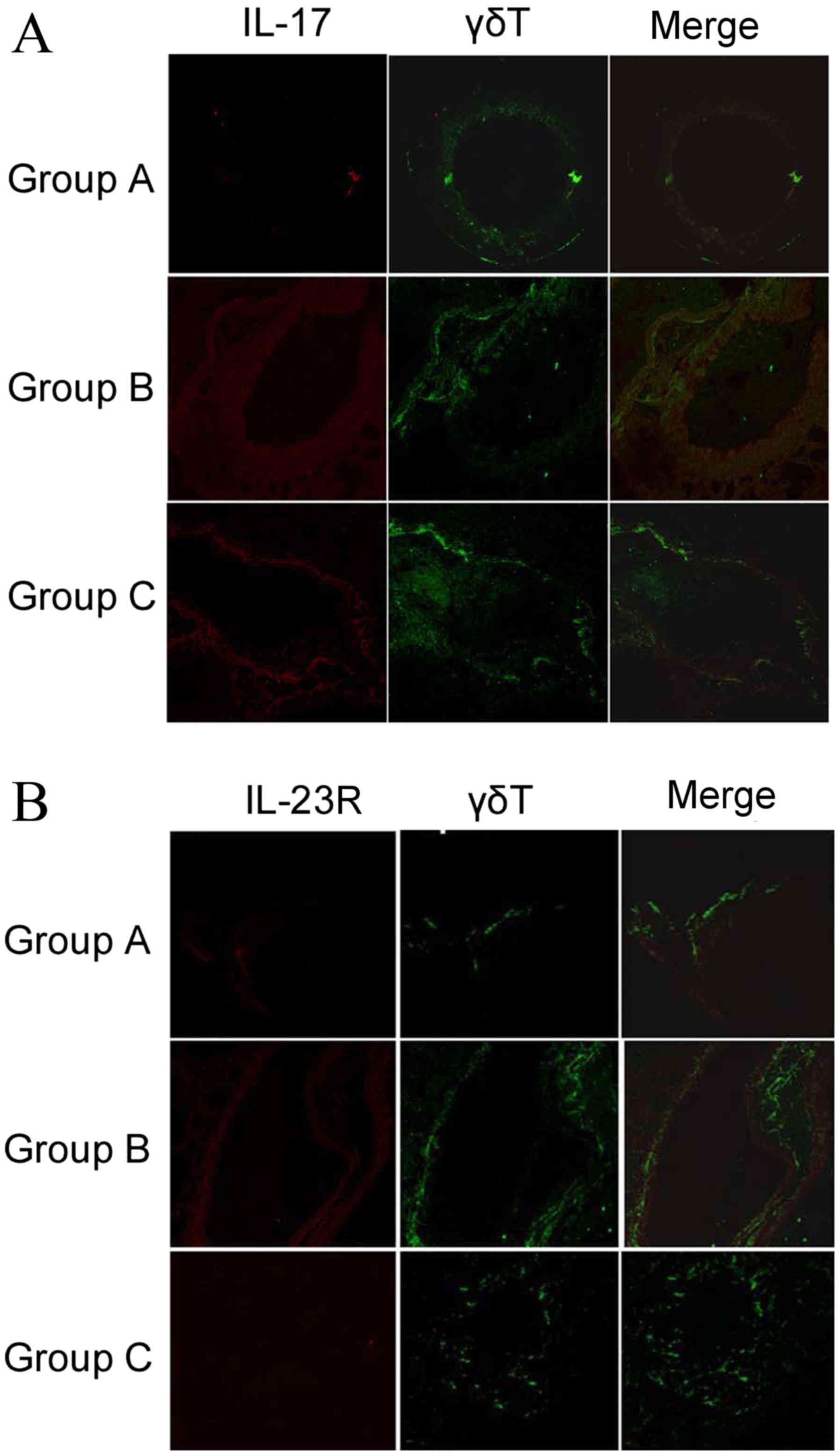
Expression of IL-17 and IL-23R in lung tissues of
the three groups was determined by immunohistochemical staining,
with images acquired using laser scanning confocal microscopy as
described in materials and methods. IL-17 and IL-23R expression
appeared to increase in the sensitized/M. phlei untreated
group, but decreased with administration of inactived M.
phlei (Fig. 4). These results
demonstrate that reduction of IL-17 and IL-23R may be related to
the antiasthmatic effect of inactived M. phlei in mice with
asthma.
