Introduction
Colon cancer is one of the most common types of
gastrointestinal cancers in China. It has the third greatest
incidence of all cancer types. Epidemiological studies of the
etiology and underlying mechanisms of colon cancer have
demonstrated significant value for its early diagnosis, clinical
treatment and prognosis. Tumor invasion and lymph node metastasis
are important factors for determining the prognosis of the disease.
At present, the primary treatment for colon cancer is chemotherapy.
However, there are a number of severe adverse effects associated
with currently available anticancer drugs, particularly
chemotherapeutic agents, including nausea, vomiting, mouth ulcers,
bone marrow suppression and hair loss. In addition, anticancer
drugs may affect the heart, kidney, liver, lungs, as well as
additional organs, which may lead to infertility or malformation.
Furthermore, resistance to therapy is a major obstacle in the
successful treatment of patients with colon cancer (1,2).
Therefore, novel individualized treatment strategies are required
to improve the survival of patients, and the prevent cancer
invasion and metastasis (3).
MicroRNA (miRNA) is a type of endogenous non-coding
RNA with an evolutionarily highly conserved structure. Previous
research involving miRNAs have revealed a role of miRNAs in the
regulation of numerous cellular functions, including development
and differentiation, cell cycle regulation, metabolism and
apoptosis (4,5). A large number of miRNAs are encoded
by genes located in regions frequently exposed to changes in cancer
cells, and alterations in miRNA expression levels have been
associated with various cancers (6,7). In
addition, specific miRNA expression signatures have been
demonstrated to correlate with the prognosis and progression of
different cancers (8). By
downregulating protein-encoding genes that promote or inhibit cell
proliferation, several miRNAs have been demonstrated to function as
tumor suppressors or oncogenes (6–8).
miRNA (miR)-34a may be an effector molecule of the tumor suppressor
gene p53, and thus inhibit the proliferation of various cancer
cells (9–11). However, the underlying mechanisms
of miR-34a and its role in colon cancer remain unclear. The present
study investigated the role of miR-34a expression in the
proliferation, invasion and metastasis of colon cancer and its
underlying mechanisms, via construction of an miR-34a expression
vector and its transfection into HCT116 colon cancer cells.
Materials and methods
Cell strains and reagents
The HCT116 human colon cancer cell line was
purchased from the Cell Bank of the Chinese Academy of Sciences
(Beijing, China). Cell culture reagents including high-sugar
Dulbecco's modified Eagle's medium (DMEM), fetal bovine serum,
penicillin and streptomycin were purchased from Gibco; Thermo
Fisher Scientific, Inc. (Waltham, MA, USA), the miRNA Extraction
kit was purchased from Qiagen, Inc. (Valencia, CA, USA), and the
Taqman MicroRNA Reverse Transcription and the TaqMan MicroRNA
Detection kit were obtained from Takara Biotechnology, Co., Ltd.
(Dalian, China). MTT reagent was purchased from Sigma-Aldrich;
Merck Millipore (Darmstadt, Germany), the miR-34a eukaryotic
expression vector (GAU GUU CUA UUG AAG AGU GUU UG), miR-34a
negative control (GGA CAC GA AAT CTA TGC GCG TG) and green
fluorescent protein (GFP)-carrying recombinant plasmid were
designed and synthesized by Shanghai GenePharma Co., Ltd.
(Shanghai, China), and the transfection reagent
Lipofectamine® 2000 was obtained from Invitrogen; Thermo
Fisher Scientific, Inc. Western blotting reagents (loading buffer,
protein marker, electrophoretic liquid, transfer buffer and
developer) were purchased from Beyotime Institute of Biotechnology
(Haimen, China), and primary and secondary antibodies were
purchased from Cell Signaling Technology, Inc. (Danvers, MA, USA).
Other common reagents were purchased from Sigma-Aldrich; Merck
Millipore.
Cell culture
HCT116 cells were cultured in high-sugar DMEM
containing 10% fetal bovine serum and 1,000 U/ml
penicillin-streptomycin, in a constant temperature incubator at
37°C and 5% CO2. The medium was refreshed every 2–3
days. Trypsin was used to digest cells when they had reached
>80% confluence, before they were used for downstream
experiments.
miR-34a transfection
Following digestion and resuspension, the HCT116
cells were diluted to 105 cells/ml with complete DMEM. Cells (2 ml)
were seeded into 6-well plates and cultured in a constant
temperature incubator at 37°C and 5% CO2 for 24 h. The
serum-free medium was replaced when cell fusion was >50% and
transfection was performed. The transfection solution was prepared
in advance according to the manufacturer's protocol. Plasmid (5 µg)
was added into 500 µl DMEM, mixed and incubated at room temperature
for 5 min. Lipofectamine 2000 (10 µl) was added into DMEM, mixed
and incubated at room temperature for 5 min. The two solutions were
subsequently mixed and incubated at room temperature for 20 min.