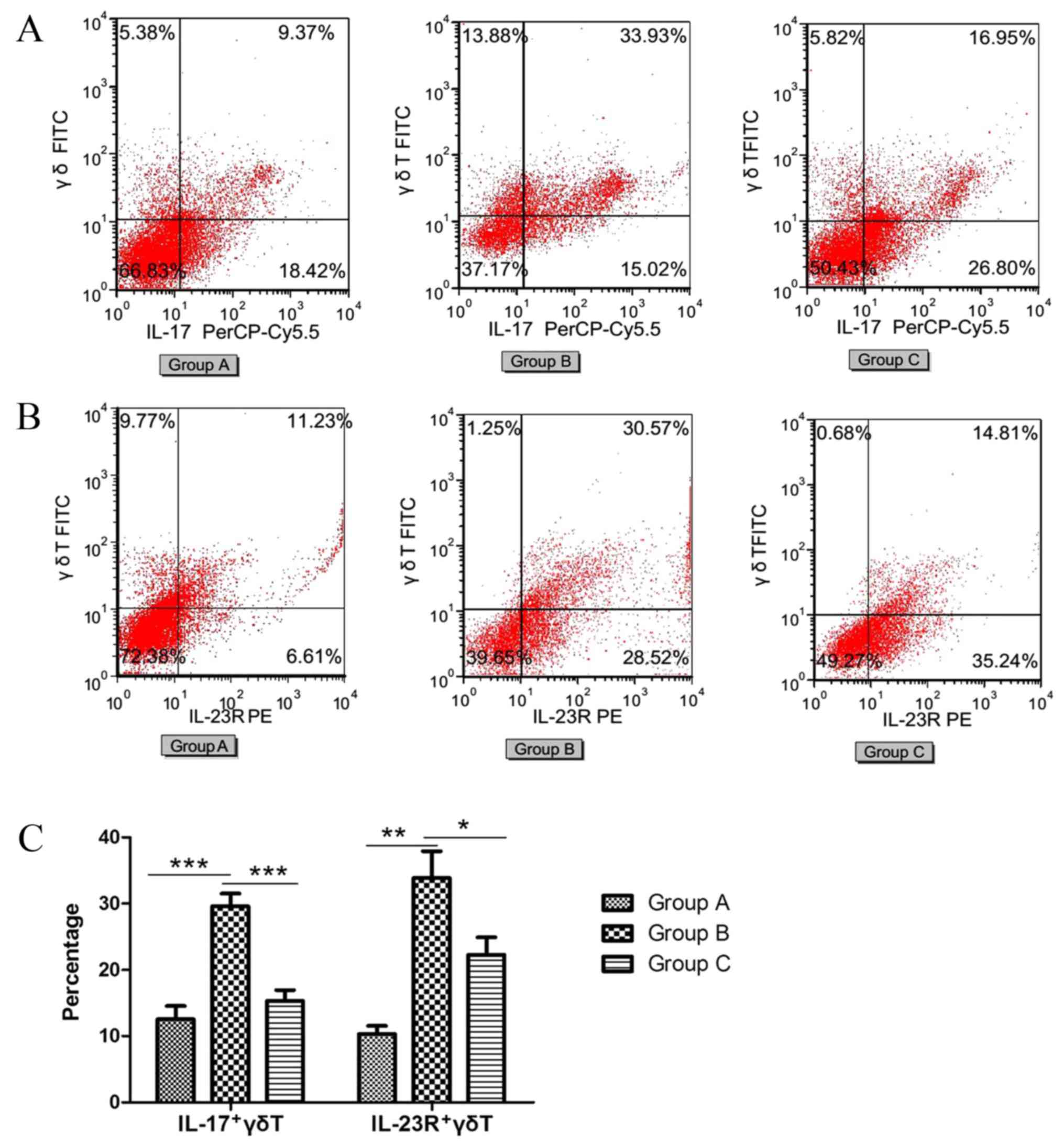
Effects of inhaled inactived M. phlei
on the production of IL-17 or IL-23R positive γδT cells with
FACS
FACS was performed to determine the ratio of IL-17
positive γδT (IL-17+γδT) cells (Fig.
5A) and IL-23R positive γδT (IL-23R+γδT) cells (Fig. 5B). The percentage of IL-17+γδT
cells and IL-23R+γδT cells significantly increased in the
sensitized/M. phlei untreated group compared with the normal
control group (P<0.0001 and P<0.0001, respectively; Fig. 5C). However, in the sensitized/M.
phlei treated group, the percentages of IL-17+γδT cells and
IL-23R+γδT cells were significantly decreased compared with the
sensitized/M. phlei untreated group (P<0.0001 and
P=0.015, respectively; Fig. 5C).
This reduction of IL-17+γδT cells and IL-23R+γδT cells indicates
that inflammation was attenuated and lung-function partially
recovered. In addition, from the immunofluorescence detection
(Fig. 4) and cell sorting
(Fig. 5) results, it is possible
to conclude that the antiasthmatic effect of inhaled inactived
M. phlei is the result of the inhibition IL-17 and IL-23R
expression, which decreases production of IL-17+γδT cells and
IL-23R+γδT cells.
Discussion
Previous studies have demonstrated that inactivated
M. phlei nebulized therapy is effective in adults and
children aged 4–12 years with moderate persistent asthma (30,31),
however the detailed mechanism remains unclear. The results of the
present study indicate that inhaled administration of inactivated
M. phlei is able to alleviate allergen-induced airway
inflammation in OVA-challenged mice. In addition,
methacholine-associated damage is prevented in these mice by
inhaled inactived M. phlei treatment, and pulmonary function
is restored to close to the level of healthy mice. Therefore,
inhaled inactived M. phlei may be an effective treatment for
asthma.
Although it is widely accepted that the
pathognomonic features of asthma are mediated mainly by Th2 cells
and their associated cytokines, increasing evidence suggest IL-17,
an important pro-inflammatory cytokine that is mainly produced by
γδT cells, is involved in the development of asthma (32). It has been demonstrated that IL-17
is expressed in the airway of patients with asthma (7,10)
and correlates with airway hyper- responsiveness (21,33,34).
The present study has clearly demonstrated that
inhaled administration of inactivated M. phlei suppresses
production of IL-17-producing γδT cells and decreased
IL-23R-producing γδT cells in the lungs of treated mice (Fig. 5).
IL-23 is important for the maintenance of IL-17
production, however, pathogen products and environmental signals
can also regulate IL-17-producing γδT cells, particularly
Mycobacterium. Therefore, IL-17 production is complicated by
the involvement of multiple immune mediators. Previous studies have
demonstrated that combining C-C motif chemokine receptor 6 and CD44
for FACS sorting of γδT cells yielded an almost 100% pure
population of IL-17-producing cells, indicating that γδT cells can
be the sole source of IL-17 (21).
Toll-like receptor triggering of γδT cells provides the first
source of IL-17 (21). Cytokine
IL-6 is responsible for the development, activation and recruitment
of IL-17+γδT cells (35). IL-21 may also be involved in the
development of IL-17+γδT cells (36). In addition, AHR-mediated
environmental signals can shape the functional capacity of
IL-17+γδT cells (21).
However, a number of mechanisms of the inhibitory effect of M.
phlei on IL-17+γδT cells remain to be
identified.
In conclusion, the current study demonstrates that
inactivated M. phlei acts as an immune regulator of the
IL-17+γδT-mediated response in the lung. Inactivated
M. phlei suppresses the IL-17+γδT-mediated immune
response, airway inflammation and airway hyperresponsiveness in the
lung, at least partially inhibiting the expression of IL-23R.
Therefore, inactivated M. phlei may be an effective strategy
for regulating IL-17+γδT-mediated airway inflammation
and airway hyperresponsiveness. This may, therefore, represent an
effective treatment strategy for asthma.
Acknowledgements
The present study was funded by the National Natural
Science Foundation of China (grant no. 81360007).