This mixture was added into 6-well plates, mixed and incubated.
Complete DMEM was replaced 12 h after transfection and cultured in
a constant temperature incubator at 37°C and 5% CO2 for
further experiments.
miR-34a expression detection
Total miRNA was extracted from cultivated HCT116
cells with an miRNA Extraction kit and the extracted miRNA was
reverse-transcribed to cDNA with the TaqMan MicroRNA Reverse
Transcription (RT) kit under the following reaction conditions:
16°C for 30 min, 42°C for 30 min and 85°C for 5 min. Quantitative
polymerase chain reaction (qPCR) was performed on the prepared cDNA
using the TaqMan MicroRNA Detection kit under the following
conditions: 95°C for 10 min, followed by 40 cycles of 95°C for 15
sec and 60°C for 1 min. Following completion of qPCR, gene
amplification was analyzed on a LightCycler® 480 (Roche
Diagnostics, Basel, Switzerland) to obtain the corresponding
quantitation cycle (Cq) value. U6 served as the internal
reference to correct the copy number of the PCR template. Primer
sequences were as follows: U6 forward, 5′-CTCGCTTCGGCAGCACA-3′ and
reverse, 5′-AACGCTTCACGAATTTGCGT-3′; miR-143a forward,
5′-GAGCTACAGTGCTTCATCTCAGCTCAGCA-3′ and reverse,
5′-GCTGAGCTGAGATGAAGCACTGTAGCTCA-3′. Each group was tested in
triplicate. Relative gene expression was calculated using the
2-ΔΔCq method (12).
Western blot analysis
HCT116 cells of the miR-34a transfection, negative
control and blank control groups were cultured for 24 h, following
which the medium was removed. Precooled PBS was added to wash
cells, followed by 10 µl radioimmunoprecipitation assay lysis
buffer (Shanghai Qiaoyu Industrial Co., Ltd., Shanghai, China).
Following 10 min, total protein was extracted and the protein
concentration was quantified using the bicinchoninic acid method.
Proteins (50 µg) from each group were subjected to 10% SDS-PAGE.
Following electrophoresis, proteins were transferred to a
polyvinylidene difluoride membrane, which was blocked with 5% milk
in TBS for 1 h. Membranes were then incubated with the following
primary antibodies at 4°C overnight: anti-Bcl-2 (cat. no. AB112;
dilution, 1:1,000), anti-Bax (cat. no. YK1165; dilution, 1:500),
anti-MMP-2 (cat. no. PA129183; dilution, 1:1,000), anti-MMP-9 (cat.
no. 3852S; dilution, 1:1,000) and anti-GAPDH (cat. no. XB-1572;
dilution, 1:1,000). The following day, the membrane was washed with
TBS containing Tween-20 (0.05%), and incubated with a horseradish
peroxidase-conjugated IgG secondary antibody (cat. no. KC-GT-035;
dilution, 1:5,000) for 1 h at room temperature. Protein bands were
visualized with 3,3-diaminobenzidine, and BandScan 5.0 (Informer
Technologies, Inc., Los Angeles, CA, USA) was used for analysis of
the protein bands.
MTT assay for cell proliferation
HCT116 cells from the miR-34a transfection, negative
control and blank control groups were seeded into a 96-well plate
at a density of 105/ml, and 200 µl complete DMEM was added.
Following transfection, the cells were incubated at 37°C and 5%
CO2 for 24, 36, 48 or 72 h. The medium was removed and
MTT was added at 10 µl/well. The cells were subsequently incubated
at 37°C for 1 h. A microplate reader (Thermo Fisher Scientific,
Inc.) was used to record the absorbance of each well at 450 nm, to
calculate the inhibition of cell proliferation.
Transwell invasion assay
HCT116 cells from the miR-34a transfection, negative
control and blank control groups were seeded into a 6-well plate.
An 8-µm diameter Corning culture chamber was used to assess the
invasiveness of HCT116 cells. The chamber membrane was coated with
a single layer of Matrigel in advance. HCT116 cells (105) from the
transfection, negative control and blank control groups were added
to the upper chamber, whereas complete DMEM was added to the lower
chamber, and plates were incubated 37°C for 24 h. The culture
medium was removed, and cells were fixed with 4% paraformaldehyde
and stained with hematoxylin. A total of five fields of view were
randomly selected under a light microscope to count cells. Cell
invasion rate = (number of cells penetrating the lower
chamber/number of cells penetrating the lower chamber in the blank
control group) × 100.
Statistical analysis
Data analysis was performed using GraphPad Prism
software version 5.0 (GraphPad Software, Inc., La Jolla, CA, USA).
The data are expressed as the mean ± standard error. A one-way
analysis of variance was performed to compare groups followed by a
Dunnett's test. P<0.05 was considered to indicate a
statistically significant difference.
Results
Upregulation of miR-34a expression
levels in miR-34a-transfected HCT116 cells
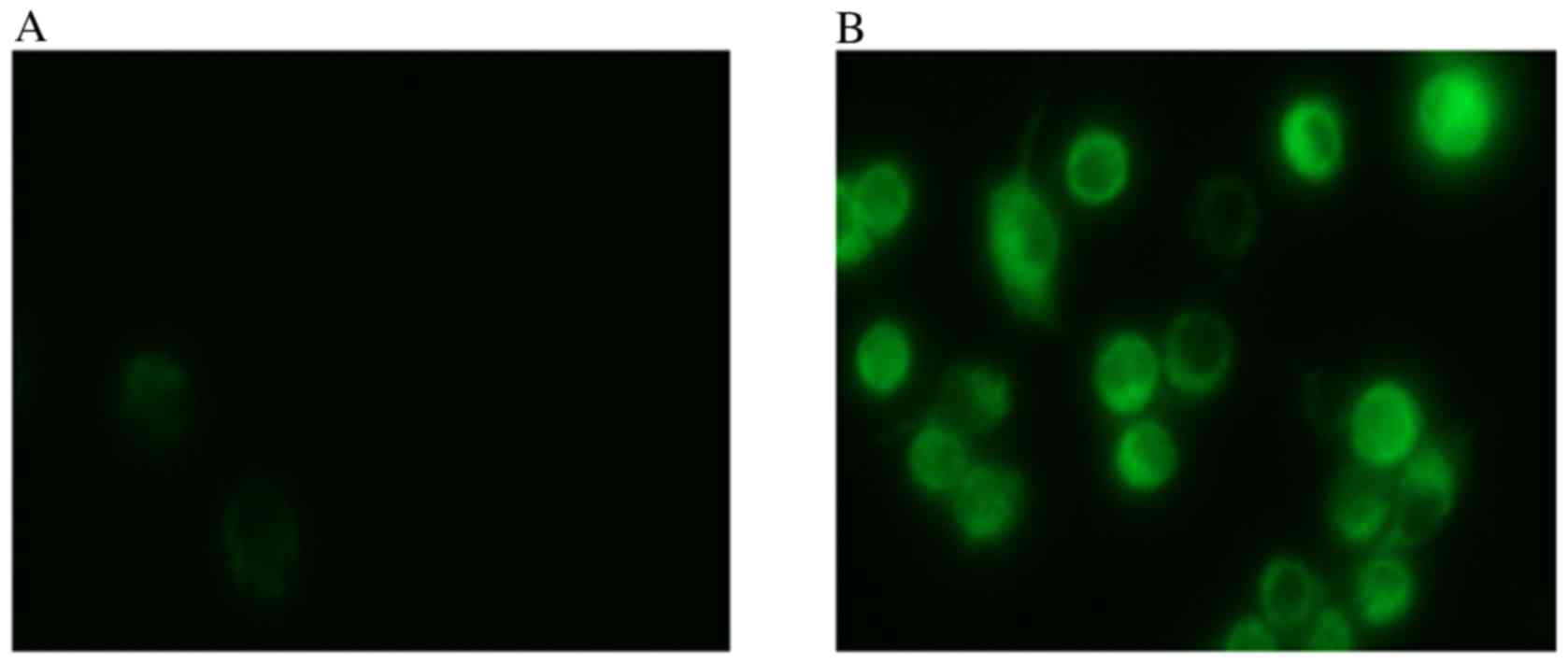
A total of 24 h after transfection of HCT116 cells
with miR-34, visible fluorescence was detected from the GFP present
in the plasmid (Fig. 1), which
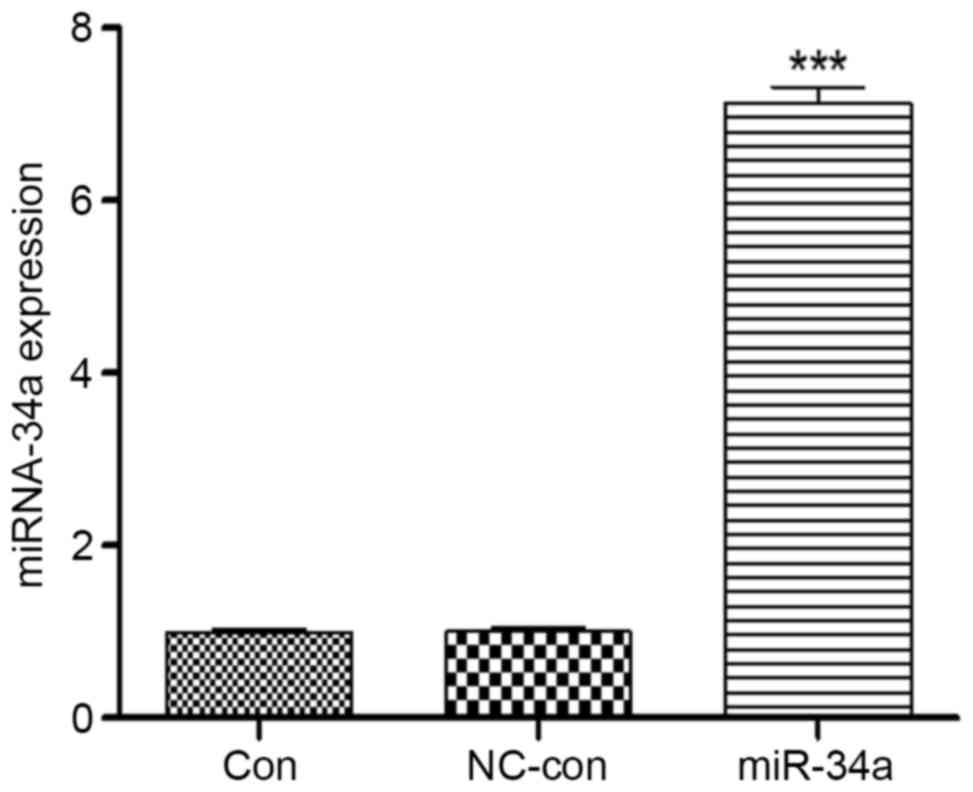
suggested a successful transfection. Total miRNA was extracted from
the cells for RT-qPCR, which demonstrated that miR-34a expression
levels in HCT116 cells increased 7.46±1.36-fold compared with the
blank control group (P<0.001), whereas no significant