References
|
1
|
Galli SJ, Tsai M and Piliponsky AM: The
development of allergic inflammation. Nature. 454:445–454. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Ming M, Luo Z, Lv S and Li C: Inhalation
of inactivated-Mycobacterium phlei prevents asthma-mediated airway
hyperresponsiveness and airway eosinophilia in mice by reducing
IL-5 and IL-13 levels. Mol Med Rep. 14:5343–5349. 2016.PubMed/NCBI
|
|
3
|
Cockcroft DW and Davis BE: Mechanisms of
airway hyperresponsiveness. J Allergy Clin Immunol. 118:551–559;
quiz 560–1. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Hofmann MA, Kiecker F and Zuberbier T: A
systematic review of the role of interleukin-17 and the
interleukin-20 family in inflammatory allergic skin diseases. Curr
Opin Allergy Clin Immunol. 16:451–457. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Kawaguchi M, Onuchic LF, Li XD, Essayan
DM, Schroeder J, Xiao HQ, Liu MC, Krishnaswamy G, Germino G and
Huang SK: Identification of a novel cytokine, ML-1, and its
expression in subjects with asthma. J Immunol. 167:4430–4435. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Hellings PW, Kasran A, Liu Z,
Vandekerckhove P, Wuyts A, Overbergh L, Mathieu C and Ceuppens JL:
Interleukin-17 orchestrates the granulocyte influx into airways
after allergen inhalation in a mouse model of allergic asthma. Am J
Respir Cell Mol Biol. 28:42–50. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Molet S, Hamid Q, Davoineb F, Nutku E,
Taha R, Pagé N, Olivenstein R, Elias J and Chakir J: IL-17 is
increased in asthmatic airways and induces human bronchial
fibroblasts to produce cytokines. J Allergy Clin Immunol.
108:430–438. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Shen F, Zhao MW, He B, Wang YZ and Yao WZ:
The levels and clinical implications of induced sputum
interleukin-17 in chronic obstructive pulmonary disease and asthma.
Zhonghua Nei Ke Za Zhi. 43:888–890. 2004.(In Chinese). PubMed/NCBI
|
|
9
|
Liang SC, Long AJ, Bennett F, Whitters MJ,
Karim R, Collins M, Goldman SJ, Dunussi-Joannopoulos K, Williams
CM, Wright JF and Fouser LA: An IL-17F/A heterodimer protein is
produced by mouse Th17 cells and induces airway neutrophil
recruitment. J Immunol. 179:7791–7799. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Barczyk A, Pierzchala W and Sozañska E:
Interleukin-17 in sputum correlates with airway hyperresponsiveness
to methacholine. Respir Med. 97:726–733. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Lockhart E, Green AM and Flynn JL: IL-17
production is dominated by gammadelta T cells rather than CD4 T
cells during Mycobacterium tuberculosis infection. J Immunol.
177:4662–4669. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Lu H, Li DJ and Jin LP: γδT Cells and
Related Diseases. Am J Reprod Immunol. 75:609–618. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Shibata K and Yoshikai Y: Functions of
IL-17-producing γδ T Cells. Open Immunology Journal. 2:151–155.
2009. View Article : Google Scholar
|
|
14
|
Stark MA, Huo Y, Burcin TL, Morris MA,
Olson TS and Ley K: Phagocytosis of apoptotic neutrophils regulates
granulopoiesis via IL-23 and IL-17. Immunity. 22:285–294. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Nakamura R, Shibata K, Yamada H, Shimoda
K, Nakayama K and Yoshikai Y: Tyk2-signaling plays an important
role in host defense against Escherichia coli through IL-23-induced
IL-17 production by gammadelta T cells. J Immunol. 181:2071–2075.
2008. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Saunus JM, Wagner SA, Matias MA, Hu Y,
Zaini ZM and Farah CS: Early activation of the interleukin-23-17
axis in a murine model of oropharyngeal candidiasis. Mol Oral
Microbiol. 25:343–356. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Aggarwal S, Ghilardi N, Xie MH, de Sauvage
FJ and Gurney AL: Interleukin-23 promotes a distinct CD4 T cell
activation state characterized by the production of interleukin-17.