differences were identified between the negative control
(0.99±0.01) and blank control (1.00±0.02) groups (Fig. 2). Therefore, transfection of HCT116
cells with miR-34a significantly increased the expression levels of
miR-34a.
miR-34a transfection inhibits HCT116
cell proliferation
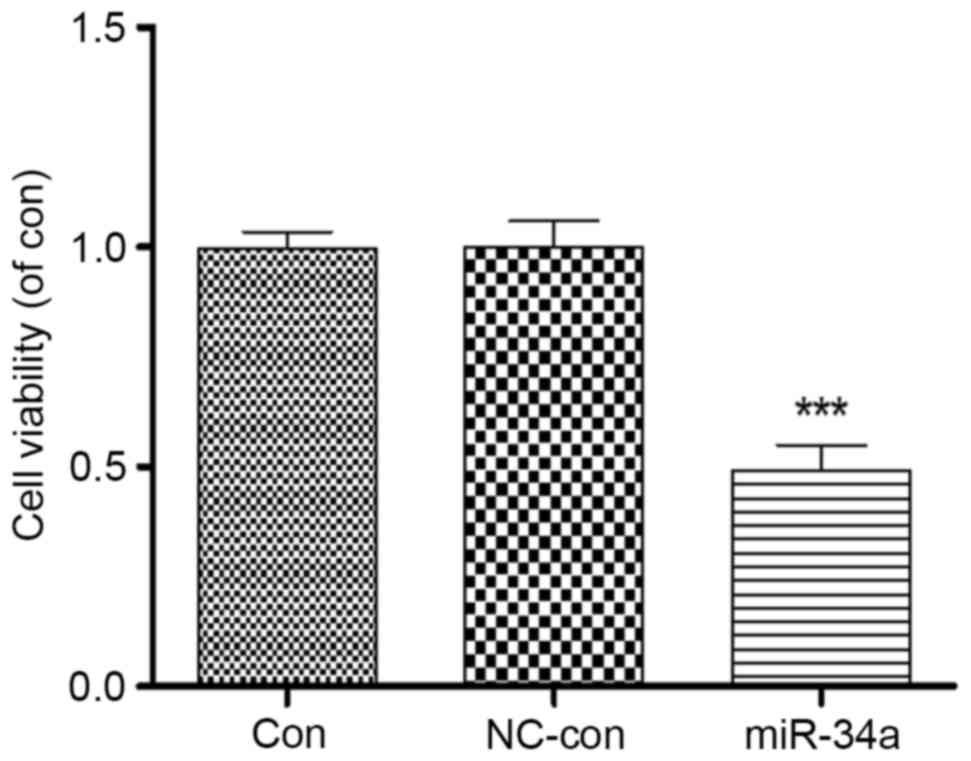
Proliferation of HCT116 cells was analyzed following
transfection with miR-34a. An MTT assay revealed that proliferation
of HCT116 cells overexpressing miR-34a was significantly inhibited
to 0.49±0.11 compared with the blank control group (P<0.001),
whereas no significant differences were identified between the
negative control (1.00±0.08) and blank control (0.99±0.06) groups
(Fig. 3). This suggested a close
association between miR-34a and the proliferation of HCT116
cells.
miR-34a transfection influences the
protein expression levels of B-cell lymphoma 2 (Bcl-2) and
Bcl-2-associated X protein (BAX)
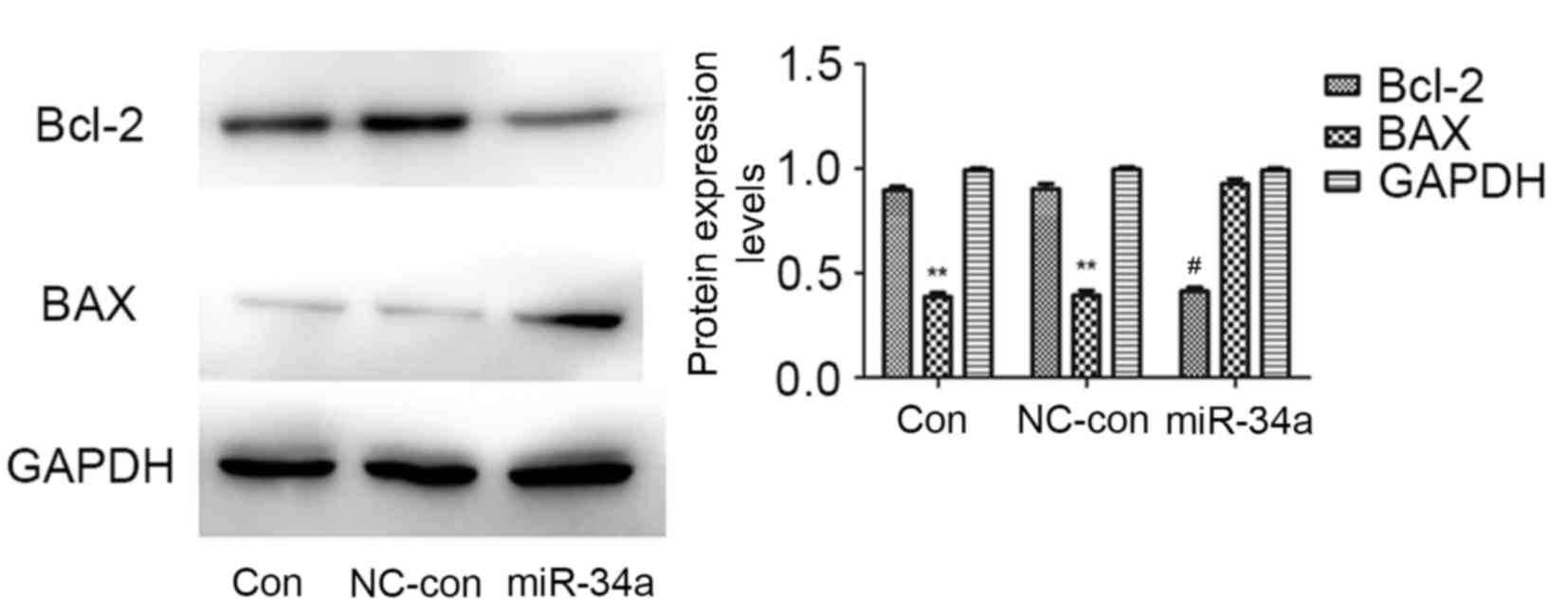
Bcl-2 and BAX are important molecules in apoptosis.
Western blotting revealed that, compared with the blank control and
negative control groups, the protein expression levels of Bcl-2
were markedly reduced in the miR-34a transfected group (P=0.002),
whereas those of BAX were significantly increased (P=0.010). No
significant differences were identified between the blank control
and negative control groups (Fig.
4). This suggested that miR-34a may inhibit the proliferation
of HCT116 cells by regulating the protein expression levels of
Bcl-2 and BAX.