J Biol Chem. 278:1910–1914. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Sutton CE, Lalor SJ, Sweeney CM, Brereton
CF, Lavelle EC and Mills KH: Interleukin-1 and IL-23 induce innate
IL-17 production from gammadelta T cells, amplifying Th17 responses
and autoimmunity. Immunity. 31:331–341. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Zhong Q, Zhou K, Liang QL, Lin S, Wang YC,
Xiong XY, Meng ZY, Zhao T, Zhu WY, Yang YR, et al: Interleukin-23
secreted by activated macrophages drives γδT cell production of
interleukin-17 to aggravate secondary injury after intracerebral
hemorrhage. J Am Heart Assoc. 5:pii: e0043402016. View Article : Google Scholar
|
|
20
|
Sutton CE, Mielke LA and Mills KH:
IL-17producing γδ T cells and innate lymphoid cells. Eur J Immunol.
42:2221–2231. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Martin B, Hirota K, Cua DJ, Stockinger B
and Veldhoen M: Interleukin-17-producing gammadelta T cells
selectively expand in response to pathogen products and
environmental signals. Immunity. 31:321–330. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Robinson DS, Hamid Q, Ying S, Tsicopoulos
A, Barkans J, Bentley AM, Corrigan C, Durham SR and Kay AB:
Predominant TH2-like bronchoalveolar T-lymphocyte population in
atopic asthma. N Engl J Med. 326:298–304. 1992. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Kon OM and Kay AB: T cells and chronic
asthma. Int Arch Allergy Immunol. 118:133–135. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Nagai H, Teramachi H and Tuchiya T: Recent
advances in the development of anti-allergic drugs. Allergol Int.
55:35–42. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Ye YL, Chuang YH and Chiang BL: Strategies
of mucosal immunotherapy for allergic diseases. Cell Mol Immunol.
8:453–461. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Zhang J, Li C and Guo S: Effects of
inhaled inactivated Mycobacterium phlei on airway inflammation in
mouse asthmatic models. J Aerosol Med Pulm Drug Deliv. 25:96–103.
2012. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Sinkora M, Sinkorová J and Holtmeier W:
Development of gammadelta thymocyte subsets during prenatal and
postnatal ontogeny. Immunology. 115:544–555. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Poole JA, Wyatt TA, Romberger DJ, Staab E,
Simet S, Reynolds SJ, Sisson JH and Kielian T: MyD88 in lung
resident cells governs airway inflammatory and pulmonary function
responses to organic dust treatment. Respir Res. 16:1112015.
View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Nakasone C, Yamamoto N, Nakamatsu M, Kinjo
T, Miyagi K, Uezu K, Nakamura K, Higa F, Ishikawa H, O'brien RL, et
al: Accumulation of gamma/delta T cells in the lungs and their
roles in neutrophil-mediated host defense against pneumococcal
infection. Microbes Infect. 9:251–258. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Zhang J, Guo S, Li C and Jiang X:
Therapeutic effects of inhaled inactivated Mycobacterium phlei in
adult patients with moderate persistent asthma. Immunotherapy.
4:383–387. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Ming M, Li C, Luo Z and Lv S: Effect of
inhaled inactivated Mycobacterium phlei in children with moderate
asthma. Immunotherapy. 5:191–197. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Nakada EM, Shan J, Kinyanjui MW and Fixman
ED: Adjuvant-dependent regulation of interleukin-17 expressing γδ T
cells and inhibition of Th2 responses in allergic airways disease.
Respir Res. 15:902014. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
O'Brien RL, Roark CL and Born WK:
IL-17-producing gammadelta T cells. Eur J Immunol. 39:662–666.
2009. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Roark CL, Simonian PL, Fontenot AP, Born
WK and O'Brien RL: gammadelta T cells: An important source of
IL-17. Curr Opin Immunol. 20:353–357. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Lochner M, Peduto L, Cherrier M, Sawa S,
Langa F, Varona R, Riethmacher D, Si-Tahar M, Di Santo JP and Eberl
G: In vivo equilibrium of proinflammatory IL-17+ and regulatory
IL-10+ Foxp3+ RORgamma t+ T cells. J Exp Med. 205:1381–1393. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Nurieva R, Yang XO, Martinez G, Zhang Y,
Panopoulos AD, Ma L, Schluns K, Tian Q, Watowich SS, Jetten AM and
Dong C: Essential autocrine regulation by IL-21 in the generation
of inflammatory T cells. Nature. 448:480–483. 2007. View Article : Google Scholar : PubMed/NCBI
|