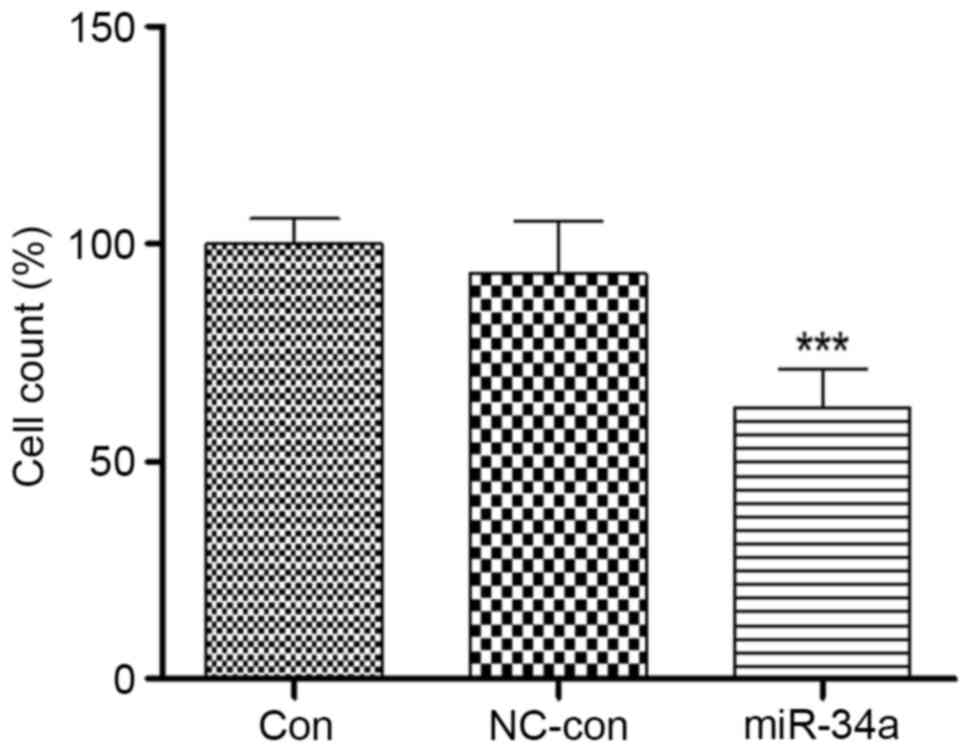
miR-34a transfection influences the
invasiveness of HCT116 cells
Colon cancer is refractory primarily due to its
ability to invade and metastasize. Therefore, the present study
assessed the invasiveness of miR-34a-transfected HCT116 cells. A
Transwell assay demonstrated that the invasiveness of HCT116 cells
overexpressing miR-34a was significantly inhibited, to 62.76±8.44%
of the level in the blank control group (P<0.001). No
significant differences were identified between the negative
control (92.98±10.13%) and blank control (100.51±8.22%) groups
(Fig. 5). This suggested that
miR-34a may be involved in HCT116 cell invasion.
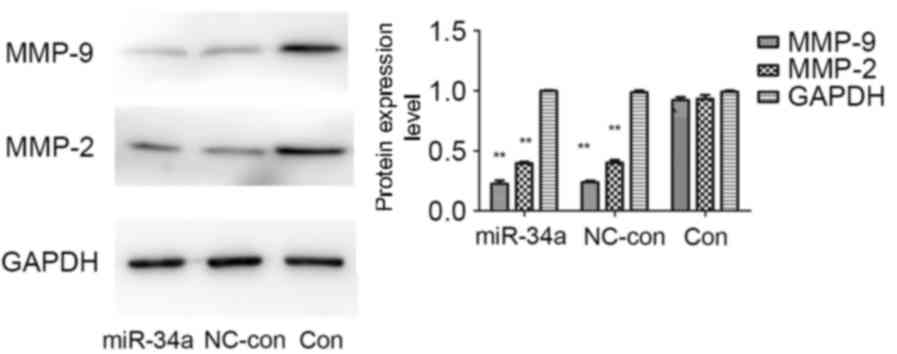
miR-34a transfection influences the
protein expression levels of matrix metalloproteinase (MMP)-2 and
−9
There is a close association between the activity of
MMPs and the invasion and metastasis of colon cancer cells. Western
blot analysis demonstrated that compared with the blank control and
negative control groups, protein expression levels of MMP-2 and
MMP-9 were markedly reduced in the miR-34a-transfected group, the
MMP-9 protein expression levels to a greater extent. No significant
differences were identified between the blank control and negative
control groups (Fig. 6). This
suggested that miR-34a may inhibit the invasion and metastasis of
HCT116 cells by regulating the protein expression levels of MMP-2
and MMP-9.
Discussion
Cancer of the colon is a common malignant digestive
tract tumor in China, and up to 8 million new cases are diagnosed
every year worldwide. Characteristics of the disease include
invasive growth, a high degree of malignancy and a high recurrence
rate. Its etiology and underlying mechanisms remain to be
understood, providing challenges for the clinical treatment of the
disease (13,14). Further investigation of the
underlying molecular mechanisms and drug targets of colon cancer is
therefore required. miRNAs are single-stranded, endogenous,
non-coding small molecule RNAs, the functions of which have become
clearer in recent years. It is highly conserved in structure, and
may alter this by specific binding to the target mRNA at the
3′-untranslated region, promoting the degradation of the target
gene mRNA or inhibiting its translation and transcription, and
therefore regulating target protein expression (15–17).
Previous studies have demonstrated that numerous miRNAs are located
in chromosomal regions of the tumor-associated genomes, which
possess different functions and serve different regulatory roles in
the malignant biological behaviors of tumor cells, particularly
proliferation, invasion and metastasis (1). Functional studies on miRNA provide
novel insights and strategies for the diagnosis and treatment of a
variety of tumors, including colon cancer. Such studies have
increased or decreased the expression levels of a specific miRNA,
via expression vectors or antisense miRNA, to inhibit the growth
and metastasis of tumor cells (16). In addition, previous studies have
demonstrated the differential expression of multiple miRNAs in
colon cancer. For example, Wang et al (18) demonstrated that miRNA-21 inhibited
the proliferation and cell cycles of colon cancer cells by targeted
regulation of CDC25a expression. In addition, miR-143 and miR-145
have been implicated in the proliferation and chemosensitivity of
colon cancer cells, thus rendering them potential diagnostic and
therapeutic targets (19,20). miR-34a may promote the apoptosis of
glioma cells, block the cell cycle at G1 phase and inhibit
proliferation; in addition, it may inhibit the invasion and
metastasis of tumor cells (21).
Furthermore, miR-34a reveals different regulatory functions in a
variety of cancers, including prostate, liver and lung. Therefore,
it is considered an important tumor suppressor (22). However, a limited number of studies
have been performed on the role of miR-34a in colon cancer.
To investigate the role of miR-34a in colon cancer,
the present study designed and synthesized a eukaryotic expression
vector of miR-34a and transfected it into HCT116 cells. RT-qPCR
demonstrated a significant increase of miR-34a expression levels in
transfected cells. Overexpression of miR-34a inhibited the
proliferation, invasion and metastasis of HCT116 cells. The results
demonstrated that miR-34a inhibited tumor-associated genes in
HCT116 cells, which indicated that miR-34a may be used as a
potential target for gene therapy of colon cancer, consistent with
the findings of previous studies (4,19).
Certain studies have identified that certain miRNAs regulate target
proteins associated with the proliferation, invasion and metastasis
of cancer cells; for example, miR-146b may inhibit the invasion of
glioma cells by regulating MMP expression levels (23). Therefore, the present study
examined the expression of proteins closely associated with cell
proliferation and metastasis, and the results were consistent with
those of previous studies. Protein expression levels of Bcl-2 were
suppressed, whereas those of BAX were upregulated following miR-34a
overexpression. Protein levels of both MMP-2 and MMP-9, which are
associated with tumor metastasis, were inhibited. This suggested
that miR-34a may reduce colon cancer cell proliferation and
invasion by regulating the protein expression levels of Bcl-2/BAX,
and MMP-2 and MMP-9.
In conclusion, the results of the present study
demonstrated that overexpression of miR-34a may inhibit the
proliferation, invasion and metastasis of HCT116 cells. This effect
may be associated with the regulation of protein expression levels
of Bcl-2/BAX, and MMP-2 and MMP-9. These findings suggested that
miR-34a may be a potential target for the diagnosis and treatment
of colon cancer, and provides a theoretical and experimental basis
for subsequent studies.
Acknowledgements
The present study was supported by the Joint Funds
of the Natural Science Foundation of Liaoning Province (grant nos.
2013023024 and 2015020324), the National Natural Science Foundation
of China (grant no. 81172052) and the Yingcai Program of Dalian
Medical University.
References
|
1
|
Adamowicz K and Zaucha R: Evaluation of
the impact of cancer treatment on the adoption and consolidation of
pro-health attitudes in the field of cancer in treated patients
with colon cancer. J Cancer Educ. 2016.(Epub ahead of print).
View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Kotelevets L, Chastre E, Desmaële D and
Couvreur P: Nanotechnologies for the treatment of colon cancer:
From old drugs to new hope. Int J Pharm. 514:24–40. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Watanabe T, Itabashi M, Shimada Y, Tanaka
S, Ito Y, Ajioka Y, Hamaguchi T, Hyodo I, Igarashi M, Ishida H, et
al: Japanese Society for Cancer of the Colon and Rectum (JSCCR)
guidelines 2010 for the treatment of colorectal cancer. Int J Clin
Oncol. 17:1–29. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Krol J, Loedige I and Filipowicz W: The
widespread regulation of microRNA biogenesis, function and decay.
Nat Rev Gene. 11:597–610. 2010.
|
|
5
|
Fabian MR, Sonenberg N and Filipowicz W:
Regulation of mRNA translation and stability by microRNAs. Annu Rev
Biochem. 79:351–379. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Flynt AS and Lai EC: Biological principles
of microRNA-mediated regulation: Shared themes amid diversity. Nat
Rev Genet. 9:831–842. 2008. View
Article : Google Scholar : PubMed/NCBI
|
|
7
|
Ventura A and Jacks T: MicroRNAs and
cancer: Short RNAs go a long way. Cell. 136:586–591. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Calin GA, Liu CG, Sevignani C, Ferracin M,
Felli N, Dumitru CD, Shimizu M, Cimmino A, Zupo S, Dono M, et al:
MicroRNA profiling reveals distinct signatures in B cell chronic
lymphocytic leukemias. Proc Natl Acad Sci USA. 101:11755–11760.
2004. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Kastl L, Brown I and Schofield AC:
miRNA-34a is associated with docetaxel resistance in human breast
cancer cells. Breast Cancer Res Treat. 131:445–454. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Rathod SS, Rani SB, Khan M, Muzumdar D and
Shiras A: Tumor suppressive miRNA-34a suppresses cell proliferation
and tumor growth of glioma stem cells by targeting Akt and Wnt
signaling pathways. FEBS Open Bio. 4:485–495. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Rodriguez-Ubreva J, Ciudad L, van Oevelen
C, Parra M and Graf T: C/EBPa-mediated activation of microRNAs 34a
and 223 inhibits Lef1 expression to achieve efficient reprogramming
into macrophages. Mol Cell Biol. 34:1145–1157. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(−Delta Delta C(T)) Method. Methods. 25:402–408. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Yothers G, O'Connell MJ, Allegra CJ,
Kuebler JP, Colangelo LH, Petrelli NJ and Wolmark N: Oxaliplatin as
adjuvant therapy for colon cancer: Updated results of NSABP C-07
trial, including survival and subset analyses. J Clin Oncol.
29:3768–3774. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Volinia S, Calin GA, Liu CG, Ambs S,
Cimmino A, Petrocca F, Visone R, Iorio M, Roldo C, Ferracin M, et
al: A microRNA expression signature of human solid tumors defines
cancer gene targets. Proc Natl Acad Sci USA. 103:2257–2261. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Plönes T, Elze M, Kayser G, Pfeifer D,
Burger M and Zissel G: mRNA and miRNA analyses in cytologically
positive endobronchial ultrasound-guided transbronchial needle
aspiration: Implications for molecular staging in lung cancer
patients. Cancer Cytopathol. 122:292–298. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Alexandrov PN, Zhao Y, Jones BM,
Bhattacharjee S and Lukiw WJ: Expression of the
phagocytosis-essential protein TREM2 is down-regulated by an
aluminum-induced miRNA-34a in a murine microglial cell line. J
Inorg Biochem. 128:267–269. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Castro RE, Ferreira DM, Afonso MB,
Borralho PM, Machado MV, Cortez-Pinto H and Rodrigues CM:
miR-34a/SIRT1/p53 is suppressed by ursodeoxycholic acid in the rat
liver and activated by disease severity in human non-alcoholic
fatty liver disease. J Hepatol. 58:119–125. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Wang P, Zou F, Zhang X, Li H, Dulak A,
Tomko RJ Jr, Lazo JS, Wang Z, Zhang L and Yu J: microRNA-21
negatively regulates Cdc25A and cell cycle progression in colon
cancer cells. Cancer Res. 69:8157–8165. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Sun R, Chen P, Li L, Sun H, Nie X, Liang
Y, Yuan F, Pu Y, Bai P, Zhang L and Gao L: A polymorphism rs4705341
in the flanking region of miR-143/145 predicts risk and prognosis
of colorectal cancer. Oncotarget. 2016.(Epub ahead of print).
|
|
20
|
Wei YS, Xiang Y, Liao PH, Wang JL and Peng
YF: An rs4705342 T>C polymorphism in the promoter of miR-143/145
is associated with a decreased risk of ischemic stroke. Sci Rep.
6:346202016. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Li N, Fu H, Tie Y, Hu Z, Kong W, Wu Y and
Zheng X: miR-34a inhibits migration and invasion by down-regulation
of c-Met expression in human hepatocellular carcinoma cells. Cancer
Lett. 275:44–53. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Gallardo E, Navarro A, Viñolas N, Marrades
RM, Diaz T, Gel B, Quera A, Bandres E, Garcia-Foncillas J, Ramirez
J and Monzo M: miR-34a as a prognostic marker of relapse in
surgically resected non-small-cell lung cancer. Carcinogenesis.
30:1903–1909. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Tazawa H, Tsuchiya N, Izumiya M and
Nakagama H: Tumor-suppressive miR-34a induces senescence-like
growth arrest through modulation of the E2F pathway in human colon
cancer cells. Proc Natl Acad Sci USA. 104:15472–15477. 2007.
View Article : Google Scholar : PubMed/NCBI
|